 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The effects of dietary supplementation of tannins mix (quebracho and chestnut) in rabbit diet were evaluated for productive performances, health parameters and digestibility in order to quantify their practical utilisation in the rearing system. One-hundred and twenty Martini group hybrid rabbits of 30 days old were fed four different diets for 60 days. The diets were formulated as: basal diet (negative control, C), basal diet supplemented by 0.3% of tannins mix (T0.3), basal diet supplemented by 0.6% of tannins mix (T0.6) and basal diet supplemented with coccidiostat (positive control, CC). Live performances did not show any significant differences, moreover, no significant differences were observed for all carcase traits except for gastrointestinal tract (p = .015, lowest values for T0.3 diet). Also, digestibility of the feed and faecal microbial load was not influenced by tannins addition. Slight differences between the diets were detected in catalase and glutathione peroxidase concentrations in plasma, as a common trend was revealed with higher values of C than the other diets. From an economical point of view, T0.3 diet showed to be more profitable than CC and T0.6. Tannins addition might be taken into account as potential feed additive in rabbit feeds, as it does not affect negatively the productive performances, digestibility and induce a slight increase of antioxidant status.
On an economical point of view tannin diet showed to be more profitable than a diet added with coccidiostat.
Addition of tannins in rabbits’ feed did not affect negatively productive performances and digestibility.
Tannins represent a valuable feed additive in rabbit farming as a slightly increase of antioxidant status was induced.
Highlights
Introduction
Tannins are a complex group of polyphenolic compounds, which are classified into three major groups as hydrolysable tannins, condensed tannins and phlorotannins (Huang et al. Citation2018). These molecules, which differ in chemical structure as well as biological characteristics, were found in terrestrial plants and algae as a defence against external attacks. Hydrolysable and condensed (also called non-hydrolysable or proanthocyanidins) are common in temperate and tropical woods respectively, while, phlorotannins are found only in marine brown algae (Chung et al. Citation1998; Mueller-Harvey Citation2006; Huang et al. Citation2018).
Consumption of tannins could have several negative effects including hepatotoxicity, toxic nephrosis, feed intake depression and growth reduction (Mueller-Harvey Citation2006), this negative effect has been related to the possible reduction of protein digestibility, digestive enzymes activity and damages of intestinal mucosa (Mueller-Harvey Citation2006).
In the last decades, tannins were largely studied for their potential use in animal nutrition both for their antimicrobial and antioxidant properties (Mueller-Harvey Citation2006; Tosi et al. Citation2013; Huang et al. Citation2018).
Generally, tannins exhibited strong antioxidant activity (Chung et al. Citation1998) and could prevent the colonisation of intestinal bacteria, protozoa and viruses (Min and Hart Citation2003; Biagi et al. Citation2010; Elizondo et al. Citation2010).
Several tannin species used as feed supplementation are derived from chestnut (Castanea sativa Mill.) and quebracho (Schinopsis spp.) woods. It has been shown that these two types of tannins, belonged respectively to the hydrolysable and condensed family, affected several different characteristics. Ranucci et al. (Citation2015) and Liu et al. (Citation2016) reported that dietary chestnut tannin reduced lipid oxidation in pigs, as well as, decrease malondialdehyde concentration in meat, serum and liver of heat-stressed lambs. Similarly, Luciano et al. (Citation2009) reported that quebracho tannins affected colour stability during refrigerated storage of sheep meat.
Tannins represent a valuable feed additive also in rabbit farming, as could positively affect rabbits in an enteropathy infected environment, reducing mortality rate and enhancing live weight as reported by Maertens and Struklec (Citation2010) and could have various effects as health or growth enhancer or on physical-chemical characteristics of meat (Dalle Zotte and Cossu Citation2009; Gai et al. Citation2009; Liu et al. Citation2009; Citation2011; Citation2012; Parisi et al. Citation2018).
Most of the previously mentioned studies reported several quantifications on dietary supplementation of one single type of tannin and no data are available on the effect of a mix of hydrolysable and condensed tannins.
