 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
An experiment was conducted to evaluate the effect of xylanase and cellulase derived from dried tomato pomace treated with Aspergillus niger and fermented juice of epiphytic of lactic acid bacteria (FJLB) on fermentative quality and in vitro digestibility of Napier grass silages. Thus, we treated Napier grass silage with or without fibrolytic enzymes (ENZ) in combination with or without FJLB. The pH values of silages treated with FJLB, but not ENZ, were found to be lower than the other silages during the ensiling process. The content of water soluble carbohydrates (WSC) of all silages was likely to decrease during fermentation in all silages. However, after 30 days of ensiling, the addition of ENZ resulted in higher WSC content when compared to the other treatments. At 30 day of ensiling, the crude protein of silage was higher in silage treated with FJLB. The gas production related to the soluble fraction was higher in silage treated with ENZ but total gas production was similar between treatments. The latter is in line the observation that the degradability of organic matter also was not different between treatments. It is concluded that neither the fermentation quality nor the in vitro digestibility of organic matter is improved when Napier grass is ensiled with both FJLB and fibrolytic enzymes compared with FJLB alone.
Ensiling Napier grass, which contains high water soluble carbohydrates, without additives results in an acceptable fermentation quality.
A combination of fermented juice of epiphytic lactic acid bacteria (FJLB) and fibrolytic enzymes has no effect on quality of Napier grass silage.
Highlights
Introduction
Napier grass (Pennisetum purpureum) is one of the most promising grasses available for ruminant production in tropical areas because of its high potential dry matter (DM) yield, i.e., around 71 tonnes DM/ha/year (Wijitphan et al. Citation2009). However, both the dry matter (DM) content and the content of water soluble carbohydrates (WSC) are considered too low for successful ensiling. Interestingly, in ruminant nutrition fibrolytic enzymes are currently applied due to their potency to improve fibre degradability and thus digestible energy intake in ruminants (Beauchemin et al. Citation2003). Indeed, enzymes such as xylanases and cellulases, can convert (hemi) cellulose into sugars (Stokes Citation1992) thereby rendering substrate available for fermentation. In line with this notion, it seems plausible that fibrolytic enzymes can also be applied in the process of ensiling and several authors (Sun et al. Citation2012; Khota et al. Citation2016; Desta et al. Citation2016) have already reported about the potency of fibrolytic enzymes to improve the quality of silage.
Apart from its low WCS content, the number of epiphytic lactic acid bacteria (LAB) in Napier grass also, may be too low for successful ensiling. Bureenok et al. (Citation2005a, Citation2006) has shown that the addition of fermented juice of epiphytic LAB (FJLB) from tropical forages such as Guinea grass and Napier grass (i.e., grasses with a relatively low WSC content), already improved the quality of the respective silages. Interestingly, Sun et al. (Citation2009) suggested that inoculants would be more effective in case the ensiling material has a high versus a low WSC content. It can thus be suggested that a combination of fibrolytic enzymes and FJLB versus FJLB alone, has an added value when ensiling grasses with a low WSC content. Currently, there is no information available on Napier silage fermentation and its digestibility when treated with fibrolytic enzymes in combination with FJLB. In the current study, we used “home-made” fibrolytic enzymes originating from Aspergillus niger. The aim of the current study is to evaluate the effects of FJLB and A. niger derived fibrolytic enzymes and their combination, on fermentation quality, chemical composition, and in vitro degradability of silage from Napier grass.
Materials and methods
Preparation of fibrolytic enzymes
A culture of A. niger was obtained from the National Centre for Genetic Engineering and Biotechnology (BIOTEC, Pathum Thani, Bangkok). Inoculum of A. niger was maintained on potato dextrose agar (PDA) at 30˚C for 5 days and stored at 4˚C until used. The spores from PDA plates were harvested by using sterile 0.01% Tween 80 (w/v) to obtain 1 × 107 spores mL−1.
