Abstract
An experiment was conducted to evaluate the effects of cyclic heat stress on phenotypic response, meat quality and muscle glycolysis of broiler. One hundred and eighty 35-day-old male Xueshan broilers with similar BW were randomly selected and exposed to either thermal neutral (TN; 23 °C, 55–60% RH, n = 6) or heat stress conditions (CHS; cyclic temperatures of 35 °C from 10:00 to 18:00, 32 °C from 18:00 to 20:00, 28 °C from 20:00 to 08:00, and 32 °C from 08:00 to 10:00 at 40–45% RH, n = 6) for 7 days. Relative to broilers under thermoneutral conditions, broilers subjected to cyclic heat stress had an overall increase in rectal temperature and respiration rate (p < .001, p < .001, respectively), and a reduced body weight, average daily gain, average feed intake and feed conversion ratio (p = .001, p < .001, p < .001, p = .002, respectively). A higher L* value was obtained from both the breast and thigh muscle of broilers under high temperatures (p = .022 and p = .009, respectively). Furthermore, the thigh muscle of broilers exposed to high temperatures showed a higher hexokinase activity than that of broilers exposed to thermal neutral conditions (p = .014). The results indicate that cyclic heat stress significantly lowers Xueshan broiler production performance and meat quality but has no significant effect on the chemical composition and glycolytic process of the meat.
Cyclic heat stress decreases body weight, average daily weight gain, average feed intake and feed conversion ratio in yellow-feather broilers.
Cyclic heat stress decreases the weight, physicochemical characteristics of breast and thigh meat in yellow-feather broilers.
Cyclic heat stress increases hexokinase activity to mobilize more glucose to supply the energy for aerobic metabolism in yellow-feather broilers.
Highlights
Introduction
The animal industry has suffered significant economic losses related to climate in many regions (Rhoads et al. Citation2010; Gao et al. Citation2013), particularly due to heat. The climate is becoming increasingly warmer, and the global average temperature will continue to increase by 0.2 °C per decade, according to the Intergovernmental Panel on Climate Change (Goor et al. 2015). Studies have shown that heat stress accounts for an estimated total annual economic loss of $1.69–$2.36 billion to the U.S. livestock production industry; of which total $128–$165 million occurs in the poultry industry, with the broiler industry alone accounting for $58.1 million (St-Pierre et al. Citation2003).
The optimal temperature for performance is called the thermoneutral zone (TNZ), and for broilers the TNZ ranges from 16 to 26 °C (Diarra and Tabuaciri Citation2014). When the ambient temperature is above the TNZ, heat stress may occur, which is known to have a detrimental effect on broiler production efficiency and meat yields. Moreover, heat stress has been considered one of the crucial environmental factors affecting meat quality, resulting in pale and exudative meat (Sandercock et al. Citation2001). These adverse effects of heat stress not only distress producers but also frustrate consumers, as a result, many studies have focussed on the effects of heat stress. Recent studies have reported that acute heat stress significantly decreases pH and a* values (redness) and increases L* values (paleness) and the shear force value of broiler breast muscles (Petracci et al. Citation2004; Akşit et al. Citation2006; Liu et al. Citation2011). Sandercock et al. (Citation2001) exposed broilers to acute heat stress and observed a lower pH and higher water loss and incidence of breast muscle haemorrhages, and they speculated that acute heat stress can induce disruptions in muscle membrane integrity and may be associated with changes in post-mortem breast muscle glycolytic metabolism leading to alterations in meat characteristics. What’s more, Owens et al. (Citation2000) stated that decreased meat quality was caused by the denaturation of sarcoplasmic proteins, which results in the scattering of light.
