Abstract
The rearing of meagre (Argyrosomus regius) up to commercial sizes, using diets of different protein/lipid ratios, was examined in two long-term trials. In the first 2 × 2 trial, four diets containing two protein (43% and 47%) and two lipid levels (15% and 20%) were evaluated in fish of 350 g initial weight. Fish were reared for 8 months in triplicate experimental cages, up to final weight of 900g. Growth performance showed that diets containing 43% protein were the most appropriate, as indicated by the better thermal growth coefficient (TGC): 0.70 and 0.71 (vs. 0.61 and 0.53 for 47/15 and 47/20 diets) and better daily growth index DGI: 0.91 and 1.00 (vs. 0.88 and 0.79 for 47/15 and 47/20, respectively). Liver fat did not differ among groups, but liver glycogen in the 43/15 dietary group was found to be significantly higher than the 47/20 (3.89% vs 1.88%). The histological examinations revealed a trend for increased lipid deposition when fish were fed high fat diets. Diets that performed best, namely 43/15 and 43/20, were used in a second trial conducted at a commercial fish farm. Fish weighing 520g were reared for 7 months up to 1100 g final weight. No significant differences were observed in the growth parameters examined. The feed conversion ratio was found to be better in the 43/20 diet compared to the 43/15 (1.58 vs. 1.68, respectively). The dietary fat levels significantly affected fillet fat content. However, such difference in nutritional content was not reflected in human-perceived sensory differences.
Introduction
Meagre (Argyrosomus regius) is receiving currently special attention due to the necessity of species diversification in Mediterranean mariculture (Duncan et al. Citation2013; Parisi et al. Citation2014). Nowadays, the annual production of meagre exceeds 14,000 tonnes (FAO Citation2014). Interestingly, this species is characterised by domestication ability, high tolerance to wide ranges of salinity (5–39‰) and temperature (13–28 °C) and exhibits high growth rates reaching 1.2 kg in less than 2 years. Its quality features include an attractive body shape for selling as whole fish, a good processing yield and nutritional value, low fillet fat, excellent taste and firm texture (Grigorakis et al. Citation2011; Duncan et al. Citation2013), which distinguish meagre as a highly marketable fish species.
Although meagre farming started in the 1990s and is nowadays considered as an established enterprise, the nutritional requirements of the species have been studied only sporadically. Diets formulated for gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax) have often been empirically used in meagre rearing (Duncan et al. Citation2013; Parisi et al. Citation2014). Research on the protein and lipid requirements of juvenile meagre, has been limited to short-term trials of 2–3 months duration (Chatzifotis et al. Citation2010, Citation2012; Velazco-Vargas et al. Citation2014), while only one 6-month evaluation exists (Martínez et al. Citation2011). Extruded diets of 45–50% protein and 20–24% fat are generally administered during rearing of this species (Duncan et al. Citation2013; Parisi et al. Citation2014).
Long-term trials conducted in larger fish reaching commercial sizes, are limited to comparisons of meagre that received variable commercial diets (Mittakos et al. Citation2012; Vargas-Chacoff et al. Citation2014) while growth results are also missing (Piccolo et al. Citation2008). Substitution of fishmeal and fish oil by vegetable raw materials and its impact on meagre growth – feed utilisation, metabolism, digestion and fatty acids profile has also been a subject of research (Emre et al. Citation2015; Ribeiro et al. Citation2015). However, to our knowledge, no studies are available in the literature on the subject of dietary protein/lipid requirements in long-term feeding experiments for fish reaching commercial size. The management impact on meagre quality has also been limited to the effects of rearing systems (Martelli et al. Citation2013), while there is no data concerning the impact of dietary protein and fat levels on the quality of this species.
Determination of the basic nutritional requirements of the species and, in particular, the optimal protein to lipid ratio, is an essential prerequisite for a well-balanced diet, specifically designed for the species. In addition, meagre is characterised as a ‘lean fish’ with low fillet fat (Piccolo et al. Citation2008; Hernández et al. Citation2009), thus making it an interesting species-model for examining the effects of dietary fat content on fillet quality. Within this framework, the aim of this study was to establish the optimal dietary protein to lipid ratio in meagre, both during the fattening phase at industrial scale and over the course of a long-term trial. In addition to growth performance and feed utilisation, technical yields, organ histology and impacts on meagre fillet quality are also evaluated.
