Abstract
The aim of the experiment was to study the effect of housing growing rabbits in enriched cages with small groups (eight rabbits/cage, C; n = 96; stocking density: 15 rabbits/m2) or in enriched pens with large groups (65 rabbits/pen, P; n = 130; stocking density: 15 rabbits/m2) on their growth performance and on slaughter and meat quality traits. The C rabbits showed higher final body weight (2540 vs. 2443 g, p < .01), better feed conversion ratio (5–11 weeks: 3.39 vs. 3.61, p < .05), lower mortality rate (5.2 vs. 31.5%, p < .001), and lower fecal corticosterone level (26.3 vs. 29.4 nmol/g, p < .05) compared to P rabbits. The increased possibility of physical activity of P compared to C rabbits resulted in more developed hind part of the reference carcase, thicker hind leg bones (34.8 vs. 33.4 g, p < .05), lower perirenal fat (15.1 vs. 20.8 g, p < .001) and hind leg meat lipid content (2.00 vs. 2.42%, p < .05), as well as higher haem iron content of the hind leg meat (5.29 vs. 4.22 mg/kg, p < .01). However, pen housing was detrimental for the dressing out percentage and for the hind leg meat to bones ratio. Physical meat quality traits were not affected by the housing system.
Housing of growing rabbits in large cages and large pens was compared.
Caged rabbits had better productive performance, lower mortality and stress.
Penned rabbits resulted in lower dressing out percentage, carcase adiposity and meat lipids content.
Most of the meat quality traits were independent of the housing system.
Highlights
Introduction
Several studies are nowadays focussing on the effect of growing rabbit’s housing and husbandry system on welfare and health (EFSA Citation2005; Trocino and Xiccato Citation2006; González-Mariscal et al. Citation2017; Turner et al. Citation2017), and on live performances (Szendrő and Dalle Zotte Citation2011). One of the important animal welfare issues are group size, and the size and equipment of cages and pens. Although, it was scientifically demonstrated that the individually housed rabbits exhibit the best live performance (Maertens and De Groote Citation1984) and favourable meat quality traits (Xiccato, Trocino, Majolini, et al. Citation2013) rabbit is considered a social animal (Held et al. Citation1995). Living in social isolation rabbits can display physiological symptoms of stress, show certain stereotypes, exhibit extreme fear towards man and new environment (Podberscek et al. Citation1991; Chu et al. Citation2004). During a long period bicellular housing was the most frequent system but recently based on the EFSA recommendation (Citation2005) the group size of seven to nine, preferably retaining littermates in groups, would be advantageous. Szendrő and Dalle Zotte (Citation2011) recommended 8–10 full-sibs as the maximum number of growing rabbits per cage or pen. There are more disadvantages than benefits of housing growing rabbits in large groups (more than 10 rabbis in a group): with increasing group size the productive performance, some carcase and meat quality traits worsened (Dal Bosco et al. Citation2002; Dalle Zotte et al. Citation2009; Princz et al. Citation2009; Xiccato, Trocino, Filiou, et al. Citation2013). At large group size the infection risk increases leading to a rise in mortality (Maertens and Van Herck Citation2000), especially when the floor type was bedded with straw litter (Dal Bosco et al. Citation2002). The proportion of injured rabbits also increases (Szendrő et al. Citation2009). Nonetheless, the Swiss Federal Council (Citation2005), Belgian Royal Decree (Citation2014), Dorning and Harris (Citation2017), the Compassion in World Farming (Citation2017), recommend housing 25–50 or more growing rabbits in a group.
In the present study, the productive performances, as well as the carcase and meat quality traits and a welfare indicator (corticosterone level) of growing rabbits housed in enriched cages with small groups or in enriched pens with large groups were compared.
Materials and methods
Animals and housing
The trial was carried out at the experimental rabbitry of Kaposvár University using Pannon Ka (maternal line) growing rabbits (n = 226). Rabbits were weaned at five weeks of age. Along the trial the room temperature varied between 15 °C and 20 °C, and natural lighting was provided. Rabbits were fed ad libitum with a commercial pelleted diet (five–nine weeks of age: digestible energy (DE) = 10.3 MJ/kg; crude protein (CP) = 16.1%; ether extract (EE) = 2.8%; crude fibre (CF) = 16.9% and coccidiostatic medication. Nine to 11 weeks of age: DE = 11.0 MJ/kg; CP = 16.1%; EE = 4.4%; CF = 16.0%), and drinking water was available from nipple drinkers.
