Abstract
The study evaluated the effects of eCG treatment prior to ovum pick up (OPU) on follicular population, oocyte and embryo yields in summer and autumn in Podolic cattle. The effects of repeated OPU on cattle wellbeing was also documented. Twenty-six animals were used, and split into two groups, treatment (OPU; n = 18) and control (CG; n = 8). The OPU cattle were subsequently split into two subgroups (n = 9) and underwent repeated OPU, without and with eCG priming, for a total of 8 sessions (4 sessions/season). Follicular population, oocyte and embryo yields were recorded in those sub-groups. CG was handled in the same manner of OPU, except for epidural anaesthesia and follicular aspiration. Biochemical profile, serum protein electrophoresis and haptoglobin levels were analysed in OPU and CG. Hormonal priming increased the number of medium follicles (1.7 ± 0.2 vs 0.9 ± 0.2, p < .05), while it decreased the recovery rate and number of cumulus-enclosed oocytes (COCs) (recovery rate: 38.3 ± 3.5 vs 60.5 ± 4.0%; COCs: 2.3 ± 0.3 vs 3.4 ± 0.4, respectively; p < .01). However, priming increased cleavage (72.9 ± 5.7 vs 49.4 ± 5.4; p < .05) and blastocyst (41.1 ± 5.7 vs 23.0 ± 4.2; p = .054) rates. With regard to season’s effect, a higher number of COCs was recorded in autumn than in summer (3.1 ± 0.4 vs 2.6 ± 0.3; p < .05) without affecting though the number of embryos produced (0.9 on average). Since haematological parameters did not vary between OPU and CG, our preliminary data suggest that repeated OPU may be used as a conservation strategy in Podolic cattle without affecting wellbeing.
Podolic cattle is an endangered Italian breed, reared in semi-extensive/extensive systems
Ovum pick-up (OPU) can be carried out in this breed without impairing animal health and welfare
The eCG treatment before OPU (priming) did not improve the number of embryos produced per donor.
Highlights
Introduction
Podolic cattle is considered an endangered breed at risk of extinction with a population size consisting of about 25,000 heads (ANABIC Citation2018). This breed is commonly bred in extensive or semi-extensive systems for production of meat and high-quality cheese, mainly in the South of Italy. This autochthonous breed, resistant and well adapted to the environment, is a key asset for many stakeholders, providing also the opportunity to utilise marginal areas otherwise abandoned with deleterious effect on the environment itself. Due to the rearing conditions and the low number of pure subjects, it is not uncommon to register the presence of hypofertile subjects, (ANABIC Citation2018). This is a concern that may impair the breed preservation. The increasing awareness of the importance of biodiversity safeguarding has boosted the interest in conservation strategies worldwide.
Ovum pick-up allows the retrieval of immature oocytes from antral follicles, and in conjunction to in vitro embryo production (IVEP) is considered the most competitive technology within conservation strategies for producing a large number of embryos from live donors, ensuring thus the increase of endangered animal population (Comizzoli et al. Citation2000; Velazquez Citation2008). This is mainly due to its repeatability, as oocyte collection can be carried out even twice per week for a long time without interfering with the reproductive status of the donors (Pieterse et al. Citation1991; Broadbent et al. Citation1997; Kruip and Den Daas Citation1997; Galli et al. Citation2001). Furthermore, it has been shown that the extension of OPU sessions up to 5 months does not affect neither follicular waves and oocyte characteristics nor IVEP efficiency (Kruip et al. Citation1994). In addition, OPU is a valid alternative to MOET for embryo production in cattle, as it can be successfully carried out regardless of the reproductive status of the donor (i.e. acyclic and hypofertile cows, animals having patent tube and those not responsive to MOET treatments) (Galli et al. Citation2014). This is particularly important for breed preservation programmes, that face the constraint of a limited availability of pure donors. Although OPU has been carried out for a long time without hormonal stimulation, the current trend is to stimulate donors with gonadotropins (priming) to increase follicular availability (Merton et al. Citation2003; Blondin et al. Citation2012; Vieira et al. Citation2014), enhancing embryo production and reducing animal handling. However, the cost benefit ratio of hormonal priming is still questionable.
