 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Due to a series of problems, such as drug resistance and tissue residue caused by adding antibiotics to feed, this research aimed to study the effects of Artemisia annua L. aqueous extract (AAE) as an alternative to antibiotics on growth performance and antioxidant capacity of broilers. A total of 240 one-day-old mixed-sex Arbour Acres broilers were randomly allotted into six groups with five replicates of eight birds each. These six diets were formulated by adding 0, 500, 1000, 1500, 2000 mg/kg AAE and 50 mg/kg chlortetracycline (CTC) to the basal diet, respectively. Average daily body weight gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were measured. Total antioxidant capacity (T-AOC), the activity of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and the concentrations of malondialdehyde (MDA) were determined, and the relative gene expression in tissues was measured. The polyphenol flavonoid contents and in vitro antioxidative results showed that: the total phenolic and flavonoid contents were 39.58 ± 6.01 mg GAE/g, 7.04 ± 0.55 mg RE/g, respectively. The inclusion of AAE increased ADG, the activities of T-AOC, CAT, SOD and GSH-Px, and the gene expression of antioxidant enzymes, the concentrations of MDA decreased in serum and tissues. In conclusion, AAE can be used as a feed additive due to its capability to improve growth performance, antioxidant function in broilers. Dietary inclusion of 1000–1500 mg/kg AAE can be used as an alternative for antibiotic growth promoter replacement without negative effect on broiler performance.
Artemisia annua L. aqueous extract can promote growth performance and antioxidant function of Broilers.
Artemisia annua L. aqueous extract is expected to become the substitute for antibiotics and promote the growth of the body.
The optimal dose range of Artemisia annua L. aqueous extract in diet of broilers was 1000–1500 mg/kg.
Highlights
Introduction
Traditionally, antibiotics have been used in poultry production for improving growth performance and preventing diseases (Sugiharto Citation2016; Attia et al. Citation2017, Citation2018, Citation2019a). However, these commercial antibiotics could engender drug-resistance in mankind as well as animals (McNamee et al. Citation2013; Neijat et al. Citation2019). Consequently, a globe ban has been imposed on antibiotic usage to abate antibiotics in poultry, and great effort has been expended to find alternatives to antibiotics (Huang et al. Citation2018). Phytogenic active substances, which are natural, are less toxic, and residue-free, were thought to be ideal growth promoters included in the ration (Prakasita et al. Citation2019). Plant products, such as cinnamaldehyde, thymol, turmeric and ginkgo biloba have many beneficial biological functions, including antimicrobial, anti-inflammatory, antioxidant, anticancer and immunomodulatory effects (Kim et al. Citation2014; Niu et al. Citation2019; Attia et al. Citation2019a). Phytogenic feed additive, as an alternative to antibiotics, improved growth performance, immune response, and intestine morphology and microflora and reduced gas emission in excreta without a detrimental effect on meat quality in chickens (Attia et al. Citation2018; Hesabi Nameghi et al. Citation2019).
Artemisia annua L. (A. annua) is one of the natural herbs belongs to Artemisia species of Asteraceae and distributed in many countries worldwide, including Argentina, China, France, Italy, Spain, USA, Vietnam and so on (Romero et al. Citation2006; Khodakov and Kotikov Citation2009; Sadiq et al. Citation2014). A. annua has abundant nutrient profiles such as amino acids, vitamins, and mineral elements, as well as antioxidant compounds including phenolics and flavonoids (Ferreira et al. Citation2010; Wan et al. Citation2016; Zhigzhitzhapova et al. Citation2019). Besides, it has immune regulation function (Zhang and Sun Citation2009; Song et al. Citation2018).
Recently, Tu’s team discovered the antimalarial activity of A. annua, and this discovery (artemisinin) gained the Nobel Prize in Medicine in 2015 (Tu Citation2016). It was reported that the supplementation of A. annua products to broilers’ feed could enhance growth performance (de Almeida et al. Citation2012; Engberg et al. Citation2012). Using A. annua leaves improved antioxidant status of laying hens (Baghban-Kanani et al. Citation2019) and improve the broilers’ immune function (Gholamrezaie Sani et al. Citation2013). Besides, the ethanol extract of A. annua could regulate the immune function of mice (Zhang and Sun Citation2009). Dietary supplementation of enzymatically treated A. annua (EA) could alleviate the intestinal inflammatory response in heat-stressed broilers (Song et al. Citation2017). Therefore, based on A. annua rich in biologically active substances and biological functions, this study from the perspective of cost, using water as a solvent, the bioactive substance of A. annua was extracted by water decoction. This study aimed to evaluate the effects of AAE on growth performance and antioxidant capacity of broilers and the feasibility of replacing antibiotics with AAE.
