Abstract
Adipose tissue has long been considered as an energy storage organ. Adipokines, produced by and secreted from adipose tissues, play an important role in regulating fat accumulation, energy balance, and glucose metabolism. Adiponectin receptors (AdipoR1 and AdipoR2) are expressed in various sheep tissues, including adipose tissue. However, the role of adiponectin receptor-mediated signalling has never been investigated in sheep adipose tissue. In this study, sheep preadipocytes were ultimately differentiated into typical adipocytes in vitro 7 days after differentiation. The expression levels of LPL (lipoprotein lipase), HSL (Hormone-sensitive lipase), FAS (fatty acid synthase), and PPARα/PPARγ (peroxisome proliferator-activated receptor alpha/gamma) were significantly changed in the sheep preadipocytes during differentiation for 7 days. After induction of preadipocyte differentiation, lipid accumulation increased significantly in the transfected preadipocytes of AdipoR1-siRNA1 but decreased in the AdipoR1-overexpression (AdipoR1-GFP) preadipocytes. In addition, the expression of HSL and PPARγ was significantly changed in preadipocytes by transfected with AdipoR1-siRNA1 or AdipoR1-GFP. However, the lipid accumulation and adipogenic gene expression were not significantly changed in preadipocytes by transfected with AdipoR2-siRNA2 and AdipoR2-GFP. Taken together, these results indicate that AdipoR1 could regulate sheep adipose metabolism via regulating HSL and PPARγ expression during adipocyte differentiation.
We first established an in vitro culture system for sheep preadipocytes derived from the paraspinal muscle adipose.
Sheep preadipocytes were ultimately differentiated into typical adipocytes after induction and differentiation.
Both suppression and overexpression of adiponectin receptor 1 expression affect lipid accumulation and adipocyte metabolism during adipocyte differentiation.
Highlights
Introduction
Adipose tissue is recognised as an important energy storage organ (Chudek et al. Citation2013) which produces and secretes a number of peptides and other factors, known as adipokines (Ma et al. Citation2015). Adipokines play an important role in regulating glucose and lipid metabolisms, as well as in inflammatory processes (Ouchi et al. Citation2011). A series of adipokines, such as adiponectin (Maeda et al. Citation2012), leptin (Zhang et al. Citation1994), resistin (Steppan et al. Citation2001), apelin (Boucher et al. Citation2005), and fibroblast growth factor 21 (FGF21) (Nishimura et al. Citation2000) were identified in human and mouse. Many studies showed that adipokines are also involved in the differentiation and maturation of adipocytes (Guilherme et al. Citation2008).
Adiponectin, also known as a 30 kDa adipocytokine hormone, has an N-terminal collagenous domain and a C-terminal globular domain (Goldstein et al. Citation2009). Adiponectin is an important in vitro signalling molecule that regulates adipocyte differentiation in adipose tissue. Adiponectin is exclusively generated by adipose tissue and secreted into the bloodstream (Chen et al. Citation2006). Many studies have found its secretion is inversely correlated with adipose tissue levels in patient populations (Ukkola and Santaniemi Citation2002; Da et al. Citation2018; Mader et al. Citation2018; Zhang et al. Citation2018). Adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) are expressed in various tissues, including adipose tissue, skeletal muscle and other tissues (Jasinski-Bergner et al. Citation2017; Nicholson et al. Citation2018) and their expression levels are influenced by insulin (Guilherme et al. Citation2008). AdipoR1 and AdipoR2 contain seven transmembrane domains, which are structurally and functionally distinct from G-protein-coupled receptors (Almabouada et al. Citation2013). Adiponectin can bind to their specific receptors through C-terminal globular domain, stimulating unique sets of signalling molecules such as peroxisome proliferator-activated receptor gamma (PPARγ), 5′-AMP-activated protein kinase (AMPK) and Phospho-p38 mitogen-activated protein kinase (p38 MAPK) activation (Fang and Sweeney Citation2006). Adiponectin receptor-mediated signalling has important effects on various physiological processes, including glucose regulation, reproduction, and fatty acid oxidation (Diez and Iglesias Citation2003; Reverchon et al. 2014).