The fibrolytic enzymes were produced as described by Saithi et al. (Citation2016). Briefly, the culture medium used for solid-state fermentation was dried tomato pomace (residue from the extraction of tomato sauce). The moisture content of dried tomato pomace was adjusted to about 50% and autoclaved (Nishio et al. Citation1979; Oriol et al. Citation1988; Hamidi-Esfahani et al. Citation2004; Hamidi-Esfahani et al. Citation2007). The substrate was cooled and mixed with 10% of an A. niger spore suspension (1 × 107 spores mL−1) (w/w). The solid-state culture was incubated at 30 °C for 72 h (Szewczyk and Myszka Citation1994). Then, the substrate was dried at 50 °C for 3–5 days before being used as fibrolytic enzyme.
Dried tomato pomace treated by A. niger was milled through a 1 mm sieve. The moisture content of the culture medium was determined by drying in a hot-air oven at 80˚C to derive a constant weight. The activity of xylanase and cellulase was according to a modified method of Bailey et al. (Citation1992) and Mandels et al. (Citation1976), respectively. The cellulase and xylanase activities from A. niger incubated with dried tomato pomace were 60,998 and 37,815 units g−1 DM, respectively.
Preparation of fermented juice of epiphytic lactic acid bacteria
Fermented juice of epiphytic lactic acid bacteria (FJLB) was prepared as described by Ohshima et al. (Citation1997) and Bureenok et al. (Citation2005a, Citation2005b). Briefly, 200 g of fresh Napier grass was macerated in 1000 mL of sterilised distilled water with a home blender. The juice was filtered through a double layer of cheesecloth; the filtrate was transferred to a glass bottle and 2% glucose was added. Then, the bottle was capped and incubated anaerobically at 30 °C for 2 days before being used as silage additive. The LAB counts of the FJLB were 5.5 × 108 colony forming unit (cfu) mL−1. Contents of mould and/or yeast, if any, were not measured in the current study because it was considered unnecessary.
Silage making
Napier grass (Pennisetum purpureum x Pennisetum americanum ‘Pak chong 1’) was harvested after 70 day of regrowth. The experimental silages were prepared from fresh Napier grass (19.27% dry matter (DM)) and the chemical composition of the Napier grass was as follows (% DM): crude ash, 12.64; crude protein (CP), 5.3; neutral detergent fibre (NDF), 74.63; acid detergent fibre (ADF), 44.6; and water soluble carbohydrate (WSC), 9.13. Napier grass was chopped to 0.5–2 cm using a crop cutter. Right after cutting, all grass was thoroughly mixed and divided into four equal portions. Then, the four experimental silages were prepared with the treatments arranged in a 2 × 2 factorial fashion, i.e., forage with or without fibrolytic enzymes (ENZ) and with or without additional FJLB. The applied doses of forage specific FJLB was log10 6.74 cfu g−1 fresh forage and ENZ was applied at a level of 0.5 g kg−1 fresh forage (contained 304,490 and 189,075 units of cellulase and xylanase activities, respectively). Thereafter, ∼100 g of the mixed portions was packed tightly into plastic pouches (20.32 × 33 cm, 120 μ thickness; M-PLASPACK, Bangkok, Thailand). Air was withdrawn from the plastic pouches by means of a vacuum sealer. For each sampling day and each experimental forage, four replicate pouches (n = 4) were prepared and all pouches were kept at ambient temperature (27–35 °C). Pouches from each treatment were opened after 1, 7, 14 or 30 days of ensiling for chemical analysis.
