Abstract
This experiment was conducted to evaluate the effects of supplementary Moringa oleifera leaf (MOL) on performance, egg quality, plasma parameters and organ histopathological indices of layers. A total of 360 27-week-old Hy-Line Grey commercial layers were randomly allotted to four groups. Each group consisted of six replicates with 15 birds and 3 birds were placed in one cage. The control group was fed a corn-soybean meal based diet and the experimental groups were fed on control diet supplemented with 5%, 10% or 15% MOL (MOL5, MOL10 and MOL15 group). The experiment lasted for 8 weeks. No significant differences were observed in egg weight or feed intake among all groups (p > .05). The birds in MOL15 group had higher feed conversation ratio and lower egg production compared with those in control group (p < .05). Layers in MOL5 had a deeper yolk colour than those in control group (p < .05). The albumen height and Haugh unit increased with increasing level of MOL when eggs were stored at 4 °C and 28 °C for 4 weeks (p < .05). Layers in MOL15 group had higher aspartate aminotransferase activity and lower uric acid concentration than other groups (p < .05). Layers in MOL10 and MOL15 groups had higher malondialdehyde content than those in control group (p < .05). Supplementary MOL increased the activity of glutathione peroxidase (p < .05). In conclusion, dietary supplementation with 5% MOL could improve yolk colour value and protein absorption without adverse effects on laying performance and egg quality.
Introduction
The poultry industry is one of the fastest growing animal industries globally, but is hampered by a heavy shortage of feed ingredients especially in developing countries (Al-Harthi et al. Citation2009). Thus, it is essential to explore the non-traditional feed resources which could be used in poultry feed formulations (El-Deek et al. Citation2010). Moringa oleifera Lam., or known as drumstick tree, is a multipurpose tree that thrives in both tropical and sub-tropical conditions (Worku Citation2016). Moringa oleifera leaf (MOL) is reported to contain 25–27% crude protein (Gadzirayi et al. Citation2012) and high levels of minerals as well as vitamins (Yang et al. Citation2006). The protein quality of MOL has been reported to be comparable to that of milk and eggs (Fahey Citation2005). Kakengi et al. (Citation2005) compared the nutritive values of different morphological components of MOL with Leucaena leucocephala leaf meal in Tanzania and they observed a high level of pepsin and total soluble protein in MOL. Previous study also showed that MOL contains high level of antioxidants such as flavonoids (Vongsak et al. Citation2013).
Several studies showed that animal performance could be improved by dietary supplementation of MOL. An improvement in egg production, yolk colour and feed conversion ratio were observed in Rhode Island Red hens supplemented with MOL (Mohammed et al. Citation2012). The addition of MOL powder to the diets was associated with increased weight in broilers (Donkor et al. Citation2013). In a recent study, Kholif et al. (Citation2015) observed an increased feed intake, nutrient digestibility and milk yield in goats fed diets including MOL. The effect of MOL was proven to be dosage-related and a higher supplementation level could lower animal performance due to the increased concentration of anti-nutrient factors (such as saponins and phenols) (Worku Citation2016). This study was conducted to investigate the effect of graded level of MOL on intake, feed conversion ratio (FCR), egg production and quality of laying hens. By determination of plasma biochemistry indices as well as liver and kidney histopathology, we aimed to further illustrate how different levels of supplementary MOL affects the performance and health of layers.
Materials and methods
Preparation of MOL powder
One batch (∼200 kg) of fresh, matured MOL was obtained from Century Love Moringa Institute and dried naturally in a sunny and ventilated place. The dried leaves were subsequently ground using a kind of large-scale pulveriser (9FQ60-30, Tengzhou Machinery Factory, Tengzhou, China), passed through a 40-mesh screen and then stored in airtight plastic bags at ambient temperature (21–27 °C) before being mixed into diets. The contents of dry matter (DM, 930.15), crude protein (CP, 990.03), ether extract (EE, 920.39), crude fibre (CF, 962.09) and crude ash (942.05) were analysed according to the Association of Official Analytical Chemists (AOAC Citation2000). The metabolisable energy and total amino acid content of MOL were calculated according to Farrell (Citation1978) and Yemm et al. (Citation1955). The nutrient level of the MOL is shown in Table .
Table 1. Chemical composition and amino acid content of the Moringa oleifera leaf (MOL) sample on dry matter basis.