Many studies showed that alteration of expression of adiponectin and its receptors in adipose tissue affects obesity in porcine (Sun et al. Citation2009) and pig (Liu et al. Citation2008; Gao et al. 2013). Recently, the expression level of adiponectin and its receptors are detected in different fat depots of sheep (Oliveira et al. Citation2017). However, little is known about the role of adiponectin receptors on adipose differentiation in sheep. In this study, we established an in vitro culture system for sheep preadipocytes derived from the paraspinal muscle adipose and examined the expression of adipokine genes during preadipocyte differentiation. To investigate the effects of AdipoR1 and AdipoR2 expression on the differentiation of sheep preadipocytes, we monitored differentiation and measured the mRNA levels of adipogenesis-related gene, including LPL, HSL, PPARα and PPARγ. Our study helps to elucidate the proliferation and differentiation of sheep preadipocytes, improves our understanding of adipose deposition in sheep and further reveals the function of the AdipoR1 and AdipoR2 gene in the process of the sheep fat deposition.
Methods
Experimental animals
The sheep used in this study were healthy Lanzhou fat-tailed sheep less than 10 days old and raised in Ruiling Breeding Science and Technology Company Limited of Gansu Province (Lanzhou, China). Experiments were performed in accordance with the Guiding principles in the use of animals, adopted by the Chinese Association for Laboratory Animal Sciences. The study was approved by Ethics Committee on the use and Care of animals, Gansu Agricultural University.
Cell culture and differentiation conditions
Paraspinal muscle adipose tissues were aseptically collected from sheep less than 10 days old, rinsed in phosphate buffer saline (PBS) containing 5% double antibiotics. adipose tissues were cut into pieces of ∼1 mm3 in size and digested with 0.2% collagenase (Sigma, Shanghai, China) for 1 h at 37 °C. The digested tissues were filtered through 200 and 80 μm nylon cell filters. The filtered solution was collected and centrifuged at 2000 rpm for 10 min. The pellet was resuspended in proliferation medium (DME/F-12) containing 20% FBS (CellMax, Beijing, China), and 1% double antibiotics at a density of 1 × 105 cells/cm2 in 6 cm cell culture dishes (Corning, New York, USA). After 48 h, the proliferation medium was removed and added to the fresh medium. The medium was changed every 2 days, and the cells were passaged once every 3 days.
Induction of cell differentiation
The fourth generation of subculture preadipocytes seed in 6-well plates and cultured in proliferation medium for one day. Induction medium (proliferation medium supplemented with 1 μmol/L dexamethasone, 5 mmol/L IBMX and 10 mg/L insulin (all from Sigma)) was added and cultured for 2 days. After two days of induction, the medium was replaced with a differentiation medium (proliferation medium supplemented with 10 mg/L insulin) and cultured for 7 days. The medium was replaced with fresh differentiation medium every two days.
Oil red O staining and optical density (OD) measurement
Cells were washed three times with PBS, and fixed with 10% formalin for 30 min. Oil red O staining solution (Solarbio, Beijing, China) was added to sufficiently cover the bottom of 6-well plates and stained for 10 min. After washing the cells three times with PBS, and re-stained with haematoxylin for 5 min, and rinsed three times with PBS. After staining, the plates were placed under a light microscope for imaging.
Triglycerides were extracted from Oil Red O-stained adipocytes with 100% isopropanol, and a blank with the same treatment served as the control. the absorbance values were measured at 490 nm in spectrophotometer. Each group included three replicates.
Transfection of siRNA
Three specific small interfering RNAs (siRNAs) targeting the AdipoR1 and AdipoR2 were designed and synthesised from BIONEER (Daegu, Korea), respectively (Table ). siRNA transfection was performed on adipocytes at 70% confluence following plating into 6-well plates. Proliferation medium was replaced with Opti-MEM I Reduced Serum Medium (Gibco, New York, USA). Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen, New York, USA) was used as a carrier according to manufacturer's instructions. Thirty nano molar siRNAs were used in a total transfection volume of 2.4 mL/well. After 8 h of transfection, the transfection media was replaced with DME/F-12 medium containing 10% FBS and 1% antibiotics for 48 h.
