Abstract
The current study was conducted to examine the pre-protection effects of vitamin A (VA) on lipopolysaccharide (LPS)-induced oxidative damage in bovine mammary epithelial cells (BMECs) and to explore the antioxidant mechanisms of VA via nuclear factor erythroid 2-related factor (Nrf2)/nuclear factor kappa-B (NF-κB)-signalling pathways. The BMECs were divided into 10 treatment groups with six replicates per treatment and were cultured with dimethyl sulphoxide (DMSO, vehicle negative control) or 0, 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the absence or presence of LPS (1 μg/mL) and VA for an additional 6 h. The results showed that exposure to LPS alone decreased the cell proliferation compared with the control. The addition of VA (from 0.05 to 4 μg/mL) promoted the proliferation of BMECs, and had positive effect on activities of glutathione peroxidase and its gene or protein expression but decreased NO and IL-1 production in a quadratic manner. Furthermore, VA significantly inhibited LPS-induced NF-κB activation and had a positive effect on Nrf2 activation in a quadratic dose-response manner and VA at a concentration of 1 μg/mL exhibited the strongest effect. The protective effect of VA dose greater than 2 μg/mL was weakened or even had no protective effect. These results suggest that VA pre-treatment protects BMECs from LPS-induced oxidative stress is due to Nrf2-signalling pathway activation and NF-κB-signalling pathway inhibition.
VA protected against LPS-induced inflammatory cytokines IL-1β production on dose dependent manner quadraticlly, and the concentration of 1 μg/mL exhibited the strongest effect.
VA significantly inhibited LPS-induced NF-κB activation and had a positive effect on Nrf2 activation in a quadratic dose-response manner.
VA might be a valuable agent for the treatment of anti-inflammatory and antioxidant.
Highlights
Keywords:
Introduction
Our previous in vitro studies indicated that vitamin A (VA) prevents the bovine mammary epithelial cells (BMECs) from oxidative stress by enhancing antioxidant enzyme activity (Jin et al. Citation2016). Multicentre studies have demonstrated the overproduction of nitric oxide (NO) influences in antioxidant system (Banan et al. Citation2000; Kielbik et al. Citation2013). Our published studies also indicated that VA reduces NO-induced oxidative stress in BMECs not only by increasing antioxidant enzyme activities but also by reducing NO content (Shi, Guo, et al. Citation2016). The key enzyme that regulates NO production is inducible nitric oxide synthase (iNOS) which is primarily responsible for NO production in the inflammatory processes (Stefano and Kream Citation2011). Pro-inflammatory cytokine interleukin-1 (IL-1) is critical for activation of iNOS (Hung et al. Citation2008), which act by way of the nuclear factor kappa-B (NF-κB) pathway (Peng et al. Citation2015). The significance of IL-1 in the development of iNOS is also supported by the investigation of BMECs (Guo et al. Citation2016). Our group previously demonstrated that VA can attenuate oxidative stress through reducing NO overproduction, and inhibited IL-1 production is probably contributed to the results (Shi, Guo, et al. Citation2016). However, the exact mechanism is still lacking.
NF-κB is normally localised in the cytoplasm and bound to a group of non-phosphorylated inhibitory proteins known as inhibitors of NF-κB (IκBα) in an inactivate form. During activation, IκB is phosphorylated and thus disassociates from NF-κB which allows NF-κB to translocate to the nucleus where it then regulates the expression of inflammatory cytokines (Arora et al. Citation2014). Glutathione (GSH), which plays a pivotal role in the detoxification of NO (Zhu et al. Citation2009; Siems et al. Citation2010), is a key antioxidant via its role as an indispensable cofactor for numerous enzymes including the glutathione peroxidases (GPx) (Marí et al. Citation2009). What important is the GSH-related redox state of cells plays an important role in the regulation of proinflammatory cytokines, including IL-1 and the activity of intracellular signalling pathways (Haddad and Harb Citation2005). Nuclear factor erythroid 2-related factor (Nrf2) is redox-sensitive responses to increased oxidative stress by regulating cellular antioxidant defences (Kansanen et al. Citation2013). Numerous studies have demonstrated that Nrf2 is critical for the activation of the cellular glutathione system and maintaining the redox state (Maes et al. Citation2012). These results implied that VA protects BMECs from oxidative stress is likely to be related to suppressed NO overproduction from IL-1 via modulating Nrf2/NF-κB-signalling pathways. LPS is endotoxins of Gram-negative bacteria and the increasing evidence suggests that LPS causes an increase in the expression of genes encoding various pro-inflammatory cytokines by the activation of the NF-κB which plays an important role in transcriptional regulation of many pro-inflammatory genes, especially IL-1β (Pei and Wang Citation2015). Therefore, LPS is commonly used as a revulsive for IL production, stimulating cellular oxidative damage. On the basis of previous studies in BMECs, LPS-induced oxidative stress model was established (Shi, Yan, et al. Citation2016). Thus, in the present study, we treated BMECs with LPS to induce IL-1 overproduction for knowing the possible underlying mechanisms by which that VA pre-treatment protects BMECs from oxidative stress by modulating Nrf2 and NF-κB-signalling pathways.
Materials and methods
Ethics statement
The experiments using mammary glands were reviewed and carried out according to the guidelines set forth by the Animal Care and Use Committee, Inner Mongolia Agricultural University, Hohhot, China.
