Abstract
The effects of feeding low dietary crude protein (CP) and/or metabolisable energy (ME) with or without supplemental protease on growth performance, carcase characteristics and physiological responses in broiler chickens were investigated under cyclic heat stress condition. A total of 350 day-old male broiler chicks were fed with one of the following seven experimental diets: (1) recommended-CP and recommended-ME (RPE, served as control); (2) recommended-CP and low-ME (RPLE); (3) recommended-CP and low-ME with protease (RPLEP); (4) low-CP and recommended-ME (LPRE); (5) low-CP and recommended-ME with protease (LPREP); (6) low-CP and low-ME (LPE) and (7) low-CP and low-ME with protease (LPEP). From 22 to 42 d of age, half of the chickens from each dietary group were exposed to 34 ± 1 °C for 7 h daily (heat stress), whereas the other half were raised at constant 23 ± 2 °C (normal temperature). Supplementation of protease to RPLE, LPRE and LPE diets had no significant effects on feed intake (FI), weight gain (WG) or feed conversion ratio (FCR). Diet had no effect on serum glucose, total protein, certain acute phase proteins (APPs), corticosterone or breast yield. Regardless of protease supplementation, heat stressed birds had significantly lower FI, WG and breast yield, and higher FCR, APPs and corticosterone compared to birds raised in normal temperature. In conclusion, dietary supplementation of protease to low CP and/or ME diets showed negligible effects on growth performance, carcase characteristic and physiological responses in broiler chickens under heat stress condition. The inclusion of microbial protease in broiler diets could be considered by poultry industry as an effective nutritional tool for reducing ME or CP, in order to decrease abdominal fat deposition, improve feed efficiency and increase the profit margin.
Highlights
Protease supplementation has no specific help for broilers under heat stress.
Feeding low CP and/or ME diet is not stressful for broiler chickens.
Introduction
Heat stress (HS) is one of the most challenging environmental stressors. Heat-stressed birds are characterised by a number of behavioural, metabolic and physiological changes to maintain body homeostasis (Gonzalez-Esquerra and Leeson Citation2006; Mack et al. Citation2013). Physiologically, HS may elevate the production of heat shock protein 70 (HSP70), serum levels of corticosterone (CORT) and certain acute phase proteins (APPs) such as alpha-1 acid glycoprotein (AGP), ovotransferrin (OVT) and ceruloplasmin (CPN) (Najafi et al. Citation2015; Olubodun et al. Citation2015; Zulkifli et al. Citation2018). For birds under HS condition, dietary CP reduction was reported to be beneficial as protein metabolism was associated with higher heat increment than carbohydrate or lipid metabolisms (Musharaf and Latshaw Citation1999; Ojano-Dirain and Waldroup Citation2002). Diets reduced in crude protein (CP) or metabolisable energy (ME) have received a lot of attention in poultry nutrition to minimise the feed cost and environmental impact in the past few decades (Rahman et al. Citation2002; Zaman et al. Citation2008). However, reduction of CP and or ME beyond certain levels may exert a negative effect on feed intake (FI) and growth rate (Zaman et al. Citation2008; Dairo et al. Citation2010; Awad et al. Citation2015, Citation2017). Although broiler chickens may adapt to diet of low-CP or ME content by eating more feed in an attempt to meet their nutrient requirements (Payne Citation1967; Leeson et al. Citation1996), reduction below a certain level may not be compensated (Attia et al. Citation2011). Moreover, for birds under HS condition, feeding low-CP diets could be detrimental to their feed conversion ratio (FCR) (Zulkifli et al. Citation2018). Therefore, optimising broilers performance under HS condition is one of the major challenges encountered by poultry producers and nutritionists (Sandercock et al. Citation2001; de Souza et al. Citation2016). Protease supplementation may improve CP and energy digestibility in low-CP diets (Angel et al. Citation2011; Fru-Nji et al. Citation2011). Despite the increase in energy digestibility (≥100 kcal/kg) in previous studies (Freitas et al. Citation2011; Fru-Nji et al. Citation2011; Kalmendal and Tauson Citation2012), researchers have not treated energy metabolisability as affected by proteases supplementation in much detail. The enzyme supplementation has been reported to enhance ME digestibility in low-ME diets (Freitas et al. Citation2011; Kamel et al. Citation2015). Earlier studies from this laboratory have demonstrated that protease supplementation significantly increased ileal digestibility of energy, CP and amino acids (AA) (Law et al. Citation2015) and improved FCR, weight gain (WG), carcase yield and intestinal absorptive surface area in broilers raised in a hot and humid tropical environment (Law et al. Citation2018). Hence, protease supplementation with an appropriate reduction in dietary CP and ME may not only improve bird’s performance under HS condition and ensure the maximum utilisation of nutrients but also reduce the cost of production. Therefore, the objective of the present study was to investigate the effects of feeding low dietary CP and/or ME with or without supplemental protease on growth performance, carcase characteristics and physiological responses in broiler chickens raised under cyclic HS conditions. The hypothesis was that protease supplementation would increase the digestibility of energy, crude protein and amino acids in diets that reduced in CP and/or ME, and that increases in nutrients digestibility would lead to the same growth performance like standard CP and ME diets. We also hypothesised that feeding proteases supplemented low CP and/or ME diets would mitigate the effects of heat stress, resulting in lower HSP70 expression, and CORT and APPs concentrations.