Table 1. siRNA sequence.
Plasmid construction and transfection
The full-length sheep AdipoR1 (GenBank accession No. KJ159212) and AdipoR2 cDNA (KF921623.1) were amplified using primers AdipoR1-F: 5′-GAATTCGATGTCGTCTCACAAGGGGCC-3′ and AdipoR1-R 5′- GTCGACGAGGAGGGAGTCACAGTAC-3′, AdipoR2-F: 5′-GAATTCGATGAACGAGACAGAGGAAAG-3′ and AdipoR2-R: 5′-GTCGACCAGTGCGTCCTCTTCGCTG-3′, respectively. The amplified products were cut with EcoR I and Sal I and inserted into the pCMV-GFP vector. The recombinant pCMV-GFP vectors were transformed into the Top 10 competent cells. Large amplification of the plasmid DNA was prepared using the Endo Free Plasmid Maxi Kit (Qiagen, Beijing, China) to remove bacterial endotoxins. Lipofectamine™ 3000 Transfection Reagent (Invitrogen, New York, USA) was used as carrier according to manufacturer's instructions. Five micrograms of plasmid DNA were used in a total transfection volume of 2.4 mL/well. After 8 h of transfection, the transfection media was replaced with DME/F-12 medium containing 10% FBS and 1% antibiotics for 48 h.
Quantitative PCR (qPCR)
RNA was extracted using PureLink™ RNA Mini Kit (Thermo Fisher Scientific, New York, USA), and oligo (dT) primers and reverse transcriptase (Thermo Fisher Scientific, New York, USA) were used to synthesise cDNA. The 20 μL reverse transcription reaction contained 5 μL total RNA (1μg), 4 μL 5 × Reaction buffer, 1 μL RiboLock RNase Inhibitor, 2 μL 10 mM dNTP, 1 μL Oligo (dT18) primer, and 1 μL RevertAid M-MuLV RT, and 6 μL RNase-free water. The reaction mixture was incubated at 42 °C for 1 h and 75 °C for 5 min. The reaction mixture was diluted 10-fold with nuclease-free water. qPCR was performed using All-in-OneTM mRNA qRT-PCR Detection Kit (Genecopoeia, Guangzhou, China). The 20 μL qPCR reaction included 2 μL of cDNA (1:5 dilution), 10 μL 2 × QuantiTect SYBR Green PCR Master Mix, 2 μL 10 × miScript Universal Primer, 2 μL 10 × miScript Primer Assay and 4 μL H2O. The qPCR mix was subjected to the following thermal profile: 95 °C for 30 s followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s. GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) was used as the reference housekeeping gene for normalising mRNA levels. The primer sequences used for PCR are listed in Table . The expression levels for each target gene were calculated using the comparative threshold cycle (CT) method. The ΔCt values were calculated according to the formula ΔCt = Ct (target gene)-Ct (GAPDH) in correlation analysis, and the ΔΔCt was calculated according to the formula ΔΔCt = ΔCt (control group)-ΔCt (experimental group). The relative expression level was calculated using the 2−ΔΔCt formula. Data used in the final analysis were collected from triplicate independent experiments.
Table 2. Primers used for real-time PCR.
Protein preparation and Western blot analysis
Total protein was extracted from sheep adipocytes using lysis buffer containing protease inhibitor cocktail, and 20 μg of protein was separated on 10% SDS-PAGE gel and transferred to PVDF membranes (Merck, Darmstadt, Germany). The membranes were blocked and then incubated at 4 °C overnight with either mouse anti-AdipoR1 (ab77611, Abcam), anti-AdipoR2 (ab77613, Abcam), and anti-actin (ab8227, Abcam). After incubating with anti-rabbit secondary antibodies at room temperature for 1 h, membranes were visualised by exposing X-ray films using Pierce ECL Western Blotting Substrate (ThermoFisher Scientific).
Statistical analysis
Data analyses were performed using GraphPad Prism 5 software (Cary, NC), a one-tailed unpaired t-test was used for comparison of two groups, and One-way ANOVA was used for comparison of three or more groups, with a p value less than .05 being considered to be significantly different.