Mammary epithelial cells isolation and culture
Primary cells were isolated from the mammary tissue of mid-lactation Holstein dairy cows at a local slaughterhouse ( Hohhot, China) according to a procedure described by Wellnitz and Kerr (Citation2004) and Qi et al. (Citation2014). Approximately 1 cm3 pieces of fresh mammary gland tissues were removed aseptically and washed with cold phosphate buffered solution (PBS) (HyClone, Logan, UT, USA) containing 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, BRL, Carlsbad, CA, USA) and then transported to the laboratory immediately. Mammary tissue fragments were minced with sterile scissors and then digested by collagenase II (Gibco, Carlsbad, CA, USA) maintained in a humidified incubator at 37 °C in an atmosphere of 5% CO2 for 1 h with shaking every 20 min. The digests were filtered through a 200 μm nylon mesh to remove the large tissue fragments, and the filtered liquid was centrifuged at 179 g for 5 min and the supernatant liquid was removed. The cell pellet which is the purified BMEC was seeded at the medium containing Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12) (Gibco, Carlsbad, CA, USA) media supplemented with 10% foetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 0.5% insulin (Gibco, Carlsbad, CA, USA), 4 µg/mL prolactin (Sigma-Aldrich, St. Louis, MO, USA), 1 μg/mL hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/mL epithelial growth factor (Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin under 5% CO2 and 95% air at 37 °C. Cells were passaged twice and subsequently cryopreserved in the DMEM/F12 medium containing 10% FBS and 10% DMSO (Sigma, St. Louis, MO, USA).
Working solution preparation and experimental design
The LPS working solution and the VA (all-trans retinoic acid (RA)) working solution were prepared as follows: LPS (Sigma-Aldrich, St. Louis, MO, USA) was prepared in DMEM/F12 at room temperature to get the concentration of 0.1 mg/mL. The resulting solution was added to the cell culture medium to obtain the desired concentration of 1 μg/mL and then sterile-filtered before the experiments. All-trans RA was dissolved in dimethylsulphoxide (DMSO, Sigma, Munich, Germany), stored as 20 mg/mL stocks at −80 °C and parcelled in the dark. Immediately before use, the stock solution was diluted to the desired concentration (0 μg/mL, 0.05 μg/mL, 0.1 μg/mL, 0.2 μg/mL, 0.5 μg/mL, 1 μg/mL, 2 μg/mL, 3 μg/mL, and 4 μg/mL) with the Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12) media (Gibco Laboratories, Grand Island, NY, USA) and subsequently sterile-filtered. The concentration of DMSO was always less than or equal to 0.5%, which was not toxic (Kurosawa et al. Citation2009). The two kinds of working solutions were stored at −4 °C before use.
The BMECs were randomly divided into 10 groups with six replicates, and three independent experiments were performed. The first group was used as control: without VA and LPS (Sigma-Aldrich, St. Louis, MO, USA, L4391) for 30 h. Group 2 was the LPS-treated group: without VA for 24 h before treated with LPS (1 μg/mL) for an additional 6 h. Groups 3–10 severed as the VA plus LPS-treated groups: each group was pre-treated BMEC with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the presence of 1 μg/mL of LPS and VA for a further 6 h. The LPS concentration (1 μg/mL) and its reaction time (6 h) were performed according a previous test (Shi, Yan, et al. Citation2016).
Cell proliferation assay
Susceptibility of cells to LPS-mediated cell injury was evaluated by the methyl thiazolyl tetrazo-lium (MTT) cytotoxicity assay using the following procedure: 5 × 104 cells/well were distributed in 96-well plates. LPS and VA were added to the cells as in the experimental design. After the 30 h incubation times indicated, cells in 96-well plates were exposed to 20 μL of MTT (5 mg/mL in 1× PBS) was added to each well and incubated at 37 °C for 4 h. Next, the formazan crystals in each well were dissolved in 100 μL DMSO for 10 min with shaking. The absorbance at 490 nm in each well was recorded immediately using an ELISA microplate reader Synergy H4 (BioTek, Winooski, VT, USA). The higher absorbance meant more cell proliferation. The net absorbance in the wells containing cells cultured with control medium was taken as the 100% viability value. The cell proliferation was expressed as a cell relative growth rate (RGR): RGR = [OD490 nm (treatment group)−OD490 nm (control group)] × 100% (OD = optical density).
Cell lysates and culture supernatant preparation
Cells from the different treatment groups were lysed on ice for 30 min with lysis buffer (Beyotime, Nanjing, China). The lysates were centrifuged at 1200 g for 10 min at 4 °C to remove cell debris. The supernatant was used for the analysis of the activities of glutathione peroxidase (GPx) and ROS as well as the concentrations of malondialdehyde (MDA). Activities of SOD, CAT, T-AOC, and iNOS, concentrations of NO, IL-1, IL-6, and TNF-α in BEMCs were determined in the cell-free supernatant.
Enzyme activity and inflammatory factor content measurement
The activity of GPx, SOD, CAT, and T-AOC content in the cells were measured using a spectrophotometric diagnostic kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions and their absorbance were both measured by a spectrophotometer at a wavelength of 412 nm (UN2CO-WFT2100; Aoyi Co. Ltd., Shanghai, China). The MDA concentration was estimated with a thiobarbituric acid and chemical fluorescence analyser test separately from a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions by Synergy H4 (BioTek, Winooski, VT, USA) instrument.