Materials and methods
General design – experimental diets
Two experimental trials were conducted. The first trial took place at the experimental cage facilities of the Hellenic Centre for Marine Research (HCMR; Souda, Crete, Greece), while the second trial at the production unit of FORKYS S.A. (Souda, Crete, Greece). The first trial screened the effects of four experimental diets (Table ), in a 2 × 2 experimental design. The two diets, which produced the best results as regards performance and feed utilisation, were chosen for evaluation at industrial scale in the second trial. The small disparities in the composition of the same diets within the two trials (Table ) are due to variability in raw material batches.
Table 1. Formulation, g/kg and proximate composition, g/100 g of the diets used for meagre in trials 1 and 2.
The diets used for the second trial, besides their total composition, were also screened for their fatty acid composition (Table ).
Table 2. Fatty acid composition, g/100g total fatty acids, of the two experimental diets used in trial 2.
The extruded experimental diets were formulated and manufactured (IRIDA S.A., Nea Artaki, Greece) in order to contain two protein levels (43% and 47%) and two fat levels (15% and 20%). The raw materials used for the production of the diets, as well as their inclusion levels, were the same in both trials.
Trial 1
Fish rearing – sampling
Meagre with an average initial body weight of 350g (weight ranges appear in Table ), were distributed in 12 floating cages of 3 × 3 × 6 m3. A total of 270 individuals were placed in each cage reaching a stocking density of 5.5 kg/m3 and each diet was administered in triplicate groups of fish. Randomization of the dietary treatments (replicates) in the 12 cages took place in order to establish statistical validity. The trial lasted 8 months (June 2012–February 2013) and water temperature followed the local seasonal fluctuation (minimum 17 °C in January–February and maximum 26 °C in August–September). Feeding was performed to satiation with automatic feeders, twice a day, at 8:30–9:30 am and at 15:30–16:30 pm with 15 min intervals, for 6 days per week. Mean daily feeding rate was 1.1% of fish biomass during the entire rearing period. During the first 5 months of the trial (period of high temperatures 22–26 °C), the feeding rate ranged between 1.1 and 1.2% of fish biomass, while for the remaining period (22–16 °C) it was decreased to a value between 0.7 and 0.9%.
Table 3. Growth and feed utilisation indexes of meagre fed the four experimental diets in trial 1.
Fish growth was recorded monthly by weighing 50% of every cage population from the different treatments. At the end of the trial, all fish were bulk-weighed. A total of 20 fish from each cage was individually weighed, fork length was measured and sampled fish were subsequently gutted. Liver was weighed for hepatosomatic index determination and samples were taken for fat and glycogen determination. All tissues were stored at −20 °C until chemical analysis was performed. 20 whole fish and 10 fillets per replicate tank were used for total body and fillet proximate composition, respectively.
Measurements – chemical analyses
The following indexes were calculated for the evaluation of growth and feed utilisation:
SGR (specific growth rate) = 100 × [ln final weight (g) – ln initial weight (g)]/trial duration (in days)
TGC (thermal growth coefficient) = final body weight.1/3 – initial body weight.1/3)/(T × t)*1000 where T is water temperature in °C and t is time in days
DGI (Daily Growth Index) = (Final BW3) − (Initial BW3) × 100/days of rearing
FCR (feed conversion ratio) = feed consumed g/weight increase g,
PER (protein efficiency ratio) = weight increase g/protein consumed g
ANPU (Apparent net protein utilization or protein retention) = 100 × (final body protein content/fish − initial body protein content/fish)/total protein consumed/fish.
HSI (Hepatosomatic Index) = [liver weight (g)/total body weight (g)] × 100
CI (condition index) = [total body weight (g)/fork length3 (cm3)] × 100
Fillet and body proximate composition analyses were carried out according to the standard AOAC (Citation2005) methodologies. A pool of liver samples from five fish was homogenised in order to analyse fat and glycogen contents. Liver fat was determined according to Folch et al. (Citation1957) and glycogen according to Alexis et al. (Citation1985).
Tissue histology
Samples of gut and liver were removed from 10 fish of each replicate cage at the end of the experiment (30 fish/treatment), fixed in 10% buffered formalin and processed for paraffin histology. Sections (5 mm; microtome Leica RM 2255, Nussloch, Germany) were stained with haematoxylin and eosin (Leica Auto Stainer XL, Nussloch, Germany) and examined under light microscopy (Olympus VANOX-T, NJ) equipped with a digital camera (Infinity, Lumenera, Ontario, Canada). Sections of the anterior, mid and distal gut were examined for the appearance of absorptive vacuoles in the mucosal enterocytes and the integrity of mucosa and submucosa. Liver sections were evaluated as regards the lipid degeneration level and the integrity of hepatocytes. Photos were processed using Image analysis software (Digital Image Systems, Athens, Greece).