To our knowledge, previous studies on heat stress have primarily investigated the effects of acute heat stress on broiler performance and meat quality, though some researchers have studied the effects of chronic heat stress on broiler performance (Petracci et al. Citation2004; Zhang et al. Citation2012), but few such studies have been undertaken in China. In addition, previous studies were primarily focussed on Arbor Acres (AA) broilers, with little work conducted on local Chinese broilers from different regions. In recent years, an increasing number of consumers is interested in purchasing organic or free-range poultry products as they believe the products have a perceived superior sensory quality and meat safety (Fanatico et al. Citation2008). Nearly 10% of Americans surveyed reported they regularly consumed organic products (Hisey Citation2004). Yellow-feathered broiler breeds are commonly used for free-range or organic chicken production in China and other Asian countries (Tong et al. Citation2015). The Xueshan broilers, bred by the Jiangsu Lihua Agri-tech Corporation (a company who takes yellow-feather broilers as the leading industry owns 21 subsidiaries around China, and provides 255 million yellow-feather broilers for the markets in 2017), are popular local yellow-feather broilers which are typically reared in free-range systems in the eastern China where the summer average temperature is around 35 °C, and they frequently get into heat stress strait.
Thus, the objective of the present study was to investigate the effects of a 7-day cyclic heat stress (based on the actual summer temperature measured by farm workers from the Jiangsu Lihua Agri-tech Corporation) on phenotypic response, meat quality and muscle glycolytic activity of breast and thigh muscles in yellow-feather broilers.
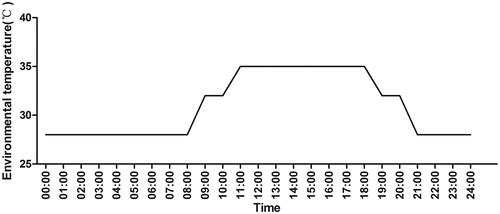
Materials and methods
Birds and housing
All procedures involving animal use and care were approved by the Institutional Animal Care and Use Committee of the Poultry Institute, Chinese Academy of Agricultural Sciences (Yangzhou; 32° 31′17.56 North, 119° 30′30.97 East, 6.4 m above sea level). According to previous experimental design (Zhang et al. Citation2012; Song et al. Citation2014), before treatment, the broilers were acclimated in an environmental chamber for 5 days under the following conditions: a 24 h photoperiod, 23 °C and 55–60% relative humidity (RH); feed and water were provided ad libitum, thereafter, one hundred and eighty 35-d-old male Xueshan broilers with similar body weight (BW, 655 ± 6 g) were randomly selected and divided into 2 groups with 90 in each group, and 15 broilers were put in one cage, with 6 cages in total in each group, and each group was in a climatic chamber (100 × 80 × 35 cm) with two column cages which were at three layers. One group was exposed to thermal neutral condition (TN; 23 °C, 55–60% RH), and the other group was exposed to heat stress conditions (CHS; cyclic temperatures of 35 °C from 10:00 to 18:00, 32 °C from 18:00 to 20:00, 28 °C from 20:00 to 08:00 and 32 °C from 08:00 to 10:00 at 40–45% RH; Figure ) for 7 days. Regardless of the environmental treatment, all birds were reared in group in cages (100 × 80 × 35 cm) with set temperature and a 24 h photoperiod and fed the same diet throughout the duration of the experimental period. What’s more, a long trough and three nipple drinkers were provided for each cage to ensure that feed and water were provided ad libitum. The composition and nutrient levels of the broiler diet are shown in Table and contained adequate nutrient levels as set by the NRC (Citation1994). During the 7-day experimental period, broilers were monitored continuously for signs of distress.
Table 1. Composition and nutrient levels of experimental diets (air-dry basis, %).
Sample collection and analytical determination
For each cage, rectal temperature, respiration rate, body weight and feed intake were measured on the day 7 of treatment when birds were 42 d of age. Rectal temperatures were recorded from 3 birds per cage with a digital thermometer (DT-610B, China Everbest Machinery Industry Co., Ltd., Guangdong, China) and respiration rates (breaths/min) were measured visually and counted with a stopwatch at 10:00 and 16:00, and an average value was then calculated. Body weights were recorded for all birds at 42 d and immediately prior to slaughter. Feed intake and broiler mortality were recorded for each cage. The feed conversion ratio was calculated by correcting for the BW of any bird which was removed during the experiment.