Enzyme-linked immunosorbent assays (ELISA)
The activities of ROS and iNOS and contents of NO, IL-1, IL-6, and TNF-α levels in culture supernatants was determined by ELISA, following manufacturer’s instructions (R&D Systems, Inc., Minneapolis, MN, USA). The standard curves of themselves were set up meanwhile. Colour changes were determined at 450 nm. The interassay coefficient of variation was lower than 11% and the intraassay coefficient of variation was lower than 9%.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from the BMECs using Trizol solution (TaKaRa, Inc., Dalian, China) following to the manufacturer’s protocols. A 2% agarose gel electrophoresis and microplate reader was used to assess RNA integrity and purity. Then cDNA was synthesised in a 10 μL reaction system using PrimeScript RT reagent Kit DRR036A (TaKaRa, Dalian, China). The reaction programme of the cDNA synthesis was as follows: 37 °C for 15 min and 85 °C for 5 s. The RT products (cDNA) were stored at −20 °C for real-time PCR assay. Real-time PCR reactions were assessed at a final volume of 20-μL reactions containing 10 μL of 2× SYBR Premix Ex TaqTMII, 2 μL cDNA, 0.4 μL of each 10 μM forward and reverse primers, and 7.2 μL RNase free water. The mRNA abundance was normalised using the geometric mean of three stable housekeeping genes (glyceraldehyde phosphate dehydrogenase (GAPDH), ubiquitously expressed transcript (UXT), and ribosomal 18S rRNA). The primer sequences are presented in . The reactions were performed in a LightCycler480 real-time PCR machine (Roche Diagnostics Ltd, Forrentrasse CH-6343 Rotkreuz, Switzerland) with an initial denaturing step of 95 °C for 30 s followed by 40 cycles of 95 °C for 30 s (denaturation), 60 °C for 30 s (annealing), and 72 °C for 20 s (extension). The quality and the specificity of the PCR products were assessed by the melt curve analysis and subsequent gel agarose electrophoresis. The quantitative real-time PCR data were calculated using the 2–ΔΔCt method.
Table 1. Sequences of primers used in real-time polymerase chain reaction (real-time PCR).
Western blot
After treatment, BMECs was washed twice with PBS and proteins were extracted from the experimental condition cultured BMECs using a lysis buffer (Beyotime, Beijing, China). After measurement of the protein concentration, the protein samples were boiled for 5 min at 100 °C; 60 μg of total protein per lane were loaded and separated by 12% SDS-PAGE and then electrophoretically transferred (Mini Trans-Blot Cell; Bio-Rad, Hercules, CA, USA) from the gel to polyvinylidene difluoride membranes (Merck Millipore, Billerica, CA, USA). The membranes were incubated overnight at 4 °C with primary polyclonal rabbit anti-GPx1 (22 kDa, 1: 1000; Abcam, Cambridge, CA, USA), anti-iNOS (130 kDa, 1:500; Novus, Centennial, CO, USA) or anti-GAPDH (36 kDa, 1: 2000; Proteintech, Chicago, IL, USA) and polyclonal rabbit anti-RARα (55 kDa, 1:750), monoclonal rabbit anti-IL-1β (31 kDa, 1:750), monoclonal rabbit anti-IKKβ (87 kDa, 1:750), monoclonal rat anti-IκBα (39 kDa, 1:1000), monoclonal rabbit anti-NF-κBp65 antibody (65 kDa, 1:750), monoclonal rabbit anti-phospho-IKKβ (87 kDa, 1:500, Ser180), monoclonal rabbit anti-phospho-IκBα (40 kDa, 1:1000, Ser32), monoclonal rabbit anti-phospho-NF-κBp65 (65 kDa, 1:500, Ser536), monoclonal rabbit anti-Nrf2 (97–100 kDa, 1:1000), and monoclonal rabbit anti-Keap1 (60–64 kDa, 1:1000) (all both from Cell Signalling Technology, Danvers, MA, USA). After washing three times with PBS/0.1% Tween 20 (PBS-T), membranes were incubated with goat anti-rabbit IgG (1:1000) (KPL Laboratories, Gaithersburg, MD, USA) or goat anti-rat IgG labelled with horseradish peroxidase for 1 h at room temperature. After incubating with secondary antibodies and washing three times with PBS-T, the immunoreactive bands were visualised using an enhanced chemiluminescence system (Beyotime Institute of Biotechnology, Beyotime, Shanghai, China). Finally, images from the radiographic film were scanned and the integrated density was determined by the software Quantity One (Bio-Rad, Hercules, CA, USA). Relative density was quantified by normalisation of the integrated density of each protein to that of the corresponding GAPDH (used as an equal loading control) according to Kang et al. (Citation2016) and our previous research in the study group (Jin et al. Citation2016). Relative protein levels were determined by ImageJ software (NIH).
Statistical analysis
The experiment data were analysed using the General Linear Model procedure of SAS software (Statistical Analysis System, Version 9.2, SAS Inc., Cary, NC, USA) to test the significance of the treatment groups by regression analysis. Regression analysis was conducted to evaluate linear and quadratic effects of VA on the all response criteria except for controls. Differences between means were determined using Duncan’s multiple comparisons to test for significance. Data are presented in the tables as the means with standard errors of the mean. Differences were considered significant at p < .05, whereas the differences were considered to be a statistical trend when .05 < p < .10.