Results
Adipose accumulation and the expression of adipogenesis-related genes in sheep preadipocytes during adipocyte differentiation
After 3–7 days, sheep preadipocytes derived from muscle adipose tissues exhibited spindle and fibroblast-like shape (Figure ). Oil Red O staining demonstrated small lipid droplets appeared in sheep preadipocytes after differentiation for 3 days (Figure ). The amount of the lipid droplets increased and they subsequently fused into large ones during differentiation for 7 days (Figure ). More than 60% of sheep preadipocytes ultimately differentiated into typical adipocytes after differentiation (Figure ). The oil red O elution analysis showed that the amount of fat accumulation increased on days 3 and 7 during the adipocyte differentiation (Figure ; p < .05).
Figure 1. Intracellular lipid accumulation and mRNA expression of adipogenesis-related genes in sheep preadipocytes during adipocyte differentiation. (A–C) Morphological characteristics of sheep preadipocytes derived from muscle adipose tissues. The morphology of sheep preadipocytes was cultured in growth medium for 1 day (d) (A), 3 days (B), and 7 days (C). Bars = 100 μm. (D–F) Intracellular lipid accumulation of the undifferentiated (D), differentiated 3 days (E), and differentiated 7 days (F) preadipocytes by Oil Red O staining. (G) Quantitative analysis of Oil Red O stained cells in preadipocytes. Bars = 25 μm. (H) Quantitative analysis of the expression level of eight adipogenesis-related genes in differentiated preadipocytes (differentiation days-7). Values are means ± SEM of duplicate independent analyses. *p < .05; **p < .01.
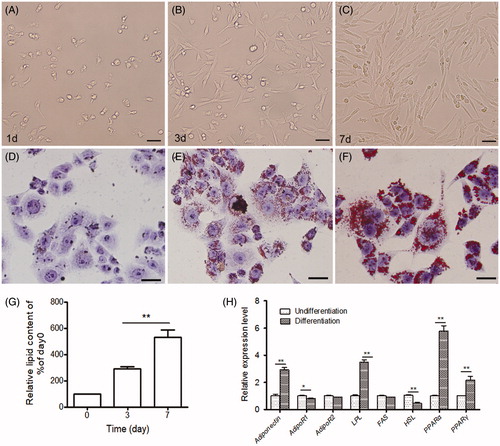
The mRNA expression level of eight genes crucial for adipose accumulation, including adiponectin, AdipoR1, AdipoR2, LPL (lipoprotein lipase), HSL (Hormone-sensitive lipase), FAS (fatty acid synthase), and PPARα/PPARγ, were detected in sheep preadipocytes during differentiation by qPCR. The results showed that HSL and AdipoR1 mRNA level were decreased significantly in sheep preadipocytes during differentiation for 7 days, whereas the rest genes (besides AdipoR2) significantly increased during this process (Figure ; p < .05). There is no significant change for AdipoR2 mRNA (Figure ; p > .05).
Suppression of AdipoR1 and AdipoR2 expression promoted adipocyte differentiation
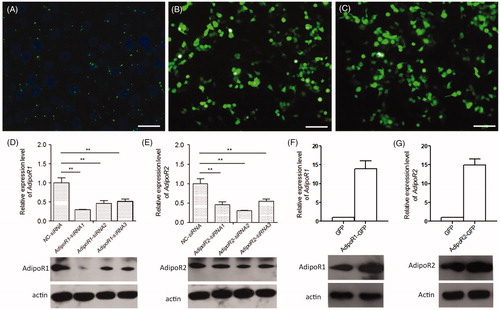
To investigate whether adiponectin receptors are involved in the process of adipocyte differentiation, either AdipoR1-siRNA or AdipoR2-siRNA were respectively transfected into sheep preadipocytes. Figure showed that the transfection efficiency of the FAM-siRNA was 72.5%, indicating that siRNAs were successfully transfected into the sheep preadipocytes. Compared with negative control-siRNA (NC-siRNA) cells, the mRNA and protein expression levels of AdipoR1 and AdipoR2 were significantly lower in the AdipoR1-siRNA1 cells or AdipoR2-siRNA2 cells, respectively (Figure ; p < .05).