Materials and methods
Preparation of Artemisia annua L. aqueous extract
Fresh aerial part of A. annua L. was harvested from Hohhot in July 2017. Plant materials were washed with distilled water and dried at room temperature in the shade. The dried materials were extracted in hot distilled water at 80 °C for 6 h (1 g:25 mL), and the supernatant of the extract liquor was collected and was concentrated using a rotary vacuum evaporator after filtrating (RE-5298, Shanghai Yarong Biochemical Instrument Factory, Shanghai, China) at 70 °C, and then the concentrated liquor was freeze-dried (ALPHA1-2LD plus, Christ, Germany) to prepare the powder, and stored at −20 °C. Two hundred and fifty grams of the powder is from one kilogram of the plant materials.
Assay of total phenolic and flavonoid contents
According to Lee et al. (Citation2017) and Wan et al. (Citation2016), the total phenolic content of AAE was measured with Folin–Ciocalteu method and gallic acid were used as a standard. Total phenolic content was expressed as gallic acid equivalents (mg GAE/g). Total flavonoid content was determined with an aluminium chloride method, and rutin was used as a reference standard. The result was expressed as rutin equivalents (mg RE/g).
In vitro antioxidant capacity
ABTS free radical scavenging assay
The ABTS (2,2′-Azino-bis (3-Ethylbenzo-Thiazoline-6-Sulfonic Acid)) method (Cherian et al. Citation2013) was used to assess total antioxidant activity. The standard curve was prepared with trolox, an aqueous soluble vitamin E analogue, and the result was expressed as μmol trolox equivalent (μmol TE/g).
DPPH free radical scavenging assay
The antioxidant potential of AAE was determined using DPPH (2,2-diphenyl-1picrylhydrazyl). Ascorbic acid was used as a standard. Absorbance was determined at 517 nm using a UV spectrophotometer (Ullah et al. Citation2017).
Assay of hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity of AAE was evaluated by the reported method (Di et al. Citation2017). Hydroxyl radical scavenging ability of AAE was calculated according to the following equation:
where A2 is the absorbance of AAE samples, A1 is the absorbance of the samples under conditions as A2 with distilled water instead of H2O2, A0 is the absorbance of the distilled water instead of samples.
Reducing power assay
The reducing power (RP) was assessed according to the method of Wan et al. (Citation2016) with some modifications. Briefly, AAE sample (0.75 mL) was blended with PBS (0.2 mmol/L, pH 6.6, 0.75 mL) and 1% potassium ferricyanide (0.75 mL). After incubation for 20 min at 50 °C, the mixture solution was added 10% trichloroacetic acid (0.75 mL), then centrifugated at 1500 × g for 10 min. The supernatant (1.5 mL) was subsequently mixed with distilled water (1.5 mL) and 0.1% ferric chloride (0.4 mL). The absorbance at 700 nm of the solution was measured. Increased absorbance of the sample indicated increased RP.
Birds, experimental design and diets
In total, 240 one-day-old mixed-sex Arbour Acres broilers were randomly divided into six groups with five replicates of eight birds each. The birds were kept in stainless-steel wire cages with 8 chickens/cage (100 × 50 × 50 cm) and maintained under optimal temperature. These six diets were formulated by adding 0, 500, 1000, 1500, 2000 mg/kg AAE and 50 mg/kg Chlortetracycline (CTC, the concentration of CTC is 100%) to the basal diet, respectively. All experimental diets were formulated based on NRC requirements 1994 (Dale Citation1994) and meet nutrients recommendations of Feeding Standard of Chicken, China (NY/T 33-2004) (Chinese Ministry of Agriculture Citation2004) (Table ). The experimental period was 42 d, including the starter period (d 1–21) and the finisher period (d 22–42). Experimental mash feed and water were available ad libitum with a light cycle of 23 h followed by 1 h of darkness. The inner temperature was kept at 32–34 °C for the first 3 d, and then gradually reduced by 2–3 °C per week to a final temperature of 22 °C. The relative humidity was maintained at about 50–60%. The vaccination procedure was conducted normally.
Table 1. Composition and nutrient levels of the basal diet (as-fed basis), %.
Growth performance measurement and sampling
On d 21 and 42, bird weight and feed intake were measured and the average body weight, average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR, feed/gain) were calculated based on per cage. In addition, five broilers from per treatment (one bird per replicate) were selected to collect 10 mL blood samples from the wing vein using a vacuum blood collection tube. After standing for 45 min at room temperature, the blood samples were centrifuged at 3000 × g for 15 min, and then serum samples were separated and stored at −20 °C for further analysis. Then, the liver and spleen were excised 3–5 g and weighed. Subsequently, a part of tissues was placed in a cryogenic vial and snap-frozen in liquid nitrogen at first and then stored at −80 °C for preparation of homogenate and total RNA isolation.