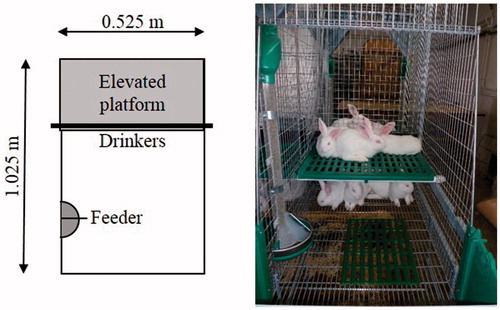
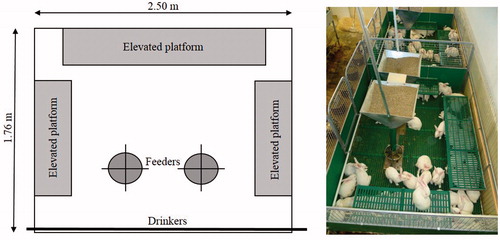
Rabbits were randomly divided into two groups. In the first group (C) full-sib rabbits were housed into 12 cages (1.025 × 0.525 m, basic area 0.54 m2, eight rabbits/cage). The floors of cages were wire mesh, equipped with footpads, and enriched with elevated plastic-mesh platforms (0.42 × 0.52 m) (Figure ). In the second group (P) rabbits were housed in two large pens (1.76 × 2.50 m, basic area 4.4 m2, 65 rabbits/pen), similar to park system. Pens were floored with plastic-mesh, and plastic-mesh elevated platforms (two 0.42 × 1.055 m and one 0.42 × 1.575 m) were installed (Figure ). Both cages and pens were made by Meneghin srl (Italy), and the stocking density was 15 rabbits/m2 in both groups (calculated on the floor space without elevated platforms). Gnawing sticks were placed both to cages and pens as environmental enrichment.
Growth performance and welfare indicators
Individual body weight of rabbits was measured at 5, 7, 9, and 11 weeks of age, whereas feed intake for 5 to 7, 7 to 9, 9 to 11, and 5- to 11-week periods was recorded on the cage or pen unit. Daily weight gain and feed conversion ratio were then calculated. Mortality was recorded daily.
Pooled feces samples of each cage and pen were collected at 6, 9, and 11 weeks of age for evaluating the corticosterone concentration (metabolites in faecal samples) as an indirect stress parameter (cage: n = 36, pen: n = 6). For collection we used mosquito net with wooden frame placed under the cages and pens. Assays were done at the Veterinary University (Hungary), according to the method of Palme et al. (Citation1999), modified by Szendrő et al. (Citation2013).
Carcase traits
At 79 days of age 180 rabbits (all healthy rabbits: n = 91 and 89 of C and P groups, respectively) were transported to a slaughterhouse located 200 km from the experimental rabbit farm. Fasting period lasted four hours, transport included. Rabbits were slaughtered by cutting the carotid arteries and jugular veins after electrical stunning. Slaughter and carcase dissection procedures were performed according to the recommendation of the World Rabbit Science Association (Blasco and Ouhayoun Citation1996) and all the steps taken to obtain offal (head, set of organs: heart + lungs + thymus + trachea + oesophagus, liver, kidneys, perirenal- and scapular fat) weights (slaughter weight, SW, chilled carcase weight, CC, and reference carcase weight, RC) and yields (carcase yield and reference carcase yield) are detailed in a previous study (Dalle Zotte et al. Citation2009). The CC was recorded after 24 h chilling in a ventilated room at 4 °C. Twenty carcases per treatment (based on the average warm carcase weight) were retained for carcase and meat quality traits measurement. The RC included meat, bones and fat depots without head. Subsequently, carcases were cut between the seventh and eighth thoracic vertebrae and between the sixth and seventh lumbar vertebrae to obtain the fore, mid, and hind parts, which were weighed separately. The Longissimus thoracis et lumborum (LTL) meat of the mid part, and the hind legs (HL) were dissected. The CC yield and the RC yield were calculated as CC weight divided by SW × 100, and as RC weight divided by CC × 100, respectively. Carcase parts and perirenal fat were calculated to the RC weight.
The HL and LTL meat samples of both sides of the carcase were weighed, individually packed in polyethylene bags and ice-cooled in portable refrigerators. The same day, samples were transported to the Department of Animal Medicine, Production and Health (MAPS) of the University of Padova (Italy) for meat quality analyses. Depending on the further analysis the samples were stored in two different way: left HL and LTL samples of carcases were stored in a professional ventilated refrigerator at 4 °C ± 1 °C (fresh samples) whereas the right HL and LTL samples were immediately stored at −40 °C (chilled samples) until further analyses.