Materials and methods
Enzyme compositions and activity
The protease used in this experiment was a purified microbial protease (Cibenza DP100; Novus International Inc., St. Charles, MO). The enzyme is an alkaline serine endopeptidase protease derived from Bacillus licheniformis with a protease activity of 600,000 units/g. The recommended inclusion rate by the manufacturer is 300 units/g of feed. The protease activity was determined using the method described by Jin et al. (Citation2000).
Birds and management
This study was undertaken following the guidelines of the Research Policy on Animal Ethics of the Universiti Putra Malaysia. A total of 350 one-day-old Cobb male broiler chicks were purchased from a local commercial hatchery. The chicks wing banded, weighed on arrival and randomly allocated in groups of 5 into 70 cages in a 3-tired battery cages (60 × 60 × 45 cm, length × width × height) with a wire mesh floor. The cages were located inside two completely identical temperature-controlled chambers (9.1 × 3.8 × 2.3 m, length × width × height). The floor space was 0.1 m2 per bird. Ambient temperature on day 1 was set at 32 ± 1 °C and gradually decreased until 23 ± 1 °C was reached by day 21. The average relative humidity during the experimental period ranged between 61 and 87%.
Experimental design and diets
The experimental design was a 2 × 7 factorial arrangement with two levels of temperature (normal and heat stress) and 7 experimental diets. From d 1, 350 birds (5 cages as 5 replicates) were allocated to one of the seven experimental diets as follow: (1) recommended-CP and recommended-ME (RPE, served as control diet); (2) recommended-CP and low-ME (PRLE); (3) recommended-CP and low-ME with protease (RPLEP); (4) low-CP and recommended-ME (LPRE); (5) low-CP and recommended-ME with protease (LPREP); (6) low-CP and low-ME (LPE) and (7) low-CP and low-ME with protease (LPEP) (Figure ). Low-CP diets were formulated to be nutritionally marginal in dietary protein with the addition of commercially available feed-grade AA to ensure that total lysine (Lys), methionine (Met), threonine (Thr), valine (Val) and tryptophan (Trp) levels met the Cobb 500 nutrient recommendations (Tables and ). Birds had free access to feed (mash form) and water and kept under continuous lighting throughout the experiment. The treatments groups were replicated in both chambers.
Figure 1. The experimental design and experimental diets. RPE: recommended-CP and recommended-ME diet; RPLE: recommended-CP and low-ME diet; LPRE: low-CP and recommended-ME diet; LPE: low-CP and low-ME diet; Diets (except RPE) compounded was subsequently divided into two, one serving as the control and the other supplemented with the enzyme to serve as the test diet; 1Heat stress was started from d 22–42 in chamber B.
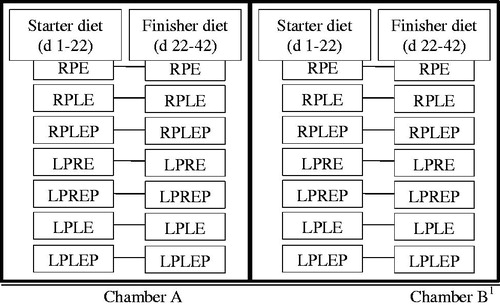
Table 1. Ingredient composition (as fed basis) of the starter and finisher diets.
Table 2. Nutrient composition of the starter and finisher diets.
Heat treatment
From day 22–42, birds in one chamber were exposed to constant 23 ± 2 °C (normal temperature), while the birds in the other chamber were exposed to 34 ± 1 °C for 7 h daily (10:00–17:00) (heat stress). The temperature increment to 34 °C took ∼45 ± 5 minutes and that was the point recorded as the start of the heat treatment.
Measurements
Body weight and FI (cage basis) data were recorded in day 1, 21 and 42. Feed conversion ratio was calculated accordingly after adjustment on mortality. Mortality was recorded upon occurrence daily. On day 42, two birds per cage were randomly selected (10 birds per diet-temperature subgroup) and removed with minimum disturbance to cage mates and killed by neck cut according to the halal method (Farouk et al. Citation2014) and exsanguination blood samples were collected. Following the blood sampling, brain samples were collected and placed in 5 mL screw-capped tube and snap frozen in liquid nitrogen for quantification of HSP 70 density expression (Soleimani et al. Citation2012). The blood samples were centrifuged at 3000 × g at 4 °C for 15 min. The obtained serum samples were stored at −80 °C until further analysis for glucose (GLU), triglycerides (TG), creatine kinase (CK), OVT, AGP, CPN and CORT. On day 43, two birds from each cage were selected (10 birds/diet-temperature subgroup) and weighed individually. Birds were slaughtered and defeathered in a rotary plucker. Non-deboned breast meat with the attached skin (both pectoralis major and minor) and abdominal fat were removed and weighed.