Figure 2. Quantitative analysis of the mRNA and protein expression levels of AdipoR1 or AdipoR2 in its silencing and overexpressing preadipocyte. (A) FAM-siRNA were transfected into the sheep preadipocytes. Bar = 50 μM. (B and C) The expression of AdipoR1-GFP and AdipoR2-GFP in preadipocytes. Bars = 100 μm. (D) The mRNA and protein expression levels of AdipoR1 in its silencing preadipocytes. (E) The mRNA and protein expression levels of AdipoR2 in its silencing preadipocytes. (F) The mRNA and protein expression levels of AdipoR1 in its overexpressed preadipocytes. (G) The mRNA and protein expression levels of AdipoR2 in its overexpressed preadipocytes. The relative expression abundance of a given gene was calculated after normalisation to GAPDH mRNA expression. Values are means ± SEM of duplicate independent analyses. *p < .05; **p < .01.
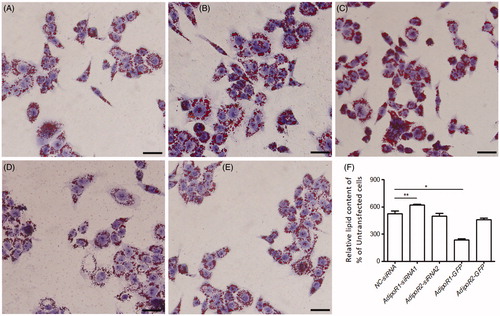
The size of lipid droplets in the AdipoR1-siRNA1 cells was larger than that of NC-siRNA cells (Figure ). The OD value of adipocytes in the AdipoR1-siRNA1 cells was also higher than that of NC-siRNA cells (Figure ). Whereas the lipid accumulation in both AdipoR2-siRNA2 and the NC-siRNA cells did not show significantly changed (Figure ).
Figure 3. Intracellular lipid accumulation in AdipoR1 and AdipoR2 silencing and overexpressing preadipocyte. (A) NC-siRNA preadipocytes; (B) AdipoR1- siRNA1 preadipocytes; (C) AdipoR2- siRNA2 preadipocytes; (D) AdipoR1-GFP overexpressed preadipocytes; (E) AdipoR2-GFP overexpressed preadipocytes; Bars = 25 μm. (F) Quantitative analysis of Oil Red O stained cells in preadipocytes during differentiation 7 days. Values are means ± SEM of duplicate independent analyses. *p < .05; **p < .01.
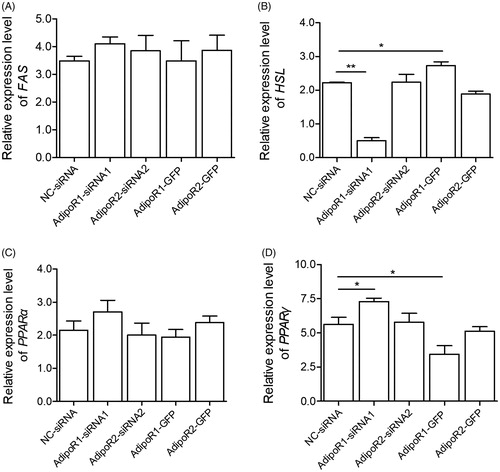
Compared with NC-siRNA cells, the expression of HSL was significantly decreased by 40% in AdipoR1-siRNA1 preadipocytes at 48 h after transfection. whereas no significant changes have been detected for HSL in preadipocytes transfected with AdipoR2-siRNA2 (data not shown). No significant changes have been detected for FAS, PPARα and PPARγ in both preadipocytes transfected with AdipoR1-siRNA1 or AdipoR2-siRNA2 (data not shown). Interestingly, the HSL mRNA expression in AdipoR1-siRNA1 cells at 7 days after differentiation was lower than NC-siRNA cells (Figure ). PPARγ were significantly upregulated by transfection of AdipoR1-siRNA1 at 7 days after differentiation, as compared with NC-siRNA cells (Figure ). There were no significant changes for the expression of FAS and PPARα in both AdipoR1-siRNA1 or AdipoR2-siRNA2 cells at 7 days after differentiation (Figure ), as compared with NC-siRNA cells.