Preparation of liver and spleen homogenate
The liver and spleen pieces were homogenised with a hand-held homogeniser (FA6/10, FLUKO, Shanghai, China) at 4 °C in ice-cold 0.9% sodium chloride solution (wt/vol, 1:9) and then centrifuged at 4000 × g for 15 min at 4 °C. The supernatant was collected for the following analysis. A Coomassie brilliant blue assay was used to determine the protein of the homogenate, according to the instructions of the commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China).
Determination of antioxidant capacities in serum, liver and spleen
The total antioxidant capacity (T-AOC), the activities of total superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT), and malondialdehyde (MDA) concentration in serum, liver and spleen were determined by spectrophotometric method according to the instructions of the commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China). The activities of SOD, GSH-Px and CAT in serum and tissue were expressed as activity unit per millilitre serum and activity unit per milligram of tissue protein (unit/mL, unit/mg protein). The concentrations of MDA were expressed as nanomole per millilitre serum and nanomole per milligram of tissue protein (nmol/mL, nmol/mg protein). T-AOC capacity was expressed as μmol trolox equivalent and μmol trolox equivalent per gram protein of homogenates (mM, μmol TE/g protein). All the above kits have been successfully used in poultry study (Wu et al. Citation2016; Wan et al. Citation2016; Song et al. Citation2018).
Total RNA extraction and reverse transcription
Total RNA from liver and spleen samples was obtained using Trizol reagent (TaKaRa Biotechnology Co. Ltd, Dalian, China). The purity and quantity of the total RNA were assessed with a spectrophotometer (Pultton P200CM, San Jose, CA, USA). Subsequently, the total RNA was treated with DNase I (TaKaRa Biotechnology Co. Ltd., Dalian, China) to remove DNA.
Total RNA was reverse transcribed to cDNA on LifeECO (TC-96/G/H(b)C, BIOER, Hangzhou, China) using TB® Green qPCR method with a Prime Script™ RT reagent kit with gDNA Eraser (TaKaRa Biotechnology Co. Ltd., Dalian, China). The reactions were incubated for 15 min at 37 °C, followed by 5 s at 85 °C.
Quantitative real-time PCR
Real-time PCR was performed using the QuantStudio® 5 real-time PCR Design & Analysis system (LightCycler® 480 II, Roche Diagnostics, Indianapolis, IN) with a TB® Premix Ex Taq™ Kit (Takara Biotechnology Co. Ltd., Dalian, China). The reactions were: 95 °C for 30 s (hold stage), followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 20 s (PCR stage), then 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s (melt-curve stage). All samples were run in duplicate and melt curve analysis was performed to validate the specificity of the PCR-amplified product. The mRNA expression of each gene was normalised to that of β-actin. The fold change relative to the control group was analysed according to the 2−ΔΔCT method (Livak and Schmittgen Citation2001). The specific sequences of primers are listed in Table .
Table 2. Primer sequences and parameter.
Statistical analysis
Data were analysed by one-way ANOVA with the GLM procedure of SAS version 9.2 (SAS Institute Inc., Cary, NC), and a pen of broilers (a replicate) served as the experimental unit for all data. The differences among treatments were tested by the Tukey–Kramer honestly significant difference test and were considered significant at p < .05. Whereas, regression analysis was used to determine the linear and quadratic dependence of growth performance and antioxidant function of broilers on AAE doses. The results were expressed as the mean and standard error of the mean (SEM).
Results
Total phenolic and flavonoid contents and in vitro antioxidant capacities of AAE
The total phenolic and flavonoid contents were, respectively, 39.58 mg GAE/g and 7.04 mg RE/g (Table ). The scavenging activities of ABTS, DPPH and hydroxyl radical, and RP of AAE are shown in Table . The results were 147.3 μmol TE/g, 73.79%, 27.59%, and 2.5663 (absorbance), respectively.
Table 3. Total phenolic and flavonoid contents and in vitro antioxidant capacities of AAE.
Growth performance
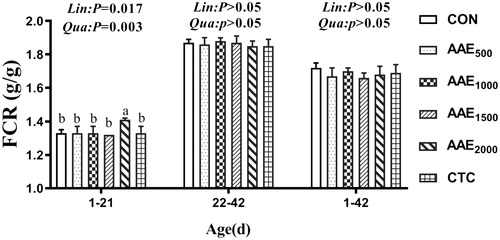
Growth performance results are presented in Figures . Diets supplemented with 1500 mg/kg AAE elevated ADG compared to the control group and CTC group (p < .05), and the ADG exhibited significant quadratic elevation effect with the increase of AAE dose on d 21 (p < .01) (Figure ). Moreover, the inclusion of 2000 mg/kg elevated FCR (p < .05), and decreased the ADG and ADFI compared to the control group and CTC group on d 21 (p < .05) (Figures ).