Determination of crude protein and amino acids
The CP content of the diets was determined following the procedure of AOAC (Citation1990). The AA content was determined using high-performance liquid chromatography, as described in details by Awad et al. (Citation2014).
Blood biochemical
The serum concentrations of blood biochemical were analysed with an automated chemistry analyser (Hitachi 902 Automatic Analyser; Hitachi, Tokyo, Japan) using commercial test kits (Roche Diagnostics, Basel, Switzerland): TP (Cat. No.: 11553836 316), TG (Cat. No.: 11488872 216), GLU (Cat. No.: 11447513 216) and CK (Cat. No.: 12132524 216).
Physiological stress indicators
The concentrations of CPN was measured using method for determining the rate of formation of a coloured product from CPN and the substrate, 1,4-phenylenediamine dihydrochloride and OVT using a radial immune diffusion method as previously described in details (Zulkifli et al. Citation2014). The AGP concentration was determined using a commercial ELISA kit specific to chicken (Life Diagnostics Inc., West Chester, PA). The CORT was measured by a commercial high sensitivity EIA kit (AC-15F1, IDS, Boldon, UK) according to the manufacturer’s instructions. The level of HSP 70 expression was determined as previously described (Soleimani et al. Citation2012) with some modifications. Briefly, brain sample (0.3 g, whole cerebrum) was homogenised using a homogeniser (IKA Ultra-Turrax, Staufen, Germany) with 1.5 mL of protein extraction buffer (20 Mm Tris, pH 7.5; 0.75 M sodium chloride) and 10 μl/mL protease inhibitor cocktail (P8340, Sigma Chemical Co., St. Louis, MO) followed by centrifugation at 20,000 × g for 30 min at 4 °C. The protein concentration and HSP 70 of the supernatants were quantified by the bicinchoninic acid protein assay kit (B9643, Sigma Chemical Co., St. Louis, MO). SDS-PAGE and Western Blotting were carried out and the final brain HSP 70 concentration was calculated as an arbitrary unit of band density relative to a total protein concentration of each sample.
Statistical analyses
All statistical analyses were carried out using the Statistical Analysis System Version 9.4 software (SAS Institute Inc., Cary, NC). One-way ANOVA was used to analyse the starter period (day 1–21) growth performance data. The growth performance data of the finisher (day 22–42) and overall (day 1–42) periods, APPs, CORT, HSP 70, serum metabolites and carcase traits data were subjected to two-way ANOVA using the General Linear Model (GLM) procedure of SAS to identify the main effects of diet, temperature and their interactions. When the interactions between the main effects were found to be significant, comparisons were made within each experimental variable. Comparison between means was done by Duncan’s multiple range test. Statistical significances are considered at p˂.05.
Results
Growth performance and mortality rate
The analysed nutrient values of CP and AA in the starter and grower diets were in close agreement with the calculated values (). The analysed protease activity in the relevant experimental diets for the RPLEP, LPREP and LPEP diets were respectively 315, 296 and 283 in the starter diets and respectively 298, 324 and 310 in the finisher diets. The protease activity was not detected in the control, LPRE, RELE or LPE diets. Diet had no effect on FI (p = .572) or WG (p = .092) in the broilers during the starter period. However, the FCR of the birds fed on LPEP diet was significantly (p = .010) greater compared with the control, RPLEP and LPREP fed counterparts but no difference with those fed with the RPLE, LPRE and LPE diets (). Diet showed no significant effect (p > .05) on mortality rate from day 1 to 21 ().
Table 3. Effect of diet on growth performance and mortality rate in broiler chickens from day 1 to 21.
There were no significant diet × temperature interactions for the FI (p = .670; p = .750), WG (p = .592; p = .794) and FCR (p = .078; p = .452) in the broilers during the finisher and overall periods (). Diets had no effect on the finisher FI (p = .446), finisher WG (p = .084) or the overall FI (p = .563). Birds fed by LPE and LPEP diets had poorer overall WG (p = .043) compared with those fed the control and RPLEP diets. However, no significant differences were found among the groups fed on RPLE, LPRE and LPREP diets. The finisher FCR (p = .003) of the birds fed on LPE diet were significantly greater than their RPLE and RPLEP counterparts but not different from the groups fed with the control, LPRE, LPREP and LPEP diets. In addition, birds fed on LPE and LPEP diets had significantly greater overall FCR (p < .001) than those fed with the control, RPLE and RPLEP diets. A similar trend of change was observed in the birds fed on diets LPRE and LPREP. Heat challenge reduced the FI (p < .001; p < .001) and WG (p < .001; p < .001) but increased the FCR (p = .001; p = .014) in the finisher and overall periods when compared to unchallenged birds. Neither diet nor heat treatment had significant effect (p > .05) on the mortality rate during the finisher period ().
Table 4. Effects of diet and temperature on growth performance and mortality rate in broiler chickens from day 22 to 42 and 1 to 42.
