Abstract
The aim of this experiment was to evaluate the influence of limestone particle size and phytase (F) on performance, physical egg parameters, mineral digestibility and microbial colonisation in the digestive tract in older hens aged 60–71 weeks. One hundred and sixty Lohmann Brown hens were housed in enriched cages. The 2 × 2 factorial experiment included two levels of 3-phytase NATUPHOS® (0 and 350 FTU/kg), two sizes of limestone grain (coarse limestone (CL) and a mix of CL and fine limestone (FL) (1:1)) in mixed feed. All of the diets contained 4.4 g/kg of total phosphorus and 34 g/kg of calcium. Coarse limestone with F addition significantly increased hen-day egg production (p = .018). The phytase supplement at 350 FTU/kg significantly increased shell quality characteristics. Haugh units (p = .039) and albumen index (p = .008) was higher when using the limestone mix, the same applies to yolk index (p = .013). The phytase addition to the mixed feed non-significantly increased calcium and phosphorus digestibility in the ileum by 3.1 and 3.9%, respectively. Intestinal microbiota from each sample did not cluster according to the treatment; however, bacteria belonging to the genera Turicibacter and Lactobacillus occurred with higher frequency in the ileum of hens that were fed mixed feed enriched with phytase. In conclusion, limestone particle size (1 to 2 mm) with F addition at 350 FTU/kg in a wheat-maize-soybean meal-based diet increased egg production while maintaining egg content and eggshell quality in older hens. Ileal microbiota was influenced by F supplementation in the diet.
Introduction
It is estimated that between 6 and 8% of the total eggs laid worldwide are either cracked or broken before they reach the final consumer. In terms of economy and in relation to the health risks associated with a cracked shell, eggshell quality remains one of the primary concerns of the poultry industry. Coarsely ground limestone can increase the utilisation of calcium (Ca) for shell formation and for increasing the eggshell thickness percentage. Towards the end of the laying period, the utilisation of Ca generally decreases. Lichovnikova and Zeman (Citation2008) examined the ratio of calcium in the eggshell to calcium intake and observed a 47.6% ratio in the cages at the beginning of the laying cycle period and a 44.2% ratio at the end of the laying cycle period. It is assumed that coarsely ground limestone remains in the small intestine longer and that Ca is released during the night when the shell is formed. Skrivan et al. (Citation2010) showed that coarse limestone with a particle size of 0.8–2.0 mm should be considered over fine limestone when formulating diets for laying hens in the early and late phases of production. The beneficial effects of coarse limestone on laying performance during the late production cycle are more pronounced than the effects on eggshell quality. A similar conclusion was reached by Wang et al. (Citation2014) in ducks. Limestone with a large particle size (0.85–2 mm) resulted in superior productive performance, egg quality and bone characteristics.
Additionally, phytase (F) can have positive effects on the shell quality. The addition of F completely counteracts the adverse effects associated with a low intake of inorganic phosphorus (P) by animals and can improve Ca bioavailability, subsequently improving eggshell quality at the marginal level of 3.4% Ca. Ahmadi and Rodehutscord (Citation2012) showed that adding 150, 300, and 400 F units (FTU)/kg to feed may decrease dietary nonphytate P (NPP) levels; optimal NPP levels were calculated as 0.18, 0.15, and 0.14%, respectively. In addition, F supplementation decreases P excretion in manure and can reduce potential environmental problems (Jalal & Scheideler Citation2001). Exogenous phytases are mainly active in an acidic pH, which can be found in the crop, proventriculus and gizzard. There are several commercial F products, such as 3-phytase NATUPHOS®, produced by a strain of Aspergillus niger.
Generally, in older hens, performance decreases, and the quality of the eggshell deteriorates. The negative effects of age can be minimised by nutrition. Many studies have assessed the effect of limestone particle size (Guinotte & Nys Citation1991; Richter et al. Citation1999; Pavlovski et al. Citation2003; Koreleski & Swiatkiewicz Citation2004; Lichovnikova Citation2007; Safaa et al. Citation2008) or F (Kozlowski & Jeroch Citation2011; Gao et al. Citation2013; Englmaierova et al. Citation2015) on performance and eggshell quality characteristics, but the combinatorial effect of these two factors in older hens has not been tested. Therefore, the aim of this study was to investigate the effect of the combinations of two limestone particle sizes and two levels of F on performance, egg quality, mineral digestibility and bacterial species representation in the ileum of older hens.