After a 12 h overnight fast, one 42-day-old broiler from each cage was randomly selected, slaughtered and subjected to a full post-mortem examination. The left side of the broiler breast meat (including the pectoralis major and pectoralis minor) and the thigh meat (including the left thigh and drumstick) were weighed and then stored at 4 °C until determination of the physico-chemical characteristics including meat colour, pH, water-holding capacity and shear force as well as key enzymes (lactic dehydrogenase, pyruvate kinase and hexokinase) and lactic acid, a product of glycolysis. The right breast and thigh muscle were sampled to determine their chemical compositions (moisture, protein and fat).
The moisture, protein and fat content of the samples were detected using official standard methods of analysis (AOAC Citation2000). The moisture contents of breast and thigh meat were determined by heating each sample in an oven at 85 °C until a constant weight was recorded. The nitrogen contents of breast and thigh meat were determined using a KjeltecTM 2300 Kjeldahl nitrogen analyser (FOSS Analytical AB, Hoganas, Sweden). The fat content was determined using a SoxtecTM 2050 Soxhlet solvent extraction system (FOSS Analytical AB, Hoganas, Sweden).
The meat colour was measured using a Minolta Chroma Meter CR-300 (Osaka, Japan). The tip of the colorimeter measuring head was placed flat against the surface of the muscle. For each measurement, 3 readings were performed; the final value for each sample was the average of these readings. The meat colour was expressed using the CIELAB dimensions of lightness (L*), redness (a*), and yellowness (b*). The higher L* values correspond to lighter colour, higher a* values correspond to greater redness, and higher b* values correspond to greater yellowness.
The pH of samples was measured 45 min post-mortem using a portable pH metre (IQ150, IQ Scientific Instruments, Inc., Carlsbad, CA). The measurements were made at the same location on individual breast and thigh muscle samples. The average pH value was calculated from 3 readings taken on the same muscle sample.
The water-holding capacity was estimated as follows: a raw meat sample weighing 1.0 g was placed between 18 pieces of 11 cm diameter filter paper and pressed at 35 kg for 5 min at 25 °C. The expressed fluid was determined as the change in the weight of the original sample (Bouton et al. Citation1971). The water-holding capacity was calculated as the ratio of expressible fluid/total moisture content (Allen et al. Citation1998).
The shear force was determined using a texture analyser and a Warner-Bratzler device (C-LM2, Northeast Agricultural University Ltd., Harbin, China). Meat samples were stored at 4 °C for 24 h and then individually cooked in a water bath at 80 °C in plastic bags to an internal temperature of 70 °C. The samples were then removed and chilled to room temperature. Strips [1.0 cm (width) × 0.5 cm (thickness) × 2.5 cm (length)] parallel to the muscle fibres were prepared from the medial portion of the muscle and sheared vertically (Molette et al. Citation2003). Shear force was expressed in kilograms.
Samples of breast and thigh meat (kept at 4 °C) were taken for analyses of lactic acid (LD), lactic dehydrogenase (LDH, pyruvate kinase (PK) and hexokinase (HK) concentrations within 24 h after slaughter. The concentration of these four compounds in skeletal muscle were measured using commercially available colorimetric diagnostic kits (Item Nos. A019-2, A020-2, A076-1 and A077-1, respectively, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
A pen of birds was the experimental unit for all data analysis. An independent-sample t Test was conducted using SPSS software (SPSS 18.0 for Windows, SPSS Inc., Chicago, IL). All data were reported as LS means; differences were considered significant at p < .05, and a trend was considered present at p < .10.
Results
Phenotypic response to heat stress
Heat-stressed broilers had an overall increase in rectal temperature (39.7 vs. 42.1 °C, p < .001; Figure ) and an ∼4-fold increase in overall respiration rate compared with the TN controls (36.3 vs. 163.7 bpm, p < .001; Figure ). Under heat stress, body weight, average daily gain and average feed intake significantly decreased (p = .001, p < .001, p < .001, respectively; Table ) whereas the feed conversion ratio increased (p = .002; Table ). No mortality was recorded during the treatment period.