Results
Effects of VA on LPS-induced BMECs proliferation
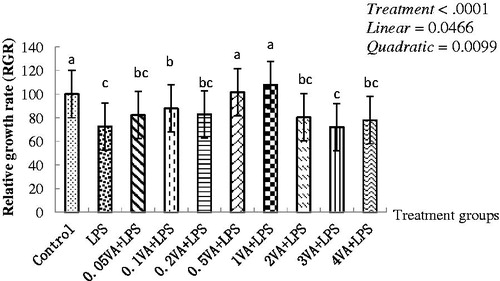
As shown in , exposure to 1 μg/mL LPS alone, a markedly decrease occurred compared with the control in the cell proliferation (p < .0001). In comparison with LPS treatment group, the cell proliferation increased significantly with increasing addition of VA in a quadratical dose-dependent manner (p = .0099), and the better effect was observed at the 0.5 μg/mL and 1 μg/mL dose of VA, and the 1 μg/mL dose of VA was the highest. However, the cell proliferation in the treatment group with VA dose from 0.05 μg/mL to 0.2 μg/mL and from 2 μg/mL to 4 μg/mL were slightly higher than the LPS treatment group except for 0.1 μg/mL VA.
Figure 1. Effect of VA on LPS-induced BMECs proliferation. Bovine mammary epithelial cells (BMECs) were randomly divided into 10 groups with six replicates. The first group was used as a control: without vitamin A (VA) and lipopolysaccharide (LPS) for 30 h. Group 2 was the LPS-treated group: without VA for 24 h before treated with LPS (1 μg/mL) alone for an additional 6 h. Groups 3–10 were eight doses of VA plus LPS-treated groups: pre-treated BMECs with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, and 4 μg/mL of VA for 24 h and then incubated in the presence of 1 μg/mL of LPS and VA for a further 6 h. The cell proliferation was evaluated by methyl thiazolyl tetrazo-lium (MTT) cytotoxicity assay. Values are means with their standard deviations depicted by vertical bars (from triplicate experiments). a–cMeans in the treatment groups not followed by the same letter differ significantly (p<.05) whereas the differences were considered to be a statistical trend when .05<p<.10.
Effect of VA on the antioxidant enzyme activity and inflammatory cytokines content in BMECs upon LPS damage
Antioxidant enzyme activities of GPx, T-AOC, SOD, and CAT in the LPS treatment group were lower than control significantly (p < .0001). Compared with LPS treatment group, VA significantly reversed these LPS-induced changes in a quadratical dose-dependent manner (p < .05) and the treatment with the addition of 1 μg of VA/mL had the highest value (). GPx activity in 1 μg/mL VA treatment group was significantly higher than that in the LPS treatment group and other groups (p < .0001), except for 0.5 μg/mL and 2 μg/mL VA. Compared to the LPS treatment group, the SOD enzyme activity and CAT activity all significantly increased in the VA treatment group (p < .0001, p < .0001, respectively), although VA treatment groups with 3 μg/mL and 4 μg/mL slightly decreased than that VA treatment groups from 1 μg/mL to 2 μg/mL; and T-AOC in the VA treatment group with dosages from 1 μg/mL to 3 μg /mL significantly increased (p < .0001). The VA treatment group with 4 μg/mL in T-AOC had no significant difference with the LPS treatment group and the other VA treatment groups.
Table 2. Effect of VA on the antioxidant enzyme activity and inflammatory cytokines content in BMECs upon LPS damageTable Footnotea.
Lipid peroxide product MDA content or cellular metabolism intermediates ROS activity and iNOS activity in the LPS treatment group were significantly higher than the control (p < .05). Compared with the LPS treatment group, VA significantly reversed those LPS-induced changes in dose-dependent manner quadratically (p < .05). Especially, the addition of 1 μg /mL VA achieved the best effect. Compared with the LPS injury group, MDA content in the 0.5–3 μg/mL VA pre-protection treatment group was significantly lower (p < .0037), but the VA dose of the 4 μg/mL treatment group was significantly higher than that of 1 μg/mL and 2 μg/mL groups. ROS activity in the VA pre-protection treatment groups from 0.2 to 4 μg/mL, and iNOS from 0.1 to 4 μg/mL were significantly lower than the LPS group, but ROS level in the 4 μg/mL VA group were significantly higher than those in the 1 μg/mL to 3 μg/mL groups, as well as iNOS level in the 3 μg/mL and 4 μg/mL groups were significantly higher than those in the 1 μg/mL and 2 μg/mL groups. Meanwhile, NO and IL-1 contents were significantly increased in the LPS treatment group versus control (p < .0001, p = .0549). In comparison to the LPS-induced group, supplementing with VA decreased NO and IL-1 concentration quadratically (p = .0139; p = .0016) and the addition of 1 μg/mL VA to the culture medium reached the minimum. Among them, compared with the LPS injury group, the NO content in the 0.2–3 μg/mL VA pre-protection treatment group and IL-1 contents in the 1–2 μg/mL VA pre-treatment groups was lower, respectively (p < .0001, p = .0549, respectively). But, the VA treatment groups from 2 μg/mL to 3 μg/mL were significantly higher than the 1 μg/mL group in NO content. The inflammatory cytokine IL-6 or TNF-α showed the same trend with IL-1 content, and their differences were significant quadratically (p = .0479) except TNF-α content.