The implications of OPU on animal health and welfare are also a matter of debate. Chastant-Maillard et al. (Citation2003) demonstrated that repeated OPU in cattle does not cause neither short-term nor long-term stress. A subsequent study on heifers confirmed that this technique is non-invasive based on physiological and behavioural responses, whereas a certain discomfort was associated to the epidural anaesthesia (Petyim et al. Citation2007). Contrary to other cattle breeds, the Podolic breed is typically bred under extensive or semi-extensive systems and therefore is not accustomed to handling. Those animals may therefore undergo stress due to OPU procedures leading to health and welfare issues.
Acute phase proteins (APPs) have been used as reliable indicators of inflammation and stress in cattle (Lomborg et al. Citation2008). The APPs consist of “negative” and “positive” proteins that show a decrease and an increase in levels, respectively, in response to a challenge. The negative APPs include albumin and transferrin. The positive APPs include haptoglobin and α, β and γ globulin (Murata et al. Citation2004). Among APPs, haptoglobin has been often used as an early detector of infection in cattle (Paulina and Tadeusz Citation2011) and it is also largely used in practice to monitor antibiotic and anti-inflammatory therapies (Murata et al. Citation2004). Electrophoresis and the Albumin/Globulin ratio are also used as stress indicators and to distinguish between acute or chronic inflammation or infection. In addition, inflammatory conditions are often associated with alterations of the total protein electrophoresis, especially regarding the α-globulin fraction (Barta Citation1993; Murata et al. Citation2004). In presence of an inflammation or infection, the biochemical profile is often used as a diagnostic tool to identify which organ may be compromised (Schalm et al. Citation1975).
To the best of the authors’ knowledge, OPU has never been performed in Podolic cattle, and hence the efficiency and its implications for health and wellbeing are unknown. The first hypothesis of this study was that priming of hypofertile Podolic donors with eCG would positively impact follicular population, improving embryo yields, hence making repeated OPU combined to IVEP a valid tool for breed preservation. The second hypothesis was that repeated OPU on a breed typically reared under extensive or semi-extensive management and therefore minimally accustomed to handling would possibly affect its wellbeing.
Therefore, the primary aim of this pilot study was to evaluate the effect of a pre-treatment of hypofertile Podolic donors with eCG on follicular population, oocyte and embryo yields in two seasons (summer and autumn). The secondary aim was to assess the influence of repeated OPU on health and wellbeing of Podolic cattle by evaluating biochemical profile, serum protein electrophoresis and haptoglobin concentration.
Material and methods
Compliance with ethical standards
Animals used in this study were treated in accordance with the European Commission recommendation 2007/526/EC and 2010/63/UE on revised guidelines for the accommodation and care of animals used for experimentation and other scientific purposes. In particular, this study was approved by the Ethical Animal Care and Use Committee of the University of Naples, “Federico II” (Naples, Italy).
Animals
The study was carried out at the “Centro Sperimentale ARSAC” of Rocca di Neto (Kr), in Calabria Region (Italy) and lasted four months (end of June–end of October). Twenty-six clinically healthy pluriparous non lactating Podolic cattle aged 6.7 ± 0.2 years, with an average body condition score of 3 (1-5 scale, as developed by Edmonson et al. Citation1989) which did not change throughout the study, were selected from a large group of animals after the interruption of sexual promiscuity (which occurred at weaning). The cows underwent clinical gynaecological examination to ascertain the reproductive status 12 days before the start of the trial: only those non pregnant, without any gross abnormalities of the genital tract and with a corpus luteum in at least one examination were enrolled in the study. All animals were kept freely in a large open-air paddock (about 30 ha). All year round, they were fed with 3 kg concentrates (11.8 MJ of metabolisable energy/kg dry matter; 180 g crude protein/kg dry matter) supplemented with 110 g mineral/vitamin (Norvite, Aberdeen, UK) daily, and ad libitum mixed hay, composed by Trifolium alexandrinum, Lolium italicum and Avena sativa, and barley straw. Water was also offered ad libitum. Both water and feed quantity and quality remained the same throughout the experiment. Before the start of the trial, all animals underwent pesticide treatment.
Experimental design
In order to assess the effects of eCG treatment on OPU, 18 Podolic cattle (OPU group) underwent repeated OPU, alternatively without and with eCG priming, for a total of 8 sessions (4 sessions/season, respectively). To assess animal health, other 8 animals were handled exactly in the same manner, except for the OPU procedure (they did not undergo epidural anaesthesia and follicular aspiration) and were used as control (CG).