Figure 2. The effects of 7 days of constant thermoneutral conditions (TN) or heat stress conditions (CHS) on (A) rectal temperature and (B) respiration rates. (A) *p < .001, n = 6/treatment; (B) *p < .001, n = 6/treatment.
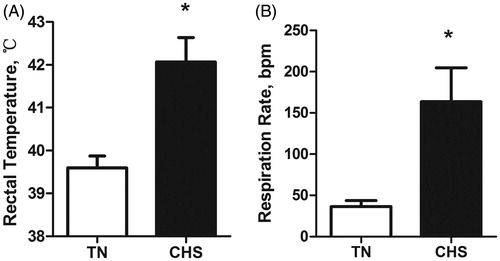
Table 2. Effects of cyclic heat stress on growth performance of broilers.
Weight, chemical composition of breast and thigh meat
Table shows weight, chemical composition of broiler breast and thigh meat on d-42. Heat exposure significantly affected the breast and thigh weight of broilers (p = .009, p = .001 respectively). Compared with the TN group, the average breast weight and thigh weight was lower by 12.99% and 16.87%, respectively. After 7 d of cyclic heat stress, CHS broilers had significantly higher breast meat moisture content (p = .047), but not thigh muscle moisture content, and there was no effect of treatment on the protein or fat content of breast or thigh meat (p > .05).
Table 3. Effects of cyclic heat stress on weight, chemical composition of broiler breast and thigh meat (n = 6).
Physico-chemical characteristics of breast and thigh meat
The effects of CHS on the physico-chemical characteristics of breast and thigh meat are described in Table . The data showed both the breast and thigh meat of broilers in the CHS group had higher L* values (p = .022, p = .009, respectively) than the TN treatment. In addition, there was a marginally lower a* value and water-holding capacity (p = .094, p = .095 respectively) in the breast meat of HS-treated broilers than in that of CHS-treated broilers.
Table 4. Effects of cyclic heat stress on physico*chemical characteristics of broiler breast and thigh meat (n = 6).
Glycolysis analysis of breast and thigh meat
The activities of LD and three key enzymes in glycolysis (LDH, PK and HK) are presented in Table . Cyclic heat stress had no significant effect on LD or the activities of LDH and PK in the breast and thigh meat (p > .05). The HK activity of thigh meat from broilers in the HS group was higher compared with that of the TN group (p = .014).
Table 5. Effects of cyclic heat stress on activity of three key enzymes in glycolysis (LDH: lactic dehydrogenase; PK: pyruvate kinase; HK: hexokinase) and lactic acid (LD) content of broiler breast and thigh meat (n = 6).
Discussion
Exposure to high ambient temperatures can have a profound effect on the physiology and health of humans and other animals (Lambert Citation2009) and is accompanied by phenotypic changes (Pearce et al. Citation2013). By design, our HS experiment resulted in marked hyperthermia as indicated by elevated rectal temperatures and respiration rates. Rectal temperature is a common index for evaluating broiler status under heat stress (Tao Citation2003). As chickens have no sweat glands, thermal regulation can fail during heat stress, as indicated by heat accumulation and increased body temperature (Yalcin et al. Citation2001). When broilers are reared under high temperatures, they are forced to increase the rate of respiration to lower body temperature (Mack et al. Citation2013).
Another immediate effect of CHS observed in the present study was the decrease in feed intake, consistent with previous reports (Zhang et al. Citation2012; Song et al. Citation2014), and this could be a strategy to minimise metabolic heat production; this is a highly conserved response among species (Collin et al. Citation2001; Baumgard and Rhoads Citation2012). Heat-stressed broilers also had significantly lower body weight and average daily gain over 7 d compared with broilers exposed to normal temperatures. Broilers are extremely sensitive to CHS because they lack functional sweat glands and produce significant quantities of metabolic heat (D’Allaire et al. Citation1996), which could reduce feed intake and increase urination. Moreover, HS significantly decreased the weight of breast and thigh muscle, which is consistent with the results from previous studies (Lu et al. Citation2007; Zhang et al. Citation2012; Angelica et al. Citation2015). As breast and thigh muscles are the most valuable parts of the broilers, decreasing their weights under HS not only has a significant impact on meat performance but also affects economic returns of broilers.