Experimental design and diets
This study was approved by the Animal Care and Use Committee of the Feed Research Institute of Chinese Academy of Agricultural Sciences. A total of 360 27-week-old Hy-Line Grey commercial layers with an initial egg production of 82.0 ± 2.0% and BW of 1.60 ± 0.2 kg were randomly allotted to four groups. The control group was fed a diet based on corn and soybean meal (Table ), and the experimental groups were fed the control diet supplemented with 5%, 10%, or 15% MOL (MOL5, MOL10 and MOL15 groups, respectively). All diets were isocaloric and isonitrogenous. Each group consisted of six replicates with 15 birds each, and three birds were placed in one cage (45 cm ×45 cm ×45 cm). Diets were formulated to meet or exceed NRC (Citation1994) guidelines. The experiment lasted for 10 weeks with 2-weeks of adaptation period and then followed by an 8-week feeding trial. During the adaptation period, all layers were fed the control diets. The birds were maintained on a 16-h light schedule and allowed ad libitum access to the diets and water. Room temperature was maintained at 15 ± 2 °C. Bird management was consistent with the recommendations of Hy-Line International Online Management Guide (The Hy-Line International Citation2009).
Table 2. Composition and nutrient levels of the basal diet on dry matter basis.
Performance, egg quality and sample collection
During the feeding trial, the number of eggs from each replicate and egg weight were recorded daily. Feed consumption in each replicate was measured on a weekly basis. The average egg weight, daily feed intake, FCR and egg production were calculated every 4 weeks. Five eggs were collected from each replicate on week 4 and 8 of the feeding trial for quality analysis. At the end of the feeding trial, ten eggs were collected from each replicate, and half was stored at 4 °C and the other half was stored at 28 °C for 4 weeks for quality analysis. Eggshell thickness and eggshell strength were measured using an Egg Shell Thickness Gauge and Egg Force Reader (ORKA Food Technology Ltd, Ramat HaSharon, Israel), respectively. The albumen height, yolk colour and Haugh unit were determined using an Egg Analyser (ORKA Food Technology Ltd, Ramat HaSharon, Israel). The egg shape index was calculated using the following formula: shape index = (width/length) × 100 (Reddy et al. Citation1979). All eggs were stored at 4 °C and analysed on the same day once all samples were collected.
At the end of the feeding trial, one bird from each replicate was randomly selected for blood and tissue sampling. Blood samples were drawn from wing veins, and then centrifuged (3,000 × g for 10 min) to obtain plasma and stored at –20 °C for biochemical analysis. After blood sampling, the birds were killed by exsanguinations of the left jugular vein and the livers and the kidneys were excised and fixed in 10% buffered neutral formalin (Sinopharm Chemical Reagent Beijing Co., Ltd, Beijing, China) for histopathological assessment.
Biochemical plasma analysis
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, and concentrations of total protein (TP), albumin (ALB) and uric acid (UA) were determined using an automatic biochemical analyser (Model 7020, Hitachi, Tokyo, Japan).
Antioxidant indices measurements
Colorimetric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to measure the enzymatic activity of the total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-PX) and level of malondialdehyde (MDA). Each sample was analysed in triplicate.
Histopathological assessment of liver and kidney
The formalin-fixed liver and kidney samples were stained with haematoxylin and eosin according to the method of Jadhav et al. (Citation2007). All reagents used were of analytical grade (Sinopharm Chemical Reagent Beijing Co., Ltd, Beijing, China). The histopathological alterations were examined using a light microscope (BX51, Olympus Corp., Tokyo, Japan).
Statistical analysis
All analyses were performed using SPSS version 19.0 for Windows (SPSS, Chicago, IL). Data were analysed using one-way analysis of variance according to the model Yik = μ + Di + eik, where Y is the single observation, u is the general mean, D is the effect of supplementation level and eik is the random error. The dose-related effects of supplemental MOL were determined using orthogonal polynomial contrast for linear and quadratic effects. Before analysis, all percentages were subjected to logarithmic transformation (log10 x + 1) to approximate normal distribution. Differences were considered statistically significant at p < .05. Data are expressed as mean and pooled SEM.
Results
Laying performance and egg quality
No difference in egg weight or feed intake was observed (p > .05, Table ). The FCR increased linearly with the increasing supplementation level of MOL (p < .05) during the initial (weeks 1–4) and overall phase (weeks 1–8), and the birds in MOL 15 had a poorer FCR compared with those in the control and MOL5 groups (p < .05). Egg production decreased linearly (p < .05) with increasing supplementary MOL from weeks 1–8. Layers in MOL15 group had a lower egg production compared with those of the control and MOL5 group (p < .05).