Figure 1. Effect of AAE on average daily body weight gain (ADG) of broilers. Note: AAE = Artemisia annua L. aqueous extract; CON: control; AAE500: 500 mg/kg AAE; AAE1000: 1000 mg/kg AAE; AAE1500: 1500 mg/kg AAE; AAE2000: 2000 mg/kg AAE; CTC: chlortetracycline. Results are presented as mean ± standard deviation (n = 5). a,b,cDifferent capital letters indicate significant differences between groups in the same period (p<.05). Dose-dependent effects of AAE are shown on the right of each figure (Lin: linear; Qua: quadratic).
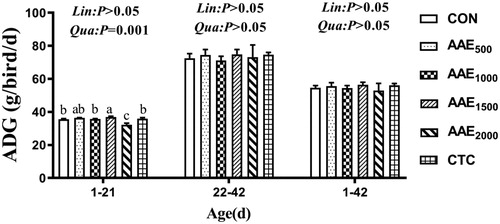
Figure 2. Effect of AAE on average daily feed intake (ADFI) of broilers. Note: AAE = Artemisia annua L. aqueous extract; CON: control; AAE500: 500 mg/kg AAE; AAE1000: 1000 mg/kg AAE; AAE1500: 1500 mg/kg AAE; AAE2000: 2000 mg/kg AAE; CTC: chlortetracycline. Results are presented as mean ± standard deviation (n = 5). a,bDifferent capital letters indicate significant differences between groups in the same period (p<.05). Dose-dependent effects of AAE are shown on the right of each figure (Lin: linear; Qua: quadratic).
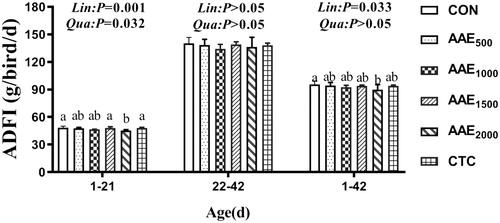
Figure 3. Effect of AAE on feed conversion ratio (FCR) of broilers. Note: AAE = Artemisia annua L. aqueous extract; CON: control; AAE500: 500 mg/kg AAE; AAE1000: 1000 mg/kg AAE; AAE1500: 1500 mg/kg AAE; AAE2000: 2000 mg/kg AAE; CTC: chlortetracycline. Results are presented as mean ± standard deviation (n = 5). a,bDifferent capital letters indicate significant differences between groups in the same period (p<.05). Dose-dependent effects of AAE are shown on the right of each figure (Lin: linear; Qua: quadratic).
The ADFI showed significant linear (p < .01) or quadratic (p < .05) reduction effect with the increase of AAE doses on d 21 (Figure ). The ADFI presented a marked linear reduction effect with the increase of AAE dose on d 1–42 (p < .05) (Figure ).
The FCR on d 21 was linearly (p < .05) increased and significantly quadratically (p < .01) increased with dietary AAE increase (Figure ).
Antioxidant capacity
The results of the serum antioxidant index are assumed in Table . Diets supplemented with 1500 mg/kg AAE significantly elevated the activity of serum SOD (p < .05), compared with the control group on d 21. Diets supplemented with 500–1500 mg/kg AAE and CTC elevated serum GSH-Px activity (p < .01), which quadratically increased with the increased additive amount AAE on d 21 (p < .01). Moreover, serum SOD activity had a significant linear or quadratic elevation effect with the increase of AAE dose on d 42 (p < .05). The addition of 1500 mg/kg AAE remarkably increased serum T-AOC (p < .05), compared with the control group on d 42. The inclusion of 500–2000 mg/kg AAE and CTC remarkably decreased serum MDA level (p < .05) compared with the control group, and MDA level showed significant linear (p = .05) or quadratic (p < .05) reduction effect with the increase of AAE doses on d 42.
Table 4. Effect of AAE on serum antioxidant capacity in broilers.