Meat quality
The pHu was measured in duplicate approximately at 32 h post-mortem in both fresh LTL (n = 20/treatment), and Biceps femoris (BF; n = 20/treatment) muscles by Testo 205 pH metre. The L*a*b* colour values (CIE Citation1976) were recorded in duplicate on a cross-section of the fresh surface of mid-LTL, between the 6th and 7th lumbar vertebrae by Minolta CR-300 colorimeter (n = 20/treatment).
Hind legs (n = 20/treatment) were allowed to thaw overnight at +4 °C, freed from plastic bags, and subsequently used for cooking loss determinations. With this purpose, they were individually vacuum-packed in PVC bags and cooked in a water bath at 80 °C for 2.5 h. Each sample was chilled with cold tap water until room temperature has been reached, then removed from its PVC bag, dried, and weighed for cooking loss determination. Cooked HL were used for Warner–Bratzler Shear Force (WBSF) measurements on cores (diameter 1.25 cm) sheared perpendicularly to muscle fibre direction with a Warner–Bratzler cell fitted on a dynamometer Texture TA-HD (SMS—Stable Micro System). WBSF was calculated by averaging four measurements per sample.
Fresh HL (n = 20/treatment) were deboned and their meat, together with that of the fresh LTL (n = 20/treatment), were prepared for the proximate composition analysis using AOAC methods (AOAC Citation1995), protein content calculated by difference. Heme iron content was analysed on HL and LTL meat samples (n = 20/treatment) using the procedure described by Hornsey (Citation1956).
Physical measurements
The chilled HLs (n = 20/treatment) were deboned in order to determine the meat to bones ratio (Blasco and Ouhayoun Citation1996). Femur and tibia were also separately weighed and measured for diameter and length (femur bone) with a dial calliper (±0.02 mm; JUWEL Digital-Schieblehre Rostfrei H4215/5X A12). Diameter was measured at the level of minor thickness at the mid-diaphysis corresponding to the breaking point. Femur fracture toughness (FT) was determined at the average bone length point by using a dynamometer Texture TA-HD (SMS—Stable Micro System) with a 6 cm wide cell and a load rate of 0.5 mm/s, and expressed in kg.
Cost factors
Cost estimation was based on the price of weaned (1.90 €/kg) and slaughtered rabbits (1.75 €/kg) from commercial system; feed price (after weaning diet: 0.28 €/kg; finishing stage of fattening diet: 0.26 €/kg); carcase without head price: 4.5 €/kg; liver and kidneys price: 2.8 €/kg; head, bone, heart, and lungs price: 0.57 €/kg (source: Rabbit Product Board and Olivia Ltd.). Feed cost was calculated as 70% of total costs (Gidenne et al. Citation2017).
Statistical analysis
Productive performances, carcase, and meat quality traits were analysed by means of one-way ANOVA (housing: cage or pen), whereas stress parameter (corticosterone concentration) were analysed by means of two-way ANOVA (age: 6, 9, and 11 weeks; housing: cage or pen). Mortality was evaluated by χ2 test. All statistical analyses were performed using SPSS 10.0 software.
Results and discussion
Productive performance
Productive performances of the C and P rabbits are summarised in Table . On overall, body weight and daily body weight gain were in favour of C group rabbits, however differences were significant only at week 9 (p < .001) and at wk 11 (p < .05) for body weight, and in the interval week 7–9 for daily body weight gain (38 vs. 33.3 g/day; p < .001).
Table 1. The performance of growing rabbits housed in cage or pen.
Several studies have proved that growth and live weight of caged rabbits (two to six rabbits/group) are higher than those of rabbits housed in pens with small groups (8–16 rabbits/pen; Lambertini et al. Citation2001; Princz et al. Citation2009) or with larger groups (24–104 rabbits/group; Martrenchar et al. Citation2001; Dal Bosco et al. Citation2002; Jehl et al. Citation2003; Combes et al. Citation2010). However, other studies did not find differences in live performance when the rabbits were kept in small (Xiccato, Trocino, Majolini, et al. Citation2013; Matics et al. Citation2014) or in large groups (Postollec et al. Citation2001; Szendrő et al. Citation2009). According to Szendrő and Dalle Zotte (Citation2011) the slower growth rate exhibited by the rabbits raised in pens can be related to their higher physical activity, as part of the ingested energy is used for this purpose.