For these reasons, the main aim of our study was to evaluate the effects of two dietary doses of chestnut and quebracho tannins mix in rabbit diet. Live performances, digestibility, carcase traits, antioxidant status, microbial load and economic value were evaluated during the growing period.
Material and methods
Animals, housing systems and diets
A total of 160 weaned hybrid rabbits (Martini Group, 30 days of age) were employed in this research study. The weaned rabbits weighing 826 ± 85.05 g were randomly allotted into the four experimental groups (30 and 10 rabbits per group for the first and the second experiment, respectively). One group was fed with a commercial pelleted diet (first control group, C), and the other groups received the same pellet supplemented by coccidiostat (second control group CC) and by a commercial mix of chestnut (Castanea sativa Mill.) and quebracho (Schinopsis spp.) tannins (Silvateam NUTRI P powder®, Ledoga S.r.l., Cuneo, Italy) at the concentrations of 0.3% (group T0.3), or 0.6% (group T0.6). Water was available ad libitum from nipple drinkers. The compositions of the diets are reported in Table .
Table 1. Diets chemical compositions.
The first trial was carried out at a local farm (located in Tuscany) with a total of 120 rabbits (30 per diet treatment). Rabbits were housed in indoor colony cages (3 rabbits/cage 60 × 40 × 32 cm) during summer (temperature: 23–30 °C, mean 27 °C; relative humidity: 43–79%, mean 60%; photoperiod of 16 h light phase) and received ad libitum feed.
Live performances were recorded, as well as, plasma collections were made. Rabbits, after 60 days of diet, were slaughtered (see section below) and carcase traits were quantified. Furthermore, economic efficiency was calculated.
The second trial was carried out at the Department of Veterinary Sciences (University of Pisa, Pisa, Italy) with a total of forty rabbits (10 per diet treatment). Animals were reared in individual cages in order to sample individual faeces used to quantify digestibility and evaluate the microbial load.
At day 35 of life, all rabbits were vaccinated by intradermal route by means of a live attenuated myxomatosis virus vaccine (Dervaximyxo SG33, Merial). A second dose was provided after 6 weeks.
In both the experiments body weights and feed intake were registered weekly, morbidity and mortality episodes were monitored daily.
The experimental protocol was designed according to the guidelines of the current European and Italian laws on the care and use of experimental animals (European directive 2010/63/UE, put into law in Italy with D. Lgs. 26/2014). Chemical composition of the tannin mix was: 750 g/kg tannins, 150 g/kg non tannin, 80 g/kg water, and 20 g/kg insolubles (pH 4, 0.1 mg/mL solution) on FM basis, ratio chestnut/quebracho tannins was under producer’s patent. The total tannin content was determined according to (ISO Citation14088:2012).
Slaughter procedures and carcases traits
Ten rabbits from each experimental group (first trial) were slaughtered at 90 days of age, at the average weight of 2784 ± 230.16, with a fasting of 6 h. Rabbits were electrically stunned and immediately bled. Slaughter and carcase dissection procedures followed the World Rabbit Science Association recommendations (Blasco and Ouhayoun Citation1996).
The pH after 45 min and 24 h of chilling was recorded on the Longissimus thoracis et lumborum (measured between 6th and 7th lumbar vertebrae) and in Biceps femoris muscle (pH meter pH 80 equipped with a S7 2 PORE SLIM electrode; XS instruments, Carpi, MO, Italy).
Plasma samples collection and enzyme quantifications
On day 0, 20, 40 and 60 of the experimental trial, 10 rabbits per group (first trial) were randomly chosen and blood samples were collected via the ear vein. Blood samples were transferred in microtube containing lithium heparin (Micro tube 1.3 mL LH, Sarstedt AG & Co., Nümbrecht, Germany) then centrifuged at 1500×g in a refrigerated centrifuge (TJ-6, Beckman Coulter, Indianapolis, US) at 4 °C for 10 min.