Table 3. Effect of dietary supplementation of Moringa oleifera leaf (MOL) on performance of laying hens.
The albumen height was higher in MOL10 group compared with that of the control group at week 4 (p < .05, Table ). Linear effect was observed in yolk colour in response to dietary MOL supplementation. Layers in MOL10 and MOL15 groups had deeper yolk colour than that in the control at week 4 (p < .05). At week 8, deeper yolk colour was observed in all MOL groups than in the control (p < .05). There was no difference in Haugh unit, egg shell strength or egg shape index among groups (p > .05).
Table 4. Effect of dietary Moringa oleifera leaf (MOL) contents on egg quality of fresh eggs of laying hens.
When eggs were stored at 4 °C or 28 °C for 4 weeks, the albumen height increased quadratically in response to the MOL supplementation (p < .05, Table ). The Haugh unit also increased quadratically in response to the MOL supplementation when eggs were stored at 4 °C for 4 weeks (p < .05), and increased linearly at 28 °C (p < .05). Laying hens fed diets containing 5% and 10% MOL showed higher albumen height and Haugh unit at 4 °C and 28 °C compared with hens fed the control diet (p < .05). Laying hens supplemented with 15% MOL also showed a higher Haugh unit compared with those fed the control diet (p < .05) when eggs were stored at 28 °C for 4 weeks. Laying hens fed the diets containing 5, 10 and 15% MOL also had deeper yolk colour than those fed the control diets when eggs were stored at 4 °C and 28 °C for 4 weeks (p < .05).
Table 5. Effect of dietary Moringa oleifera leaf (MOL) contents on egg quality of eggs during storage at different temperature.
Plasma biochemical parameters and plasma redox status
No difference in ALT activity was observed among groups (p > .05, Table ). The AST activity increased quadratically as the dietary supplemental level of MOL increased (p < .05). Laying hens fed the diets containing 15% MOL had a higher AST activity than those fed the control diet (p < .05). The TP concentration decreased quadratically with the increasing level of MOL (p < .05) and was the highest in MOL5 group (p < .05). The concentration of ALB decreased linearly and the concentration of UA decreased linearly in response to the increase in MOL supplementation (p < .05). Laying hens in MOL15 had a lower concentration of ALB and UA than those in the control group (p< .05).
Table 6. Effect of dietary Moringa oleifera leaf (MOL) contents on plasma biochemical parameter and plasma redox status of laying hens.
Compared with the birds in the control group, higher activity of GSH-PX was observed in groups supplemented with MOL (p = .008), but lower plasma MDA content was observed in MOL10 and MOL15 groups (p = .008). No difference in T-SOD activity was observed among groups. There were linear and quadratic increases in the activity of GSH-PX in response to the MOL supplementation (p< .05).
Histopathological assessment of liver and kidney
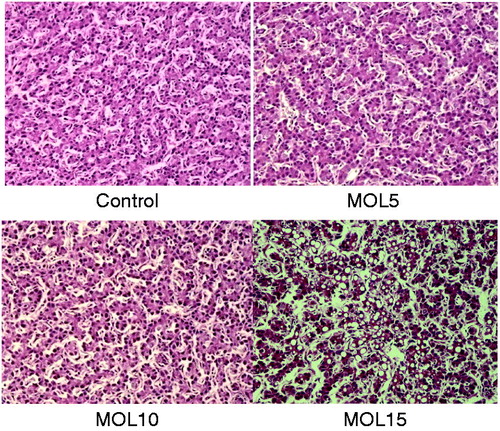
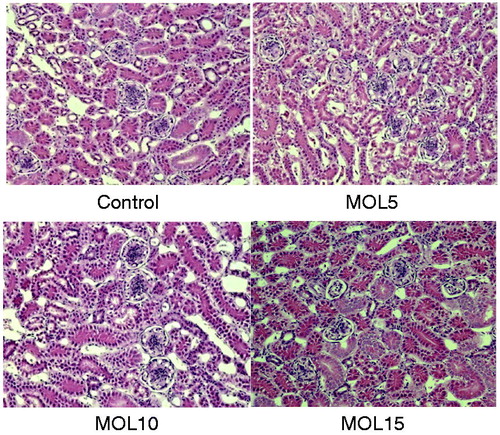
Histological lesions were not observed in the liver and kidney tissues of hens in the control, MOL5 and MOL10 groups (Figures and ). Compared with the control group, there were no obvious changes in the liver structure of layers in MOL5 and MOL10 groups. MOL5 and MOL10 groups showed normal histological appearance, and fatty changes were not observed in the hepatocytes of these groups. Kidneys of the layers of MOL5 and MOL10 groups maintained intact epithelium of tubules. Glomeruli did not show any dilatation or accumulation of material in the Bowman’s spaces. The histopathology of the livers in the MOL15 group showed large number of lipid droplets can be found in liver cells. The fatty liver cell counts rose 30–60% and inflammation was also observed in kidney tissues of MOL15 group.