Chemical analysis
After opening of the pouches on the specified days, subsamples (∼20 g fresh material) were treated with 70 mL of distilled water and stored in a refrigerator at 4 °C for 12 h (Bureenok et al. Citation2006). Then, the extract was filtered (filter paper no.5; Whatman, England) and the pH of the extract was recorded. Thereafter, the filtrate was stored at −20 °C until the analysis of lactic acid, volatile fatty acids and NH3–N (Cai Citation2004). Lactic acid and volatile fatty acids were determined by means of HPLC. The dry matter (DM) content and CP (N-Kjeldahl × 6.25) and ash were determined as described by the AOAC (Citation1999, methods no. 935.29, 990.03 and 942.05, respectively). NDF and ADF were determined according to the method of Van Soest et al. (Citation1991) and were expressed exclusive of residual ash (NDFom and ADFom). WSC content was determined by the method of Dubois et al. (Citation1956). The numbers of lactic acid bacteria (LAB) were measured by the plate count method on MRS agar. Colonies were counted from the plates at appropriate dilutions and the number of colony forming units (cfu) was expressed per gram of fresh weight.
In vitro gas production
The sampling of rumen fluid was approved by the Ethical Committee of the University of Technology Isan, Thailand. The rumen fluid was used as inoculant and was obtained from two adult goats. The animals were fed Napier grass silage supplemented with commercial concentrate. Kinetics of degradation and gas production were evaluated after 30 days of fermentation using semi-automatic equipment to measure in vitro gas production. The in vitro digestibility was determined as described by Menke and Steingass (Citation1988).
Briefly, samples of each grass silage (0.2 g DM) were accurately weighed into a 100 mL syringe. Subsequently, a mixture composed of 20 mL of anaerobic buffer solution and 10 mL of ruminal fluid was added to each syringe. Blank (rumen fluid without sample) were run in triplicate in each series. Then, the syringes were placed into a water bath and automatically stirred at 39 °C. Gas production (GP) was measured at distinct incubation times (0, 1, 2, 4, 6, 8, 12, 18, 24, 36, 48, 60, 72 84 and 96 h). Total gas productions were corrected for the blank incubation. Cumulative gas production data were fitted as described by ∅rskov and McDonald (Citation1979);
where: a = the gas production from the soluble fraction (mL), b = the gas production from the insoluble fraction (mL), c = the gas production rate constant for the insoluble fraction b (mL/h), t = the incubation time (h). a + b = the potential gas production (mL), gas production at the time “t”.
The organic matter digestibility (OMD) was calculated according to Kamalak et al. (Citation2005):
Statistical analysis
All data of Napier grass silage were subjected to ANOVA using SAS (Citation1994), based on the model:
where Yij = response variable, µ = overall mean, ENZi = fibrolytic enzyme (i = with or without), FJLBj = fermented juice of lactic acid bacteria (j = with or without), (ENZ × FJLB)ij = interaction term between ENZ and FJLB and eij = residual error. Tukey test was used to determine the significant difference between means. The level of statistical significance was declared at p < .05.
Results
Fermentation profile and chemical composition
After 30 days of ensiling, the fermentation profile was not affected (p ≥ .071) by an interaction between the fibrolytic enzymes and FJLB (Table ). Addition of ENZ had not affect (p > .120) the values of pH, AA, LA, and PA contents of Napier grass silages were not significantly different from the untreated silage. The FJLB treated silages had significantly (p < .05) decreased pH, AA content and PA content and significantly (p < .05) increased LA content and LA:AA ratio of Napier grass silage compared with the other treatment. The addition of ENZ caused a decrease (p = .017) in the LA:AA ratio of silages. The butyric acid contents of all silages were less than 0.5%, and not significantly different among treatments. The use of FJLB caused lower (p = .001) NH3–N content. The WSC contents were not affected by ENZ × FJLB (p = .185). The addition of FJLB caused a 46% decrease in WSC contents in silages (p < .001).
Table 1. Fermentation profile and chemical composition of Napier grass silages treated with fibrolytic enzyme (ENZ), fermented juice of epiphytic lactic acid bacteria (FJLB) and its combination at 30 day of ensiling (n = 4; data are given in %DM, unless otherwise stated).