Serum metabolites
There were no significant diet × temperature interactions for GLU (p = .696), TG (p = .105) or TP (p = .355). Diet had no effect on serum concentrations of GLU (p = .173) and TP (p = .068). Birds fed on the control, RPLE and RPLEP diets had lower (p < .001) TG as compared with those fed on LPRE, LPREP, LPE and LPEP diets. Regardless of diet, heat stressed birds had significantly higher GLU (p < .001), TG (p < .001) and TP (p < .001) levels than their counterparts maintained in normal ambient temperature. At 42 days of age, there were significant diet × temperature interactions for CK (p = .002). Diet had no significant effect on CK levels among the heat stressed chickens (Table ). However, under normal temperature, birds fed with the control and RPLE diets had lower CK levels than other groups (Table ). In addition, temperature produced no significant effect on CK levels among the control, LPRE, LPREP, LPE and LPEP fed birds.
Table 5. Effects of diet and temperature on serum metabolites in broiler chickens at 42 days of age.
Table 6. Creatine kinase where the interaction between diet and temperature were significant.
Breast meat yield and abdominal fat
There were no diet × temperature interactions for the percentage of breast meat yield and abdominal fat (). The breast meat yield (p = .136) was not affected by diet. Birds fed with RPLEP diet showed lesser (p < .001) abdominal fat when compared to other groups. Birds fed using LPRE, LPREP and LPE diets had greater abdominal fat compared to those fed control and RPLE. Regardless of diet, the abdominal fat was not affected (p = .328) by heat treatment. However, heat treatment had a significant effect on the breast meat yield as breast meat yield was lower in heat stressed birds compared to their counterparts kept in normal temperature.
Table 7. Effects of diet and temperature on the percentage of breast yield and abdominal fat in broiler chickens at 43 days of age.
Physiological stress indicators
There were significant diet × temperature interactions for HSP70 (p = .002), but not for CPN (p = .100), OVT (p = .792), AGP (p = .254) or CORT (p = .884) in broilers at 42 days of age (). Diet showed no significant effect on HSP70 expression among birds raised in a normal temperature condition. However, under HS condition, birds fed control and RPLE diets had significantly lower HSP70 expression than those fed other diets (). Diet had no significant effect on CPN (p = .424), OVT (p = .814), AGP (p = .083) and CORT (p = .465). Irrespective of diet, serum levels of CPN, OVT, AGP and CORT were greater (p < .001) in heat challenged birds compared to their unchallenged counterparts.
Table 8. Effects of diet and temperature on serum ceruloplasmin, ovotransferrin, α1-acid glycoprotein, corticosterone and brain heat shock protein70 in broiler chickens at 42 days of age.
Table 9. Heat shock protein 70 where the interaction between diet and temperature were significant.
Discussion
The present findings suggested that protease supplementation had a negligible effect on the performance of broilers compared to those without enzyme supplementation. This outcome is on the contrary to those of Abudabos (Citation2012) who found that enzyme supplementation was able to restore the nutritional value of diets with low CP and ME in broilers. These discrepancies could be attributed to the type of enzyme supplemented. Abudabos (Citation2012) used a multi-enzyme supplement containing an acidic protease, α-amylase, pectinase, phytase, glucoamylase, cellulase and Aspergillus awamori cells, the present experiment used a mono-component protease. Data on the effect of mono-component protease on growth performance of broilers are limited and inconsistent. Several studies (Angel et al. Citation2011; Freitas et al. Citation2011; Cowieson et al. Citation2017; Mahmood et al. Citation2017a,Citationb) have shown that supplementation of protease improved BW, FI and FCR in broilers. The improvement in growth performance was mainly attributed to the enhancement of CP and AA digestibility following protease supplementation. Protease used in the present study enhanced apparent ileal digestible energy (AIDE), CP and AA in a previous study (Law et al. Citation2015). However, the same improvements were not observed in this study. The present results concur with Ghazi et al. (Citation2002) and Kaczmarek et al. (Citation2014) who reported no improvement or poorer growth performance in broilers fed diets supplemented with protease. The authors attributed their observation to the possible negative effects of exogenous protease supplementation on secretion of the endogenous proteolytic enzymes. Consistent with this hypothesis, the protease used in the current experiment noted to decrease the endogenous pancreatic protease secretion in an earlier experiment (Law et al. Citation2018).
In the present experiment, a significantly lower WG and poorer FCR was observed when birds were given LPE and LPEP diets compared to those received the control diet. These effects were more obvious during the overall period rather than the starter phase. Thus, it appears that nutrient density is more critical during the finisher rather than the starter period (Kamran et al. Citation2008; Cowieson et al. Citation2017). Interestingly, no reduction in FI and WG, and elevation in FCR were observed when either protein (LPRE and LPREP) or energy (RPLE and RPLEP) was reduced. These results are in agreement with previous findings that a reduction of dietary CP (in essential AA fortified diet) up to 3% (Bregendahl et al. Citation2002; Si et al. Citation2004; Namroud et al. Citation2008; Awad et al. Citation2014) or energy up to 100 kcal/kg ME (Zaman et al. Citation2008; Dairo et al. Citation2010) had no adverse effects on growth performance in broiler chickens.