Ovum pick-up (OPU) donors (n = 18) were synchronised prior to OPU in order to carry out oocyte retrieval on day 4 of the cycle in both eCG and non-eCG groups. For non-eCG group synchronisation of oestrus was carried by progesterone treatment (Walsh et al. Citation2007) and OPU was carried out on day 4 after oestrus. For the eCG group the first OPU session served as dominant follicle removal to reset the follicular population, then eCG (400 IU, Ciclogonina, Zooetis, Rome, Italy) was administered the day after and OPU was carried out on day 4 of the cycle. This schedule, illustrated in , was repeated with a 2-week interval during summer (June-July), for a total of 4 sessions.
Figure 1. Schedule used prior to OPU in order to carry out oocyte retrieval with and without eCG.
PGF2(α): Prostaglandin F2-α; GnRH: gonadotropin-releasing hormone; OPU: Ovum Pick-Up; eCG: Equine chorionic gonadotropin.
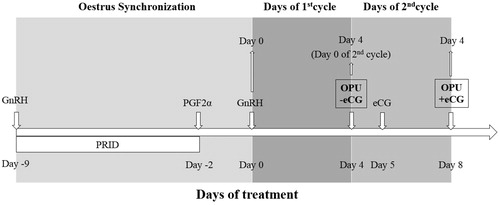
After these sessions donors were allowed to rest for 40 days, and following this rest period, additional 4 OPU sessions (2 non-eCG and 2 eCG-treated) were carried out as described above in the autumn season (September-October).
For each session, the number of aspirated follicles and recovered cumulus-enclosed oocytes (COCs) were recorded and COCs were used for IVEP. Blood samples were collected from the coccygeal vein once a month from all the animals (TG and CG). Blood samples were collected the day before the beginning of the trial (T0, June), after the first 4 OPU sessions (T1, end of July), after the 40 days rest and beginning of the second season (T2, mid-September) and at the end of the second 4 OPU sessions (T3).
Climatic parameters were collected by a weather station (Kestrel: 4000 Pocket Weather Tracker, Nielsen-Kellerman, USA) located close to the animals’ paddocks.
Ovum pick-up
The OPU procedure was performed as previously described (Galli et al. Citation2001) by the same expert operator on animals restrained in a chute under epidural anaesthesia (4 ml of 2% lidocaine hydrochloride – Gellini, Italy). A 5 MHz sector scanner was employed to scan ovaries (Aloka SSD-500, Aloka Co., Tokyo), and a 17 gauge needle properly allocated in a vaginal guide was used to aspirate antral follicles, using a −40 mmHg constant vacuum pressure (K-MAR-5100, Cook IVF Co., Australia). The aspiration line was continuously rinsed with 25 mM hepes-buffered TCM 199 supplemented with 100 USP units/ml of heparin (Eparina Vister, Teva Italia s.r.l., Milano, Italy) and 10% foetal calf serum (FCS) during follicular aspiration. Aspirated follicular fluid and COCs were kept constantly at 37 °C. The COCs were recovered immediately following OPU session and graded from 1 to 4 as previously described (Marquant-Le Guienne Citation1999). COCs from each donor were maintained separated throughout the procedure until embryo culture and final evaluation.
In vitro embryo production
Within 20 min from follicle aspiration, recovered COCs were washed twice in HEPES-buffered TCM 199 (H 199) with 10% FCS and then allocated in a 15 mL Falcon tube containing the same medium supplemented with 0.5 μg/ml FSH, 5 μg/ml L LH, 0.8 mM L-glutammine, 50 μg/ml di gentamycin and 15% of bovine serum (BS) and kept in a portable incubator at 39 °C for in vitro maturation.
After 20-22 h, in vitro–matured COCs were washed and transferred into 300 µl of IVF medium covered with mineral oil, consisting of Tyrode’s modified medium (Parrish et al. 1986), without glucose and BSA, supplemented with 5.3 USP/ml heparin (H3149, Sigma Aldrich/Merck, Milano, Italy), 30 µM penicillamine, 15 µM hypotaurine, 1 µM epinephrine and 1% BS. Frozen semen straws from Podolic bulls were thawed at 37 °C for 40 s and spermatozoa selected by Percoll discontinuous gradient (45% and 80%) and centrifugation (25 min at 300 g). The pellet was reconstituted into 2 ml of IVF medium and centrifuged twice, at 160 and 108 g for 10 min. The pellet was diluted with IVF medium and added in the fertilisation drops (50 µl) at the concentration of 1 × 106 sperm/ml.