The results of the antioxidant index in the liver are shown in Table . Diets supplemented with 500–1500 mg/kg AAE and CTC significantly elevated the activity of liver CAT (p < .05) compared with the control group on d 21, which significantly quadratically increased with the increased additive amount of AAE on d 21 (p < .01). The activity of liver SOD showed a remarkable linear (p = .05) or quadratic (p < .05) elevation effect with the increase of AAE dose on d 21. Supplementation with 500–2000 mg/kg AAE and CTC had higher liver GSH-Px activities (p < .01), compared with the control, which showed significant linear or quadratic elevation effect with the increase of AAE dose on d 21 (p < .01). Diets supplemented with 1000–2000 mg/kg AAE and CTC significantly enhanced liver T-AOC (p = .05) compared with the control, which significantly linearly or quadratically increased with the increased additive amount of AAE on d 21 (p < .01). The concentration of liver MDA significantly linearly (p = .05) or quadratically (p < .05) decreased with the increased additive amount of AAE on d 21. Furthermore, on d 42, the activity of liver CAT presented a notable quadratic elevation effect (p < .05), and the concentration of liver MDA showed a significant linear or quadratic reduction effect with the increase of AAE doses (p < .05).
Table 5. Effect of AAE on hepatic antioxidant capacity in broilers.
The results of the antioxidant index in spleen are displayed in Table . The activity of spleen SOD significantly quadratically increased with the increased additive amount of AAE on d 21 (p < .05). Diets supplemented with 1000 and 1500 mg/kg AAE and CTC significantly elevated spleen GSH-Px activity (p < .01), which showed a significant quadratic elevation effect with the increase of AAE doses on d 21 (p < .01). In addition, T-AOC quadratically increased and MDA quadratically depleted with the increased additive amount of AAE on d 21 (p < .05). Moreover, the activities of spleen CAT and GSH-Px quadratically increased with the increased additive amount of AAE on d 42(p < .05). The inclusion of 1000 and 1500 mg/kg AAE remarkable increased spleen T-AOC (p < .01) compared with the control group, and the spleen T-AOC significantly linearly or quadratically elevated with the increase of AAE dose on d 42 (p < .01). The inclusion of 1500 and 2000 mg/kg AAE and CTC remarkably decreased spleen MDA levels (p < .05) compared with the control group, and the concentration of MDA had a significant linear and quadratic reduction effect with the increase of AAE dose on d 42 (p < .05).
Table 6. Effect of AAE on spleen antioxidant capacity in broilers.
Antioxidant gene mRNA expression levels
The mRNA expression levels of the antioxidant gene in the liver are shown in Table . Diets supplemented with 1000–2000 mg/kg AAE significantly enhanced the mRNA expression levels of SOD in the liver compared with the control group (p < .05), and the mRNA expression levels of SOD in the liver significantly linearly and quadratically increased with the increased additive amount of AAE on d 21 (p < .01).
Table 7. Effect of AAE on relative mRNA expression in liver of broilers.
The mRNA expressions of antioxidant enzymes in spleen are shown in Table . The mRNA expression levels of CAT and SOD in spleen showed a significant quadratic elevation effect with the increase of AAE dose on d 21 (p < .05). Diets supplemented with 1000–2000 mg/kg AAE markedly increased the mRNA expression levels of GSH-Px in spleen compared with the control group (p < .01) on d 21, and the mRNA expression of GSH-Px in spleen had a significant quadratic elevation effect with the increase of AAE dose on d 21 (p < .01).
Table 8. Effect of AAE on relative mRNA expression in spleen of broilers.
Discussion
This study indicated that feeding birds with 1500 mg/kg AAE notably elevated ADG on d 21 compared with the control and CTC group. This result was consistent with the report of Gholamrezaie Sani et al. (Citation2013), who reported supplemented with A. annua methanolic extract (2 and 4 g/kg) or A. annua leaf powder (5, 10 and 15 g/kg) increased ADG and reduced FCR of broilers. The previous study reported that chickens feeding A. annua or extracts from A. annua could promote growth (Song et al. Citation2018). This may be related to the fact that A. annua has abundant bioactive components to promote the digestion and absorption of nutrients in the small intestine, and increase antioxidant capacity (Song et al. Citation2018). Nevertheless, Cherian et al. (Citation2013) found diet adding 20 or 40 g/kg A. annua leaves did not affect growth performance in Cobb chicks. In this study, the ADG had suppressed and the ratio of F/G augmented in 2000 mg/kg group on d 21, and the results were identical with Engberg et al. (Citation2012), which might be related to the palatability of A. annua. Besides, Wan et al. (Citation2018) reported that enhanced antioxidant capacity was a factor in promoting growth.
The T-AOC includes the effects of several antioxidant enzymes and related biomolecules with the ability to remove free radicals of a specific organ or a living organism. The level of T-AOC reflects the total antioxidant ability (Wu et al. Citation2016; Attia et al. Citation2017, Citation2018, Citation2019a). In this study, supplemented AAE boosted the T-AOC of serum, liver and spleen to some degree, which might be associated with obvious in vitro antioxidant activity of AAE and increased activity of antioxidant agents and expression of the relevant gene, and decreased levels of lipid peroxidation in vivo. A. annua rich in phenolic compounds and flavonoids has been reported to show eminent antioxidant activity (Ferreira et al. Citation2010; Cherian et al. Citation2013; Lee et al. Citation2017), and this explains a higher antioxidant status of broilers fed diets added with AAE.