Materials and methods
Hens, husbandry and diets
One hundred and sixty 60-week-old Lohmann Brown hens were randomly assigned to four treatments with 4 replicate cages at 10 hens per cage. The hens were housed in three-floor enriched cages in the same air-conditioned facility. The cage was 7,560 cm2 in area. A nest box, feeder (120 cm) and 3 nipple water dispensers were included in each cage. Additionally, the cages were equipped with a perch (150 cm), a dust bath and equipment for claw abrasion, which conformed to the European Council Directive 1999/74 EC (European Union Council Directive Citation1999). Room temperature was maintained at 20–22 °C, and the light cycle was 16 h of light and 8 h of darkness. The light intensity was approximately 10 lx in the central storey. A 2 × 2 factorial experiment was initiated: 2 levels of phytase (F; 0 and 350 FTU/kg) and 2 sizes of limestone grain (coarse limestone (CL; 1.00–2.00 mm) and a mix of CL and fine limestone (FL; 0.09–0.50 mm) at the ratio 1:1) in mixed feed. Natuphos® (BASF, Ludwigshafen, Germany), a preparation of 3-phytase (EC 3.1.3.8) produced by Aspergillus niger, was chosen as the source of F. The diets (Table ) contained (per kg) approximately 11.5 MJ of apparent metabolisable energy (AMEN), 170 g of crude protein, 34 g of Ca and 4.4 g of total phosphorus (TP). Feed and fresh water were supplied ad libitum. The experiment lasted 12 weeks. The Ethical Committee of the Institute of Animal Science approved the study protocol.
Table 1. Composition of the hen diets.
The numbers of eggs and hens and their health status were monitored daily. The hen-day egg production and feed intake were calculated weekly on a per-cage basis. The egg weights were determined once per week.
Egg quality determination
For the physical parameter determination, eggs were collected in the 65th, 68th, and 70th weeks of the hens’ age. Twice within each collection period, a whole day of egg production was analysed. A total of 753 eggs were analysed. The albumen, yolk, and shell percentages were determined based on the individual weight of each egg and the weights of its components. The yolk and albumen height were measured using a digital micrometre head IP54 (Swiss Precision Instruments, Inc., Garden Grove, CA). The yolk index (YI) was calculated as YI = (yolk height/yolk diameter) × 100. The albumen index (AI) was determined using the following formula: AI = {albumen height/[(long diameter of albumen + short diameter of albumen)/2]} × 100. The Haugh units (HU) were calculated according to Haugh (Citation1937). The egg shell index (SI) was calculated as follows (Sauveur Citation1988): SI = (SW/S) × 100, S = 4.68 × EW2/3, where SW = shell weight, S = shell surface, and EW = egg weight. The shell breaking strength was determined on the vertical axis using an Instron 3360 apparatus (Instron, Norwood, MA). The shell thickness (values were measured at the sharp and blunt ends and the equator, and the three obtained values were averaged) was measured using a micrometer after removing the shell membranes.
The ash, Ca and P contents of the eggshells were determined once during the experiment in the 66th week of the hens’ age; 128 eggs were analysed (8 eggs per cage; 4 eggs per sample; 4 cage per treatment; n = 8). The shells were dried at 105 °C for 24 h, placed in a desiccator and weighed, and the dried homogenised eggshells were ashed in a muffle furnace at 500 °C for 12 h. The total P in the dried eggshells was analysed using a vanadate-molybdate reagent (AOAC Citation2005; method 965.17). The calcium content was determined by atomic absorption spectrometry using a Solar M6 instrument (TJA Solutions, Cambridge, UK).
Diet analysis
Crude protein content of the feed was measured using a Kjeltec Auto 1030 instrument (Tecator, Höganäs, Sweden). Analyses of the total P and Ca contents of the diets were conducted in a manner similar to that described previously for the analysis of these elements in the eggshells. The phytate P contents of the diets were determined using a capillary isotachophoretic method (Duskova et al. Citation2001).