Discussion
The results of this study showed that the FCR of laying hens increased in response to the increase of dietary MOL supplementation, but egg weight and feed intake were not affected. The detrimental effect of MOL on FCR might be contributed to low available energy and protein when supplementary MOL was high in the diets, which were probably due to low digestibility of fibre component of leaves. Energy and protein content have an influence on intake and egg production (Kakengi et al. Citation2007). In accordance with Bhatnagar et al. (Citation1996) and Olugbemi et al. (Citation2010), supplementation of MOL up to 10% did not affect egg weight. Supplementary 5% or 10% MOL did not affect feed intake of layers in the current study, which is similar to that reported in broilers (Gadzirayi et al. Citation2012). The decrease in feed intake could be explained by the impaired palatability due to the existance of higher amount of saponins when MOL was supplemented above 10%. On the other hand, Kakengi et al. (Citation2007) observed an increase in feed intake in layers when 15% or 20% of dehulled sunflower seed meal was replaced by MOL. The discrepancy in feed intake could be further explained by the lower or higher energy content of MOL relative to the dietary components it replaced in different studies. The current results suggested that a supplementation level of MOL up to 10% did not have negative effect on feed intake. Supplementation of MOL at 15% had detrimental effect on FCR of layers in the current study, indicating a lower supplementation level is suitable for their use in layers’ diet. Kakengi et al. (Citation2007) also suggested that less than 15% MOL inclusion is recommended in laying hens’ diet without hampering FCR. We observed a decrease in egg production when 15% MOL was supplemented to the control diet. Similarly, Abou-Elezz et al. (Citation2011) reported that egg production decreased when the inclusion level of MOL in diets was 15%. Regardless of the possible anti-nutritional factors, the decrease in egg production could be associated with the increase in bulkiness of diets due to the increasing level of MOL as observed in other forage leaves (Kakengi et al. Citation2007; Al-Harthi et al. Citation2009). These results indicated that supplementary level of MOL incorporated into the diets of laying hens could not exceed 10%.
The albumen height and Haugh unit are important items in evaluating albumen quality and egg freshness. The albumen height and Haugh unit of laying hens increased in response to the increase in dietary MOL supplementation. The Haugh unit tended to increase in MOL10 group compared with the other groups in the current study, suggesting that supplementary MOL at 10% had beneficial effect on albumen height as well as Haugh unit. Yolk colour value increased linearly with the increasing level of supplementary MOL, which is in agreement with the finding of Abou-Elezz et al. (Citation2011). Yolk colour is a preferable trait for consumers and MOL has high concentration of xanthophylls (167.1 ± 6.1 μg/g; Pasaporte et al. Citation2014), which is associated with the colouration of body parts. Donkor et al. (Citation2013) also reported that incorporation of MOL led to the intensified colouration of combs, beaks and legs in broilers. In agreement with Ebenebe et al. (Citation2013), adding MOL had no effect on egg shape index. These results indicated that MOL could be incorporated at 15% into the diets of laying hens as it could improve egg albumen quality and yolk colour.
Storage temperature is the most profound factor that affects the quality deterioration rate of eggs (Torrico et al. Citation2014). Stadelman (Citation1995) suggested that deteriorating rate of egg quality slows down when the storage temperature is closer to the freezing point. The present study showed that the albumen height and Haugh unit of eggs stored at 4 °C were 2.5 and 1.9 times higher than those stored at 28 °C. Supplementary MOL at 5% and 10% improved the albumen height of eggs stored at 4 °C. The albumen height was also improved when the eggs were stored at 28 °C compared with that of the control group, and this improvement was also observed in the Haugh unit. As the loss of albumen quality was largely attributed to the movement of water from albumen to yolk (Mueller Citation1959; Al-Harthi et al. Citation2011), it is possible that supplementary MOL could decrease the rate of water movement from albumen to yolk and the underlying mechanism needs further investigation. Yolk colour of storage eggs was also improved by supplementary MOL at both 4 °C and 28 °C in the current study. The antioxidants contained in MOL could have a beneficial effect on improving egg yolk colour as described by Saunders et al. (Citation1967). These results indicated that 5% or 10% MOL could slow down the deteriorating rate of storage egg quality, which could be related to plasma MDA, T-SOD and GSH-Px. The MDA is an indicator of lipid peroxidation and T-SOD as well as GSH-Px are the main parameters used to assess oxidative status in the enzymatic system. Our study suggested that MOL could prevent free radical production and consequently cell damage.