Feed intake was not affected by the housing system, but the higher body weight gain of caged rabbits significantly improved their feed conversion ratio between seven and nine weeks of age, and on the overall fattening period (3.39 vs. 3.61 for cage and pen, respectively; p < .05; Table ). Similar results were reported by Maertens and Van Herck (Citation2000), Dal Bosco et al. (Citation2002), and Princz et al. (Citation2009), due to the lower feed intake in large group of rabbits. When rabbits are housed in large group chronic stress is triggered due to constant aggressiveness and fighting, resulting in lower feed intake (Maertens and Van Herck Citation2000; Szendrő and Dalle Zotte Citation2011). This was also demonstrated by stress hormone level. The faecal corticosterone level was significantly higher in penned than in caged rabbits (29.4 vs. 26.3 nmol/g, respectively; p < .05) indicating aggressiveness. The corticosterone level increased with the age but only the value of 11 weeks differed significantly compared to values of six and nine weeks (24.1, 25.6, and 30.4 nmol/g, at 6, 9, and 11 weeks, respectively; p < .001). The corticosterone level was 8.8%, 9.0%, and 13.4% higher in the pens compared to that in the cages at 6, 9, and 11 week, respectively (p < .1 in case of 6 and 11 weeks, p < .5 in case of 9 weeks). In contrast with our results Buijs et al. (Citation2011) and Trocino et al. (Citation2014) could not show elevation in the corticosterone concentration of hard faeces when the rabbits were kept in pens. When the corticosterone concentration was measured in the hair of rabbits Trocino et al. (Citation2014) found significantly higher values in penned rabbits than in caged ones. Similarly to our results Trocino et al. (Citation2014) find that the corticosterone concentration measured in hair of rabbits increased with the age, but only in case of collective pens. The best feed conversion ratio was mainly obtained with caged-rabbits (Rommers and Meijerhof Citation1998; Postollec et al. Citation2001; Princz et al. Citation2009), even though some authors did not observe significant differences between cage and pen-housed rabbits (Maertens and Van Herck Citation2000; Xiccato, Trocino, Majolini, et al. Citation2013; Matics et al. Citation2014).
Compared to caged-rabbits, penned-rabbits showed a significantly higher mortality from seven- to nine-week period onward, leading to a 31% mortality when considering the whole fattening period (p < .001; Table ). In pens, despite the large size of the floor and platforms, most rabbits stayed under the platforms, because it is considered a safe place for the rabbits (Beja et al. Citation2007; Princz et al. Citation2008). Because of this behaviour the stocking density increased under the platforms, with a lot of rabbits defaecating and urinating, thus soiling the plastic-mesh floor of the pens. The positioning of the platforms on the sides of the pens could have favoured this negative result. The rabbits could lick the floor as a part of their exploration activity, and so they quickly infect themselves and their pen-mates. The floors of cages were wire-meshed, so the risk of contamination was lower than in the pens. As for live performance, also for mortality literature data are not univocal; whereas some authors (Dal Bosco et al. Citation2002; Jehl et al. Citation2003) found large differences in mortality between caged and penned rabbits, others (Rommers and Meijerhof Citation1998; Maertens and Van Herck Citation2000; Xiccato, Trocino, Majolini, et al. Citation2013) observed low mortality in both groups.
Carcase and physical traits
Results of carcase traits are summarised in Table . Slaughter weight (SW), chilled carcase (CC) weight, reference carcase (RC) weight, and carcase parts weight were significantly higher in cage than in pen-housed rabbits. These findings are related to the significant difference in body weight at the end of fattening period (Table ). These results confirm those found previously (Dalle Zotte et al. Citation2009, Szendrő et al. Citation2009).
Table 2. Carcase traits of growing rabbits housed in cage or pen.