Gametes were co-incubated for 20 h at 39 °C, in 5% CO2 in air, after which presumptive zygotes were vortexed for 2 min to remove cumulus cells in HEPES TCM with 5% BS, washed and cultured in synthetic oviduct fluid (SOF) medium (Holm et al. Citation1999), with 30 µl/mL essential amino acids, 10 µl/mL nonessential amino acids and 5% BS in a humidified mixture of 5% CO2, 7% O2, and 88% N2 in air at a temperature of 39 °C. For IVC, to take advantage of the potential benefits of group embryo culture, while keeping zygotes from each animal separated, special dishes (Embryo corral, GPS Dishware, LifeGlobal, Connecticut) were used, designed with a central well divided into four quadrants separated by posts to permit media exchange among quadrants without allowing movement of embryos. On Day 7 (Day 0 = IVF), cleavage and blastocyst rates were recorded.
Blood parameters: biochemical parameters, electrophoresis and haptoglobin
Immediately after collection, serum was obtained by centrifugation at 1800 g for 20 min, kept at 4 °C for 4 h, and then stored at −80 °C until analyses.
Serum biochemical parameters were measured as previously described (Campanile et al. Citation2003). Briefly, the enzymatic colorimetric method was used to assess: urea (BU) (urease method), creatinine, total cholesterol, HDL cholesterol, triglycerides, total bilirubin, direct bilirubin. Colorimetric methods were used to measure total proteins, and serum protein fractions were evaluated by electrophoresis. Serum enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT) (kinetic at 37 °C) were measured using the enzymatic colorimetric method. Serum concentration of haptoglobin was measured by a colorimetric method using Phase Haptoglobin Colorimetric Assay kit (Tridelta Development, Ireland), based on the difference in peroxidase activity of the free haemoglobin that stably binds to haptoglobin in acid environment. The peroxidase activity of bound-haemoglobin is directly proportional to the quantity of haptoglobin present in the sample.
Statistical analysis
Descriptive statistics was performed on the environmental, reproductive and haematological data. Difference between the environmental parameters in summer and autumn were tested using t-test.
Reproductive parameters were further analysed by repeated measures ANOVA, evaluating the treatment (non eCG-treated/eCG-treated), the season (summer/autumn) and the interaction treatment*season as fixed factors, with animal as random factor. A Tukey test was used for post-hoc testing.
Haematological parameters were analysed by mixed linear model using PROC mixed procedure with random factors utilised to account for multiple records per animal. Models were developed using the group (OPU/Control), the time (T0, T1, T2, T3) and the interaction group*time as fixed factors, with animal as random factor. A separate model was developed with each of the blood parameters as the outcome. A Tukey test was used for post-hoc testing. Statistical analyses were performed using SAS (SAS, version 9, 1999) and statistical significance (p) was set at 0.05. All results are expressed as means ± standard error (SE).
Results
Table shows the difference among the environmental parameters recorded during summer and autumn.
Table 1. Environmental parameters recorded during the trial conducted in Calabria (Italy), mean values ± standard deviation for summer and autumn (paired-t test).
Reproductive parameters
Table shows descriptive statistics of the reproductive data for TG animals. A high individual variability was observed in the number of follicles, total number of COCs, Grade 1 + 2 COCs and blastocysts.
Table 2. Individual variability in the number of total follicles, total COCs, superior quality COCs, i.e. Grade 1 + 2 COCs and blastocysts among Podolic cattle.
There was an effect of treatment (p < .001) and season (p < .01) on reproductive parameters, while no effect of the interaction treatment *season (p = .758) was detected. Treatment with eCG increased (p < .05) the number of medium follicles, without affecting neither the number of small and large follicles, nor the total number of follicles (Table ). The eCG priming decreased (p < .01) the recovery rate, leading to lower number of both total number of COCs (p < .01) and Grade 1 + 2 + 3 COCs (p < .05). Although the treatment was effective in increasing both cleavage (p < .05) and blastocyst (p = .054) rates, the mean number of embryos produced per donor per session was similar in non eCG and eCG-treated animals (Table ).
Table 3. Effect of eCG priming on follicular population, oocyte recovery and embryo yields in Podolic cattle subjected to OPU.