Trial 2
Fish rearing – sampling
Trial 2 consisted of a large-scale industrial feeding facility, where the two best performing diets of trial 1 were used. Fish with an initial average body weight of 520 g were distributed in two circular cages, 19.1 m in diameter. A total of 13,000 individuals were placed in each cage, reaching a fish density of 5.5 kg/m3. The trial lasted 7 months (August 2013–February 2014) and water temperatures followed natural seasonal fluctuations (minimum 16 °C in February and maximum 26 °C in August–September). Feeding was performed as % of fish body weight according to the results obtained from trial 1. The initial populations were bulk-weighed and subsequently 10% of each cage population was weighed every month. At the end of the trial, the whole population was bulk-weighed and 20 fish per cage were sampled for fillet proximate composition and fillet fatty acid analysis. Additionally, 12 fish from each cage were harvested on ice and filleted in order to be used for sensory analysis.
Measurements – chemical analyses
The SGR and FCR indexes of the two populations were evaluated as previously described. For the calculation of CI, as well as other somatometric indices (dressing yield, viscerosomatic, hepatosomatic and gonadosomatic indices and fillet yield), the weight of whole and gutted fish was recorded along with their total length, and visceral, liver, gonad and fillet weight. All indices were expressed as percentages of fish total body weight. Analyses for fillet proximate composition were conducted according to the methods described by AOAC (Citation2005).
For fatty acid extraction and purification, 1 g of dorsal fish muscle (or 1 g of fish feed) was homogenised with chloroform: methanol (2/1 v/v), according to Folch et al. (Citation1957). The extracted fatty acids were trans-esterified by treatment with anhydrous methanol containing 2% sulphuric acid for 16 h, at 50 °C in the presence of nitrogen. Fatty acid analysis took place according to the procedure detailed by Fountoulaki et al. (Citation2003). The instrument used was a GC-FID (Varian 3300, Walnut Creek, CA) equipped with a flexible fused silica Megabore column (Length: 30 m, Inner diameter: 0.32 mm, Film thickness: 1 μm), with a bonded stationary phase of CP-WAX. Helium (purity 99.999%) was the carrier gas.
Taste panel
Taste panels were conducted in HCMR’s taste panel room (Agios Kosmas, Athens, Greece). The two dietary groups were evaluated using the triangle test procedure in order to identify potential difference in their sensory attributes. The panel consisted of 15 trained panellists. Each panellist received three steam-cooked samples of identically looking fish pieces (5 × 5 cm2), two originating from the same dietary treatment and one different; the panellists had to taste them in a certain order and indentify the odd sample. Preparation of the panel, training of panellists and conduct of test (coding and randomisation of samples) were carried out according to ISO (Citation2004).
Statistical analysis
Differences in growth, somatometric and chemical parameters among the four dietary groups in trial 1, were assessed by one-way analysis of variance (ANOVA) and Tukey’s test with confidence levels of 5%. Respective differences between the two dietary groups in trial 2 were evaluated by Student’s t test and with the same confidence levels. A two-way Pearson’s correlation was used for correlating dietary fatty acids with fillet fatty acids. The SPSS 13.0 statistical package was used for all statistical analyses. The analysis for the triangle test taste panel was based on the ISO (Citation2004) standard statistical tables for the minimum correct answers required to establish perceptible differences.
Results
Trial 1
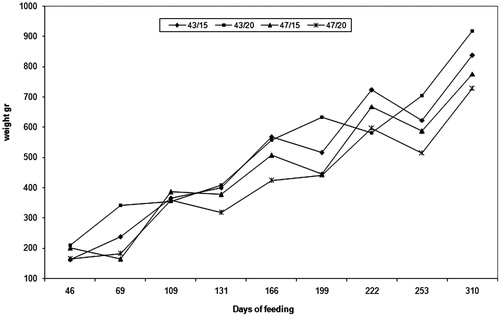
The growth and feed conversion parameters of trial 1 are shown in Table and Figure . A tendency (p = .100) for higher final weight, weight increase and SGR of the fish fed on diet 43/20 was observed. TGC and DGI, on the other hand, were significantly higher (p = .005) for the groups fed on 43% protein diets irrespective to dietary fat. Although feed conversion did not exhibit significant differences among dietary groups, the 43% protein-containing diets showed a tendency (p < .10) for a better FCR index. The differences were more pronounced for protein retention (ANPU), where the 43/20 diet showed significantly higher (p = .018) retention than the 47/20 diet, while both 43% protein diets showed a better retention than the 47/15 diet; the differences, however, were not significant. Feed consumption ranged between 1.3 and 1.0% of fish body weight during the high water temperature period (25 °C). Fish body weight in this period ranged from 350 to 600g. Feed consumption decreased when water temperature started to drop (20 °C), ranging from 0.9 to 0.7%, and fish average weight ranged from 600 to 900 g, respectively. A further drop in water temperature (17 °C) during winter was followed by a concomitant decrease in feed consumption (0.6–0.4%) for fish weighing more than 1000 g. Differences in feed consumption between diets were not significant.