Digestibility determination
Two groups of hens, which had the CL supplemented diet either with or without F Natuphos® addition (350 FTU/kg), were used to determine the digestibility of Ca and P in the ileum (n = 12). Titanium dioxide, as a dietary marker, was added to the diet at a rate of 5 g/kg. The hens were fed these diets for seven days; then, the hens were starved for 1 h, given access to the feed for 2 h and slaughtered. The hens were dissected to reveal the lower gastro-intestinal tract between Meckel's diverticulum and the ileo-caecal-colonic junction. The digesta were gently squeezed into small plastic containers, and the samples were immediately frozen and then freeze-dried. Digestibility was determined according to the modified methodology of Short et al. (Citation1996).
Microbial colonisation determination
The two groups of hens that were used to determine digestibility were used for the molecular analyses of microbial colonisation. Ileal samples were collected from 48 animals (24 animals from each treatment group) for molecular analyses. The samples of each group were randomly paired, and each pair of samples was pooled to obtain 24 samples (12 samples for each treatment). The samples were collected and immediately stored at −20 °C for molecular analyses of the bacteria.
For molecular analysis (PCR-DGGE), DNA was extracted from the cells using a QIAGEN QIAamp® DNA stool mini kit (Qiagen, Hilden, Germany). After extraction, the concentration and the A260/A280 ratio were measured using a NanoDrop 1000 instrument (Thermo Fisher Scientific, Waltham, MA) to check the quality of the DNA.
The fragments of the 16S rRNA genes were amplified from the extracted DNA with PCR using the "DGGE" bacterial primers 5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG–3′ and 5′-ATT ACC GCG GCT GCT GG-3′. The PCR conditions were as follows: 1 cycle (94 °C for 5 min, 55 °C for 1 min, and 72 °C for 1 min), 35 cycles (94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min), and 1 cycle (94 °C for 1 min, 55 °C for 1 min, and 72 °C for 7 min) (Muyzer et al. Citation1993). The primers were synthesised by Generi Biotech (Hradec Králové, Czech Republic). The PCR reaction (30 μl) was performed using REDTaq® ReadyMix with MgCl2 (Sigma-Aldrich, St. Louis, MO).
The amplicon resolution was performed using denaturing gradient gel electrophoresis (DGGE) with the Bio-Rad Dcode Universal Mutation Detection System (Bio-Rad, Hercules, CA) following the manufacturer guidelines. The PCR products (25 μl) were loaded into 9% TAE polyacrylamide gels, which contained a 35–60% denaturant gradient (100% denaturant: 77 M urea and 40% deionised formamide). The electrophoresis was performed in 1× TAE (40 mmol/l Tris, 20 mmol/l acetic acid, and 1 mmol/l EDTA) buffer at a constant voltage of 55 V and a temperature of 60 °C for 19 h. The gels were stained for 30 min with SYBR Green I dye at 10 ppm (Mrazek et al. Citation2008), and the gel image was saved with an EC3 gel documentation system (UVP Bioimaging Systems, Upland, CA). The standard ladder was included with each DGGE gel electrophoresis as a positive control (Michiels et al. Citation2012).
The DGGE profiles of the ileum samples were compared using the Ward cluster analysis based on pairwise similarities with the following settings: Ochiai band-based similarity coefficient; optimisation, 1%; tolerance, 0.5%; and fuzzy logic on (BioNumerics, Version 7.5, Applied Maths, Sint-Martens-Latem, Belgium).
The bands of interest (12 bands) were aseptically incised from the gels under UV light, and the bands were amplified and identified by sequencing. For PCR, the conditions and primers were similar to the PCR reaction previously described. The only exception was the absence of a CG-clump in the forward primer. The PCR products were further purified with a QiaQuick Purification kit (Qiagen, Hilden, Germany) and were sequenced on an ABI 3130 Genetic Analyser (Applied Biosystems, Foster City, CA). The corrected sequences were compared using BLASTn (query vs. nucleotide database) (Altschul et al. Citation1997).
Statistical analyses
The results were analysed using the general linear models (GLM) procedure in the SAS statistical software package (Statistical Analysis System, Version 9.3; SAS Citation2003). The data for hens’ performance and egg quality were analysed using a two-way analysis of variance (ANOVA), and the data from the digestibility studies were evaluated with a t-test. The main effects were the limestone grain size (L), phytase supplementation (F) and the interaction between those two factors (LxF). The differences were considered significant at p ≤ .05. The results in the tables are presented as the means and the standard error of the means (SEM).