Antioxidant capacity is associated with various antioxidant systems in the body, which include both enzymatic and non-enzymatic systems and maintain the body redox balance in animals (Attia et al. Citation2019b). Besides, the overproduction of free radicals can be quenched by antioxidants, like GSH, and/or converted into hydrogen peroxide by SOD, and then hydrogen peroxide can further be degraded to water and oxygen by GSH-Px and CAT. SOD, GSH-Px, and CAT are three main the antioxidant enzymes of antioxidant system. The concentration of MDA is generally determined to evaluate the state of lipid peroxidation. Wan et al. (Citation2017a, Citation2018) reported that MDA concentration was reduced in breast muscles and liver of broilers fed diets supplemented EA, and lipid peroxidation was decreased via elevating the activity of CAT, SOD, GSH-Px, and inhibiting the production of MDA. Similarly, in our study, AAE elevated T-SOD, GSH-Px, and CAT activities and reduced MDA concentration in serum, liver and spleen, indicating that A. annua could improve the antioxidant ability of broilers. Besides, our results also found that the gene expression of CAT, SOD GSH-Px was improved in the liver and spleen to some extent. The analogous results show that added EA to the diet can improve the activity and relative gene expression of the chicken in the jejunum, and regulate by activating the Nrf2 signal pathway (Song et al. Citation2018). Thus, it is assumed that AAE promotes the growth of broilers via activating the Nrf2 signalling pathway, and raising the gene expression of CAT, SOD, and GSH-Px, hence, increasing the activity of the body’s antioxidant enzymes and improving the body’s antioxidant abilities. It is a pity about the Nrf2 related gene expression not measured in our research. However, Song et al. (Citation2018) confirmed this point by supplement EA to broilers diets. In all cases, it may be attributed to the positive role played by bioactive compounds such as polyphenols and flavonoids in the A. annua. Moreover, it might also be ascribed to the plenty of chemical constituents in A. annua enhancing digestive enzyme activities and improving intestinal morphology, promoting intestinal development and accelerating body development (Song et al. Citation2018). However, the exact causes need further research to confirm.
Several reports illustrated that there was a positive correlation between the antioxidant ability and total phenolic or flavonoid contents of natural herbs, indicating that phenolic compounds and flavones were the predominant antioxidant components (Wan et al. Citation2016; Attia et al. Citation2018; Habibian et al. Citation2019). Coincidentally, our results demonstrated that the AAE has rich polyphenols and flavonoids, and eminent in vitro antioxidant ability. Simultaneously, Panaite et al. (Citation2018) and Wan et al. (Citation2018) found polyphenols and flavonoids improved growth performance and antioxidant capacity of broilers, which was consistent with our study. The abundant polyphenols and flavonoids in vegetables are accountable for the antioxidant capacity because they can be used as electron or hydrogen suppliers to reduce free radical formation and scavenge free radicals. In this regard, the ABTS and DPPH are two kinds of the free radicals usually and used to estimate the antioxidant capacity, and largely overproduced OH• and O2−• are two kinds of pernicious free radicals under stress (Wan et al. Citation2018). This in vitro study suggested that the AAE had prominent scavenging activities to ABTS, DPPH and hydroxyl radical, and RP, which showed that AAE could improve the antioxidant capacity of serum, hepatic and spleen. Similar results were obtained by Cherian et al. (Citation2013) and Wan et al. (Citation2017b), who found that diets supplemented with herbs improved meat quality and free radical scavenging capacity in chickens. All these results could be attributed to the antioxidant capacity of bioactive compounds such as polyphenols and flavonoids contained in A. annua and other plants (Brisibe et al. Citation2009; Attia et al. Citation2017, Citation2018, Citation2019a, Citation2019b).
Attia et al. (Citation2018) reported that thyme powder, a novel phytogenic feed additive from Thyme vulgaris L., has the potential to replace antibiotics as a growth promoter to improve growth performance and health status. In this study, a small part of the antioxidant parameters and the growth performance numerically had elevated in CTC group, compared with the control group, but no significant difference with AAE group. Therefore, we preliminarily speculated that AAE could act as an alternative of antibiotic growth promoters, to facilitate growth and antioxidant capacity of broilers. Besides, the CTC did not exert a prominent growth-promoting effect, which may be attributed to the good experimental conditions, clean chicken house, no produce extra harmful bacteria. In other studies, the effect of antibiotics seems to be related to improving immune function of antibiotics rather than to its antibiotic effect (Attia et al. Citation2017). Moreover, some reports showed that the functions of antibiotics might be related to the body in a sub-healthy state (Sugiharto Citation2016; Jha et al. Citation2019). The specific mechanism needs further research.