Heat stress during the growth period of broilers has been associated with undesirable meat attributes and a loss of quality (Sandercock et al. Citation2001; Zhang et al. Citation2012), which is an important consideration for consumers (Lara and Rostagno Citation2013). In the present study, breast meat of broilers from cyclic high-temperature environment showed a significant increase in moisture content compared with the thermoneutral group, but no difference between treatments was found in the protein or fat content of either breast or thigh meat. Zhang et al. (Citation2012) obtained similar results following a cyclic heat stress treatment, but when subjected to constant HS there was lower protein and fat content in the breast muscle. Decreased protein and fat content were also found by Arruda et al. (Citation2016). The difference in meat composition in the current study and others could be due to differences in genetics, age of the broilers and muscle type as well as the pattern of heat stress, which all are key factors affecting the chemical composition of meat (Olivo Citation2006).
Meat characteristics, including shear force, water-holding capacity, pH and meat colour are important indices for evaluating meat quality (Tong et al. Citation2012). Among physico-chemical characteristics, shear force is considered the most important by consumers, with higher quality meat having a lower shear force. Consistent with previous results (Zhang et al. Citation2012), the shear force of CHS broilers was not different to that of the TN broilers, and this can be primarily attributed to the similar level of intramuscular fat deposition between the treatments (Han et al. Citation2003). Muscle pH is an important parameter affecting preservation; a higher muscle pH decreases shelf life stability and increases microbial growth (Alberle et al. Citation2001). No difference in pH was observed between the CHS and TN groups, possibly because there was no difference in lactic content (Arruda et al. Citation2016). Meat colour is an important quality attribute, both for the consumers’ final evaluation and for acceptance of a meat product at the time of consumption. In the present study, heat-exposed broilers had a higher L* value in breast and thigh meat, which is consistent with that reported by Lu et al. (Citation2007). The tendency for a decreased a* value in breast meat suggested that higher levels of oxidised myoglobin occurred in broiler muscles under CHS conditions (Mancini and Hunt Citation2005). The water-holding capacity is significant for both whole meat and processed meat and can be an important index for distinguishing PSE meat (Owens et al. Citation2000) and thus affects the economic value of broiler meat products. This study showed a tendency for decreased water-holding capacity in CHS broilers. It has been reported that acute and chronic heat stress can result in poorer water-holding properties (Molette et al. Citation2003), primarily due to a high metabolic rate, resulting in a pronounced denaturation of proteins (Deng et al. Citation2002). The meat quality measures indicate that Xueshan broilers may have a strong resistance to heat stress and may produce meat that satisfies the requirements of local consumers.
After slaughter, adiemorrhysis arrests the supply of both nutrients and oxygen to the muscle cells, which promotes the process of anaerobic glycolysis and thus results in the accumulation of lactic acid (Pösö and Puolanne Citation2005; Zhu et al. Citation2011). There is a negative correlation between muscle lactic acid content and pH (Puolanne and Kivikari Citation2000; Kylä-puhju et al. Citation2004). Consistent with Zhang et al. (Citation2012) report, cyclic heat stress had no significant impact on the lactic acid content of either breast or thigh meat, indicating that no additional glycogen was converted to lactic acid; lactic acid results and pH results were in good agreement. Two key enzymes HK and PK play important roles in anaerobic glycolysis, as does LDH, a key enzyme in the conversion of pyruvate to lactate under anaerobic conditions in muscle (Scartozzi et al. Citation2012). There were no differences due to the treatment in meat LDH and PK contents, but an increased HK content in the thigh muscle of HS broilers, similarly to a previous study (Zhang et al. Citation2012). These findings indicate that the heat stress resulted in the mobilisation of more glucose to supply the energy required for aerobic metabolism in the broilers. Other studies have demonstrated that heat stress increases LDH and PK activities not only in meat but also in plasma (Sandercock et al. Citation2006; Xie et al. Citation2015); in contrast, Lin et al. (Citation2006) found no obvious meat damage without a change in plasma LDH after acute heat exposure.