Results
As is evident from Table , CL with F addition at 350 FTU/kg significantly (p = .018) increased hen-day egg production (88.6%) compared to CL alone (82.5%) or the mix of CL and FL with F supplement (82.7%). Other interactions were recorded in feed intake and feed conversion. Significantly, the highest daily feed intake (p = .001), feed intake per egg (p = .001) and feed conversion ratio (p = .005) values were seen in the hens fed with the mix of CL and FL together with F addition. Egg weight and egg mass production were not influenced.
Table 2. The effect of phytase and limestone particle size on performance.
In regards to the physical parameters of egg quality (Table ), F addition increased eggshell quality. Phytase supplement at 350 FTU/kg significantly increased the shell percentage (p = .007); shell index (p = .003); shell breaking strength (p = .017) and shell thickness measured at the blunt end (p = .015), in the middle (p = .009), and at the sharp end (p = .015); and the average value of shell thickness (p = .006). In addition, the mix of CL and FL with F addition significantly increased (p = .047) the ash content in shells compared to the group of hens fed CL together with F. Limestone particle size influenced the egg content quality. The mix of CL and FL increased the yolk and albumen indexes (p = .013 and p = .008), the Haugh units (p = .039) and the Ca content in the shells (p ≤ .001). Albumen and yolk percentages were not influenced by limestone particle size or by F supplementation.
Table 3. The effect of phytase and limestone particle size on egg quality.
To determine the effect of F on Ca and P digestibility and microbial colonisation in the ileum, only two groups with CL in their diet were chosen. Phytase addition at 350 FTU/kg increased, although not significantly, the digestibility of these two minerals by 3.1% and 3.9%, respectively (Table ). Figure shows the pattern from the DGGE analysis on the analysed ileal samples. Among the analyses, no specific patterns emerged in the groups within a particular treatment. Although no clustering was identified with the DGGE analysis, two groups of bands were identified (with the unaided eye) as more frequently pronounced in the group of hens with F supplementation compared to the control. After sequencing, the bands (n = 6) were identified as genera Lactobacillus and Turicibacter. The bands that were identified as Lactobacillus spp. were present in all cases in the group with F (n = 12) compared to the control, where the same band was present in only 6 of the 12 cases. The bands that were identified as Turicibacter spp. were present in only eight cases in the group with F compared to six cases in the control group.
Figure 1. DGGE analysis of the ileum* compared by the Ward cluster analysis**. *Sample nos. 1–12: control group (CL without F); sample nos. 13–24: treatment (CL with F Natuphos®); **Ochiai band-based similarity coefficient; optimisation, 1%; tolerance, 0.5%; and fuzzy logic on (BioNumerics, Applied Maths, Sint-Martens-Latem, Belgium).
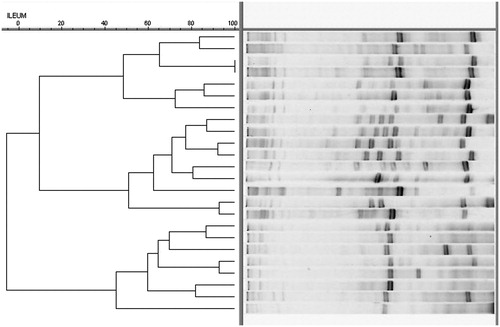
Table 4. The effect of phytase and limestone particle size on calcium and phosphorus digestibility in the ileum.
Discussion
In the current study, CL with a 3-phytase addition increased egg production in older hens, but effects from L or F alone were not found. This observed increase could be caused by higher ileal digestibility of P, N, Ca, and amino acids, which was observed by Liu et al. (Citation2007), and by suitable L particle size. The positive effect of CL on performance has been seen in a number of studies. Olgun et al. (Citation2013) showed that L particles (<2 mm) coupled to 0.44% available phosphorus in diets was the optimal combination for sustaining performance, egg production and quality and limiting mineral excretion in laying. In addition, Koreleski and Swiatkiewicz (Citation2004) observed a non-significant increase in hen-day egg production (from 90.1 to 91.8%) when one half of the FL (<0.4 mm) was replaced with L grit with a particle size of 2.0 to 4.0 mm. The results of Skrivan et al. (Citation2010) also correspond with these data when L with a particle size ranging from 0.8 to 2 mm significantly increased egg production (p = .001) and egg weight (p = .001) and decreased feed conversion (p = .001) in younger and older hens compared to FL (<0.5 mm).