Table shows the effects of season on reproductive parameters. While the number of total follicles and large follicles did not vary between summer and autumn, the number of small follicles was lower (p < .05) and the number of medium follicles (p < .05) was higher in summer compared to autumn. Furthermore, the number of COCs, Grade 1 + 2 COCs and Grade 1 + 2 + 3 COCs was lower (p < .05) in summer than in autumn. Nevertheless, the percentage of good quality COCs, cleavage and blastocyst rates, as well as the number of embryos produced per donor were similar in the two seasons.
Table 4. Effect of season on follicular population, oocyte recovery and embryo yields in Podolic cattle undergone OPU.
Table shows the effects of the interaction on the studied parameters.
Table 5. Effect of the interaction treatment (T)*season (S) on follicular population, oocyte recovery and embryo yields in Podolic cattle undergone OPU.
Haematological parameters
The effect of the interaction Group*Time was not significant on any blood parameter (data not shown). As shown in Table , no differences were observed in hematological parameters between the TG and CG animals.
Table 6. Effect of OPU on haematological parameters.
Discussion
The first aim of this work was to evaluate the feasibility of OPU and IVEP for preservation programmes tailored to Calabrian Podolic cattle. This pilot study is the first report on the use of a combined OPU/IVEP programme on Calabrian Podolic hypo-fertile cows to assess the feasibility of these technologies for breed preservation. It was demonstrated that the eCG-priming of the donors was not effective in increasing the number of embryos obtained per donor, non-supporting thus our first hypothesis. Our findings did not also support the hypothesis that repeated OPU sessions would interfere with the wellbeing of animals ordinarily kept at minimum human handling. In fact, no variation in hematological parameters indicative of infection or stress was observed.
The first finding of the study was the reduced number of follicles present on the ovary and, hence, the lower number of COCs recovered per donor. Indeed, regardless of the eCG-priming and collection season, the number of total aspirated follicles was much lower than the average value reported in the literature for Holstein cows (Galli et al. Citation2014). In addition, both the numbers of follicles and COCs were lower than those recorded in problem donors (i.e. cows that had not produced a viable embryo for 1 or more years in a conventional MOET programme) of different breeds in an earlier trial (Looney et al. Citation1994). In contrast, these parameters were similar to those recorded in suckling beef cows (Aller et al. Citation2012). It is known that oocytes recovery depends on the number of antral follicles with diameter > 2 mm, and that follicular population is affected by genetics, breeding system, nutrition, body condition score, and stage of lactation (Lucy et al. Citation1992; Pushpakumara et al. Citation2003; Kouamo et al. Citation2015). A high individual variability in the number of follicles, COCs, good quality COCs and embryos was also recorded, confirming previous observations (Tamassia et al. Citation2003).
One of the objectives of this work was to evaluate whether a pre-treatment of donors with eCG would promote follicular growth, increasing embryo yields. Equine chorionic gonadotropin is a glycoprotein with FSH and LH-like activity that, due to its long half-life (Siddiqui et al. Citation2002), requires a single administration to super-stimulate donors. The reasons for choosing eCG for priming were the low cost, the availability on the market, and especially its more practical use in extensive and semi-extensive breeding systems. The rationale for applying this protocol was that eCG would recruit gonadotropin-responsive follicles from the pool of small follicles present on the ovary after follicular ablation and that, due to its long action, would promote their growth up to few days later, when OPU would be carried out. Indeed, eCG increased the number of medium follicles, without influencing though neither the total follicular population, nor the follicles of other sizes. The significant reduction of the recovery rate in eCG-treated cows resulted in a decreased number of both total COCs and Grade 1 + 2 + 3 COCs, i.e. those considered suitable for in vitro embryo production (IVEP). However, the treatment improved oocyte developmental competence, as demonstrated by higher cleavage and blastocyst rates obtained in eCG-treated cows compared to non eCG-treated cows. The improvement of oocyte competence was likely related to the presence of a higher number of medium follicles, as a clear relationship between follicle size and oocyte quality exists in cattle, with more competent oocytes corresponding to medium-size follicles (Lonergan et al. Citation1994; Blondin and Sirard Citation1995; Machatkova et al. Citation2004). The most relevant finding was that, despite the increased blastocyst yields, the mean number of embryos produced per donor per session was similar to that recorded in non-eCG-treated cows. This is certainly due to the reduced recovery rate in eCG-treated group, that was also previously reported (Pieterse Citation1992; Ribas et al. Citation2018), likely due to the higher incidence of medium size follicles (Seneda et al. Citation2001). It has been speculated that the larger volume, associated with increased viscosity and higher intra-follicular pressure of larger follicles may in part interfere with oocyte collection during the insertion and rotation of the needle inside the follicle. In contrast, in suckling beef cows recovery rate was not affected by the treatment with a higher dose of eCG, thus the increased number of follicles resulted in increased number of oocytes and blastocysts (Aller et al. Citation2012). It can be concluded that the treatment with eCG, although increasing the number of medium follicles and oocyte competence, was not effective in maximising either the number of oocytes for IVEP or the overall number of embryos produced. Therefore, other strategies should be followed to make OPU more feasible in Podolic cattle. Priming of OPU donors with multiple FSH administration over 3 days in a progestin-based protocol and a coasting period of about 36-48 h (Blondin et al. Citation2002) was proven successful in increasing follicle number, oocyte competence and embryo yields in cattle. However, this protocol is expensive and far less practical in extensive breeding systems like those in which our study was carried out. In future perspective it could be worth assessing the effect of FSH combined with hyaluronan, which allows a slow and sustained release of the hormone for several days.