Plasma was harvested and stored at −20 °C until assayed. Plasma samples were analysed for the determinations of malondialdehyde (MDA), enzyme activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). Malondialdehyde content was quantified by TBARS method assay kit (Cayman Chemical Company, USA; No.700870), superoxide dismutase was determined using Superoxide Dismutase Assay kit (Cayman Chemical Company, USA; No.706002), catalase was determined using Catalase Assay kit (Cayman Chemical Company, USA; No.707002) and glutathione peroxidase was determined using Glutathione Peroxidase Assay kit (Cayman Chemical Company, USA; No.703102). Kits were performed as per manufacturer’s instructions.
Faeces collection and microbial determinations
From rabbits of the second trial (10 rabbits of each group, individually caged), 5 g of fresh hard faeces were aseptically collected in a sterile plastic tube and immediately processed. Collection of samples was performed at day 0, and every two weeks (days 14, 28, 42 and 56 of the trial). Each individual sample was diluted in 45 ml of sterile saline solution (1:10 w:v) and homogenised for further dilutions (1:100–1:10,000,000). Clostridium perfringens was enumerated on Tryptose Sulphite Cycloserine (TSC) agar added with egg yolk and Perfringens Selective Supplement containing D-cycloserine (37 °C for 48 h, anaerobic conditions); Escherichia coli on Tryptone Bile X-Glucuronide agar (TBX) (44 °C for 48 h, aerobic conditions); Enterobacteriaceae on Violet Red Bile Glucose Agar (VRBGA) (37 °C for 24 h, aerobic conditions); Bifidobacterium spp. on TOS-propionate agar base added with Lithium-Mupirocin (MUP) selective supplement (TOS-MUP) (37 °C for 72 h, anaerobic conditions), and Bacteroides spp. belonging to the Bacteroides fragilis group on Bacteroides Bile Esculin agar (BBE) (35 °C for 48 h, anaerobic conditions). All media and supplements were purchased from Thermo Fisher Scientific (Milan, Italy), except for TOS-MUP and BBE, which were purchased from Laboratorios Conda (Madrid, Spain). Agar plates were spreaded with 0.2 mL of each dilution and incubated at the above-mentioned temperatures. Only for TSC, the pour plate technique was employed adding to the plates 1 mL of each dilution. After incubation, colonies with typical morphologies were enumerated. Results were expressed as log CFU/g of faecal sample.
Digestibility trial
Rabbits of the second trial were used to determine the total tract apparent digestibility (TTAD), according to the European standardised method (Pérez et al. Citation1995). At the 46th day, individual faeces were collected for four consecutive days and dried in an oven (quantification of dry matter, DM) and maintained frozen (−20 °C) until analysis. Then, individual samples were pooled and the TTAD of dry matter, organic matter, crude protein, ether extract, neutral detergent fibre, acid detergent fibre, cellulose, hemicelluloses and gross energy of the experimental diets was measured. Feed and faeces were analysed according to the AOAC (Citation1995) methods (protocol numbers 930.15, 934.01, 976.06, 920.39, 942.05 and 962.09, respectively for dry matter, organic matter, crude protein, ether extract, ash and crude fibre) and Van Soest et al. (Citation1991) for neutral detergent fibre, acid detergent fibre, acid detergent lignin and hemicelluloses.
Economic efficiency
The economic efficiency for all experimental diets was calculated as the ratio between income (price of weight gain) and cost of feed consumed during the experimental period (Abdella et al. Citation1988), these evaluations were based on farmed animals data.
In particular, economic efficiency was calculated from the equation of Asar et al. (Citation2010):
where
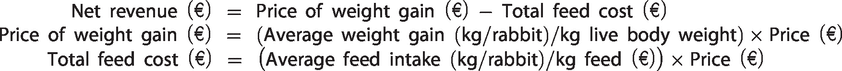
It is important to specify that each individual rabbit received the same costs including labour, veterinary supervision, housing, water and electricity and miscellaneous cost. Hence, all of these parameters were considered fixed costs for all the diets. For the price/kg live body weight was chosen the price on the market of Forlì (Italy) as reference market for the Italian wholesale in the rabbit sector (price referred to December 2017).