In contrast to the findings of the present study, the supplementation of a normal maize-soybean diet with microbial F Natuphos (500 FTU/kg) improved egg production in a study by Um and Paik (Citation1999). The positive effect of F derived from Aspergillus niger or Escherichia coli at 300 FTU/kg on the laying rate and egg mass was also evident in diets that had reduced levels of Ca (3.18%) and P (0.15%) (Liu et al. Citation2007). Three sources of F were compared (Gao et al. Citation2013), and all three sources significantly increased laying production and egg quality (p ≤ .05) in hens at 50 to 66 weeks of age.
Phytase addition improved eggshell quality in the present study. It is known that F mobilises phytate, Ca, and other nutrients. Moreover, a higher concentration of Ca at the end of the laying period can improve eggshell quality and increase hen-day egg production. Similar to these findings, Sohail and Roland (Citation2000) and Liu et al. (Citation2007) stated that F supplementation (300 FTU/kg) significantly improved egg shell quality. In addition, Englmaierova et al. (Citation2015) found that F supplementation at 350 FTU/kg to a diet with 1.8 g/kg of NPP increased shell quality to a level that was comparable to eggs from hens fed diets with only 2.1 g/kg of NPP.
In our study, CL in the diet negatively influenced Haugh units and albumen and yolk indexes. Other parameters of egg quality were not influenced by L particle size. Additionally, a 50% replacement of CL by FL had no effect on eggshell quality. Conversely, the CL used in the study by Skrivan et al. (Citation2010), with a particle size from 0.8 to 2 mm, significantly increased the strength, weight and thickness of the shell and Haugh units as a characteristic of the albumen quality compared to FL (<0.5 mm). A large L particle, as a source of Ca, may therefore be beneficial to the hen during the dark period when feed is not consumed but the Ca requirement is high due to eggshell formation (Etches Citation1987). In addition, Swiatkiewicz et al. (Citation2015) showed that the substitution of FL (0.2–0.6 mm) with CL (1.0–1.4 mm) did not affect eggshell quality at 30, 43 and 53 weeks of age (p > .05); however, it increased (p ≤ .05) the eggshell percentage, thickness, density and breaking strength in older hens (69 weeks of age). The CL in our study decreased Ca content in the eggshell (p ≤ .001). This result is consistent with the findings of Olgun et al. (Citation2013), who also stated that large particles significantly decreased the eggshell Ca amount. Conversely, Skrivan et al. (Citation2010) observed an increase in the shell Ca content by 2 mg/g DM (p = .003) in the LC diet fed to older hens. No effect of L particle size on eggshell Ca content in laying ducks was determined by Wang et al. (Citation2014), but particle size significantly increased shell P content (p ≤ .01). In addition, larger particle size clearly improved the mammillary layer of the duck eggs. This finding could explain the significant increase in their mechanical strength, which was also observed in our results.
A non-significant increase in Ca and P digestibility in the ileum was observed after F addition into the mixed feed. The increase in P retention (by 5.02%) was also seen by Bougouin et al. (Citation2014) in hens receiving exogenous phytase at 371 FTU/kg. Similarly, Um and Paik (Citation1999) observed that Ca and P retention was greater (p ≤ .05) in F-supplemented groups, whereas the excretion of crude ash and P was significantly reduced. The reduction of P excretion is particularly important in relation to pollution and poultry manure.