As the trial lasted 4 months from end-June to end-October the effect of season on follicular and oocyte population, oocyte competence and embryo yields was also evaluated. In summer a lower number of small follicles and a higher number of medium follicles were recorded. The decreased number of small follicles resulted in lower number of total and good quality COCs. Nevertheless, the average number of embryos produced per donor per session did not vary with season. In addition, the percentage of good quality oocytes and the oocyte competence were not influenced, as shown by similar cleavage and blastocyst rates. In contrast, a reduced oocyte developmental competence has been recorded in cattle during seasons characterised by high environmental temperatures (Zeron et al. Citation2001; Al-Katanani et al. Citation2002; Sartori et al. Citation2002). Decreased fertility has also been observed in Holstein multiparous cows and heifers when temperatures exceed respectively 30 °C and 35 °C (Badinga et al. Citation1985). It is worth specifying that during the trial the average daily environmental temperatures were never over 30 °C and that the animals were not lactating. Furthermore, it is known that a higher sensitivity to heat stress is described in breeds highly selected for milk production and during early lactation compared to heifers or autochthonous breeds (Badinga et al. Citation1985; Al-Katanani et al. Citation2002).
The second objective of this work was to evaluate whether repeated OPU affected the health and welfare status of Podolic cattle bred in a semi-extensive system and not used to being handled. It was demonstrated that the procedure did not negatively affect either the metabolic profile or the serum protein electrophoresis, as no variations were observed between cattle undergoing repeated OPU and control group. In particular, no differences were observed regarding the α and β globulins fractions, known to undergo variations in response to inflammatory conditions (Schalm et al. Citation1975). In addition, no differences were recorded in haptoglobin levels, indicating that transvaginal follicular aspiration did not cause acute stress or infection. Skinner et al. (Skinner et al. Citation1991) reported that a haptoglobin concentration >0.4 g/l indicates significant infection and that a concentration of 0.2 g/l and above may indicate early or mild infection. Since the values of haptoglobin were by far lower of those thresholds in both groups, we may conclude that repeated OPU did not lead to any infection or acute stress in the treated animals.
The findings of this preliminary study should be interpreted with cautions because the study was limited by several factors. The number of animals was low and therefore the study should be repeated with a larger sample size. The blood samples were not carried out immediately after the OPU procedures, consequently small shifts of the APPs may have not been recorded, and important parameters such as cortisol and glucose were not assessed. The animals were reared in semi-extensive system, so feed intake and quality could not be standardised and could have affect the results. Notwithstanding these limitations, this study is the first reporting the effects of repeated OPU sessions on the reproductive performance, the health and the welfare of Podolic cattle. Our data may be useful to promote this technique as a conservation strategy in this endangered breed.
Conclusions
The results of this preliminary investigation suggest that OPU may be performed on Podolic cattle as a conservation strategy to enhance their reproductive performance, without impairing the health and wellbeing of those animals. The repeated OPU sessions were indeed well tolerated by the animals which did not show any alterations in their haematological parameters. In contrast, the priming with eCG treatment was found not to be worth at the tested dosage, improving only some of the reproductive parameters investigated. Further studies should be carried out testing different eCG dosages or different hormonal therapy to optimise OPU results in this breed.