Chilled carcase yield (%SW) and reference carcase yield (%CC) of C group were significantly higher than that of P group (p < .05 and p < .001, respectively). Also for these traits, literature cited results are not consistent, Dal Bosco et al. (Citation2002), Jehl et al. (Citation2003), Szendrő et al. (Citation2009), and Combes et al. (Citation2010) did not find highly significant differences. In 10 out of 14 studies, the dressing out percentage of rabbits kept in larger groups decreased numerically by 0.3%–2.1%, however, the differences were not significant (Szendrő and Dalle Zotte Citation2011). Lambertini et al. (Citation2001) and Metzger et al. (Citation2003) received a significant difference between the two groups with better results in cage. Differences in carcase yield could be related to the increased movement possibilities in pens (Szendrő and Dalle Zotte Citation2011). It needs to be considered that elevated platforms were also installed into the cages and the rabbits could jump up and down. However, as the size of pens was larger, the distance between the feeders and drinkers was longer, and due to the higher stress hormone level, it can be assumed that aggressive behaviour was more frequent in pens resulting in a larger number of rabbits forced to run from the attacker, the rabbits in pens could have to move more. As a result of presumably higher physical activity of P rabbits, they showed larger ratio of hind part to reference carcase (p < .001) and lower percentage of perirenal fat (p < .001) compared to C rabbits. With exception of the study of Xiccato, Trocino, Majolini, et al. (Citation2013), where no difference in hind legs ratio was found between rabbits housed in bicellular and collective cages, according to most studies with the increasing group size, an increase in ratio of hind part and a decrease in fat depots was observed (Dal Bosco et al. Citation2002; Dalle Zotte et al. Citation2009; Gondret et al. Citation2009; Szendrő et al. Citation2009; Combes et al. Citation2010; Matics et al. Citation2014; Dalle Zotte et al. Citation2015). In our study, similarly to Dalle Zotte et al. (Citation2015), the ratio of the mid part to reference carcase was lower in P than C rabbits (p < .05), but for this trait, contradictory results were observed (Dal Bosco et al. Citation2002; Dalle Zotte et al. Citation2009; Szendrő et al. Citation2009; Combes et al. Citation2010). When authors compare cage and pen, it should be noted that in each experiment, their size, group size, installation, flooring, stocking density, etc., may be different, which may explain the contradictory results.
The more intense physical activity of pen-housed rabbits resulted in heavier and thicker bones of HL (Table ). Significant differences were found in femur and tibia weight, their percentage to HL, and their minor diameter; however, no statistical difference was found in femur length and fracture toughness. As a result, the HL meat to bones ratio was higher in C rabbits than in the P group (p < .001), confirming previous research in physical traits (Matics et al. Citation2014; Dalle Zotte et al. Citation2009, Citation2015).
Table 3. Effect of housing system on hind leg (HL) bones traits.
Meat quality
Experimental treatment did not modify the pHu and L*a*b* colour values of meat samples (Table ). Generally, the meat pHu values tended to decrease with increasing group size (Szendrő and Dalle Zotte Citation2011), because the more intense agonistic behaviour and the higher stress of rabbits housed in pens could induce an adaptive stress response in the muscle to control the greater amount of free radicals produced by metabolism, and the reduced consumption of the glycogen available for subsequent post-mortem glycolysis, hence lowering pHu values. At the same time the greater locomotory activity of pen housed rabbits should have increased muscle oxidative metabolism and consequently higher pHu values (Ouhayoun Citation1998). So there are two contradictory phenomena in the larger groups (in pens), which can lead to conflicting results. In five studies the pHu of Biceps femoris (BF) was independent of the group size (Dalle Zotte et al. Citation2009; Szendrő et al. Citation2009; Combes et al. Citation2010; Xiccato, Trocino, Majolini et al. Citation2013; Dalle Zotte et al. Citation2015). Dal Bosco et al. (Citation2002) and Dalle Zotte et al. (Citation2009) found higher pHu values in LTL muscle in caged rabbits, whereas no difference in LTL muscle pHu was observed by Combes et al. (Citation2010) and Xiccato, Trocino, Majolini, et al. (Citation2013).
Table 4. Meat quality and rheological traits of hind leg (HL) meat of rabbits housed in cage or pen.
As for the L*a*b* colour values, Dal Bosco et al. (Citation2002), Dalle Zotte et al. (Citation2009) and Szendrő et al. (Citation2009) found higher values in caged rabbits, whereas Combes et al. (Citation2010) observed opposite results, and Xiccato, Trocino, Majolini et al. (Citation2013) and Xiccato, Trocino, Filiou (Citation2013) observed no differences. A relationship can be found between the pH and L* values of meat (Szendrő and Dalle Zotte Citation2011), so if the pH was not affected by the housing system, then it was expected to find no change in the L* value. Based on the contradicting results until now collected from the literature, the effect of group size on redness (a*) and yellowness (b*) values is still unclear (Szendrő and Dalle Zotte Citation2011). In larger groups the a* value either decreased (Dal Bosco et al. Citation2002; Dalle Zotte et al. Citation2009) or increased (Combes et al. Citation2010). As for the b* value, it was found to increase (Dal Bosco et al. Citation2002; Xiccato, Trocino, Majolini, et al. Citation2013) but also to decrease (Dalle Zotte et al. Citation2009) with group size. The possibility of jumping exercise also did not modify the colour of the meat (Gondret et al. Citation2009).