In our study, dietary supplementary levels of MOL (≤ 10%) appeared safe, as they did not adversely affect birds’ physiology. No difference was found in plasma activities of AST and ALT and levels of ALB and UA among the control, MOL5 and MOL10 groups. But plasma TP levels were increased by dietary 5% MOL supplementation compared with that in control group. Plasma TP levels is a reliable indicators of the liver’s synthetic function (West Citation1990), and plasma UA is a major metabolite of nitrogen catabolism in birds and can be used to evaluate protein breakdown and amino acid utilisation (Donsbough et al. Citation2010). These results indicate that birds fed MOL at the 5% level might increase protein retention, which would improve laying performance and egg quality. In contrast, 15% MOL supplementation seems to cause a negative effect on liver and kidney function, evidenced by higher AST activities, lower ALB and UA levels in MOL15 group than the control. However, AST is considered less specific indicator of liver function than other enzymes since it can also be found in many peripheral tissues (in particular the muscles) and hence has a very wide variability (Bovera et al. Citation2007). Consistent with this speculation, the histopathological assessments showed that laying hens fed with 15% MOL developed pathological lesions, but 5% and 10% supplementation of MOL did not develop histological lesions in the livers and kidneys.
Conclusions
Dietary supplementation with 5% or 10% MOL could improve yolk colour, albumen height and Haugh unit of eggs during storage, and without adverse effects on laying performance and egg quality of layers. Thus, 5% MOL could be added to the diets of laying hens.
Disclosure statement
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study was supported by the earmarked fund for Modern Agro-industry Technology Research System [CARS-41-K13], the Agricultural Science and Technology Innovation Programme (ASTIP), National Key Technology Research and Development Programme [2011BAD26B03 and 2014BAD13B04], and China Agriculture Research System-Beijing Team for Poultry Industry.
References
- Abou-Elezz FMK, Sarmiento-Franco L, Santos-Ricalde R, Solorio-Sanchez F. 2011. Nutritional effects of dietary inclusion of Leucaena leucocephala and Moringa oleifera leaf meal on Rhode Island Red hens’ performance. Cuban J Agr Sci. 45:163–169.
- Al-Harthi MA, El-Deek AA, Attia YA, Bovera F, Qota EM. 2009. Effect of different dietary levels of mangrove (Laguncularia racemosa) leaves and spices supplementations on productive performance, egg quality, lipids metabolism and metabolic profiles in laying hens. Br Poult Sci. 50:700–708.
- Al-Harthi MA, El-Deek AA, Attia YA. 2011. Impacts of dried whole eggs on productive performance, quality of fresh and stored eggs, reproductive organs and lipid metabolism of laying hens. Br Poult Sci. 52:333–344.
- AOAC. 2000. Official methods of analysis of AOAC International. 17th ed. Gaithersburg (MD): AOAC.
- Bhatnagar R, Kataria M, Verma SVS. 1996. Effect of dietary leucaena leaf-meal (LLM) on the performance and egg characteristics in White Leghorn hens. Indian J Anim Sci. 66:1291–1294.
- Bovera F, Moniello G, de Riu N, Di Meo C, Pinna W, Nizza A. 2007. Effect of diet on the metabolic profile of ostriches (Struthio camelus var. domesticus). Trop Anim Health Prod. 39:265–270.
- Donkor AM, Glover RLK, Addae D, Kubi KA. 2013. Estimating the nutritional value of the leaves of Moringa oleifera on poultry. Food Nutr Sci. 4:1077–1083.
- Donsbough AL, Powell S, Waguespack A, Bidner TD, Southern LL. 2010. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult Sci. 89:287–294.
- Ebenebe CI, Anigbogu CC, Anizoba MA, Ufele AN. 2013. Effect of various levels of Moringa leaf meal on the egg quality of ISA Brown breed of layers. J Adv Life Sci Tech. 14:45–49.