Author contributions
Conceptualisation was done by GAP, GN, AS, DV, EDC, BG; Methodology was formulated GAP, GN, AS, VL, DV, BG; Formal Analysis was performed by BP; Data Curation was performed by GAP, GN, BP, EDC, BG; Writing-Original Draft Preparation was done by GAP, GN, BP, BG; Writing-Review & Editing was done by GAP, GN, BP, EDC, BG; Supervision was done by BG; Funding Acquisition was done by BG.
Acknowledgments
The authors are thankful to Prof Katherine Houpt for editing the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Al-Katanani Y, Paula-Lopes F, Hansen P. 2002. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J Dairy Sci. 85:390–396.
- Aller J, Mucci NC, Kaiser GG, Callejas SS, Alberio RH. 2012. Effect of repeated eCG treatments and ovum pick-up on ovarian response and oocyte recovery during early pregnancy in suckling beef cows. Anim Reprod Sci. 133:10–15.
- ANABIC (Associazione Nazionale Allevatori Italiani Carne). 2018. [accessed]. http://www.anabic.it/.
- Badinga L, Collier R, Thatcher W, Wilcox C. 1985. Effects of climatic and management factors on conception rate of dairy cattle in subtropical environment. J Dairy Sci. 68:78–85.
- Barta O. 1993. Veterinary clinical immunology laboratory. Blacksburg, VA: BAR-LAB, Inc.
- Blondin P, Bousquet D, Twagiramungu H, Barnes F, Sirard M-A. 2002. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol Reprod. 66:38–43.
- Blondin P, Sirard MA. 1995. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Develop. 41:54–62.
- Blondin P, Vigneault C, Nivet A, Sirard M. 2012. Improving oocyte quality in cows and heifers – What have we learned so far. Anim Reprod. 9:281–289.
- Broadbent P, Dolman D, Watt R, Smith A, Franklin M. 1997. Effect of frequency of follicle aspiration on oocyte yield and subsequent superovulatory response in cattle. Theriogenology. 47:1027–1040.
- Campanile G, Di Palo R, Infascelli F, Gasparrini B, Neglia G, Zicarelli F, Michael J. 2003. Influence of rumen protein degradability on productive and reproductive performance in buffalo cows. Reprod Nutr Dev. 43:557–566.
- Chastant-Maillard S, Quinton H, Lauffenburger J, Cordonnier-Lefort N, Richard C, Marchal J, Mormede P, Renard J. 2003. Consequences of transvaginal follicular puncture on well-being in cows. Reproduction. 125:555–563.
- Comizzoli P, Mermillod P, Mauget R. 2000. Reproductive biotechnologies for endangered mammalian species. Reprod Nutr Dev. 40:493–504.
- Edmonson A, Lean I, Weaver L, Farver T, Webster G. 1989. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 72:68–78.
- Galli C, Crotti G, Notari C, Turini P, Duchi R, Lazzari G. 2001. Embryo production by ovum pick up from live donors. Theriogenology. 55:1341–1357.
- Galli C, Duchi R, Colleoni S, Lagutina I, Lazzari G. 2014. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: from the research laboratory to clinical practice. Theriogenology. 81:138–151.
- Kouamo J, Tidjou SGD, Zoli AP, Mfopit YM. 2015. Effect of nutritional status on the ovarian follicular population, yield and quality of oocytes in the Ngaoundere Gudali zebu (Bos indicus). Vet World. 8:502.
- Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. 1999. High bovine blastocyst development in a static in vitro production system using sofaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology. 52:683–700.
- Kruip TA, Boni R, Wurth Y, Roelofsen M, Pieterse M. 1994. Potential use of ovum pick-up for embryo production and breeding in cattle. Theriogenology. 42:675–684.
- Kruip TA, Den Daas J. 1997. In vitro produced and cloned embryos: effects on pregnancy, parturition and offspring. Theriogenology. 47:43–52.
- Lomborg SR, Nielsen LR, Heegaard PM, Jacobsen S. 2008. Acute phase proteins in cattle after exposure to complex stress. Vet Res Commun. 32:575–582.
- Lonergan P, Monaghan P, Rizos D, Boland M, Gordon I. 1994. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 37:48–53.