Cooking loss and Warner-Bratzler shear force (WBSF) measurements were not affected by the experimental treatments (Table ). The values of WBSF in different meat samples were independent of group size in all studies (Dalle Zotte et al. Citation2009; Combes et al. Citation2010; Xiccato, Trocino, Filiou, et al. Citation2013; Matics et al. Citation2014). Notwithstanding that the water content of P HL and LTL meat were significantly higher than that of C ones (p < .01 and p < .05, respectively; Table ), the cooking loss did not differ, indicating that other factors come into play for the water holding capacity. Since there is a close negative correlation between water content and fat content, the meat lipid content was higher in C group than in P rabbits, however, the difference was statistically significant in HL meat, only (Table ). These results were consistent with the results of percentage of perirenal fat and expected, as the lipid content of meat is in close connection with the physical activity (Szendrő and Dalle Zotte Citation2011). However, in the literature, some similar studies did not observe significant differences, just trends (Dal Bosco et al. Citation2002; Szendrő et al. Citation2009; Combes et al. Citation2010; Matics et al. Citation2014; Dalle Zotte et al. Citation2015).
Table 5. Proximate composition and haem iron content of meat samples.
The protein content, similarly to ash content, in rabbit meats is difficult to change with varying the housing conditions (Dal Bosco et al. Citation2002; Szendrő et al. Citation2009; Combes et al. Citation2010; Matics et al. Citation2014; Dalle Zotte et al. Citation2015), unless to use extreme conditions, thus the absence of significant difference in protein content between meat of rabbits housed in cage or pen was expected.
The haem iron content in HL meat was higher in pen housed rabbits (5.29 vs. 4.22 mg/kg, for P and C, respectively; p < .01), whereas it did not differ in LTL meat (Table ). However, some authors did not find significant differences in haem iron content among experimental groups (Dal Bosco et al. Citation2002; Matics et al. Citation2014; Dalle Zotte et al. Citation2015).
Cost evaluation
Cost evaluation did not observe large differences between the C and P groups. Considering one thousand rabbits, the price of weaned rabbits was identical (1824 €), the cost of feed was 1442 €, and 1355 € for C and P, respectively, and the total cost (weaned rabbits + feed + 30% feed cost) was 3884 € and 3760 € for C and P, respectively.
On the contrary, the high mortality in P group differentiated the income when considering the slaughtered rabbits (4209 € and 2950 €, for C and P, respectively). This is why production in large cages is more profitable compared to large pens. Considering slaughterhouse incomes, small difference was found between the two groups (6854 € and 6786 €, for C and P, respectively, referred to 1000 2.5 kg live weight rabbits).
Conclusions
Compared to large, enriched pen with large groups, housing growing rabbits in large enriched cage with small groups has been shown to be advantageous, resulting in better growth performance, hind leg meatiness and economically, whereas large pen housing lead to lower carcase fatness through the worse live performance but to higher haem iron in hind leg meat. No differences were observed for the other analysed meat quality traits. Rearing rabbits in large groups challenges animal welfare especially at increasing age and pen design also may play an important role.
Ethical approval
The study was approved by the Ethical Committee of Kaposvár University (Hungary). All animals were handled according to the principles stated in the EC Directive 86/609/EEC regarding the protection of animals used for experimental and other scientific purposes.
Disclosure statement
No potential conflict of interest was reported by the authors.
The authors confirm that the manuscript has been read and approved by all named authors and that there are no other people who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Additional information
Funding
References
- AOAC. 1995. Official methods of analysis. 15th ed. Washington (DC): Association of Official Analytical Chemists.
- Beja P, Pais M, Palma L. 2007. Rabbit Oryctolagus cuniculus habitats in Mediterranean scrubland: The role of scrub structure and composition. Wildl Biol. 13:28–37.
- Blasco A, Ouhayoun J. 1996. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 4:93–99.
- Buijs S, Keeling LJ, Rettenbacher S, Maertens L, Tuyttens FAM. 2011. Glucocorticoid metabolites in rabbit faeces-influence of environmental enrichment and cage size. Physiol Behav. 104:469–473.
- Chu L, Garner JP, Mench JA. 2004. A behavioral comparison of New Zealand White rabbits (Oryctolagus cuniculus) housed individually or in pairs in conventional laboratory cages. Appl Anim Behav Sci. 85:121–139.