Statistical analyses
Rabbit live performances (body weights on individual data, feed intake and feed conversion ratio on cage), carcase traits, enzymes quantifications, microbial determinations, digestibility were statistically analysed via one-way ANOVA. Tukey’s test was used to determine the statistical differences between diets when p < .05. The variability was expressed as root mean square error (RMSE). Statistical analyses were performed with R software (R Core Team Citation2015).
Results and discussion
Live performances and carcase traits
No significant differences were found in average daily weight gain, feed intake, feed conversion ratio and final live weight (Table ). Indeed, the rabbit fed with diets supplemented with tannins showed similar performance to those of rabbits fed with C and CC diets. In the past tannins were described as anti-nutritive substance and studied for their anti-nutritional effects which reduced the performance in growing animals (Smulikowska et al. Citation2001; Mueller-Harvey Citation2006). In contrast, several Authors showed lack of differences, or even increased ones, on the performances of rabbit and other species fed with diets supplemented by tannins (Liu et al. Citation2009; Citation2011; Omnes et al. Citation2017; Rivera-Méndez et al. Citation2017). Similar results were observed by Liu et al. (Citation2009) in rabbits fed with chestnut tannins who hypothesised that the reason might be related to the small amount of tannins supplemented in the diet. Furthermore, at low concentration seems that tannins played a role as a protective factor of the intestinal mucosa and as a control of peristaltic activity in presence of digestive disorders.
Table 2. Productive performance of rabbits.
Slaughter traits and carcase yields are reported in Table . No significant differences were observed for all carcase traits except for gastrointestinal tract, which showed lowest values in rabbits fed diet supplemented with 0.3% of tannin mix. This might be due to the fact that the rabbits of group T0.3 showed a lower feed intake than the other groups which could determine a lower development of gastrointestinal tract, even if this difference did not influence significantly the growth performances.
Table 3. Effect of diets on slaughter traits.
No differences in carcase traits were also highlighted by Liu et al. (Citation2009) in rabbits fed inclusion of natural extract of chestnut wood at 0.5% and 1.0%. As reported before, tannins could be anti-nutritive substances in relation to their concentration. For example, rabbits fed chestnut tannins raised under high ambient temperature (33 °C) showed an improvement in the live weight and in the hot carcase weight (Liu et al. Citation2012), on the other hand, Priolo et al. (Citation2000) reported that dietary condensed tannins affected negatively the weight, the yield and the fatness of lambs’ carcases.
Enzymes quantifications
At the beginning of the trial plasma parameters of rabbits were in the range of 4.43 ± 0.49, 33.85 ± 19.60, 237.50 ± 38.40 and 0.41 ± 0.27, for SOD, CAT, GPX and MDA, respectively. Plasma parameters of the following times collection were reported in Table .
Table 4. Effects of chestnut and quebracho tannins mix on superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and malondialdehyde (MDA) concentrations of plasma in rabbits.
Both SOD activity and MDA quantification did not show significant differences among diets. Catalase activity differed significantly among diets only at day 20 of the trial: T0.3 showed the highest catalase activity while CC the lowest value, intermediate values were reported for C and T0.6.
Glutathione peroxidase activity showed significant differences at all the sampling times: C showed always the highest value as well as T0.6 the lowest one. T0.3 values were ranged between C and T0.6, while CC showed at 60 days value comparable to T0.6.
SOD and MDA values are not in accordance with the results reported by Liu et al. (Citation2011), in which rabbits were fed with chestnut tannins (0.5% and 1%) for 21 days with the initial age of 40 days. They observed SOD activity increasing and MDA decreasing in plasma of rabbit fed with both the two different concentrations of tannin.