Figure 1. Growth curves of meagre feeding on the four experimental diets in trial 1 during the whole experimental period.
The CI did not differ among the four dietary groups, unlike the HSI that was significantly lower (p = .05) for the two low fat diets (43/15 and 47/15).
Total body and fillet composition of the four dietary groups in trial 1 did not differ (Table ), although there was a tendency (p = .10) for higher fat content in the two high fat diets (43/20 and 47/20). The results did not show significant dietary effects on liver fat, but liver glycogen levels were significantly (p = .039) affected by dietary treatment since the 43/15 diet group showed higher values than the 47/20 diet group (Table ).
Table 4. Total body and fillet composition and liver glycogen and fat (g/100g) of trial 1 meagre dietary groups.
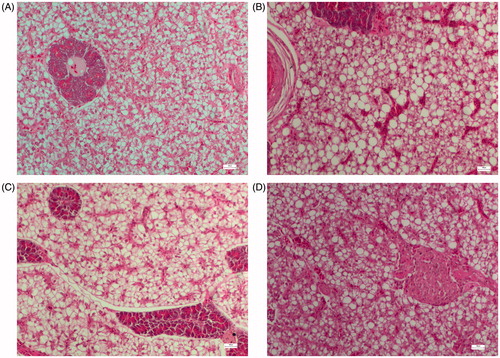
No severe intestinal abnormalities were observed among different dietary groups. Gut sections from fish fed all test diets showed low accumulation of lipid vacuoles in the mucosal enterocytes, irrespective of the diet received. Concerning liver morphology, a moderate steatosis, related to dietary treatments, was observed. In some individuals, an increasing lipid deposition in hepatocytes trend was observed when fed both the diets with 20% lipid levels (Figure ) compared to 15% (Figure ).
Figure 2. Liver histology of fish fed diets containing: (A) 43% protein and 15% lipid, (B) 43% protein and 20% lipid, (C) 47% protein and 15% lipid and (D) 47% protein and 20% lipid. In fish from groups fed with 20% lipid, hepatocyte lipid deposition was observed that was slightly improved as protein levels increased in the diets. Bars 100 μm, 20×.
Trial 2
Based on the performance results from the first trial, the two most optimal diets were selected and used for the large-scale trial (trial 2). The diets were those contained the lowest level of protein: 43/15 and 43/20.
The examined growth parameters did not reveal any significant differences between the two groups of fish fed on the different diets, with the exception of the FCR index that was better in the 43/20 dietary group (Table ). The HSI and GSI indexes and the technical characteristics of the fish (CI, dressing yield and fillet yield) did not differ either (Table ).
Table 5. Growth, feed utilisation and somatometric quality indexes of meagre that received the two experimental diets in trial 2.
The fish fed on the 43/20 diet exhibited significantly higher (p = .016) fillet fat than the 43/15 group (Table ), thus indicating the significant impact of dietary fat on the muscle fat content of the fish.
Table 6. Fillet proximate composition, g/100g, of trial 2 meagre dietary groups.
The fillet fatty acid composition of fish groups fed the 43/15 and 43/20 diets in trial 2 is given in Table . A strong positive correlation between the fillet fatty acids profile and the dietary fatty acids profile was evident (r = .98). There was a tendency for higher (p < .10) EPA and DHA contents in the fillets of the fish that received the 43/15 diet compared to the 43/20 diet (14.4% vs 12.4%). This is worth noting, especially when considered that the respective diet had lower accumulative EPA + DHA contents (12.8 vs. 13.6% for diets 43/15 and 43/20, respectively). Another important observation is that both dietary groups exhibited higher levels of fillet arachidonic acid (ARA, 20:4ω6) than their dietary ARA levels.
