Abstract
To investigate the effects of Selenium (Se) deficiency on testis development and autophagy in chicks. 20 1-day old cocks were randomly divided into two groups, named the control check (CK) group [a basic diet (containing 0.333 mg Se/kg) was fed] and the Se-deficiency (Low Se) group [a selenium deficiency diet (containing 0.033 mg Se/kg) was supplied]. After 30 days, the body and testicle weights of the chicks were measured, and the organ coefficient was calculated. While haematoxylin–eosin staining, enzyme-linked immunosorbent assay, colorimetric method was used to assess the histopathological changes of testis, the changes of sex hormone levels, and antioxidant enzyme activities in two groups of chicks, respectively, the mRNA and protein expression of autophagy-related factors in the testes was determined by using quantitative PCR and western blotting. We found that Se deficiency resulted in a significant decrease in body and testicle weights of the chicks, a reduction in organ coefficients, and damaged testis tissue. The levels of sex hormone testosterone, oestradiol, the activities of serum antioxidant enzymes T-AOC, GSH-ST and GSH-PX in the Low-Se group were significantly lower than those in the CK group. Furthermore, the level of autophagy was significantly increased in the Low-Se group compared with the CK counterpart. The mRNA levels of Beclin1, Dynein, ATG-4B, ATG5, LC3-A and LC3-B genes in the Low-Se group were significantly higher than those in the CK group, while the protein expression of ATG5 and LC3-B was significantly increased in the Low-Se group compared with the CK counterpart. These data indicates dietary selenium-deprived chicks exhibit poor testicular development, impaired sex hormone synthesis, reduced antioxidant enzyme activity, and increased mRNA and protein expression of autophagy-related factors.
Se-deprived chicks display poor testicular development, impaired sex hormone synthesis, reduced activity of antioxidant enzymes, and increased mRNA and protein levels of autophagy-related factors.
HIGHLIGHTS
Introduction
Non-metal element selenium (Se) is an ultra-trace element in geochemistry, and China is one of the countries with severe Se deficiency. Se was originally considered a highly toxic substance, and it was not until that Schwarz and Mertz (Citation1957) discovered the nutritional role of Se and proposed that Se is an indispensable trace element in biological organisms. Se has been shown to play an important role in maintaining the normal physiological functions of the vast majority of species (Roberta et al. Citation2015; Hu et al. Citation2020) and is closely related to the occurrence of diseases such as Keshan disease, liver necrosis and white muscle disease in livestock (Oropeza-Moe et al. Citation2015; Chen et al. Citation2017). Meanwhile, Se has an important biological role in the growth and development, reproduction, immune function and disease resistance of livestock (Huang Y et al. Citation2016; Zhang Z et al. Citation2020). Se-deficiency may lead to declined reproductive function in livestock and poultry, while improper Se supplementation could very easily cause poisoning. Thus, the vast majority of animal nutrition professionals have paid high attention to the research on Se. So far, scholars at home and abroad have done a lot of research on the epidemiology, aetiology, clinical symptoms, necropsy changes and pathogenesis of livestocks with Se deficiency (Sun et al. Citation2015; Chen et al. Citation2017). However, there are relatively few studies on the mechanism underlying testicular degeneration caused by Se deficiency in roosters.
Se is a known component of spermatozoa and essential for spermatogenesis (Qazi et al. Citation2019). Previous studies have demonstrated that Se deficiency or low selenium status is related to numerous reproductive disorders including abnormal testicular morphology, lower semen quality, impaired sperm structure and fertilisation ability (Ahsan et al. Citation2014; Varlamova Citation2016). It has been reported that in the case of supplementing Se, four infertile dogs improved sperm motility within 1 month (Domoslawskia et al. Citation2019). The production, differentiation and maturation of normal sperm are dependent on the precise coordination among spermatogenic cells, support cells and interstitial cells in the testis, which is regulated mainly by the hypothalamus-pituitary-gonadal axis. Androgens play a key role in the development of the male reproductive system and maintain normal physiological functions (Kaftanovskaya et al. Citation2012), while oestrogen is important for synergizing spermatogenesis (Bharti et al. Citation2011). The balance in action between oestrogens and androgens is very important for maintaining male gonad development and reproductive function, especially the quantity and quality of sperm cells and maturation of the sperm in the epididymis (Popp and Walcher Citation2015). Moreover, Se exerts antioxidant effects in the pathogenesis of body damage caused by free radical metabolism disorder mainly through affecting the activity of antioxidant enzymes. It has been reported that Se-deficiency can reduce the activity of major endogenous antioxidant enzymes (Gpx) in the body, ultimately resulting in a damage in testis tissue (Graupner et al. Citation2015). However, there are few research reports on the effects of Se deficiency on sex hormone levels and antioxidant enzyme activity in chick testes.
Autophagy is a lysosome-dependent degradation pathway characterised by cytoplasmic vacuoleization and is a universal life phenomenon unique to eukaryotic cells. Autophagy is extensively involved in a variety of physiological and pathological processes (Wang et al. Citation2017; Wang et al. Citation2020). It has been shown that testicular damage occurs concurrently with an increase in autophagosomes and lysosomes (Huang et al. Citation2019). However, it remains to be determined whether autophagy is involved in regulating the occurrence and development of testicular degeneration induced by Se deficiency.