The CAT activity results at days 40 and 60 are in accordance with Liu et al. (Citation2011), who highlighted no significant differences in relation to the diet.
Further discordant data with the previously published research article was highlighted by GPX activity. In fact, Liu et al. (Citation2011) reported that rabbits fed chestnut tannin increased GPX activity in relation to the presence of the substance. This lack of concordance could be related to the different rearing condition as well as to the differences of the experimental protocol (i.e. type of tannins, dose, age of the animals, sampling times, length of diet).
At the best of our knowledge, the molecular mechanisms by which tannins could interact with the antioxidant enzymes is not clear. In rabbit it is reported the presence of proline-rich proteins (PRPs), tannin-binding salivary proteins (TBSPs), that are effective precipitators of tannins and their production is induced by ingestion of tannins (Shimada Citation2006). Furthermore, the metabolites 4-O-methyl gallic acid and pyrogallol were identified in the urine of rabbits fed a diet containing 0.5% gallic acid (Booth et al. Citation1959).
Digestibility and microbial determinations
No differences in dry matter intake and TTAD were highlighted in relation to the diet (Table ). These findings could play an important role in future formulation of rabbit feed as probably tannins do not affect the palatability of the feed. In facts, tannins could form complexes with salivary glycoproteins that generate an astringency sensation and subsequently affect the feed intake (Gidenne et al. Citation1998). Results on palatability of tannins in rabbit are contradictory. As reported by Dalle Zotte and Cossu (Citation2009) and Celia et al. (Citation2016) quebracho tannins or Digestarom® feed additive (that in part contained tannins) affected negatively the palatability of the feed, on the other hand, Dalle Zotte et al. (Citation2012) and Gai et al. (Citation2010) reported that chestnut tannins did not influence growth performances. As in our trial, a quebracho-chestnut tannins mix was employed we could hypothesise that the partial negative effects of the quebracho tannins were mitigated by the chestnut ones.
Table 5. Total tract apparent digestibility (TTAD, %) of the four experimental diets.
Figure shows microbial determinations. No statistical differences among diets for Bacteroides spp. (p-Value=.759), Escherichia coli (p-Value=.170), Enterobacteriaceae (p-Value=.112) and Bifidobacterium spp. (p-Value=.237) were detected. Furthermore, Clostridium perfringens loads were always under the detection limit (<2 log CFU-spores/g).
Figure 1. Microbiological determinations of hard faeces. C (control diet): black line; CC (control diet + coccidiostat): dark grey line; T0.3 (control diet +0.3% tannins mix): grey line; T0.6 (control diet +0.6% tannins mix): light grey line.
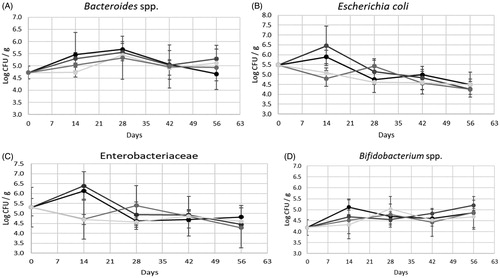
This is the first study reporting on the influence of tannins diet supplementation on rabbit hard faeces microorganism. In general, data on rabbit hard faeces microbial populations are scarce, especially those based on culture-dependent methods. This is mainly due to the fact that most of the Authors looked at the caecum flora instead, as a better indicator of rabbit health. However, Michelland et al. (Citation2010) detected no significant differences between bacterial populations of soft and hard faeces from those of caecal content. Later, Schoster et al. (Citation2013), studying a population of horses, another hindgut-fermenting species, highlighted the stability of microbial profile from caecal content samples to faecal samples. These findings lead to hypothesise that the microbial profile of faecal samples could be representative of distal gastrointestinal tract. Thus, as suggested by Kylie et al. (Citation2018) hard faeces samples could allow studying the caecal health status in an accurate and non-invasive way. Our findings show that 0.3% and 0.6% of tannin supplementation to rabbit diet did not affect the targeted microorganisms loads. As concerns, Clostridium perfringens enumeration, for all groups at all sampling times, loads were lower than the detection limit. This data reflected the good health status of animals included in the study and the optimal environmental management. As expected, E. coli and Enterobacteriaceae loads showed similar trends with no significative differences among groups or sampling times. Loads were always higher than 4 log CFU/g. However, these data are not representative of an E. coli infection since, as reported by Blanco et al. (Citation1996), E. coli from healthy rabbits generally display different characteristics from those associated with diarrhoeic phenomena. Looking at beneficial microorganisms, Bacteroides spp. represented the most abundant population, with loads always higher than 4.5 log CFU/g. As reported by Zeng et al. (Citation2015), this genus seems to be among those overrepresented in hard faeces and correlated to the healthy growth of rabbits. Finally, Bifidobacterium spp., also remained stable among different groups with concentration always higher than 4 log CFU/g.