Figure 4. Quantitative analysis of the mRNA expression level of FAS (A), HSL (B), PPARα (C), and PPARγ (D) in sheep preadipocyte after silencing and overexpressing of AdipoR1 or AdipoR2. The fold change in expression of each gene was calculated relative to in undifferentiated preadipocytes (day 0). The relative expression abundance of a given gene was calculated after normalisation to GAPDH mRNA expression. Values are means ± SEM of duplicate independent analyses. *p < .05; **p < .01.
Overexpression AdipoR1 and AdipoR2 affected lipid accumulation and adipogenesis-related gene expression
To further characterise the function of AdipoR1 and AdipoR2 in adipocyte differentiation, we generated sheep preadipocytes stably expressing AdipoR1-GFP or AdipoR2-GFP (Figure ). qPCR analysis showed that the mRNA and protein expression levels of AdipoR1 or AdipoR2 were significantly upregulated in preadipocytes expressing AdipoR1-GFP or AdipoR2-GFP, respectively (Figure ; p < .05).
Furthermore, Oil Red O staining and quantitative analysis revealed that the lipid accumulation in preadipocytes expressing AdipoR1-GFP decreased significantly compared with NC-siRNA cells (Figure ; p < .05), whereas the lipid accumulation in preadipocytes expressing AdipoR2-GFP was slightly changed (Figure ; p > .05). The alteration of adipogenesis-related gene expression was investigated during this process. In general, the mRNA expression levels of HSL in AdipoR1-GFP cells at 7 days after differentiation were higher than that of in NC-siRNA cells (Figure ; p < .05), whereas no significant change for HSL was detected in AdipoR2-GFP cells (Figure ; p > .05). PPARγ was significantly downregulated in AdipoR1-GFP cells at 7 days after differentiation, as compared with the cells transfected with pCMV-GFP (Figure ). The expression level of FAS and PPARα were not changed in both AdipoR1-GFP and AdipoR2-GFP cells at 7 days after differentiation (Figure ; p > .05).
Discussion
In this study, we successfully induced the in vitro differentiation of sheep preadipocytes into mature adipocytes when treated with an induction medium containing insulin, IBMX, dexamethasone and serum. More than 60% of sheep preadipocytes ultimately differentiated into typical adipocytes, with large and round fat droplets in cells. In addition, six adipogenic genes were typically changed in preadipocytes after differentiation for 7 days. This model is an effective tool for studying the mechanism of adipocytokine signalling in metabolic disorders and insulin resistance.
Adiponectin, produced and secreted exclusively by adipose tissue, is one of the multiple adipocytokines and has been shown to modulate both glucose and lipid metabolism (Jasinski-Bergner et al. Citation2017). Many studies have examined the metabolic effect of adiponectin in muscle and liver (Little 2011; Patel et al. Citation2012). Previous study showed overexpression of the adiponectin in 3T3-L1 preadipocytes promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation (Fu et al. Citation2005; Yang et al. 2018). Adiponectin knockout mice exhibit insulin resistance and diabetes (Kadowaki et al. Citation2006). Two adiponectin receptors (AdipoR1 and AdipoR2) promoted lipid and glucose metabolism in mouse skeletal muscle and liver tissues through AMPK and PPARs (Yamauchi et al. Citation2003). Whether AdipoR1/2 receptor-mediated adiponectin signalling acts as an adipocyte differentiation factor to regulate adipocyte differentiation remain to be fully elucidated in sheep. In this study, we found the expression of the adiponectin gene is upregulated in sheep preadipocytes during adipocyte differentiation, but the level of AdipoR1 mRNA expression was downregulated in preadipocytes during adipocyte differentiation. Above results suggested sheep preadipocytes can be used as a model to study the important role for AdipoR1 mediated adiponectin signalling in skeletal muscle adipocyte differentiation.