As expected, irrespective of dietary treatment, high ambient temperature adversely affected growth performance of birds (Temim et al. Citation2000; Lin et al. Citation2006). However, feeding LPRE, LPREP, RPLE or RPLEP diet had no detrimental effects on the bird’s performance under both normal and heat stress conditions. Zaman et al. (Citation2008) reported a beneficial effect of feeding low-CP at recommended ME diet under HS, outcomes which disagree with many other works when low-CP (Alleman and Leclercq Citation1997; Zulkifli et al. Citation2018) or low-CP and low-ME diets (Attia and Hassan Citation2017) were fed to broilers under the same conditions. These inconsistencies could be associated with differences in the levels of dietary CP, supplemented AA and/or the severity of heat challenge involved.
Diet showed no effect on serum GLU and TP in the present study. Swennen et al. (Citation2007) and Hada et al. (Citation2013) reported that carbohydrate metabolism in broiler chicken was not affected by CP and ME levels in the diets. This may be due to the strict regulation of carbohydrate metabolism in the same birds to maintain the blood GLU level (Hada et al. Citation2013). Corzo et al. (Citation2005) and Hernández et al. (Citation2012) fed broiler chickens with low-CP diets (3–4% in CP) and observed no change in serum TP. Corzo et al. (Citation2009) and (Ahmadi et al. Citation2015) commented that TP will only be affected when diets ingested by the animals are deficient in AA. Thus, it appears that meeting the AA requirement could be more important than the CP per se. However, feeding low-CP diets, as demonstrated in the current experiment lead to elevation of liver lipogenesis and thus TG. Similarly, Swennen et al. (Citation2006) and Dehghani-Tafti and Jahanian (Citation2016) reported that irrespective of energy density, birds grown on low-CP diets had higher TG. It appears that the TG level is associated with calorie/protein and the excessive energy intake above the requirement level resulted in higher TG and therefore higher abdominal fat deposition (Rosebrough and Steele Citation1985; Sterling et al. Citation2002; Malheiros et al. Citation2003; Swennen et al. Citation2007). Abdominal fat is an unfavourable trait in carcase quality and reduces its acceptability by the consumers. The increase in abdominal fat deposition is a major disadvantage of feeding low-CP diet (Sklan and Plavnik Citation2002). However, the percentage of breast meat yield was not affected by diet in the present experiment. Similar results have been reported previously by van Nguyen and Bunchasak (Citation2005) and Infante-Rodríguez et al. (Citation2016). On the other hand, the percentage of breast meat yield was decreased in the heat stressed birds. Such reduction can be attributed to lower ribosomal capacity under HS condition that leads to a decreased rate of protein synthesis and deposition (Temim et al. Citation1998). Similar results have been reported by Geraert et al. (Citation1996) and Zhang et al. (Citation2012).
It is interesting to note that birds received low-CP diet had higher CK level than those of controls under normal temperature. Moreover, the heat challenge resulted in higher CK in the birds fed low-ME diets than those provided control diet. Serum level of CK is considered a myopathy (muscle breakdown) marker when there is cell membrane damage and permeability changes (Sandercock et al. Citation2001). In this study, unlike other diets, heat stress resulted in significantly higher levels of CK in birds fed RPLE and RPLEP diets. It is possible that the variations in dietary CP and energy among the dietary groups caused the higher CK level by increasing the muscle breakdown at the expense of muscle synthesis. The phenomenon could be attributed to the lower calorie/protein in the mentioned diets, and consequently lower availability of energy under such energy demanding stressful condition (Fagan et al. Citation1992; Gaine et al. Citation2006).
The current study demonstrated that HS elevated CORT and APPs (CPN, OVT and AGP) in broilers. It is well documented that heat exposure can elevate CORT (Mahmoud et al. Citation2004; Soleimani et al. Citation2011; Najafi et al. Citation2015), APPs (Najafi et al. Citation2015; Zulkifli et al. Citation2018) and HSP 70 expression (Mahmoud et al. Citation2004; Najafi et al. Citation2015; Zulkifli et al. Citation2018) in broilers. In the present study, reducing dietary CP and ME did not affect CORT level. Similarly, (Houshmand et al. Citation2012) showed that low-CP diet (reduction of 3% in dietary CP) did not influence CORT in broiler chickens under hot and humid environment. Little information is available on the effect of low-CP diet on APPs. Recently, Zulkifli et al. (Citation2018) reported that AGP and OVT levels were reduced in broilers fed diets with more than 3% reduction in CP. On the contrary, the present findings suggest that diet had a negligible effect on APPs. The inconsistent results could be attributed to the lower levels of dietary CP reduction (2.3%) in the current experiment. However, Awad (Citation2016) indicated that 5% reduction in dietary CP did not affect the levels of APPs (CPN, OVT and AGP) if the diet was supplemented with all essential AA and Glycine (Gly). Dietary CP and ME levels with and without protease supplementation had no effect on HSP70 expression under normal temperature. However, low-CP irrespective of ME and supplemental protease significantly elevated HSP 70 expression under the same condition. It is possible that the observed higher HSP70 in low-CP fed birds is attributed to the higher calorie/protein ratio in these diets. The higher calorie/protein may provide extra energy needed for the metabolic functions related to coping mechanisms such as HSP 70 synthesis to maintain the homeostasis and cell integrity (Mallouk et al. Citation1999).