Conclusions
In summary, these results demonstrated that dietary AAE supplementation improved growth performance via elevating the activity of CAT, SOD, GSH-Px and T-AOC, and reducing the concentration of MDA in serum, hepatic and spleen, and improving gene expression levels of CAT, SOD and GSH-Px in the liver and spleen of broilers to some extent. In addition, the phytochemical feed additive AAE may be used as a reliable and potential alternative to antibiotics growth promoters in healthy chickens, and the optimal dose range of AAE in diet of broilers was 1000–1500 mg/kg.
Ethic approval
The care and use of laboratory animals reported in this study were approved by the Inner Mongolia Agricultural University’s Animal Care and Use Committee and the Ministry of Agriculture of China (GB/T 35892-2018).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Attia Y, Al-Harthi M, El-Kelawy M. 2019a. Utilization of essential oils as natural growth promoter for broiler chickens. Ita J Anim Sci. 18(1):1005–1012.
- Attia YA, Al-Harthi MA, Hassan SS. 2017. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Rev Mex Cienc Pecu. 8(1):11–21.
- Attia YA, Bakhashwain AA, Bertu NK. 2018. Utilisation of thyme powder (Thyme vulgaris L.) as a growth promoter alternative to antibiotics for broiler chickens raised in a hot climate. Europ Poult Sci. 82(238):1–15.
- Attia YA, Hamed RS, Bovera F, Al-Harthi MA, El-Hamid A, Esposito L, Shahba HA. 2019b. Milk thistle seeds and rosemary leaves as rabbit growth promoters. Anim Sci Pap Rep. 37(3):277–295.
- Baghban-Kanani P, Hosseintabar-Ghasemabad B, Azimi-Youvalari S, Seidavi A, Ragni M, Laudadio V, Tufarelli V. 2019. Effects of Using Artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. J Poult Sci. 56(2):120–127.
- Brisibe EA, Umoren UE, Brisibe F, Magalhäes PM, Ferreira JFS, Luthria D, Wu X, Prior RL. 2009. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 115(4):1240–1246.
- Cherian G, Orr A, Burke IC, Pan W. 2013. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult Sci. 92(4):1085–1090.
- Chinese Ministry of Agriculture. 2004. Feeding standard of chicken, China (NY/T 33-2004). Hunan Feed. 4:19–27.
- Dale N. 1994. National research council nutrient requirements of poultry – ninth revised edition (1994). J Appl Poult Res. 3(1):101–101.
- de Almeida GF, Horsted K, Thamsborg SM, Kyvsgaard NC, Ferreira JF, Hermansen JE. 2012. Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamics and performance. Vet Parasitol. 186(3–4):178–187.
- Di T, Chen G, Sun Y, Ou S, Zeng X, Ye H. 2017. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J Funct Foods. 28:64–75.
- Engberg RM, Grevsen K, Ivarsen E, Frette X, Christensen LP, Hojberg O, Jensen BB, Canibe N. 2012. The effect of Artemisia annua on broiler performance, on intestinal microbiota and on the course of a Clostridium perfringens infection applying a necrotic enteritis disease model. Avian Pathol. 41(4):369–376.
- Ferreira JF, Luthria DL, Sasaki T, Heyerick A. 2010. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 15(5):3135–3170.
- Gholamrezaie Sani L, Mohammadi M, Jalali Sendi J, Abolghasemi SA, Roostaie AMM. 2013. Extract and leaf powder effect of Artemisia annua on performance, cellular and humoral immunity in broilers. Iran J Vet Res. 14(1):15–20.
- Habibian M, Sadeghi G, Karimi A. 2019. Comparative effects of powder, aqueous and methanolic extracts of purslane (Portulaca oleracea L.) on growth performance, antioxidant status, abdominal fat deposition and plasma lipids in broiler chickens. Anim Prod Sci. 59(1):89–100.
- Hesabi Nameghi A, Edalatian O, Bakhshalinejad R. 2019. Effects of a blend of thyme, peppermint and eucalyptus essential oils on growth performance, serum lipid and hepatic enzyme indices, immune response and ileal morphology and microflora in broilers. J Anim Physiol Anim Nutr. 103(5):1388–1398.
- Huang P, Zhang Y, Xiao K, Jiang F, Wang H, Tang D, Liu D, Liu B, Liu Y, He X, et al. 2018. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 6(1):211–227.
- Jha R, Singh AK, Yadav S, Berrocoso JFD, Mishra B. 2019. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front Vet Sci. 6:82.