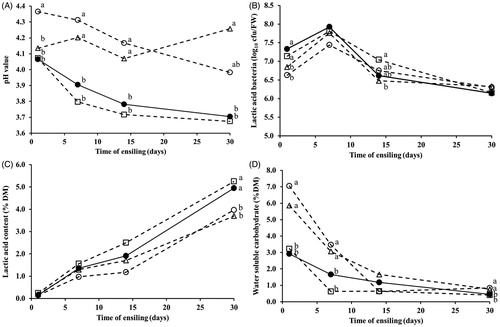
In the course time (Figure ), after the first day of ensiling, the FJLB and ENZ combined with FJLB treated silages gradually decreased the pH value throughout the ensiling period and the values were lower than the ENZ treated silage at the end of ensiling (panel A). At the first day of ensiling, LAB colonies of untreated silages and ENZ alone were lower (p < .05) than the FJLB and ENZ combined with FJLB treated silages. LAB counts were reached to log10 8 cfu g−1 fresh weight after 7 days of ensiling and declined to approximately log10 6 cfu g−1 fresh weight in all silage and were not different (p > .05) among the silage groups (panel B). Lactic acid contents gradually increased during the first day of fermentation in all silages (panel C). Although the levels of lactic acid were not significantly different during the early ensiling, pH values were significantly different among silages. The WSC content of all silages tended to decrease in the beginning of ensiling day (panel D). All FJLB additives rapidly decreased WSC content and reached the lowest value within 7 days of the start of fermentation, as compared to the control and ENZ additive.
Figure 1. Time courses on pH values, (panel a), lactic acid bacteria counts (panel b), lactic acid contents (panel c), and water soluble carbohydrate contents (panel d) during the ensiling of Napier grass. symbols: o: untreated silage (no fibrolytic enzyme (ENZ) or fermented juice of epiphytic lactic acid bacteria (FJLB)); □: silage treated with FJLB; Δ: silage treated with ENZ; •: silage treated with ENZ and FJLB.
The combination of ENZ and FJLB tended to increase the DM content of silages (p = .051). Addition of FJLB caused higher (p = .03) CP contents in silages, but the addition of both ENZ and FJLB decreased the CP contents of silages (p = .038). The NDF contents of silages did not show significant (p > .06) difference among different treatments. The ADF contents was not affected by ENZ × FJLB (p = .870). The addition of FJLB decreased in ADF contents (p = .006).
In vitro kinetics of degradation and gas production
The gas production kinetics and some estimated parameters in all silages are given in Table . As can be seen from the result, addition of ENZ caused an increase in (p = .009) gas production of the immediate soluble fractions (a) but the addition of both ENZ and FJLB decreased the gas production from these fractions of silages (p = .014). Gas production from slowly fermentable fraction (b), the potential gas production (a + b) and the gas production rate (c) were not significantly different among treatments. The organic matter digestibility (OMD) were not different in all silages.
Table 2. In vitro degradability coefficients of the dry matter (DM) of 30-days Napier grass silages treated with fibrolytic enzyme (ENZ) and fermented juice of epiphytic lactic acid bacteria (FJLB) (n = 4).