Conclusions
The present findings indicated that, regardless of protease supplementation, dietary CP and ME can be reduced to 18.5% and 2985 kcal/kg, respectively with no adverse effect on the FI, WG and survivability rates in broilers. However, unlike the single reduction of dietary CP or ME, the combined reduction of CP and ME was detrimental to FCR in broilers during 1–42 days of age. Irrespective of dietary CP and ME, supplementation of protease had negligible influence on growth performance. Heat stress adversely affects the growth performance of broiler chickens, regardless of protease supplementation and dietary CP or ME. These findings suggest that protease supplementation is a potential nutritional strategy for the poultry industry to minimise the adverse effects of decreasing nutrient density and potentially reducing the feed cost without affecting the growth performance of broilers, particularly in reduced ME or CP diet.
Ethical Approval
The experimental protocol used in this study was conducted in full compliance with the Research Policy and Code of Practice for the Care and Use of Animal for Scientific Purposes of Universiti Putra Malaysia.
Disclosure statement
We certify that there is no conflict of interest with any financial organisation regarding the material discussed in the manuscript.
Additional information
Funding
References
- Abudabos AM. 2012. Effect of enzyme supplementation to normal and low density broiler diets based on corn-soybean meal. Asian J Anim Vet Adv. 7:139–148.
- Ahmadi M, Yaghobfar A, Tabatabaei SH. 2015. Study of effects difference levels of crude protein and amino acid of diet on intestinal morphological and blood biological parameters of poultry. Biological Forum. 7:666–670.
- Alleman F, Leclercq B. 1997. Effect of dietary protein and environmental temperature on growth performance and water consumption of male broiler chickens. Br Poult Sci. 38:607–610.
- Angel C, Saylor W, Vieira S, Ward N. 2011. Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poult Sci. 90:2281–2286.
- AOAC. 1990. Official methods of analysis. 15th edition. Arlington, VA: AOAC Inc.
- Attia YA, Hassan RA, Tag El-Din AE, Abou-Shehema BM. 2011. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Anim Nutr. 95:744–755.
- Attia YA, Hassan SS. 2017. Broiler tolerance to heat stress at various dietary protein/energy levels. Eur Poult Sci. 81.
- Awad EA. 2016. Effects of feeding low-protein diets fortified with amino acids on broiler chickens under high environmental temperatures. PhD Thesis, Universiti Putra Malaysia.
- Awad EA, Zulkifli I, Soleimani AF, Aljuobori A. 2017. Effects of feeding male and female broiler chickens on low-protein diets fortified with different dietary glycine levels under the hot and humid tropical climate. Ital J Anim Sci. 16:453–461.
- Awad EA, Zulkifli I, Soleimani AF, Loh TC. 2015. Individual non-essential amino acids fortification of a low-protein diet for broilers under the hot and humid tropical climate. Poult. Sci. 94:2772–2777.
- Awad EA, Zulkifli I, Soleimani AF, Loh TC. 2014. Amino acids fortification of low-protein diet for broilers under tropical climate. 2. Nonessential amino acids and increasing essential amino acids. Ital J Anim Sci. 13:631–636.
- Bregendahl K, Sell JL, Zimmerman DR. 2002. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult Sci. 81:1156–1167.
- Corzo A, Fritts CA, Kidd MT, Kerr BJ. 2005. Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim Feed Sci Technol. 118:319–327.
- Corzo A, Loar R, Kidd M. 2009. Limitations of dietary isoleucine and valine in broiler chick diets1. Poult Sci. 88:1934–1938.
- Cowieson A, Zaefarian F, Knap I, Ravindran VR. 2017. Interactive effects of dietary protein concentration, a mono-component exogenous protease and ascorbic acid on broiler performance, nutritional status and gut health. Anim Prod Sci. 57:1058–1068.
- Dairo FAS, Adesehinwa AOK, Oluwasola TA, Oluyemi JA. 2010. High and low dietary energy and protein levels for broiler chickens. Afr J Agric Res. 5:2030–2038.
- de Souza LFA, Espinha LP, de Almeida EA, Lunedo R, Furlan RL, Macari M. 2016. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest Sci. 192:39–43.
- Dehghani-Tafti N, Jahanian R. 2016. Effect of supplemental organic acids on performance, carcass characteristics, and serum biochemical metabolites in broilers fed diets containing different crude protein levels. Anim Feed Sci Technol. 211:109–116.
- Fagan JM, Wajnberg EF, Culbert L, Waxman L. 1992. ATP depletion stimulates calcium-dependent protein breakdown in chick skeletal muscle. Am J Physiol Endocrinol Metab. 262:E637–E643.