Conclusions
The present study indicated that cyclic heat stress, designed to simulate the high-temperature summer of eastern China, resulted in increased Xueshan broilers’ rectal temperature and respiration rate, poorer growth performance, decreased physico-chemical quality of breast and thigh muscle and mobilisation of more glucose to supply the energy required by aerobic metabolism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aksit M, Yalin S, Özkan S, Metin K, Özdemir D. 2006. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult Sci. 85:1867–1874.
- Alberle ED, Forrest JC, Gerrard DE, Mills EW. 2001. Principles of Meat Science. 4th ed. Dubuque, IA: Kendall/Hunt Publ. Co.
- Allen CD, Fletcher DL, Northcutt JK, Russell SM. 1998. The relationship of broiler breast color to meat quality and shelf-life. Poult Sci. 77:361–366.
- Angelica VG, Bolek KJ, Ashwell CM, Persia ME, Rothschild MF, Schmidt CJ, Lamo SL. 2015. Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress. Genet Sel Evol. 47:96.
- AOAC. 2000. Official methods of analysis. 17th ed. Gaithersburg, MD: Association of Official Analytical Chemists.
- Arruda AMV, Melo AS, Marinho JBM, Fernandes RTV, Figueiredo LC. 2016. Chemical composition and pH of the meat of broilers submitted to pre-slaughter heat stress. JABB. 4:93–95.
- Baumgard LH, Rhoads RP. 2012. Effects of environment on metabolism. In: Collier RJ, editor. Environmental physiology. New Jersey: John Wiley and Sons, Inc.
- Bouton PE, Harris PV, Shorthose WR. 1971. Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. J Food Sci. 36:435–439.
- Collin A, Milgen J, Dividich LJ. 2001. Modeling the effect of high, constant temperature on food intake in young growing pigs. Anim Sci. 72:519–527.
- D'Allaire S, Drolet R, Brodeur D. 1996. Sow mortality associated with high ambient temperatures. Can Vet J. 37:237–239.
- Deng Y, Rosenvold K, Karlsson AH, Horn P, Hedegaard J, Steffensen CL, Andersen HJ. 2002. Relationship between thermal denaturation of porcine muscle proteins and water-holding capacity. J Food Sci. 67:1642–1647.
- Diarra S, Tabuaciri P. 2014. Feeding management of poultry in high environmental temperatures. Int J of Poult Sci. 13:657–661.
- Fanatico AC, Pillai PB, Hester PY, Falcone C, Mench JA, Owens CM, Emmert JL. 2008. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult Sci. 87:1012–1021.
- Gao Z, Liu F, Yin P, Wan C, He S, Liu X, Zhao H, Liu T, Xu J, Guo S. 2013. Inhibition of heat-induced apoptosis in rat small intestine and IEC-6 cells through the AKT signaling pathway. BMC Vet Res. 9:241.
- Han JZ, Sang YZ, Zhou TQ. 2003. Effects of feeding patterns and feeding levels on muscle fiber characteristics and meat quality. Chinese Anim Husbandry Veteri Medi. 35:17.
- Hisey P. 2004. Subject: Organic consumption is rising, survey says. http://www.meatingplace.com.
- Kylä-Puhju M, Ruusunen M, Kivikari R, Puolanne E. 2004. The buffering capacity of porcine muscles. Meat Sci. 67:587–593.
- Lambert GP. 2009. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 87:E101–E108.
- Lara LJ, Rostagno MH. 2013. Impact of heat stress on poultry production. Animals (Basel). 3:356–369.
- Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol Part A Mol Integr Physiol. 144:11–17.