- Khodakov GV, Kotikov IV. 2009. Component composition of essential oil from Artemisia annua and A. scoparia. Chem Nat Compd. 45(6):909–912.
- Kim WS, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD, Shin HS, Kim W. 2014. Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol. 19(1):21–27.
- Lee AR, Niu KM, Kang SK, Han SG, Lee BJ, Kim SK. 2017. Antioxidant and antibacterial activities of lactobacillus-fermented Artemisia annua L. as a potential fish feed additive. J Life Sci. 27(6):652–660.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 25(4):402–408.
- McNamee SE, Cunningham R, Elliott CT. 2013. Simultaneous immunochemical detection of four banned antibiotic growth promoters in raw and cooked poultry tissue. Food Addit Contam Part A. 30(7):1270–1278.
- Neijat M, Shirley RB, Welsher A, Barton J, Thiery P, Kiarie E. 2019. Growth performance, apparent retention of components, and excreta dry matter content in Shaver White pullets (5 to 16 week of age) in response to dietary supplementation of graded levels of a single strain Bacillus subtilis probiotic. Poult Sci. 98(9):3777–3786.
- Niu Y, Zhang JF, Wan XL, Huang Q, He JT, Zhang XH, Zhao LG, Zhang LL, Wang T. 2019. Effect of fermented Ginkgo biloba leaves on nutrient utilisation, intestinal digestive function and antioxidant capacity in broilers. Br Poult Sci. 60(1):47–55.
- Panaite TD, Criste RD, Saracila M, Tabuc C, Turcu RP, Olteanu M. 2018. The use of ascorbic acid and Artemisia annua powder in diets for broilers reared under heat stress. Rom Biotechnol Lett. 23(5):13976–13985.
- Prakasita VC, Asmara W, Widyarini S, Wahyuni AETH. 2019. Combinations of herbs and probiotics as an alternative growth promoter: an in vitro study. Vet World. 12(4):614–620.
- Romero MR, Serrano MA, Vallejo M, Efferth T, Alvarez M, Marin JJ. 2006. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 72(13):1169–1174.
- Sadiq A, Hayat MQ, Ashraf M. 2014. Ethnopharmacology of Artemisia annua L.: a review.//artemisia annua-pharmacology and biotechnology. Berlin, Heidelberg, Germany: Springer; p. 9–25.
- Song Z, Cheng K, Zhang L, Wang T. 2017. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol. 69:184–190.
- Song ZH, Cheng K, Zheng XC, Ahmad H, Zhang LL, Wang T. 2018. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. 97(2):430–437.
- Sugiharto S. 2016. Role of nutraceuticals in gut health and growth performance of poultry. J Saudi Soc Agric Sci. 15(2):99–111.
- Tu Y. 2016. Artemisinin-A gift from traditional Chinese medicine to the world (Nobel Lecture). Angew Chem Int Ed Engl. 55(35):10210–10226.
- Ullah F, Iqbal N, Ayaz M, Sadiq A, Ullah I, Ahmad S, Imran M. 2017. DPPH, ABTS free radical scavenging, antibacterial and phytochemical evaluation of crude methanolic extract and subsequent fractions of chenopodium botrys aerial parts. Pak J Pharm Sci. 30(3):761–766.
- Wan X, Ahmad H, Zhang L, Wang Z, Wang T. 2018. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J Sci Food Agric. 98(10):3715–3721.
- Wan X, Zhang J, He J, Bai K, Zhang L, Wang T. 2017a. Dietary enzymatically treated Artemisia annua L. supplementation alleviates liver oxidative injury of broilers reared under high ambient temperature. Int J Biometeorol. 61(9):1629–1636.
- Wan XL, Niu Y, Zheng XC, Huang Q, Su WP, Zhang JF, Zhang LL, Wang T. 2016. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim Feed Sci Technol. 221:27–34.
- Wan XL, Song ZH, Niu Y, Cheng K, Zhang JF, Ahmad H, Zhang LL, Wang T. 2017b. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult Sci. 96(4):844–850.
- Wu Y, Zhou Y, Lu C, Ahmad H, Zhang H, He J, Zhang L, Wang T. 2016. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS One. 11(4):e0154410.
- Zhang YX, Sun HX. 2009. Immunosuppressive effect of ethanol extract of Artemisia annua on specific antibody and cellular responses of mice against ovalbumin. Immunopharmacol Immunotoxicol. 31(4):625–630.
- Zhigzhitzhapova SV, Dylenova EP, Gulyaev SM, Randalova TE, Taraskin VV, Tykheev ZA, Radnaeva LD. 2019. Composition and antioxidant activity of the essential oil of Artemisia annua L. Nat Prod Res. 2019:1–4.