- El-Deek AA, Attia YA, Al-Harthi MA. 2010. Including whole inedible date in grower-finisher broiler diets and the impact on productive performance, nutrient digestibility and meat quality. Animal. 4:1647–1652.
- Fahey JW. 2005. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 1:1–15.
- Farrell DJ. 1978. Rapid determination of metabolisable energy of foods using cockerels. Br Poult Sci. 19:303–308.
- Gadzirayi CT, Masamha B, Mupangwa JF, Washaya S. 2012. Performance of broiler chickens fed on mature Moringa oleifera leaf meal as a protein supplement to soyabean meal. Int J Poult Sci. 11:5–10.
- The Hy-Line International. 2009. Hy-Line Variety W-36 Commercial Management Guide. Dallas Centre (IA): Hy-Line Int. Inc.
- Jadhav SH, Sarkar SN, Patil RD, Tripathi HC. 2007. Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Arch Environ Con Tox. 53:667–677.
- Kakengi AMV, Shem MN, Sarwatt SV, Fujihara T. 2005. Can Moringa oleifera be used as a protein supplement for ruminants?. Asian Austral J Anim Sci. 18:42–47.
- Kakengi AMV, Kaijage J, Sarwatt S, Mutayoba SK, Shem MN, Fujihara T. 2007. Effect of Moringa oleifera leaf meal as a substitute for sunflower seed meal on performance of laying hens in Tanzania. Livest Res Rural Dev. 19. Article #120.
- Kholif AE, Gouda GA, Morsy TA, Kholif AM, López S, Kholif AM. 2015. Moringa oleifera leaf meal as a protein source in lactating goat's diets: feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin Res. 129:129–137.
- Mohammed KAEF, Sarmiento-Franco L, Santos-Ricalde R, Solorio-Sanchez JF. 2012. The nutritional effect of Moringa oleifera fresh leaves as feed supplement on Rhode Island Red hen egg production and quality. Trop Anim Health Pro. 44:1035–1040.
- Mueller WJ. 1959. Factors affecting the quality loss in egg albumen during storage. Poult Sci. 38:843–846.
- NRC. 1994. Nutrient requirements of poultry. 9th rev. ed. Washington, DC: National Academy Press.
- Olugbemi TS, Mutayoba SK, Lekule FP. 2010. Evaluation of Moringa oleifera leaf meal inclusion in cassava chip based diets fed to laying birds. Livest Res Rural Dev. 22. Article #118. http://www.lrrd.org/lrrd22/6/olug22118.htm
- Pasaporte MS, Rabaya FJR, Toleco MRM, Flores DM. 2014. Xanthophyll content of selected vegetables commonly consumed in the Philippines and the effect of boiling. Food Chem. 158:35–40.
- Reddy PM, Reddy VR, Reddy CV. 1979. Egg weight, shape index and hatch ability in Khaki Campbell duck egg. Int J Poult Sci. 14:26–31.
- Saunders CF, Hayes JP, Erasmus J, Jooste JP, Plessis PHCD, Venter EL. 1967. Egg yolk pigmentation as influenced by BHT, vitamin E and sunflower oil. S Afr J Agri Sci. 10:373–386.
- Stadelman WJ. 1995. The preservation of quality in shell eggs. In: Stadelman WJ, Cotterill OJ, editors. Egg science and technology. 4th ed. New York: The Haworth Press; p. 67–79.
- Torrico DD, Wardy W, Carabante KM, Pujols KD, Xu ZM, No HK, Prinyawiwatkul W. 2014. Quality of eggs coated with oil–chitosan emulsion: combined effects of emulsifier types, initial albumen quality, and storage. LWT-Food Sci Technol. 57:35–41.
- Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. 2013. Maximising total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crop Prod. 44:566–571.
- West HJ. 1990. Effect on liver function of acetonaemia and the fat cow syndrome in cattle. Res Vet Sci. 48:221–227.
- Worku A. 2016. Moringa oleifera as a potential feed for livestock and aquaculture industry. Afr J Agr Sci Technol. 4:666–676.
- Yang RY, Tsou S, Lee TC, Chang LC, Kuo G, Lai PY. 2006. Moringa, a novel plant rich in antioxidants, bioavailable iron, and nutrients. In: ACS Symposium Series (Vol. 925, pp. 224–239). UK: Oxford University Press;p. 224–239.
- Yemm EW, Cocking EC, Ricketts RE. 1955. The determination of amino-acids with ninhydrin. Analyst. 80:209–214.