Effect of VA on the gene expression of selenoproteins, inflammatory cytokines, Nrf2 and NF-κB pathway of BMECs upon LPS damage
Compared with the control, the addition of LPS alone to the culture medium down-regulated mRNA expressions of GPx1, GPx4 () significantly (p = .0090, p < .0001, respectively). VA significantly enhanced the mRNA expression of these two selenoprotein enzymes in a quadratic dose-dependent manner (p = .0004, p = .0004, respectively) than the LPS group, and the addition of VA 1 μg/mL caused the greatest mRNA expression. As shown in , the mRNA expression levels of GPx1 and GPx4 were significantly higher in the 0.2–2 μg/mL VA treatment group than in the LPS treatment group and in the 0.05 μg/mL VA treatment group (p = .0090, p < .0001, respectively). However, there was no significant difference between the four VA treatment groups (0.05, 0.1, 3, and 4 μg/mL) and the LPS injury group. LPS in the culture medium up-regulated the mRNA expression of IL-1β, IL-6, TNF-α, and iNOS of BMECs significantly (p < .05) versus control group. In comparison to the LPS group, supplementation with VA decreased those inflammatory cytokines gene expression above quadratically (p < .05). Especially, the treatment with the addition of 1 μg of VA/mL had the lowest inflammatory cytokines mRNA expressions compared with LPS treatment. Among them, the gene expression levels of IL-1β, IL-6, TNF-α, and iNOS were significantly decreased in those VA pre-protection groups including 0.1–3 μg/mL, 1–2 μg/mL, 0.5–2 μg/mL, and 0.5–3 μg/mL compared with the LPS injury group, respectively (p < .0001, p = .0002, p < .0001, p < .0001, respectively). However, compared to the LPS treatment group, there was no significant difference in the gene expressions of IL-1β at 0.05 μg of VA/mL and at 4 μg of VA/mL, of IL-6 at 0.05–0.5 μg of VA/mL and at 3–4 μg of VA/mL, of TNF-α at 0.05–0.2 μg of VA/mL, of iNOS at 0.05 and 0.2 μg of VA/mL, and at 4 μg of VA/mL.
Table 3. Effect of VA on the gene expression of selenoprotein, inflammatory cytokines, Nrf2, and NF-κB pathway of BMECs upon LPS damageTable Footnotea.
The NF-κBp50 and NF-κBp65 mRNA expression had the similar trend like IL-1β gene expression in a quadratic dose-dependent manner (p = .0126, p = .0020). The gene expression of Nrf2, a key factor in the antioxidant signalling pathway, was lower in the LPS damage group than in the control significantly (p < .0001). Compared to the LPS group, supplementing VA from 0.05 to 4 μg/mL resulted in higher Nrf2 mRNA expression in a quadratic dose-dependent manner (p = .0013). Meanwhile, the addition of VA at a concentration of 1 μg/mL achieved the highest effect, which is significantly higher than the supplementing VA from 0.05–0.1 μg/mL and 4 μg/mL, but there was no significant difference compared with other treatment groups.
Effect of VA on LPS-induced selenoproteins, RARα, IL-1β protein expression, and phosphorylation of Nrf2 and NF-κB pathways in BMECs
As shown in and , LPS decreased the protein expression levels of RARα, Nrf2, and GPx1 than the vehicle-treated cells significantly (p = .0003, p < .0001, p < .0001, respectively). In comparison to the LPS group, the supplementation with VA significantly quadratically increased RARα and Nrf2 (p = .0003, p < .0001, respectively) protein levels of BMECs and GPx1 has a quadratic trend. In addition, 1 μg /mL VA achieved the highest effect. Among them, RARα protein expression was significantly higher in the doses from 0.5 to 2 μg/mL VA pre-treated group than in the LPS group (p = .0003), but other VA pre-treated group had no significant difference with the LPS group in RARα protein expression compared with the LPS group, Nrf2 protein expression was significantly higher except in the 0.05–1 μg/mL VA pre-protection group, and one in the 4 μg/mL VA group is significantly lower than in 1 μg/mL VA group (p < .0001). The GPx1 protein expression was significantly increased in the whole VA treatment group compared to the LPS injury group, but in 0.05 μg/mL VA group and those VA groups at the levels higher than 1 µg/mL was less than the other VA treatment groups (p < .0001).
Figure 2. Effect of VA on LPS-induced selenoproteins, RARα, IL-1β protein expression, and phosphorylation of Nrf2 and NF-κB pathways in BMECs. The cells were randomly divided into 10 groups with six replicates. The first group was used as control: without vitamin A (VA) and lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) for 30 h. Group 2 was the LPS-treated group: without VA for 24 h before treated with LPS (1 μg/mL) for an additional 6 h. Groups 3–10 were eight doses of VA plus LPS treated groups: pre-treated bovine mammary epithelial cells (BMECs) with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the presence of 1 μg/mL of LPS and VA for a further 6 h. Expressions of retinoic acid receptor α (RARα) (A), nuclear factor E2-related factor 2 (Nrf2) (B), glutathione peroxidase1 (GPx1) (C), Kelch-like ECH2 associated protein 1 (Keap1) (D), interleukin-1β (IL-1β) (H), inducible nitric oxide synthase (iNOS) (I), and phosphorylated IκB kinase β (P-IKKβ) (E), inhibition kappa-B α (P-IκBα) (F), nuclear factor kappa-Bp65 (P-NF-κBp65) (G) protein levels were detected by Western blotting and normalised to glyceraldehyde phosphate dehydrogenase (GAPDH) levels.
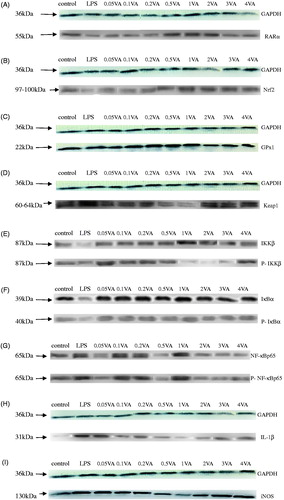
Table 4. Effect of VA on LPS-induced selenoproteins, RARα, IL-1β protein expression, and phosphorylation of Nrf2 and NF-κB pathways in BMECsTable Footnotea.