A change in the nutrient supply after F administration might influence the digestive tract microflora. In our previous study (Englmaierova et al. Citation2015), Lactobacillus spp. was more frequent in the ileum and caecum of hens fed a diet enriched with 350 FTU/kg 3-phytase Natuphos®. In this study, a higher frequency of bacteria belonging to the Lactobacillus genera were also found in the ileum of hens fed a diet enriched with F. In addition, bacteria belonging to the Turicibacter genera were observed. Similar results were seen by Ptak et al. (Citation2015), where 6-phytase also increased Lactobacillus sp. and Enterococcus sp. in the ileum of chickens. Moreover, these researchers showed that F plays a role in modulating the gut microbiota; however, this phenomenon is clearly linked to the levels of P and Ca in the diet. In contrast, Lu et al. (Citation2009) found that supplemental enzymes (F and xylanase) had no effect on intestinal morphology or Lactobacillus and anaerobic bacterial counts in the intestinal digesta of broilers. However, in a study by Bhuiyan et al. (Citation2010), microbial enzymes (Avizyme 1502 and Phyzyme XP) decreased the lactobacilli population, which may be due to carbohydrates that remained undigested in diets without microbial enzymes being fermented in the hind gut and leading to an increase in microbial populations, as explained by the authors. In addition, supplementation with 750 FTU of F significantly reduced the log10 counts for total anaerobic bacteria, Escherichia coli, and coliform bacteria in the ileal digesta of chicks (0.28, 0.22, and 0.59 log10, respectively). Phytase supplementation might effectively decrease ileal pathogenic bacterial populations and beneficially affect gut health (Aydin et al. Citation2010). As evident from the aforementioned facts, the effect of F on intestinal microbial composition undoubtedly merits further investigation.
Conclusions
Feeding older hens a wheat-maize-soybean meal-based diet with 4.4 g/kg of TP, 34 g/kg of Ca, and CL with a 1–2 mm grain size enriched with 3-phytase at 350 FTU/kg led to an increase in performance without a loss of egg content or eggshell quality. The addition of 3-phytase alone improved eggshell quality, increased Ca and P digestibility and changed bacterial colonisation of the ileum.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Ahmadi H, Rodehutscord M. 2012. A meta-analysis of responses to dietary nonphytate phosphorus and phytase in laying hens. Poult Sci. 91:2072–2078.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402.
- AOAC. 2005. Official methods of analysis. 18th ed. Gaithersburg (MD): AOAC International.
- Aydin A, Pekel AY, Issa G, Demirel G, Patterson PH. 2010. Effects of dietary copper, citric acid, and microbial phytase on digesta pH and ileal and carcass microbiota of broiler chickens fed a low available phosphorus diet. J Appl Poult Res. 19:422–431.
- Bhuiyan MM, Islam AF, Iji PA. 2010. Response of broiler chickens to diets containing artificially dried high-moisture maize supplemented with microbial enzymes. South Afr J Anim Sci. 40:348–362.
- Bougouin A, Appuhamy JADRN, Kebreab E, Dijkstra J, Kwakkel RP, France J. 2014. Effects of phytase supplementation on phosphorus retention in broilers and layers: a meta-analysis. Poult Sci. 93:1981–1992.
- Duskova D, Marounek M, Brezina P. 2001. Determination of phytic acid in feeds and faeces of pigs and poultry by capillary isotachophoresis. J Sci Food Agric. 81:36–41.
- Englmaierova M, Skrivan M, Skrivanova E, Bubancova I, Cermak L, Vlckova J. 2015. Effects of a low-phosphorus diet and exogenous phytase on performance, egg quality, and bacterial colonisation and digestibility of minerals in the digestive tract of laying hens. Czech J Anim Sci. 60:542–549.
- Etches RJ. 1987. Calcium logistics in the laying hen. J Nutr. 117:619–628.
- European Union Council Directive. 1999. Council Directive 1999/74/EC of July 19th, 1999. Official Journal of the European Communities L. 203:53–57.
- Gao CQ, Ji C, Zhang JY, Zhao LH, Ma QG. 2013. Effect of a novel plant phytase on performance, egg quality, apparent ileal nutrient digestibility and bone mineralization of laying hens fed corn–soybean diets. Anim Feed Sci Technol. 186:101–105.
- Guinotte F, Nys Y. 1991. Effects of particle size and origin of calcium sources on eggshell quality and bone mineralization in egg laying hens. Poult Sci. 70:583–592.
- Haugh RR. 1937. The Haugh unit for measuring egg quality. US Egg Poultry Mag. 43:552–555.
- Jalal MA, Scheideler SE. 2001. Effect of supplementation of two different sources of phytase on egg production parameters in laying hens and nutrient digestibility. Poult Sci. 80:1463–1471.