- Farouk MM, Al-Mazeedi HM, Sabow AB, Bekhit AED, Adeyemi KD, Sazili AQ, Ghani A. 2014. Halal and kosher slaughter methods and meat quality: a review. Meat Sci. 98:505–519.
- Freitas D, Vieira S, Angel C, Favero A, Maiorka A. 2011. Performance and nutrient utilization of broilers fed diets supplemented with a novel mono-component protease. J Appl Poult Res. 20:322–334.
- Fru-Nji F, Kluenter A-M, Fischer M, Pontoppidan K. 2011. A feed serine protease improves broiler performance and increases protein and energy digestibility. J Poult Sci. 48:239–246.
- Gaine PC, Pikosky MA, Martin WF, Bolster DR, Maresh CM, Rodriguez NR. 2006. Level of dietary protein impacts whole body protein turnover in trained males at rest. Metab Clin Exp. 55:501–507.
- Geraert P, Padilha J, Guillaumin S. 1996. Metabolic and endocrine changes induced by chronic heatexposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 75:195–204.
- Ghazi S, Rooke JA, Galbraith H, Bedford MR. 2002. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br Poult Sci. 43:70–77.
- Gonzalez-Esquerra R, Leeson S. 2006. Physiological and metabolic responses of broilers to heat stress-implications for protein and amino acid nutrition. Worlds Poult Sci J. 62:282–295.
- Hada F, Malheiros RD, Silva JDT, Marques RH, Gravena RA, Silva VK, Moraes VMB. 2013. Effect of protein, carbohydrate, lipid, and selenium levels on the performance, carcass yield, and blood changes in broilers. Rev Bras Cienc Avic. 15:385–394.
- Hernández F, López M, Martínez S, Megías MD, Catalá P, Madrid J. 2012. Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1- to 48-day-old broilers. Poult Sci. 91:683–692.
- Houshmand M, Azhar K, Zulkifli I, Bejo M, Kamyab A. 2012. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult Sci. 91:393–401.
- Infante-Rodríguez F, Salinas-Chavira J, Montaño-Gómez MF, Manríquez-Nuñez OM, González-Vizcarra VM, Guevara-Florentino OF, JAR DL. 2016. Effect of diets with different energy concentrations on growth performance, carcass characteristics and meat chemical composition of broiler chickens in dry tropics. SpringerPlus. 5:1937–1942.
- Jin L, Ho Y, Abdullah N, Jalaludin S. 2000. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult Sci. 79:886–891.
- Kaczmarek SA, Rogiewicz A, Mogielnicka M, Rutkowski A, Jones RO, Slominski BA. 2014. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult Sci. 93:1745–1753.
- Kalmendal R, Tauson R. 2012. Effects of a xylanase and protease, individually or in combination, and an ionophore coccidiostat on performance, nutrient utilization, and intestinal morphology in broiler chickens fed a wheat-soybean meal-based diet. Poult Sci. 91:1387–1393.
- Kamel NF, Ragaa M, El-Banna RA, Mohamed FF. 2015. Effects of a monocomponent protease on performance parameters and protein digestibility in broiler chickens. Agric Agric Sci Procedia. 6:216–225.
- Kamran Z, Sarwar M, Nisa M, Nadeem M, Mahmood S, Babar M, Ahmed S. 2008. Effect of low-protein diets having constant energy-to-protein ratio on performance and carcass characteristics of broiler chickens from one to thirty-five days of age. Poult Sci. 87:468–474.
- Law FL, Zulkifli I, Soleimani AF, Liang JB, Awad EA. 2018. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian-Australas J Anim Sci. 31:1291–1300.
- Law L, Zulkifli I, Soleimani A, Hossain M, Liang J. 2015. Nutrient digestibility of broiler chickens fed on a low-protein diet supplemented with mono-component proteases. Eur Poult Sci. 81:1–15.
- Leeson S, Caston L, Summers JD. 1996. Broiler response to diet energy. Poult Sci. 75:529–535.
- Lin H, Jiao H, Buyse J, Decuypere E. 2006. Strategies for preventing heat stress in poultry. Worlds Poult Sci J. 62:71–86.
- Mack L, Felver-Gant J, Dennis R, Cheng HW. 2013. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult Sci. 92:285–294.
- Mahmood T, Mirza MA, Nawaz H, Shahid M. 2017a. Effect of different exogenous proteases on growth performance, nutrient digestibility, and carcass response in broiler chickens fed poultry by-product meal-based diets. Livest Sci. 200:71–75.
- Mahmood T, Mirza MA, Nawaz H, Shahid M, Athar M, Hussain M. 2017b. Effect of supplementing exogenous protease in low protein poultry by-product meal based diets on growth performance and nutrient digestibility in broilers. Anim Feed Sci Technol. 228:23–31.
- Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. 2004. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp Biochem Physiol A Mol Integr Physiol. 137:35–42.
- Malheiros RD, Moraes VM, Collin A, Janssens GP, Decuypere E, Buyse J. 2003. Dietary macronutrients, endocrine functioning and intermediary metabolism in broiler chickens: pair wise substitutions between protein, fat and carbohydrate. Nutr Res. 23:567–578.