Table 7. Fillet fatty acid composition, g/100g total fatty acids, of trial 2 meagre dietary groups.
In the taste panel triangle test, only 4 out of the 15 panellists gave the correct answer (found the odd sample). A minimum of correct nine answers are required to establish statistical difference according to the respective statistical tables (for instance Appendix 1 of Huss (Citation1988). This implies that there was no difference (p > .05) in flavour between the two groups as regards their sensory attributes.
Discussion
This is the first attempt to optimise protein/fat ratios for meagre, based on long-term feeding trials and large-scale production. The initial reasoning behind choosing these two dietary protein levels (43% and 47%) was the good response of smaller fish (100–200 g), in a range of 45–47% protein (Martínez et al. Citation2011; Velazco-Vargas et al. Citation2014) and the general rule that dietary protein requirements are lower for larger fish. Respectively, for dietary fat, we took into account that a 20% diet produced better results than higher lipid diets (Martínez et al. Citation2011) and we also wanted to check a lipid level that is lower than the 17–20% range used in the aforementioned studies.
The overall growth and feed utilisation results from trial 1 indicate that fish respond better to low protein diets (43% dietary protein content), while the best performance as regards the TGC, DGI and PER indexes was obtained in combination with the highest dietary fat level (20%), thus suggesting a slight protein sparing by dietary lipids. However, this dietary lipid effect was related to the dietary protein level. This is indicated by the lower growth and feed utilisation in fish fed the high-protein/high-fat (47/20) diet. Therefore, it appears that there is an optimum dietary fat and protein level that, when exceeded, can cause suppression of growth in meagre. This has been confirmed for various species such as grass carp (Ctenopharyngodon idella), white weakfish (Atractoscion nobilis) (López et al. Citation2006) and, more importantly, juvenile meagre (Chatzifotis et al. Citation2010). Based on the aforementioned observations of this study, the two diets with the low protein levels (43/15 and 43/20) were chosen for the large-scale experiment (trial 2).
In growth studies on young meagre, Chatzifotis et al. (Citation2012) and Martínez et al. (Citation2011), found optimum protein levels of 50% and 47% for 23g and 95g fish, respectively. Velazco-Vargas et al. (Citation2014) examined the growth of larger meagre (final weight of 150–190g) fed isolipidic diets (17% dietary lipid) with variable digestible protein levels and found an optimum digestible dietary protein level of 430g/kg corresponding to 45% total dietary protein. In this study, the higher performance of the diets containing 43% protein, indicated that meagre of larger sizes exhibit lower protein requirements than fish at earlier life stages, as has been reported for other fish species (Wilson Citation2002).
Total body composition in this work was in accordance with previously reported studies on the same species (Martelli et al. Citation2013). The low fillet fat content observed for all dietary treatments, is within the typical 0.73–3% range reported for commercially sized meagre (Giogios et al. Citation2013; Martelli et al. Citation2013). Liver fat was not affected by different dietary levels of protein and fat in trial 1, thus showing that retention of this macronutrient is lower in this tissue. Similar findings have been reported by Chatzifotis et al. (Citation2010) for meagre juveniles. However, the hepatosomatic index of fish fed the highest lipid levels were found to be higher compared to the lowest lipid level groups. Guerreiro et al. (Citation2012) found similar results for the Senegalese sole (Solea selegalensis) when fed high-fat diets, with liver not being affected by dietary lipid content, while Dias et al. (Citation2004) observed for the same species that the HSI index was higher with high energy content diets.
Furthermore, liver glycogen levels were found to be affected by dietary treatment, and more specifically the highest levels were found in the group fed the 43/15 diet. Due to the fact that the diets were balanced with carbohydrate sources (Table ), it is possible that these differences are due to the different levels of these macronutrients in the diets. Kaushik and Teles (Citation1985) and Fountoulaki (Citation2003) reported that increasing carbohydrate levels in the diets can lead to higher glycogen content in the liver of gilthead sea bream juveniles. The same result was observed by Hemre et al. (Citation1995) in Atlantic salmon (Salmon salar) when fed increasing levels of dietary starch. Diet 43/15 contained the highest level of dietary starch compared to the other groups, which may have led to higher glycogen deposition.