- Liu GQ, Yang XJ, Jing XU, Zong K, Zhang L. 2011. Effect of acute heat stress on blood biochemical parameters and meat quality of broiler. Chinese Poult Sci. 3:10–14.
- Lu Q, Wen J, Zhang H. 2007. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. 86:1059–1064.
- Mack LA, Felver-Gant JN, Dennis RL, Cheng HW. 2013. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult Sci. 92:285–294.
- Mancini RA, Hunt MC. 2005. Current research in meat color. Meat Sci. 71:100–121.
- Molette C, Remignon H, Babile R. 2003. Maintaining muscles at a high post-mortem temperature induces PSE-like meat in turkey. Meat Sci. 63:525–532.
- NRC. 1994. Nutrient requirements of poultry. 9th rev. ed. Washington, DC: Natl. Acad. Press.
- Olivo RO. 2006. Mundo do frango: cadeia produtiva da carne de frango. Ed. do Autor, Criciúma, pp. 240–272.
- Owens CM, Mckee SR, Matthews N, Sams A. 2000. The development of pale, exudative meat in two genetic lines of turkeys subjected to heat stress and its prediction by halothane screening. Poult Sci. 79:430–435.
- Owens CM, Hirschler EM, McKee SR, Martinez-Dawson R, Sams AR. 2000. The characterization and incidence of pale, soft, exudative turkey meat in a commercial plant. Poult Sci. 79:553–558.
- Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. 2013. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PloS One. 8:e70215.
- Petracci M, Betti M, Bianchi M, Cavani C. 2004. Color variation and characterization of broiler breast meat during processing in Italy. Poult Sci. 83:2086–2092.
- Pösö AR, Puolanne E. 2005. Carbohydrate metabolism in meat animals. Meat Sci. 70:423–434.
- Puolanne E, Kivikari R. 2000. Determination of the buffering capacity of postrigor meat. Meat Sci. 56:7–13.
- Rhoads ML, Kim JW, Collier RJ, Crooker BA, Boisclair YR, Baumgard LH, Rhoads RP. 2010. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J Dairy Sci. 93:170–179.
- Sandercock DA, Hunter RR, Mitchell MA, Hocking PM. 2006. Thermoregulatory capacity and muscle membrane integrity are compromised in broilers compared with layers at the same age or body weight. Br Poult Sci. 47:322–329.
- Sandercock DA, Hunter RR, Nute GR, Mitchell MA, Hocking PM. 2001. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult Sci. 80:418–425.
- Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, Del Prete M, Loretelli C, Belvederesi L, Svegliati Baroni G, et al. 2012. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. Plos One. 7:e32653.
- Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, Shi B, Zou XT. 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci. 93:581–588.
- St-Pierre N, Cobanov B, Schnitkey G. 2003. Economic losses from heat stress by US livestock industries. J Dairy Sci. 86:E52–E77.
- Tao XP. 2003. Effects of temperature-humidity-velocity conditions on the sensitive physiological and biochemical indices of broilers. Chinese Acad Agric Sci. 56:34–36.
- Tong HB, Cai J, Lu J, Wang Q, Shao D, Zou JM. 2015. Effects of outdoor access days on growth performance, carcass yield, meat quality, and lymphoid organ index of a local chicken breed. Poult Sci. 94:1115–1121.
- Tong HB, Lu J, Zou JM, Wang Q, Shi SR. 2012. Effects of stocking density on growth performance, carcass yield, and immune status of a local chicken breed. Poult Sci. 91:667–673.
- Xie J, Tang L, Lu L, Zhang L, Lin X, Liu HC, Odle J, Luo X. 2015. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult Sci. 94:1635–1644.
- Yalcin S, Ozkan S, Turkmut L, Siegel PB. 2001. Responses to heat stress in commercial and local broiler stocks. II. Developmental stability of bilateral traits. Br Poult Sci. 42:153–160.
- Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, Feng DY. 2012. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. 91:2931–2937.
- Zhu X, Ruusunen M, Gusella M, Zhou G, Puolanne E. 2011. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler pectoralis major muscles. Meat Sci. 89:181–188.