Discussion
Fermentation quality and nutritive value
Usually, good silage should have pH value of 4.20 or less, butyric acid content less than 10 g kg−1 DM, and NH3-N content less than 100 g kg−1 total nitrogen (McDonald et al. Citation1991). Therefore, these silage were well preserved. Since the FJLB used in our study contained LAB with producing lactic acid to inhibit growth of clostridia and aerobic bacteria, it improved silage quality (Wang et al. Citation2009). The significantly higher CP contents in FJLB treated silages could be attributable to a high LA content. As a result, the pH dropped sharply, which inhibited the growth of Clostridium spp. (Nadeau et al. Citation2000; Tian et al. Citation2014). Clostridium spp. usually produce NH3-N from protein in the silage materials (Xing et al. Citation2009). In this study, the ENZ treatment had no effect on degradation of plant fibre to increase sugar for promoting lactic acid fermentation. This could be attributed to the level of WSC (91.3 g kg−1 DM) in the material. Generally, the content of WSC available in forage materials is greatly related to silage fermentation and good silage quality is readily made from forage crops with high WSC content. Zhang et al. (Citation2016) suggested that a WSC content of about 60–70 g kg−1 DM should be sufficient for the fermentation process. Thus, the WSC contents of the Napier grass used in the current study may have been enough to stimulate the growth of LAB for producing LA. Therefore, the addition of ENZ may not have beneficial effects on promoting the propagation of LAB. Previous studies, have suggested that addition of cellulase could improve the fermentation quality by degrading NDF and ADF of tropical crop and by-product silages (Khota et al. Citation2016; Li et al. Citation2017; Wang et al. Citation2019). This result was not evident in our study, this could be attributed to the fibrolytic enzyme activity which depends on the temperature and pH condition (Colombatto et al. Citation2004). Cellulase requires a pH of 5.0–6.5 and temperature of 39 °C–50 °C for optimal activity (Chung et al. Citation2012; Kung et al. Citation2002). Therefore, the rapid decrease in pH from the fresh Napier grass (5.5) to below 4.2 within first day after ensiling in all treated silages (Figure ) could have inhibited the activity of cellulase. This study agreed with Khota et al. (Citation2017) who found that fibrolytic enzyme did not significantly decrease fibre contents in sorghum silages which caused by the rapid decreasing pH value to below 4 at the early ensiling process.
In vitro gas production
Nagadi et al. (Citation2000) suggested that the in vitro gas production is highly dependent on the availability of fermentable carbohydrate and nitrogen. Addition of FJLB resulted in a higher content of crude protein in silages compared with the silage without any additive. However, the gas production from soluble fraction was not different. This is in line with Akinfemi et al. (Citation2009) who suggested that protein fermentation gives a relatively small gas production when compared to carbohydrate fraction. The ENZ treated silages had a higher residual of WSC content, but the gas production of soluble fraction was higher in ENZ silages. This may have been caused by the non-soluble carbohydrate fraction in ENZ treated silage. Although, lower in WSC content, the gas production of soluble fraction in silages treated with FJLB was not different from the ENZ treated silages. The most plausible explanation is the high content of lactic acid in the FJLB treated silages. Kondo et al. (Citation2004) reported that the lactic acid content showed a positive relationship with gas production which silage could preserve more fermentable nutrients at low pH and high lactic acid and suggested that lactic acid itself might be metabolised in vitro rumen system. The combination of ENZ and FJLB did not improve the nutrient digestion. A possible reason for this is that fibre-degrading enzymes predigested the readily digestible fibre leaving a slower and less degradable fraction. Moreover, the LAB also used WSC to produce the lactic acid. Therefore, the leftover of readily digestible fraction was smaller than the others.
Conclusions
This study confirmed that addition of fibrolytic enzyme and its combined with lactic acid bacteria (FJLB) did not improve the fermentation and chemical composition of Napier grass silage which contained enough WSC. The FJLB improved the fermentation quality and inhibited protein degradation of Napier grass silage.
Ethical Approval
The experimental protocol was approved by the Ethical Committee of the University of Technology Isan, Thailand.
Disclosure statement
The authors declare no conflict of interest in this paper.
References
- Akinfemi A, Adesanya AO, Aya VE. 2009. Use of an in vitro gas production technique to evaluate some Nigerian feedstuff. Am-Euras J Sci Res. 4:240–245.
- AOAC. 1999. Official methods of analysis. 16th ed. Arlington (VA): Association of Official Analytical Chemists.
- Bailey JA, O’Connell RJ, Pring RJ, Nash C. 1992. Infection strategies of Colletotrichum species, Colletotrichum species. In: Bailey JA, Jeger MJ, editors. Colletotrichum: biology, pathology and control. Wallingford (UK): CAB International; p. 1–26.
- Beauchemin KA, Colombatto D, Morgavi DP, Yang WZ. 2003. Use of fibrolytic enzymes to improve feed utilization by ruminants. J Anim Sci. 81:E37–E47.