Concerning the histological observations, the different dietary levels did not affect intestinal morphology. Moderate steatosis was observed in the liver in groups fed the highest levels of dietary fat (43/20 and 47/20), showing increased lipid deposition in hepatocytes, though without nuclear displacement. Higher fat accumulation in hepatic cells has also been found in gilthead sea bream fed high-lipid content diets (Caballero et al. Citation1999). Inevitably, when dietary lipid or energy exceed the capacity of the hepatocytes to oxidise fatty acids, steatosis is very likely to occur, as a result of increased synthesis and deposit of triacylglycerols (Caballero et al. Citation2004). It is possible that a higher dietary protein level improved hepatocyte structure by decreasing lipid deposition as observed for the 47/20 diet (Figure ) and as demonstrated by a slight decrease in the lipid concentration in the liver of those fish. The hepatic morphology observed in meagre fed high-lipid content diets possibly indicates that there are no pathological consequences, as reflected by the values of the growth parameters.
The second trial (trial 2) was performed in order to obtain additional information on the growth performance of meagre at large-scale production, to allow growth to commercial weights of >1kg, and to estimate the end product quality features. Trial 2 was carried out under similar conditions with trial 1 with a few exceptions, i.e. same cage culture technique, same geographical area, similar time period and fish from the same broodstock. However, larger cages and larger fish were involved in trial 2. Concerning fish growth performance, TGC values in trial 2 were lower, potentially due to the different fish size. A negative correlation of TGC with fish size has been observed for various fish species (Strand et al. Citation2011); therefore, the decrease of TGC with fish size indicates that the growth rate decreases as fish become larger.
Likewise, for both dietary groups, the CI index at the end of trial 2 was lower than that of trial 1 fish. CI was similar to the values reported by Poli et al. (Citation2003) and Martelli et al. (Citation2013) for meagre of the same size. These observations probably indicate that the CI index decreases with meagre size, i.e. fish weight does not follow the length increase rate of the fish and fish become leaner with growth. This finding is also confirmed by Vargas-Chacoff et al. (Citation2014) who found a decrease in the CI index with fish growth in a meagre population.
On the other hand, feed efficiency in trial 2 was better than in trial 1, as indicated by the lower FCR index. This can probably be explained by the fact that, feeding in trial 1 was to satiation unlike in trial 2, where feeding was performed as % of body weight. The most optimal FCR value was obtained with the 43/20 diet and this is in agreement with previous studies for juvenile meagre (Chatzifotis et al. (Citation2010). Besides, the feed intake difference between the two trials appeared to have an impact on the HSI of the fish; thus, trial 2 fish exhibited lower HSI values, thus confirming previous findings relating feeding regime to HSI in Atlantic cod (Gadus morhua L.) (Lambert and Dutil Citation2001).
The fillet fatty acid profile found in this study is in accordance with the existing literature on meagre (Poli et al. Citation2003; Piccolo et al. Citation2008; Grigorakis et al. Citation2011; García Mesa et al. Citation2014). Palmitic acid (16:0), oleic acid (18:1ω9) and linolenic acid (18:2ω6) were found to be the most abundant saturated (SFA), monounsaturated (MUFA) and ω6 fatty acids, respectively. The fillet fatty acid profile was strongly correlated with the dietary fatty acid profile, following the rule for all cultured fish species (Tocher Citation2003).
Fillet fat in the first trial was not affected by dietary fat, contrary to the second trial where fillet fat increased in the 43/20 dietary group. A possible explanation for this differentiation could be the difference in fish size, since the fish in the second trial reached higher final weights. It is generally observed that fat accumulation increases with fish size (Grigorakis Citation2010) and that, therefore, the dietary effect on fat deposits might have been more pronounced for larger fish. The elevated fillet fat content in fish that received higher dietary fat was anticipated, since this is a general rule for marine Mediterranean and other fish species (Grigorakis Citation2007, Citation2010). However, the differences in fillet fat were not reflected in the fish sensory properties. Muscular fat is an important determinant of the mouth sensation during fish fillet consumption and in particular of the ‘juiciness’ perception (Grigorakis Citation2007). The fact that muscular lipid difference reflects into mouth sensation has been previously identified in a comparison between wild and farmed gilthead sea bream (Grigorakis et al. Citation2003). Love (Citation1992) implied that in fish species with low fillet fat contents even small changes of lipid levels can result in organoleptic quality alterations. However, in this case no sensory differences were observed in overall, between the two fish groups.
Conclusions
The rearing of meagre up to commercial size, using diets with different protein/energy ratios, showed that 43% protein combined with either 15 or 20% dietary fat is the most suitable protein level for rearing meagre. Dietary fat significantly impacts on the muscular fat of the fish and on some of the fillet fatty acids. However, these chemical differences were not translated into human-perceived sensory differences.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Alexis MN, Papaparaskeva-Papoutsoglou E, Theochari V. 1985. Formulation of practical diets for rainbow trout (Salmo gairdneri) made by partial or complete substitution of fish meal by poultry by-products and certain plant by-products. Aquaculture. 50:61–73.