- CIE. 1976. Recommendations on uniform color spaces, color differences, equations. Psychometric color terms. Suppl. 2, CIE Publications n.15, Paris, France: Commission Internationale de l'Eclairage, Colorimétry.
- Combes S, Postollec G, Cauquil L, Gidenne T. 2010. Influence of cage or pen housing on carcass traits and meat quality of rabbit. Animal 4:295–302.
- Compassion in World Farming. 2017. Good rabbit award. URL: https://www.ciwf.org.uk/news/2015/06/good-rabbit-award-launched [accessed 2018 January 31] https://www.compassioninfoodbusiness.com/our-work/
- Compassion in World Farming. 2017. Progress for Europe’s rabbits. URL: https://www.ciwf.org.uk/news/2017/01/progress-for-europes-rabbits [accessed 2018 January 31].
- Dal Bosco A, Castellini C, Mugnai D. 2002. Rearing rabbits on a wire net floor or straw litter: behaviour, growth and meat quality traits. Livestock Product Sci. 75:149–156.
- Dalle Zotte A, Princz Z, Metzger S, Szabó A, Radnai I, Biró-Németh E, Orova Z, Szendrő Z. 2009. Response of fattening rabbits reared under different housing conditions. 2. Carcass and meat quality. Livestock Sci. 122:39–47.
- Dalle Zotte A, Szendrő K, Gerencsér Z, Szendrő Z, Cullere M, Odermatt M, Radnai I, Matics Z. 2015. Effect of genotype, housing system and hay supplementation on carcass traits and meat quality of growing rabbits. Meat Sci. 110:126–134.
- Decree BR. 2014. Concerning the welfare of rabbits in breeding units (C-2014/24303). J Belgian Govern. 19.08.2014, 6061–6064. URL: http://www.vvsg.be/veiligheid/brandweer/brandweerhervorming/Documents/KB%202014.06.29%20personeelsplan.pdf [accessed 2017 January 8].
- Dorning J, Harris S. 2017. The welfare of farmed rabbits in commercial production systems. https://www.ciwf.org.uk/media/7430014/the-welfare-of-farmed-rabbits-in-commercial-production-systems-a-scientific-review-february-2017.pdf. (accessed 2018 October 10)
- EFSA. 2005. The impact of the current housing and husbandry systems on the health and welfare of farmed domestic rabbits. EFSA J. 267:1–140.
- Gidenne T, Garreau H, Drouilhet L, Aubert C, Maertens L. 2017. Improving feed efficiency in rabbit production, a review on nutritional, technico-economical, genetic and environmental aspects. Anim Feed Sci Technol. 225:109–122.
- Gondret F, Hernandez P, Rémignon H, Combes S. 2009. Skeletal muscle adaptations and biomechanical properties of tendons in response to jump exercise in rabbits. J Anim Sci. 87:544–553.
- González-Mariscal G, Burt AS, Nowak R. 2017. Behavioral and neuroendocrine indicators of well-being in farm and laboratory mammals. In: Pfaff DW, Joëls M, editors. Hormones, brain and behavior. 3rd ed. Amsterdam (The Netherlands): Elsevier Inc.; p. 453–485.
- Held SDE, Turner RJ, Wooton RJ. 1995. Choice of laboratory rabbits for individual or group-housing. Appl Anim Behav Sci. 46:81–91.
- Hornsey HC. 1956. The colour of cooked cured pork. I. Estimation of the nitric oxide haem pigments. J Sci Food Agric. 7:534–540.
- Jehl N, Meplain E, Mirabito L, Combes S. 2003. Incidence de trois modes de logement sur les performances zootechniques et la qualité de la viande de lapin. INRA-ITAVI 2003. Proceedings of the 10ème Journées de la Recherche Cunicole, November 19–20; Paris, France; p. 181–184.
- Lambertini L, Vignola G, Zaghini G. 2001. Alternative pen housing system for fattening rabbits: Effect of density and litter. World Rabbit Sci. 9:141–147.
- Maertens L, De Groote G. 1984. Influence of the number of fryer rabbits per cage on their performance. J Appl Rabbit Res. 7:151–155.
- Maertens L, Van Herck A. 2000. Performance of weaned rabbits raised in pens or in classical cages: First results. Proceedings of the 7th World Rabbit Congress; July 4–7; Valencia, Spain; p. 435–440.
- Martrenchar A, Boilletot E, Cotte JP, Morisse JP. 2001. Wire floor pens as an alternative to metallic cages in fattening rabbits: Influence on some welfare traits. Anim Welf. 10:153–161.