- Koreleski J, Swiatkiewicz S. 2004. Calcium from limestone meal and grit in laying hen diets-effect on performance, eggshell and bone quality. J Anim Feed Sci. 13:635–645.
- Kozlowski K, Jeroch H. 2011. Efficacy of different levels of Escherichia coli phytase in hens fed maize-soyabean meal based diets with a decreased non-phytase phosphorus content. J Anim Feed Sci. 20:224–235.
- Lichovnikova M. 2007. The effect of dietary calcium source, concentration and particle size on calcium retention, eggshell quality and overall calcium requirement in laying hens. Br Poult Sci. 48:71–75.
- Lichovnikova M, Zeman L. 2008. Effect of housing system on the calcium requirement of laying hens and on eggshell quality. Czech J Anim Sci. 53:162–168.
- Liu N, Liu GH, Li FD, Sands JS, Zhang S, Zheng AJ, Ru YJ. 2007. Efficacy of phytases on egg production and nutrient digestibility in layers fed reduced phosphorus diets. Poult Sci. 86:2337–2342.
- Lu M, Li D, Gong L, Ru Y, Ravindran V. 2009. Effects of supplemental microbial phytase and xylanase on the performance of broilers fed diets based on corn and wheat. J Poult Sci. 46:217–223.
- Michiels J, Skrivanova E, Missotten J, Ovyn A, Mrazek J, de Smet S, Dierick N. 2012. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: effects on performance, gut bacteria and morphology and plasma oxidative status. J Anim Physiol Anim Nutr (Berl). 96:1101–1111.
- Mrazek J, Strosova L, Fliegerova K, Kott T, Kopecny J. 2008. Diversity of insect intestinal microflora. Folia Microbiol (Praha). 53:229–233.
- Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 59:695–700.
- Olgun O, Yildiz AO, Cufadar Y. 2013. Effects of limestone particle size and dietary available Phosphorus (AP) contents on performance, eggshell quality and mineral excretion in laying hens. Rev Med Vet. 164:464–470.
- Pavlovski Z, Vitorovic D, Lukic M, Spasojevic I. 2003. Improving eggshell quality by replacement of pulverised limestone by granular limestone in the hen diet. Acta Vet-Beogr. 53:35–40.
- Ptak A, Bedford MR, Swiatkiewicz S, Zyla K, Józefiak D. 2015. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS One. 10:e0119770.
- Richter G, Kiessling G, Ochrimenko W, Luedke H. 1999. Influence of particle size and calcium source on limestone solubility in vitro, performance and eggshell quality in laying hens. Arch Geflugelkd. 63:208–213.
- SAS. 2003. SAS/STAT User's Guide (release 9.3). Cary (NC): SAS Institute.
- Safaa HM, Serrano MP, Valencia DG, Frikha M, Jimenez-Moreno E, Mateos GG. 2008. Productive performance and egg quality of brown egg-laying hens in the late phase of production as influenced by level and source of calcium in the diet. Poult Sci. 87:2043–2051.
- Sauveur B. 1988. Reproduction des volailles et production d'oeufs [Reproduction of poultry and egg production]. Paris: INRA; 472 p.
- Short FJ, Gorton P, Wiseman J, Boorman KN. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 59:215–221.
- Skrivan M, Marounek M, Bubancova I, Podsednicek M. 2010. Influence of limestone particle size on performance and egg quality in laying hens aged 24-36 weeks and 56-68 weeks. Anim Feed Sci Technol. 158:110–114.
- Sohail SS, Roland DA. 2000. Influence of phytase on calcium utilization in commercial layers. J Appl Poult Res. 9:81–87.
- Swiatkiewicz S, Arczewska-Wlosek A, Krawczyk J, Puchala M, Józefiak D. 2015. Effects on performance and eggshell quality of particle size of calcium sources in laying hens’ diets with different Ca concentrations. Arch Tierz-Arch Anim Breed. 58:301–307.
- Um JS, Paik IK. 1999. Effects of microbial phytase supplementation on egg production, eggshell quality, and mineral retention of laying hens fed different levels of phosphorus. Poult Sci. 78:75–79.
- Wang S, Chen W, Zhang HX, Ruan D, Lin YC. 2014. Influence of particle size and calcium source on production performance, egg quality, and bone parameters in laying ducks. Poult Sci. 93:2560–2566.