The general lack of a decrease in microbial loads of faecal samples from rabbit groups fed with tannins-supplemented diets seems to contrast with the numerous reports on tannins antimicrobial activity (Huang et al. Citation2018). However, it is important to consider that most of the studies carried out in vitro analysis. On the other hand, the observed stability in microbial population of hard faeces could reflect the ability of gastrointestinal microorganisms to adapt to tannins presence in the diet, as reported by Smith and Mackie (Citation2004). Indeed, these Authors observed that after 3 weeks of intake, the proportion of tannins-resistant microorganisms, especially Gram negative, such as Enterobacteriaceae and Bacteroides spp., increased significantly in rat’s faecal bacterial population.
Economic efficiency
No statistical differences were highlighted for morbidity rate and mortality rate; thus, these two parameters were not taken into account for the calculation of economic efficiency.
The results of the economic evaluation are summarised in Table . Regarding total feed cost, control group C showed the lowest value followed by group T0.6, group T0.3 and group CC. Concerning the price of weight gain, the value/rabbit obtained for group T0.6 is the highest, followed by C, CC, and finally T0.3. Feeding dietary treatments with a commercial mix of chestnut and quebracho tannins resulted in a negative effect of improving net revenue, especially with concentrations of 0.3%. This disadvantage, in comparison with the control diet, has been due both to a higher feed intake (+3.43%) and a higher feed price (1.95%), together with a lower weight gain (−0.78%).
Table 6. Economic efficiency of the experimental diets.
On the other side, the T0.6 group presents just the same feed intake with a higher weight gain (1.14%), but higher feed price (3.89%): in this way the net revenue is almost the same of the control group. Finally, the best economic efficiency is for group C, followed by group T0.6, CC, T.03. The relative economic efficiency is lower for all the experimental diets in comparison with the control group: group T0.6 followed by groups T0.3 and CC.
These results indicate that inclusion of coccidiostat in rabbit diet was not profitable, from the economic point of view, where compared with other diets. At the same time, tannins can be used effectively as a feed ingredient in the diet of growing rabbits without a relevant negative effect on the economic efficiency only at a level of 0.6%.
Conclusions
Under the conditions assayed, no modifications were highlighted on growth, carcase traits, digestibility and economic efficiency of the rabbits, without inducing modification in microbial load of hard faeces. On the other hand, a positive effect was observed on enzyme concentration in plasma of rabbits fed the two diets added with tannins mix with profitable revenue than the coccidiostat added diet. However, due to limited literature on the topic, further research is needed to evaluate the potential practical application of this mix in rabbit rearing.
Acknowledgements
Authors thank Silvateam (Ledoga S.r.l.) for supplying tannins mix.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdella HM, Shalash SMM, Bouls NZ, Selim AD. 1988. Effect on growing rabbits of feeding different levels of crude protein. J Appl Rabbit Res. 11:252–256.
- AOAC. 1995. Official methods of analysis of the Association of Official Analytical Chemists, 15th ed. Arlington, Virgina, USA: the Association of Official Analytical Chemists, Inc.