- Matics Z, Szendrő Z, Odermatt M, Gerencsér Z, Nagy I, Radnai I, Dalle Zotte A. 2014. Effect of housing conditions on production, carcass and meat quality traits of growing rabbits. Meat Sci. 96:41–46.
- Metzger S, Kustos K, Szendrő Z, Szabó A, Eiben C, Nagy I. 2003. The effect of housing system on carcass traits and meat quality of rabbit. World Rabbit Sci. 11:1–11.
- Ouhayoun J. 1998. Influence of the diet on rabbit meat quality. In: De Blas C, Wisemann J. editors. The nutrition of the rabbit. Oxon (UK): CABI Publishing, 177–196.
- Palme R, Robia C, Messmann S, Hofer J, Möstl E. 1999. Measurement of faecal cortisol metabolites in ruminants: non-invasive parameter of adrenocortical function. Wiener Tierärztliche Monatsschrift 86:237–241.
- Podberscek AL, Blackshaw JK, Beattie AW. 1991. The behaviour of group penned and individually caged laboratory rabbits. Appl Anim Behav Sci. 28:353–363.
- Postollec G, Boilletot E, Maurice R, Michel V. 2001. The effect of housing system on the behaviour and growth parameters of fattening rabbits. Anim Welf. 15:105–111.
- Princz Z, Dalle Zotte A, Metzger S, Radnai I, Biró-Németh E, Orova Z, Szendrő Z. 2009. Response of fattening rabbits reared under different housing conditions. 1. Live performance and health status. Livestock Sci. 121:86–91.
- Princz Z, Radnai I, Biró-Németh E, Matics Z, Gerencsér Z, Nagy I, Szendrő Z. 2008. Effect of cage height on the welfare of growing rabbits. Appl Anim Behav Sci. 114:284–295.
- Rommers J, Meijerhof R. 1998. Effect of group size on performance, bone strength and skin lesions of meat rabbits housed under commercial conditions. World Rabbit Sci. 6:299–302.
- Szendrő Z, Dalle Zotte A. 2011. Effect of housing conditions on production and behaviour of growing meat rabbits: a review. Livestock Sci. 137:296–303.
- Szendrő Z, Mikó A, Odermatt M, Gerencsér Z, Radnai I, Dezséry B, Garai É, Nagy I, Szendrő K, Matics Z. 2013. Comparison of performance and welfare of single-caged and group-housed rabbit does. Animal 7:463–468.
- Szendrő Z, Princz Z, Romvári R, Locsmándi L, Szabó A, Bázár G, Radnai I, Biró-Németh E, Matics Z, Nagy I. 2009. Effect of group size and stocking density on productive, carcass and meat quality traits and aggression of growing rabbits. World Rabbit Sci. 17:153–162.
- Swiss Federal Council. 2005. Animal Welfare Act https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiwtLeTxPvdAhXC_qQKHW2-BqIQFjAAegQIARAC&url=https%3A%2F%2Fwww.blv.admin.ch%2Fdam%2Fblv%2Fen%2Fdokumente%2Ftiere%2Frechts-und-vollzugsgrundlagen%2Fanimal-welfare-act-tschg.pdf.download.pdf%2FAnimal_Welfare_Act_(TSchG)_position_as_at_1.1.2011.pdf&usg=AOvVaw1lGnWxg6t3aGE7qd7hp9nv (accessed 2018 October 10).
- Trocino A, Xiccato G. 2006. Animal welfare in reared rabbits: A review with emphasis on housing systems. World Rabbit Sci. 14:77–93.
- Trocino A, Filiou e, Tazzoli M, Bertotto D, Negrato E, Xiccato G. 2014. Behaviour and welfare of growing rabbits housed in cage and pen. Livestock Sci. 167:305–314.
- Turner VP, Buijs S, Rommers J, Tessier M. 2017. Code of practice for the care and handling of rabbits: Review of scientific research on priority issues. Québec, Canada: National Farm Animal Care Committee.
- Xiccato G, Trocino A, Filiou E, Majolini D, Tazzoli M, Zuffellato A. 2013. Bicellular cage vs. collective pen housing for rabbits: Growth performance, carcass and meat quality. Livestock Science. 155:407–414.
- Xiccato G, Trocino G, Majolini D, Tazzoli M, Zuffellato A. 2013. Housing of growing rabbits in individual, bicellular and collective cages: growth performance, carcass traits and meat quality. Animal. 7:627–632.