- Bureenok S, Namihira T, Tamaki M, Mizumachi M, Kawamoto Y, Nakada T. 2005a. Fermentative quality of guineagrass silage by using fermented juice of the epiphytic lactic acid bacteria (FJLB) as a silage additive. Asian Australas J Anim Sci. 18:807–811.
- Bureenok S, Namihira T, Kawamoto Y, Nakada T. 2005b. Additive effects of fermented juice of epiphytic lactic acid bacteria on the fermentative quality of guinea grass (Panicum maximum Jacq.) silage. Grassl Sci. 51:243–248.
- Bureenok S, Namihira T, Mizumachi S, Kawamoto Y, Nakada T. 2006. The effect of epiphytic lactic acid bacteria with or without different byproduct from defatted rice bran and green tea waste on napiergrass (Pennisetum purpureum Schumach) silage. J Sci Food Agric. 86:1073–1077.
- Cai Y. 2004. Methods for feed evaluation of forages: Silage Analyses. In: Japan Society of Grassland Science (Eds.). Proceedings of field and laboratory methods for grassland science. Tokyo (Japan): Japan Livestock Technology Association. p. 279–283.
- Chung YH, Zhou M, Holtshausen L, Alexander TW, McAllister TA, Guan LL, Oba M, Beauchemin KA. 2012. A fibrolytic enzyme additive for lactating Holstein cow diets: ruminal fermentation, rumen microbial populations, and enteric methane emissions. J Dairy Sci. 95:1419–1427.
- Colombatto D, Mould FL, Bhat MK, Phipps RH, Owen E. 2004. In vitro evaluation of fibrolytic enzymes as additives for maize (Zea mays L.) silage. Anim Feed Sci Technol. 111:111–128.
- Desta ST, Yuan X, Li J, Shao T. 2016. Ensiling characteristics, structural and nonstructural carbohydrate composition and enzymatic digestibility of napier grass ensiled with additives. Bioresource Technol. 221:447–454.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Calorimetric method for determination of sugars and related substances. Anal Chem. 28:350–356.
- Hamidi-Esfahani Z, Hejazi P, Shojaosadati SA, Hoogschagen M, Vasheghani-Farahani E, Rinzema A. 2007. A two-phase kinetic model for fungal growth in solid-state cultivation. Biochem Eng J. 36:100–107.
- Hamidi-Esfahani Z, Shojaosadati SA, Rinzema A. 2004. Modelling of simultaneous effect of moisture and temperature on A. niger growth in solid-state fermentation. Biochem Eng J. 21:265–272.
- Kamalak A, Canbolat O, Gurbuz Y, Ozay O. 2005. Comparison of in vitro gas production technique with in situ nylon bag technique to estimate dry matter degradation. Czech J Anim Sci. 50:60–67.
- Khota W, Pholsen S, Higgs D, Cai Y. 2016. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculant. J Dairy Sci. 99:9768–9781.
- Khota W, Pholsen S, Higgs D, Cai Y. 2017. Fermentation quality and in vitro methane production of sorghum silage prepared with cellulase and lactic acid bacteria. Asian-Australas J Anim Sci. 30:1568–1574.
- Kondo M, Kita K, Yokota H. 2004. Effects of tea leaf waste of green tea, oolong tea, and black tea addition on Sudangrass silage quality and in vitro gas production. J Sci Food Agric. 84:721–727.
- Kung L, Cohen MA, Rode LM, Treacher RJ. 2002. The effect of fibrolytic enzymes sprayed onto forages and fed in a total mixed ratio to lactating dairy cows. J Dairy Sci. 85:2396–2402.
- Li M, Zhou H, Zi X, Cai Y. 2017. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim Sci J. 88:1531–1537.
- Mandels M, Andreotti R, Roche C. 1976. Measurement of saccharifying in cellulase. Biotechnol Bioeng Symp. 6:21–33.