- Mallouk Y, Vayssier-Taussat M, Bonventre JV, Polla BS. 1999. Heat shock protein 70 and ATP as partners in cell homeostasis (Review). Int J Mol Med. 4:463–537.
- Musharaf NA, Latshaw JD. 1999. Heat increment as affected by protein and amino acid nutrition. Worlds Poult Sci J. 55:233–240.
- Najafi P, Zulkifli I, Jajuli NA, Soleimani AF, Ramiah SK, Amir AA, O’Reily E, Eckersall D. 2015. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int J Biometeorol. 59:1577–1583.
- Namroud NF, Shivazad M, Zaghari M. 2008. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poult Sci. 87:2250–2258.
- Ojano-Dirain CP, Waldroup PW. 2002. Protein and amino acid needs of broilers in warm weather: a review. Int J Poult Sci. 1:40–46.
- Olubodun J, Zulkifli I, Hair-Bejo M, Kasim A, Soleimani AF. 2015. Physiological response of glutamine and glutamic acid supplemented broiler chickens to heat stress. Eur Poult Sci. 79:1–12.
- Payne CG. 1967. Environmental control in poultry production, layer response to energy. London, UK: Longmans.
- Rahman MS, Pramanik MAH, Basak B, Tarafdar SU, Biswas SK. 2002. Effect of feeding low protein diets on the performance of broilers during hot humid season. Int J Poult Sci. 1:35–39.
- Rosebrough RW, Steele NC. 1985. Energy and protein relationships in the broiler. 1. Effect of protein levels and feeding regimens on growth, body composition, and in vitro lipogenesis of broiler chicks. Poult Sci. 64:119–126.
- Sandercock DA, Hunter RR, Nute GR, Mitchell MA, Hocking PM. 2001. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult Sci. 80:418–425.
- Si J, Fritts CA, Burnham DJ, Waldroup PW. 2004. Extent to which crude protein may be reduced in corn-soybean meal broiler diets through amino acid supplementation. Int J Poult Sci. 3:46–50.
- Sklan D, Plavnik I. 2002. Interactions between dietary crude protein and essential amino acid intake on performance in broilers. Br Poult Sci. 43:442–449.
- Soleimani AF, Zulkifli I, Omar AR, Raha AR. 2011. Physiological responses of 3 chicken breeds to acute heat stress. Poult Sci. 90:1435–1440.
- Soleimani AF, Zulkifli I, Omar AR, Raha AR. 2012. The relationship between adrenocortical function and Hsp70 expression in socially isolated Japanese quail. Comp Biochem Physiol Part A Mol Integr Physiol. 161:140–144.
- Sterling KG, Costa EF, Henry MH, Pesti GM, Bakalli RI. 2002. Responses of broiler chickens to cottonseed- and soybean meal-based diets at several protein levels. Poult Sci. 81:217–226.
- Swennen Q, Janssens GPJ, Collin A, Le Bihan-Duval E, Verbeke K, Decuypere E, Buyse J. 2006. Diet-induced thermogenesis and glucose oxidation in broiler chickens: influence of genotype and diet composition. Poult Sci. 85:731–742.
- Swennen Q, Laroye C, Janssens G, Verbeke K, Decuypere E, Buyse J. 2007. Rate of metabolic decarboxylation of leucine as assessed by al [1‐13C1] leucine breath test combined with indirect calorimetry of broiler chickens fed isocaloric diets with different protein: fat ratio. J Anim Physiol Anim Nutr. 91:347–354.
- Temim S, Chagneau AM, Guillaumin S, Michel J, Peresson R, Tesseraud S. 2000. Does excess dietary protein improve growth performance and carcass characteristics in heat-exposed chickens?. Poult Sci. 79:312–317.
- Temim S, Chagneau AM, Peresson R, Michel J, Guillaumin S, Tesseraud S. 1998. Muscle protein turnover in broiler chickens: effects of high ambient temperatures and dietary protein intake. Reprod Nutr Dev. 38:190–190.
- van Nguyen T, Bunchasak C. 2005. Effects of dietary protein and energy on growth performance and carcass characteristics of Betong chicken at early growth stage. Songklanakarin J Sci Technol. 27:1172–1178.
- Zaman QU, Mushtaq T, Nawaz H, Mirza MA, Mahmood S, Ahmad T, Babar ME, Mushtaq MMH. 2008. Effect of varying dietary energy and protein on broiler performance in hot climate. Anim Feed Sci Technol. 146:302–312.
- Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, Feng DY. 2012. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. 91:2931–2937.
- Zulkifli I, Akmal AF, Soleimani AF, Hossain MA, Awad EA. 2018. Effects of low-protein diets on acute phase proteins and heat shock protein 70 responses, and growth performance in broiler chickens under heat stress condition. Poult Sci. 97:1306–1314.
- Zulkifli I, Najafi P, Nurfarahin AJ, Soleimani AF, Kumari S, Aryani AA, O’Reilly EL, Eckersall PD. 2014. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult Sci. 93:3112–3118.