However, protein expressions of Keap1 (; p = .0562), IL-1β (; p = .0025), and iNOS (; p < .0001) and phosphorylation levels of IKKβ () (p < .0001), IκBα () (p = .0123), NF-κBp65 () (p < .0001) were opposite to levels of GPx1, Nrf2, and RARα with LPS and VA. Compared with the control group, the expression of proteins of iNOS, IL-1β and Keap1 were significantly increased in the LPS group (p < .0001, p < .0001, p < .0001, respectively). And the three protein expressions were significantly lower in all the VA pre-protection groups except iNOS at a dose of 0.05 μg/mL and 4 μg/mL VA than in the LPS group, respectively (p < .0001, p < .0001, p < .0001, respectively). Those protein expressions in 2 μg/mL, 3 μg/mL, and 4 μg/mL VA treatment groups were significantly higher than 1 μg/mL group. Phosphorylation levels of related factors including IKKβ, IκBα, and NF-κBp65, which belong to the NF-κB-signalling pathway, showed a similar pattern to those parameters above. Among them, the phosphorylation level of IKKβ, IκBα, and NF-κBp65 in the whole VA groups was significantly lower than the LPS groups (p < .0001, p < .0001, p < .0001, respectively), especially reached the lowest in the VA group with 1 μg/mL. And the VA group with 1 μg/mL significantly lower than 3 μg/mL and 4 μg/mL VA groups especially IKKβ in those three phosphorylation levels above.
Discussion
NO is an important biological mediator in the living organism that is synthesised from L-arginine using NADPH and molecular oxygen. However, the overproduction of NO which is regulated by iNOS act as damaging effects (Aktan Citation2004). Tarassishin et al. (Citation2014) found iNOS was induced only by IL-1/IFNγ in human astrocytes. Inflammatory cytokines played an important role in the development of stress damage (Rainard and Riollet Citation2006) and led to the injury of mammary tissues (Zhao and Lacasse Citation2008). It has been known that inflammatory cytokines mainly depend on the activation of NF-κB (Karin and Greten Citation2005). These results implied that IL-1-induced iNOS activity up-regulation and NO overproduction via the activation of NF-κB resulted in cell injuries. LPS could result in the activation of NF-κB, regulating the expression of inflammatory cytokines in mice embryonic fibroblast (Yamamoto et al. Citation2003). LPS is one of the main stress sources used in the study of oxidative damage in BMECs and goat mammary epithelial cells to induce IL-1 production (Bulgari et al. Citation2017; Wang et al. Citation2017). Our previous research has established LPS-induced oxidative stress model of BMECs (Shi, Yan, et al. Citation2016). Typically, NF-κB is localised to the cytosol and bound to its inhibitor called IκB, in un-stimulated cells. Once the cells are treated with various inducers, IκB is degraded by the IκB kinase (IKK) complex, and phosphorylation NF-κBp65 and NF-κBp50 translocate into the nucleus (Solt and May Citation2008). Finally, NF-κBp65 promotes the transcription of target genes, like TNF-α, IL-1β, and IL-6 (Hsing et al. Citation2011). The current study indicated that LPS activated the phosphorylation of NF-κBp65, NF-κBp50, IκBα, and IKKβ, and the addition of VA in advance exhibited changes that were reversed compared to the changes associated with the parameters described above for the LPS treatment group, as well as upregulating the antioxidant enzyme activities suggesting that VA had the potential to counter measure the oxidative damage of BMECs and to improve antioxidative function. But the mechanism is not clear.
Our previous in vivo studies indicated that addition of VA to the diet enhanced antioxidant activity in dairy cows (Jin et al. Citation2014), and in vitro research demonstrated that VA could attenuate oxidative stress through reducing NO overproduction (Shi, Guo, et al. Citation2016). However, the exact mechanism is still lacking. Therefore, we carried out this experiment to investigate that whether VA showed protective effects on LPS-induced injury and whether the protective mechanism was attributed to suppressed IL-1-induced NO overproduction via modulating Nrf2/NF-κB-signalling pathways. The results showed that VA (from 0.05 to 4 μg/mL) pre-treatment with LPS showed the opposite changes in those parameters above in a dose-dependent manner quadratically, promoting GPx, and their gene and protein expressions but decreased NO and IL-1 production, suppressing the phosphorylation of NF-κBp65, NF-κBp50, IκBα, and IKKβ quadratically compared to alone LPS treatment group, indicating that VA-protected BMEC from LPS-induced oxidative stress was probably attributed to inhibited IL-1β expression by suppressing NF-κB-signalling pathway.
It is obviously that the antioxidant effect of VA was increased as the increase of dosage and the addition of 1 µg/mL VA showed the best effect, while effect of VA at the levels higher than 2 µg/mL was reduced in the current study. Low doses of VA also showed reduced protective effect. The genes expression and proteins expression of IL-1 and iNOS, the MDA content and NO content, and the activities of ROS and iNOS showed the opposite changes. Based on the results of several indicators, the protective effect of VA on LPS-induced oxidative damage was dose-dependent, with 0.5–2 μg/mL having more effective protective effect, with the addition of 1 µg/mL VA best. It may be because a small amount of VA has a weak protective effect on cells, while excessive VA has a weakened protective effect on cells and is even toxic (Wu et al. Citation2016).