- Menke KH, Steingass H. 1988. Estimation of energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim Res Dev. 28:7–55.
- McDonald P, Henderson AR, Heron S. 1991. The biochemistry of silage. 2nd ed. Marlow Bucks (UK): Chalcombe Publications.
- Nadeau EMG, Buxton DR, Russell JR, Allison MJ, Young JW. 2000. Enzyme, bacterial inoculant, and formic acid effects on silage composition of orchardgrass and alfalfa. J Dairy Sci. 83:1487–1502.
- Nagadi S, Herrero M, Sessop NS. 2000. The effect of fermentable nitrogen availability on in vitro gas production and degradability of NDF. Anim Feed Sci Technol. 87:214–251.
- Nishio N, Tai K, Nagai S. 1979. Hydrolase production by Aspergillus niger in solid state cultivation. Euro. J Appl Microbiol Biotechnol. 8:263–270.
- Ohshima M, Kimura E, Yokota H. 1997. A method of making good quality silage from direct cut alfalfa by spraying previously fermented juice. Anim Feed Sci Technol. 67:129–137.
- Oriol E, Raimbault M, Roussos S, Viniegra-Gonzales G. 1988. Water and water activity in the solid state fermentation of cassava starch by Aspergillus niger. Appl Microbiol Biotechnol. 27:498–503.
- Ørskov ER, McDonald I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 92:499–503.
- Saithi S, Borg J, Nopharatana M, Tongta A. 2016. Mathematical modeling of biomass and enzyme production kinetics by Aspergillus niger in solid-state fermentation at various temperatures and moisture contents. J Microbiol Biochem Technol. 8:123–130.
- SAS. 1994. Users guide, Version 6. 4th ed. Carry (NC): SAS Institute Inc.
- Stokes MR. 1992. Effects of an enzyme mixture, an inoculant, and their interaction on silage fermentation and dairy production. J Dairy Sci. 75:764–773.
- Sun Q, Gao F, Yu Z, Tao Y, Zhao S, Cai Y. 2012. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. J Anim Sci. 83:305–309.
- Sun ZH, Liu SM, Tayo GO, Tang SX, Tan ZL, Lin B, He ZX, Hang XF, Zhou ZS, Wang M. 2009. Effects of cellulase or lactic acid bacteria on silage fermentation and in vitro gas production of several morphological fractions of maize stover. Anim Feed Sci Technol. 152:219–231.
- Szewczyk KW, Myszka L. 1994. The effect of temperature on the growth of A. niger in solid state fermentation. Bioprocess Eng. 10:123–126.
- Tian J, Yu Y, Yu Z, Shao T, Na R, Zhao M. 2014. Effects of lactic acid bacteria inoculants and cellulase on fermentation quality and in vitro digestibility of Leymus chinensis silage. Grassl Sci. 60:199–205.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Wang J, Wang JQ, Zhou H, Feng T. 2009. Effects of addition of previously fermented juice prepared from alfalfa on fermentation quality and protein degradation of alfalfa silage. Anim Feed Sci Technol. 151:280–290.
- Wang S, Guo G, Li J, Chen L, Dong Z, Shao T. 2019. Improvement of fermentation profile and structural carbohydrate compositions in mixed silages ensiled with fibrolytic enzymes, molasses and Lactobacillus plantarum MTD-1. Ital J Anim Sci. 18:328–335.
- Wijitphan S, Lorwilai P, Arkaseang C. 2009. Effects of plant spacing on yields and nutritive values of Napier grass (Pennisetum purpureum Schum.) under intensive management of nitrogen fertilizer and irrigation. Pak J Nutr. 8:1240–1243.
- Xing L, Chen LJ, Han LJ. 2009. The effect of an inoculant and enzymes on fermentation and nutritive value of sorghum straw silages. Bioresour Technol. 100:488–491.
- Zhang Q, Li X, Zhao M, Yu Z. 2016. Lactic acid bacteria strains for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 71:472–481.