- Asar MA, Osman M, Yakout HM, Safoat A. 2010. Utilization of corn-cob meal and faba bean straw in growing rabbits diets and their effects on performance, digestibility and economical efficiency. Egypt Poult Sci. 30:415–442.
- Biagi G, Cipollini I, Paulicks BR, Roth FX. 2010. Effect of tannins on growth performance and intestinal ecosystem in weaned piglets. Arch Anim Nutr. 64:121–135.
- Blanco JE, Blanco M, Blanco J, Mora A, Balaguer L, Mouriño M, Juarez A, Jansen WH. 1996. O serogroups, biotypes, and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J Clin Microbiol. 34:3101–3107.
- Blasco A, Ouhayoun J. 1996. Harmonization of criteria and terminology in rabbit meat research. Revised proposal. World Rabbit Sci. 4:93–99.
- Booth AN, Masri MS, Robbins DJ, Emerson OH, Jones FT, De Eds F. 1959. The metabolic fate of gallic acid and related compounds. J Biol Chem. 234:3014–3016.
- Celia C, Cullere M, Gerencsér Z, Matics Z, Giaccone V, Kovács M, Bónai A, Szendrő Z, Dalle Zotte A. 2016. Dietary supplementation of Digestarom® herbal formulation: effect on apparent digestibility, faecal and caecal microbial counts and live performance of growing rabbits. World Rabbit Sci. 24:95–105.
- Chung K-T, Wong TY, Wei C-I, Huang Y-W, Lin Y. 1998. Tannins and human health: a review. Crit Rev Food Sci Nutr. 38:421–464.
- Dalle Zotte A, Cossu M. 2009. Dietary inclusion of tannin extract from red quebracho trees (Schinopsis spp.) in the rabbit meat production. Ital J Anim Sci. 8:784–786.
- Dalle Zotte A, Matics Z, Bohatir P, Sartori A, Gerencsér Z, Szendrő Z. 2012. Effect of dietary supplementation of chestnut hydrolysable tannin on digestive efficiency, growth performance and meat quality in growing rabbits. In: 10th World Rabbit Congress; Sept 3-6, Sharm El- Sheikh, Egypt. p. 961–965.
- Elizondo AM, Mercado EC, Rabinovitz BC, Fernandez-Miyakawa ME. 2010. Effect of tannins on the in vitro growth of Clostridium perfringens. Vet Microbiol. 145:308–314.
- Gai F, Gasco L, Liu HW, Lussiana C, Brugiapaglia A, Masoero G, Zoccarato I. 2009. Effect of diet chestnut tannin supplementation on meat quality, fatty acid profile and lipid stability in broiler rabbits. Ital J Anim Sci. 8:787–789.
- Gai F, Gasco L, Schiavone A, Zoccarato I. 2010. Nutritional effects of chestnut tannins in poultry and rabbit. In: Petridis GK, editor. Tannins: Types, Foods Containing and Nutrition.: Hauppauge, NY: Nova Science Publishers, Inc.
- Gidenne T, Carabaño R, García J, de Blas C. 1998. Fibre digestion. In: De Blas C, Wiseman J, editors. Nutr Rabbit. Wallingford Oxon, UK: CABI Publishing; p. 241–253.
- Huang Q, Liu X, Zhao G, Hu T, Wang Y. 2018. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 4:137–150.
- ISO (14088:2012). Leather – Chemical tests – Quantitative analysis of tanning agents by filter method; [accessed 2017 Apr 05]
- Kylie J, Weese JS, Turner PV. 2018. Comparison of the fecal microbiota of domestic commercial meat, laboratory, companion, and shelter rabbits (Oryctolagus cuniculi). BMC Vet Res. 14:143.
- Liu H, Gai F, Gasco L, Brugiapaglia A, Lussiana C, Guo KJ, Tong JM, Zoccarato I. 2009. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 83:678–683.
- Liu H, Li K, Mingbin L, Zhao J, Xiong B. 2016. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 116:236–242.