- AOAC. 2005. Official methods of Analysis of AOAC International. AOAC. Available from: http://www.aoac.org/omarev1/front_18th_ed.pdf.
- Caballero MJ, Izquierdo MS, Kjørsvik E, Fernández AJ, Rosenlund G. 2004. Histological alterations in the liver of sea bream, Sparus aurata L., caused by short- or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J Fish Dis. 27:531–541.
- Caballero MJ, López-Calero G, Socorro J, Roo FJ, Izquierdo MS, Férnandez AJ. 1999. Combined effect of lipid level and fish meal quality on liver histology of gilthead seabream (Sparus aurata). Aquaculture. 179:277–290.
- Chatzifotis S, Panagiotidou M, Divanach P. 2012. Effect of protein and lipid dietary levels on the growth of juvenile meagre (Argyrosomus regius). Aquac Int. 20:91–98.
- Chatzifotis S, Panagiotidou M, Papaioannou N, Pavlidis M, Nengas I, Mylonas CC. 2010. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture. 307:65–70.
- Dias J, Rueda-Jasso R, Panserat S, Conceição LECd, Gomes EF, Dinis MT. 2004. Effect of dietary carbohydrate-to-lipid ratios on growth, lipid deposition and metabolic hepatic enzymes in juvenile Senegalese sole (Solea senegalensis, Kaup). Aquacult Res. 35:1122–1130.
- Duncan NJ, Estévez A, Fernández-Palacios H, Gairin I, Hernández-Cruz CM, Roo J, Schuchardt D, Vallés R. 2013. Aquaculture production of meagre (Argyrosomus regius): Hatchery techniques, ongrowing and market. In: Alan G and Gavin B, editors. Advances in Aquaculture Hatchery Technology. Cambridge UK: Woodhead Publishing, p. 519–41.
- Emre Y, Kurtoğlu A, Emre N, Güroy B, Güroy D. 2015. Effect of replacing dietary fish oil with soybean oil on growth performance, fatty acid composition and haematological parameters of juvenile meagre, Argyrosomus regius. Aquac Res. 47:2256–2265.
- FAO. 2014. “Cultured Aquatic Species Information Programme. Argyrosomus regius.” FAO. Available from: http://www.fao.org/fishery/culturedspecies/Argyrosomus_regius/en.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Fountoulaki E. 2003. Effects of feeding different protein/energy levels and essential fatty acids on growth, physiology and metabolism of gilthead sea bream Sparus aurata fingerlings. [PhD Thesis]. National Capodestrian University of Athens.
- Fountoulaki E, Alexis MN, Nengas I, Venou B. 2003. Effects of dietary arachidonic acid (20:4n-6), on growth, body composition, and tissue fatty acid profile of gilthead bream fingerlings (Sparus aurata L.). Aquaculture. 225:309–323.
- García Mesa S, Suárez MD, Rincón Cervera MA, Guil Guerrero JL, González G, Cárdenas S, García Gallego M. 2014. Time course of muscle fatty acid composition of cultured meagre (Argyrosomus regius) during the first sixteen months of a cage culture. Grasas Aceites. 65:1–9.
- Giogios I, Grigorakis K, Kalogeropoulos N. 2013. Organoleptic and chemical quality of farmed meagre (Argyrosomus regius) as affected by size. Food Chem. 141:3153–3159.
- Grigorakis K. 2007. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: a review. Aquaculture. 272:55–75.
- Grigorakis K. 2010. Effects of nutrition and aquaculture practices on fish quality. In: Alasalvar Cesarettin, Miyashita Kazuo, Shahidi Fereidoon and Udaya W, editors. Handbook of seafood quality, safety and health applications. Oxford, UK: Wiley-Blackwell, p. 82–95.
- Grigorakis K, Fountoulaki E, Vasilaki A, Mittakos I, Nathanailides C. 2011. Lipid quality and filleting yield of reared meagre (Argyrosomus regius). Int J Food Sci Technol. 46:711–716.
- Grigorakis K, Taylor KDA, Alexis MN. 2003. Organoleptic and volatile aroma compounds comparison of wild and cultured gilthead sea bream (Sparus aurata): sensory differences and possible chemical basis. Aquaculture. 225:109–119.