Short-interfering RNAs (siRNAs) can be efficiently transfected into preadipocytes and is a useful tool to study adipocyte differentiation (Xu et al. Citation2006). Fluorescent siRNA has proven to be an effective approach for monitoring transfection efficiency and optimising transfection methods. In this study, we investigated the function of adiponectin receptor in adipocyte differentiation by transfecting target-specific siRNA. qRT-PCR and Western blotting analysis show that the mRNA and protein expression levels of AdipoR1 and AdipoR2 were significantly decreased in AdipoR1-siRNA1 and AdipoR2-siRNA2 transfection groups, respectively. This result suggested that the siRNA method can effectively block the gene expression in sheep adipocytes. The results of the morphological and the OD value studies confirm that silence of AdipoR1 could promote preadipocyte differentiation, whereas overexpression of AdipoR1 could inhibit preadipocyte differentiation. However, AdipoR2-siRNA did not show significant differences in lipid accumulation compared with the NC-siRNA groups. It is possible that AdipoR2 is predominantly expressed in the liver. HSL, a key neutral fat enzyme, expresses predominantly in adipose tissue, where it plays an important role in controlling lipid and energy metabolism (Xia et al. Citation2017). HSL is regulated by various hormones, such as catecholamine, insulin and adrenaline (Nakatsuji et al. Citation2010). HSL null mice decreased expression level of adipogenic transcription factor and lipogenic enzymes, suggesting that impairment of adipogenesis (Ström et al. 2008). The mRNA expression of HSL were significantly downregulated in AdipoR1-siRNA1 preadipocytes, whereas it was upregulated in AdipoR1-GFP preadipocytes, suggesting that AdipoR1 plays an important role in adipogenesis by regulating HSL in sheep preadipocytes. PPARγ is another important adipocytokines in adipocyte differentiation and silence the expression of PPARγ gene can result in a significant inhibition of adipogenesis in human preadipocytes (Xu et al. Citation2006). HSL modulates adipogenesis through regulating the expression level of PPARγ in mice (Shen et al. Citation2011). Thus, the acceleration of adipogenesis in AdipoR1-siRNA1 cells was accompanied by an increase in the expression level of key transcription factors PPARγ during adipocyte differentiation, which could promote the adipocyte differentiation.
Conclusions
In conclusion, AdipoR1 plays an important role in adipogenesis possibly by regulating HSL and PPARγ mRNA expression in sheep preadipocytes during adipocyte differentiation.
Ethical approval
Experiments were performed in accordance with the Guiding principles in the use of animals, adopted by the Chinese Association for Laboratory Animal Sciences. The study was approved by Ethics Committee on the use and Care of animals, Gansu Agricultural University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almabouada F, Diaz-Ruiz A, Rabanal-Ruiz Y, Peinado JR, Vazquez-Martinez R, Malagon MM. 2013. Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties. J Biol Chem. 288:3112–3125.
- Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, et al. 2005. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 146:1764–1771.
- Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS. 2006. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 49:1292–1302.
- Chudek J, Adamczak M, Nieszporek T, Wiecek A. 2013. The adipose tissue as an endocrine organ–a nephrologists' perspective. Semin Nephrol. 33:2–13.
- Da ST, Costa-Silva M, Correa CG, Denardin G, Mla A, Msph C, Muraro-Wildner L, Luiza-Bazzo M, González-Chica DA, Dantas-Correa EB. 2018. Clinical significance of serum adiponectin and resistin levels in liver cirrhosis. Ann Hepatol. 17:286–299.
- Diez JJ, Iglesias P. 2003. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 148:293–300.
- Fang X, Sweeney G. 2006. Mechanisms regulating energy metabolism by adiponectin in obesity and diabetes. Biochm Soc Trans. 34:798–801.
- Fu Y, Luo N, Klein RL, Garvey WT. 2005. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 46:1369–1379.
- Gao Y, Li F, Zhang Y, Dai L, Jiang H, Liu H, Zhang S, Chen C, Zhang J. 2013. Silencing of ADIPOQ efficiently suppresses preadipocyte differentiation in porcine. Cell Physiol Biochem. 31:452–461.
- Goldstein BJ, Scalia RG, Ma XL. 2009. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 6:27–35.