- Liu H, Zhou D, Tong J, Vaddella V. 2012. Influence of chestnut tannins on welfare, carcass characteristics, meat quality, and lipid oxidation in rabbits under high ambient temperature. Meat Sci. 90:164–169.
- Liu HW, Dong XF, Tong JM, Zhang Q. 2011. A comparative study of growth performance and antioxidant status of rabbits when fed with or without chestnut tannins under high ambient temperature. Anim Feed Sci Technol. 164:89–95.
- Luciano G, Monahan FJ, Vasta V, Biondi L, Lanza M, Bella M, Pennisi P, Priolo A. 2009. Lamb meat colour stability as affected by dietary tannins. Ital J Anim Sci. 8:507–509.
- Maertens L, Struklec M. 2010. Technical note: preliminary results with a tannin extract on the performance and mortality of growing rabbits in an enteropathy infected environment. World Rabbit Sci. 14:189–192.
- Michelland RJ, Combes S, Monteils V, Cauquil L, Gidenne T, Fortun-Lamothe L. 2010. Molecular analysis of the bacterial community in digestive tract of rabbit. Anaerobe. 16:61–65.
- Min BR, Hart SP. 2003. Tannins for suppression of internal parasites. J Anim Sci. 81:E102–E109.
- Mueller-Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric. 86:2010–2037.
- Omnes M-H, Le Goasduff J, Le Delliou H, Le Bayon N, Quazuguel P, Robin JH. 2017. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquac Reports. 6:21–27.
- Parisi F, Mancini S, Mazzei M, Forzan M, Turchi B, Perrucci S, Poli A, Paci G. 2018. Effect of dietary supplementation of a mix of chestnut and quebracho tannins on intestinal morphology, bacterial load, Eimeria spp oocyst excretion and immune response after vaccination in rabbits. Am J Anim Vet Sci. 13:94–103
- Pérez JM, Lebas F, Gidenne T, Maertens L, Xiccato G, Parigi-Bini R, Dalle Zotte A, Cossu ME, Carazzolo A, Villamide MJ, et al. 1995. European reference method for in vivo determination of diet digestibility in rabbits. World Rabbit Sci. 3:41–43.
- Priolo A, Waghorn GC, Lanza M, Biondi L, Pennisi P. 2000. Polyethylene glycol as a means for reducing the impact of condensed tannins in carob pulp: Effects on lamb growth performance and meat quality. J Anim Sci. 78:810–816.
- R. Core Team 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Ranucci D, Beghelli D, Trabalza-Marinucci M, Branciari R, Forte C, Olivieri O, Badillo Pazmay GV, Cavallucci C, Acuti G. 2015. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 100:319–326.
- Rivera-Méndez C, Plascencia A, Torrentera N, Zinn RA. 2017. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J Appl Anim Res. 45:199–203.
- Schoster A, Arroyo LG, Staempfli HR, Weese JS. 2013. Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism. BMC Res Notes. 6:91.
- Shimada T. 2006. Salivary proteins as a defense against dietary tannins. J Chem Ecol. 32:1149–1163.
- Smith AH, Mackie RI. 2004. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl Environ Microbiol. 70:1104–1115.
- Smulikowska S, Pastuszewska B, Święch E, Ochtabińska A, Mieczkowska A, Nguyen V, Buraczewska L. 2001. Tannin content affects negatively nutritive value of pea for monogastrics. J Anim Feed Sci. 10:511–523.
- Tosi G, Massi P, Antongiovanni M, Buccioni A, Minieri A, Marenchino L, Mele M. 2013. Efficacy test of a hydrolysable tannin extract against necrotic enteritis in challenged broiler chickens. Ital J Anim Sci. 12:e62.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Zeng B, Han S, Wang P, Wen B, Jian W, Guo W, Yu Z, Du D, Fu X, Kong F, et al. 2015. The bacterial communities associated with fecal types and body weight of rex rabbits. Sci Rep. 5:9342