- Guerreiro I, Peres H, Castro-Cunha M, Oliva-Teles A. 2012. Effect of temperature and dietary protein/lipid ratio on growth performance and nutrient utilization of juvenile Senegalese sole (Solea senegalensis). Aquacult Nutr. 18:98–106.
- Hemre GI, Sandnes K, Lie ø, Waagbø R. 1995. Blood chemistry and organ nutrient composition in Atlantic salmon, Salmo salar L., fed graded amounts of wheat starch. Aquacult Nutr. 1:37–42.
- Hernández MD, López MB, Álvarez A, Ferrandini E, García García B, Garrido MD. 2009. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 114:237–245.
- Huss HH. 1988. Fresh fish. Quality and quality changes. Roma: FAO.
- ISO. 2004. ISO Standard 4120: Sensory analysis - Methodology - Triangle test. 15. Geneva, Switzerland: International Standards Organization.
- Kaushik SJ, Teles AD. 1985. Effect of digestible energy on nitrogen and energy-balance in rainbow-trout.. Aquaculture. 50:89–101.
- Lambert Y, Dutil J-D. 2001. Food intake and growth of adult Atlantic cod (Gadus morhua L.) reared under different conditions of stocking density, feeding frequency and size-grading. Aquaculture. 192:233–247.
- López LM, Torres AL, Durazo E, Drawbridge M, Bureau DP. 2006. Effects of lipid on growth and feed utilization of white seabass (Atractoscion nobilis) fingerlings. Aquaculture. 253:557–563.
- Love RM. 1992. Chap. 1 Biochemical dynamics and the quality of fresh and frozen fish. In: edited by Hall GM Fish Processing Technology, 1–30. London, UK: Blackie Academic.
- Martelli R, Parisi G, Lupi P, Bonelli A, Dalle Zotte A, Franci O. 2013. Effect of rearing system on body traits and fillet quality of meagre (Argyrosomus regius, Asso 1801) chilled for a short time. Ital J Anim Sci. 12:186–195.
- Martínez LS, Espert RJ, Moya V, Moya SV, Jover CM, Tomas VA. 2011. Growth and nutrient efficiency of meagre (Argyrosomus regius, Asso, 1801) fed extruded diets with different protein and lipid levels. Int J Fish Aquac.. 3:195–203.
- Mittakos I, Ayala MD, López-Albors O, Grigorakis K, Lenas D, Kakali F, Nathanailides C. 2012. Muscle cellularity, enzyme activities, and nucleic acid content in meagre (Argyrosomus regius). Can J Zool. 90:1270–1277.
- Parisi G, Terova G, Gasco L, Piccolo G, Roncarati A, Moretti VM, Centoducati G, Gatta PP, Pais A. 2014. Current status and future perspectives of Italian finfish aquaculture. Rev Fish Biol Fish. 24:15–73.
- Piccolo G, Bovera F, De Riu N, Marono S, Salati F, Cappuccinelli R, Moniello G. 2008. Effect of two different protein/fat ratios of the diet on meagre (Argyrosomus regius) traits. Ital J Anim Sci. 7:363–371.
- Poli BM, Parisi G, Zampacavallo G, Iurzan F, Mecatti M, Lupi P, Bonelli A. 2003. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac Int. 11:301–311.
- Ribeiro L, Moura J, Santos M, Colen R, Rodrigues V, Bandarra N, Soares F, et al. 2015. Effect of vegetable based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture. 447:116–128.
- Strand Å, Magnhagen C, Alanärä A. 2011. Growth and energy expenditures of Eurasian perch perca fluviatilis (Linnaeus) in different temperatures and of different body sizes. J Aquac Res Dev. 2:114–122.
- Tocher DR. 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 11:107–184.
- Vargas-Chacoff L, Ruiz-Jarabo I, Páscoa I, Gonçalves O, Mancera JM. 2014. Yearly growth and metabolic changes in earthen pond-cultured meagre Argyrosomus regius. Sci Mar. 78:193–202.
- Velazco-Vargas J, Tomás-Vidal A, Hamdan M, Moyano López FJ, Jover Cerda M, Martínez-Llorens S. 2014. Influence of digestible protein levels on growth and feed utilization of juvenile meagre Argyrosomus regius. Aquac Nutr. 20:520–531.
- Wilson R. 2002. Aminoacids and protein. In: Halver JE and Hardy WR, editors. Fish nutrition. London: Academic Press, p. 142–179.