- Guilherme A, Virbasius JV, Puri V, Czech MP. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
- Jasinski-Bergner S, Buttner M, Quandt D, Seliger B, Kielstein H. 2017. Adiponectin and its receptors are differentially expressed in human tissues and cell lines of distinct origin. Obes Facts. 10:569–583.
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 116:1784–1792.
- Liu BH, Wang YC, Wu SC, Mersmann HJ, Cheng WTK, Ding ST. 2008. Insulin regulates the expression of adiponectin and adiponectin receptors in porcine adipocytes. Domest Anim Endocrinol. 34:352–359.
- Little S. 2011. The cat: clinical medicine and management. Elsevier Health Sciences. Elsevier Saunders, St. Louis, MO, USA. pp 1195–1227.
- Ma X, Lee P, Chisholm DJ, James DE. 2015. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol. 6:1.
- Mader R, Novofastovski I, Schwartz N, Rosner E. 2018. Serum adiponectin levels in patients with diffuse idiopathic skeletal hyperostosis (DISH). Clin Rheumatol. 37(10):2839–2845.
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. 2012. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) 1996. Biochem Biophys Res Commun. 425:556–559.
- Nakatsuji H, Maeda N, Hibuse T, Hiuge A, Hirata A, Kuroda Y, Kishida K, Kihara S, Funahashi T, Shimomura I. 2010. Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem Biophys Res Commun. 392:100–105.
- Nicholson T, Church C, Baker DJ, Jones SW. 2018. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond). 15:9.
- Nishimura T, Nakatake Y, Konishi M, Itoh N. 2000. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 1492:203–206.
- Oliveira BSP, Costa JAS, Gomes ET, Silva DMF, Torres SM, Monteiro PLJ, Jr, Santos Filho AS, Guerra MMP, Carneiro GF, Wischral A, et al. 2017. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in goat ovary and its effect on oocyte nuclear maturation in vitro. Theriogenology. 104:127–133.
- Ouchi N, Parker JL, Lugus JJ, Walsh K. 2011. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 11:85–97.
- Patel SA, Hoehn KL, Lawrence RT, Sawbridge L, Talbot NA, Tomsig JL, Turner N, Cooney GJ, Whitehead JP, Kraegen EW, et al. 2012. Overexpression of the adiponectin receptor adipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology. 153:5231–5246.
- Reverchon M, Rame C, Bertoldo M, Dupont J. 2014. Adipokines and the female reproductive tract. Int J Endocrinol. 2014:232454.
- Shen W-J, Yu Z, Patel S, Jue D, Liu L-F, Kraemer FB. 2011. Hormone-sensitive lipase modulates adipose metabolism through PPARγ. Biochim Biophys Acta. 1811:9–16.
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. 2001. The hormone resistin links obesity to diabetes. Nature. 409:307–312.
- Ström K, Hansson O, Lucas S, Nevsten P, Fernandez C, Klint C, Movérare-Skrtic S, Sundler F, Ohlsson C, Holm C, et al. 2008. Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice. Plos One. 3:e1793.
- Sun YG, Zan LS, Wang HB, Guo HF, Yang DP, Zhao XL, Gui LS. 2009. Insulin inhibits the expression of adiponectin and AdipoR2 mRNA in cultured bovine adipocytes. Asian-Australas J Anim Sci. 22:1429–1436.
- Ukkola O, Santaniemi M. 2002. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 80:696–702.
- Xia B, Cai GH, Yang H, Wang SP, Mitchell GA, Wu JW. 2017. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Gen. 13:e1007110.
- Xu Y, Mirmalek-Sani SH, Yang X, Zhang J, Oreffo ROC. 2006. The use of small interfering RNAs to inhibit adipocyte differentiation in human preadipocytes and fetal-femur-derived mesenchymal cells. Exp Cell Res. 312:1856–1864.
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 423:762–769.
- Yang W, Yang C, Luo J, Wei Y, Wang W, Zhong Y. 2018. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol Med Rep. 17:428–435.
- Zhang Y, Chen RM, Lin XQ, Yuan X, Yang XH. 2018. The correlation between serum adipokines levels and metabolic indicators in girls with Turner syndrome. Cytokine. 113: 139–143.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature. 372:425–432.