RA is the major bioactive metabolite of retinol or vitamin A. The RA activity is mediated primarily by the members of the retinoic acid receptor (RAR) subfamily, namely RARα, RARβ, and RARγ, which belong to the nuclear receptor (NR) superfamily of transcription factors. RARs form heterodimers with the members of the retinoid X receptor (RXR) subfamily and act as ligand-regulated transcription factors through binding specific RA response elements (RAREs) located in target genes promoters. In our current study, protein expression of RARα was in a dose-dependent manner quadratically, suggesting that VA played a role by receptors. RARs also have non-genomic effects and activate kinase signalling pathways, which fine-tune the transcription of the RA target genes (Di et al. Citation2015). The Nrf2 protein can binds to RARα and activates the Nrf2 activity and induce Nrf2 target genes in vivo mouse model (Tan et al. Citation2008).
Pivotal to antioxidant response is the transcription factor Nrf2. Under basal conditions, Nrf2 is mainly found sequestered in the cytosol as an inactive complex bound to a repressor molecule known as Kelch-like ECH-associating protein (Keap1). Oxidants can induce Nrf2 releasing from Keap1. The free Nrf2 translocates to the nuclear protein and binds to the antioxidant response element (ARE) sequences of the target gene promoters in conjunction with small Maf protein, resulting in up-regulation of antioxidant and phase II detoxifying enzymes, such as SOD, GPx and haem oxygenase-1 (HO-1) (Chen and Kunsch Citation2004). In the current study, the results showed that VA significantly quadratically improved Nrf2 gene expression and protein expression and its downstream antioxidant enzymes level through reducing Keap1 protein expression. These results suggested that VA protect against LPS-induced inflammation by suppressing NF-κB-signalling pathway as well as enhancing the activation of the Nrf2-signalling pathway in dose-dependent manner.
Brigeliusflohe suggested that overexpressed phospholipid hydroperoxide glutathione peroxidase (PHGPx) is sufficient to inhibit NF-κB activation (Brigeliusflohe et al. Citation1997). Kretz-Remy and Arrigo (Citation1996) found the similar result that nuclear translocation of NF-κB as well as IκBα degradation were inhibited in seleno-glutathione peroxidase (GPx)-overexpressing human breast carcinoma T47D cells exposed to oxidative stress. To sum up, we hypothesised that VA increased a large number of downstream GPx production by promoting Nrf2 gene expression which was for inhibiting the NF-κB-signalling pathway ultimately and achieved its pre-protection effect. Therefore, silence or overexpression of GPx to validate the hypothesis above is necessary in the next study.
Conclusions
In conclusion, these results showed that VA protected against LPS-induced inflammatory cytokines IL-1β production on dose dependent manner quadratically, and the mechanism was attributed to reduced IL-1β production and NO overproduction via inhibiting NF-κB-signalling pathway and mediating Nrf2 antioxidant signalling pathway. VA might be a valuable agent for the treatment of anti-inflammatory and antioxidant.
Geolocation information
Hohhot (E: 111.65°, N: 40.82°) is the capital of Inner Mongolia which located in the north of China. The climate in Hohhot is based off a four-season, monsoon climate.
Disclosure statement
The authors H. S., X. G., S. Y., Y. G., B. S., and Y. Z. declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this article. H. S. and X. G. contributed equally to this work and should be regarded as co-first authors.
Additional information
Funding
References
- Aktan F. 2004. iNOS-mediated nitric oxide production and its regulation. Life Sci. 75:639–653.
- Arora R, Yates C, Gary BD, McClellan S, Tan M, Xi Y, Reed E, Piazza GA, Owen LB, Dean-Colomb W. 2014. Panepoxydone targets NF-kB and foxm1 to inhibit proliferation, induce apoptosis and reverse epithelial to mesenchymal transition in breast cancer. Plos One. 9:e98370.
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. 2000. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 294:997–1008.
- Brigeliusflohe R, Friedrichs B, Maurer S, Schultz M, Streicher R. 1997. Interleukin-1-induced nuclear factor KB is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J. 328:199–203.
- Bulgari O, Dong X, Roca AL, Caroli AM, Loor JJ. 2017. Innate immune responses induced by lipopolysaccharide and lipoteichoic acid in primary goat mammary epithelial cells. J Anim Sci Biotechnol. 8:29–29.
- Chen XL, Kunsch C. 2004. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 10:879–891.
- Di MA, Leboffe L, De ME, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C. 2015. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 41:1–115.
- Guo YM, Zhang BQ, Shi HY, Yan SM, Shi BL, Guo XY. 2016. Establishment of oxidative damage model of bovine mammary epithelial cells induced by diethylenetriamine/nitric oxide adduct. Chin J Anim Nutri. 28:2378–2384.
- Haddad JJ, Harb HL. 2005. L-gamma-glutamyl-l-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol. 42:987–1014.
- Hsing CH, Lin MC, Choi PC, Huang WC, Kai JI, Tsai CC, Cheng YL, Hsieh CY, Wang CY, Chang YP, et al. 2011. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS One. 6:e17598.
- Hung LF, Lai JH, Lin LC, Wang SJ, Hou TY, Chang DM, Liang CC, Ho LJ. 2008. Retinoid acid inhibits IL-1-induced iNOS, COX-2 and chemokine production in human chondrocytes. Immunol Invest. 37:675–693.