- Looney CR, Lindsey BR, Gonseth VL, Johnson DL. 1994. Commercial aspects of oocyte retrieval and in vitro fertilization (IVF) for embryo production in problem cows. Theriogenology. 41:67–72.
- Lucy M, Savio J, Badinga L, De La Sota R, Thatcher W. 1992. Factors that affect ovarian follicular dynamics in cattle. J Anim Sci. 70:3615–3626.
- Machatkova M, Krausova K, Jokesova E, Tomanek M. 2004. Developmental competence of bovine oocytes: effects of follicle size and the phase of follicular wave on in vitro embryo production. Theriogenology. 61:329–335.
- Marquant-Le Guienne B. 1999. Atlas of the bovine oocyte. AETE Newslett. 10:6–8.
- Merton J, De Roos A, Mullaart E, De Ruigh L, Kaal L, Vos P, Dieleman S. 2003. Factors affecting oocyte quality and quantity in commercial application of embryo technologies in the cattle breeding industry. Theriogenology. 59:651–674.
- Murata H, Shimada N, Yoshioka M. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 168:28–40.
- Paulina J, Tadeusz S. 2011. Acute phase proteins in cattle. In: Veas F, editor. Acute phase proteins as early non-specific biomarkers of human and veterinary diseases. London, UK: InTech.
- Petyim S, Båge R, Madej A, Larsson B. 2007. Ovum pick‐up in dairy heifers: does it affect animal well‐being? Reprod Domest Anim. 42:623–632.
- Pieterse M, Vos P, Kruip TA, Wurth YA, van Beneden H, Willemse A, Taverne M. 1992. Repeated transvaginal ultrasound-guided ovum pick-up in egg-treated cows. Theriogenology. 37:273.
- Pieterse M, Vos P, Kruip TA, Willemse A, Taverne M. 1991. Characteristics of bovine estrous cycles during repeated transvaginal, ultrasound-guided puncturing of follicles for ovum pick-up. Theriogenology. 35:401–413.
- Pushpakumara P, Gardner N, Reynolds C, Beever D, Wathes D. 2003. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology. 60:1165–1185.
- Ribas BN, Missio D, Roman I, Neto NA, Claro I, dos Santos Brum D, Leivas FG. 2018. Superstimulation with eCG prior to ovum pick-up improves follicular development and fertilization rate of cattle oocytes. Anim Reprod Sci. 195:284.
- Sartori R, Sartor-Bergfelt R, Mertens S, Guenther J, Parrish J, Wiltbank M. 2002. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci. 85:2803–2812.
- Schalm OW, Jain NC, Carroll EJ. 1975. Veterinary hematology. 3rd edition. Philadelphia, USA: Lea & Febiger.
- Seneda MM, Esper CR, Garcia JM, de Oliveira JA, Vantini R. 2001. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim Reprod Sci. 67:37–43.
- Siddiqui M, Shamsuddin M, Bhuiyan M, Akbar M, Kamaruddin K. 2002. Effect of feeding and body condition score on multiple ovulation and embryo production in zebu cows. Reprod Domest Anim. 37:37–41.
- Skinner J, Brown R, Roberts L. 1991. Bovine haptoglobin response in clinically defined field conditions. Vet Record. 128:147–149.
- Tamassia M, Heyman Y, Lavergne Y, Richard C, Gelin V, Renard J, Chastant-Maillard S. 2003. Evidence of oocyte donor cow effect over oocyte production and embryo development in vitro. Reproduction. 126:629–637.
- Velazquez MA. 2008. Assisted reproductive technologies in cattle: applications in livestock production, biomedical research and conservation biology. Ann Rev Biomed Sci. 10:36–62.
- Vieira L, Rodrigues C, Netto AC, Guerreiro B, Silveira C, Moreira R, Sá Filho M, Bó G, Mapletoft R, Baruselli P. 2014. Superstimulation prior to the ovum pick-up to improve in vitro embryo production in lactating and non-lactating Holstein cows. Theriogenology. 82:318–324.
- Walsh RB, Leblanc SJ, Duffield TF, Kelton DF, Walton JS, Leslie KE. 2007. The effect of a progesterone releasing intravaginal device (PRID) on pregnancy risk to fixed-time insemination following diagnosis of non-pregnancy in dairy cows. Theriogenology. 67:948–956.
- Zeron Y, Ocheretny A, Kedar O, Borochov A, Sklan D, Arav A. 2001. Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction. 121:447–454.