- Jin L, Yan SM, Shi BL, Bao HY, Gong J, Guo XY, Li JL. 2014. Effects of vitamin a on the milk performance, antioxidant functions and immune functions of dairy cows. Anim Feed Sci Tech. 192:15–23.
- Jin L, Yan SM, Shi BL, Sheng R, Shi HY, Zhao YL, Li JL. 2016. Effects of retinoic acid on the synthesis of selenoprotein and the antioxidative indices of bovine mammary epithelial cells in vitro. Czech J Anim Sci. 61:194–202.
- Kang S, Lee JS, Lee HC, Petriello MC, Kim BY, Do JT, Lim DS, Lee HG, Han SG. 2016. Phytoncide extracted from pinecone decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J Microbiol Biotechnol. 26:579–587.
- Kansanen E, Kuosmanen SM, Hanna L, Anna-Liisa L. 2013. The keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 1:45–49.
- Karin M, Greten FR. 2005. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 5:749–759.
- Kielbik M, Klink M, Brzezinska M, Szulc I, Sulowska Z. 2013. Nitric oxide donors: spermine/NO and diethylenetriamine/NO induce ovarian cancer cell death and affect STAT3 and AKT signaling proteins. Nitric Oxide. 35:93–109.
- Kretz-Remy CP, Arrigo AP. 1996. Inhibition of IKB – a phosphorylation and degradation and subsequent NF-κB activation by glutathione peroxidase overexpression. J Cell Biol. 133:1083–1093.
- Kurosawa T, Nakamura H, Yamaura E, Fujino H, Matsuzawa Y, Kawashima T, Murayama T. 2009. Cytotoxicity induced by inhibition of thioredoxin reductases via multiple signaling pathways: role of cytosolic phospholipase A(2)alpha-dependent and -independent release of arachidonic acid. J Cell Physiol. 219:606–616.
- Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. 2012. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates-Nrf2 activators and GSK-3 inhibitors . Inflammopharmacology. 20:127–150.
- Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. 2009. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 11:2685–2700.
- Pei Z, Wang J. 2015. Propofol attenuates LPS-induced tumor necrosis factor-α, interleukin-6 and nitric oxide expression in canine peripheral blood mononuclear cells possibly through down-regulation of nuclear factor (NF)-κB activation. J Vet Med Sci. 77:139–145.
- Peng XX, Zhang SH, Wang XL, Ye TJ, Li H, Yan XF, Wei L, Wu ZP, Hu J, Zou CP, et al. 2015. Panax notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in raw264.7 macrophages. Chin Med. 10:1526.
- Qi L, Yan S, Sheng R, Zhao Y, Guo X. 2014. Effects of saturated long-chain fatty acid on mRNA expression of genes associated with milk fat and protein biosynthesis in bovine mammary epithelial cells. Asian-Australas J Anim Sci. 27:414–421.
- Rainard P, Riollet C. 2006. Innate immunity of the bovine mammary gland. Vet Res. 37:369–400.
- Robinson TL, Sutherland IA, Sutherland J. 2007. Validation of candidate bovine reference genes for use with real-time PCR. Vet Immunol Immunopathol. 115:160–165.
- Shi HY, Guo YM, Liu Y, Shi BL, Guo XY, Jin L, Yan SM. 2016. The in vitro effect of lipopolysaccharide on proliferation, inflammatory factors and antioxidant enzyme activity in bovine mammary epithelial cells. Anim Nutri. 2:99–104.
- Shi HY, Yan SM, Jin L, Shi BL, Guo XY. 2016. Vitamin A affects the expression of antioxidant genes in bovine mammary epithelial cells with oxidative stress induced by diethylene triamine-nitric oxide polymer. Czech J Anim Sci. 61:117–126.
- Siems W, Crifo C, Capuozzo E, Uchida K, Grune T, Salerno C. 2010. Metabolism of 4-hydroxy-2-nonenal in human polymorphonuclear leukocytes. Arch Biochem Biophys. 503:248–252.
- Solt LA, May MJ. 2008. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 42:3–18.
- Stefano GB, Kream RM. 2011. Reciprocal regulation of cellular nitric oxide formation by nitric oxide synthase and nitrite reductases. Med Sci Monit. 17:RA221–RA226.
- Tarassishin L, Suh HS, Lee SC. 2014. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. 62:999–1013.
- Tan KP, Kosuge K, Yang M, Ito S. 2008. NRF2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic Biol Med. 45:1663–1673.
- Wang W, Hu X, Shen P, Zhang N, Fu Y. 2017. Sodium houttuyfonate inhibits LPS-induced inflammatory response via suppressing TLR4/NF-ĸB signaling pathway in bovine mammary epithelial cells. Microb Pathog. 107:12–16.
- Wellnitz O, Kerr DE. 2004. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet Immunol Immunopathol. 101:191–202.
- Wu X, Chen Q, Washio Y, Yokoi H, Suzuki T. 2016. Excess retinoic acid induces fusion of centra by degenerating intervertebral ligament cells in Japanese flounder, Paralichthys olivaceus. J Exp Zool Part B. 326:464–473.
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643.
- Zhao X, Lacasse P. 2008. Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci. 86:57–65.
- Zhu X, Gallogly MM, Mieyal JJ, Anderson VE, Sayre LM. 2009. Covalent cross-linking of glutathione and carnosine to proteins by 4-oxo-2-nonenal. Chem Res Toxicol. 22:1050–1059.