The health status of testicles at the early stage of development has important significance and influence on the reproductive success at the later stage. From 2 to 15 weeks of age, the growth and development of testis are mainly in the state of cell division, the growth and reproduction of spermatogonia cells in testis at this stage is very important for the future fertility. Thus, in the present study, we examined the organ coefficients, changes in testicular histopathology, serum content of sex hormones, antioxidant enzyme activity, and mRNA and protein expression of autophagy-related genes to determine the effects of Se deficiency on the development of testis tissue and autophagy in chicks at the early stage of development.
Material and methods
Animals
A total of sixteen 1-day-old ISA male chicks were purchased from Weiwei Co., Ltd. (Harbin, China). Chicks were randomly divided into two groups: the control check (CK) group and the Se-deficiency (Low-Se) group. The Se content in basal diet of the chicks in the Low-Se group was 0.033 mg/kg, while the chicks in the CK group were fed a basal diet containing 0.333 mg/kg of Se (Table ). According to commercial practice, chicks were reared under the same management conditions and provided ad libitum food and water during the trial. On day 30 of the experiment, samples were collected. Each chick was euthanized with sodium pentobarbital, and the specimens of testis tissue were taken and rinsed with ice-cold sterile deionised water. Each sample was divided into two halves. One part was used for paraffin section preparation, while the remaining part was immediately frozen in liquid nitrogen and stored at −80 °C for further gene expression analysis. All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University under the approved protocol number SRM-06.
Table 1. Composition of basal diet fed to chicks.
Weighing and organ coefficient calculation
The chicks were weighed prior to being sacrificed. After sacrifice, the intact testicles on both sides were carefully removed from the chicks, washed in normal saline solution, and sucked dry with normal filters. An electronic balance was used to accurately weigh the chick testes, and the organ coefficient was calculated according to the following formula: Organ coefficient = organ weight (mg)/chick weight (g).
Haematoxylin–eosin staining (HE staining)
The testis tissue specimen of the chicks was collected, and after the sample was embedded and fixed, the dewaxed sections were then soaked for 3 min, respectively, in 100% alcohol, 95% alcohol, 85% alcohol, 75% alcohol, and double-distilled water. After the distilled water was placed, the sections were placed in haematoxylin aqueous solution for several minutes, and the aqueous solution was separated for several seconds. After rinsing for 1 h in running water and being dehydrated, the mixture was stained with alcoholic eosin staining solution for 2–3 min, and the stained sections were dehydrated with the pure alcohol and then placed in xylene for 3 min twice. Images were taken through the microscope. All experiments were performed in triplicate.
Enzyme-linked immunosorbent assay (ELISA)
The level of testosterone and oestradiol in the chick serum were detected using an ELISA kit (Beijing Chenglin Biotechnology, Co. Ltd). In brief, 50 μl of capture mAb was applied to the ELISA plate for incubation and washed five times with PBST. Following this, the plate was treated with 50 μL of bovine serum albumin (BSA) blocking solution (10 mg/mL). Serum (50 μL) and biotin-conjugated detector mAb (50 μL) were added to the wells and the plate was incubated at 37 °C for 30 min, and probed with avidin-HRP solution (10 mg/mL). 100 μLof tetramethylbenzidine (TMB) substrate solution (10 mg/mL) was added to trigger the colour reaction of the Ag–Ab complex, and absorbance was measured using the automated ELISA reader (Bio-Rad model 550, Irvine, CA) at 450 nm after final rinsing with PBST. The concentration is determined by comparing the optical density to a standard curve. All experiments were performed in triplicate.
Determination of serum antioxidant enzyme activity
Colorimetric methods were used to determine the changes in serum antioxidant enzyme activities, including total antioxidation capacity (T-AOC), glutathione-S transferase (GSH-ST) and glutathione peroxidase (GSH-PX). The procedure was performed strictly according to the manufacturer’s instructions. The kits were purchased from Nanjing Jiancheng Bioengineering Co. Ltd. Jiangsu, China. All experiments were performed in triplicate.
Quantitative PCR detecting system
Total RNA was isolated by TRIzol reagent (CWBio Beijing Kangwei Century Biotechnology Co., Ltd.), and cDNA was obtained by reverse transcription after DNA elimination. The prepared cDNA was amplified using SYBR Green PCR kit (Thermo Fisher Scientific, Inc.), of which the results were calculated by ABI Prism 7300 SDS Software (Applied Biosystems; Thermo Fisher Scientific, Inc.). β-actin was included as the internal control, the relative expression level of Beclin1, Dynein, ATG-4B, ATG5, LC3-A and LC3-B genes was expressed by the ratio of the corresponding gene to the β-actin optical density. The primers used (Sangon Biotech Co., Ltd., Shanghai, China) are listed in Table . All experiments were performed in triplicate.
Table 2. Gene-special primers for Beclin-1, Dynein, ATG-4B, ATG-5, LC3-A, LC3-B and β-actin used in the qPCR.
Western blot
Tissue protein was extracted from the ipsilateral testis of each chick. According to the instruction manual of the RIPA lysate, about 40 mg of testis tissue pieces were excised, and quickly placed into 400 μL of RIPA lysate containing protease and phosphatase inhibitors at 4 °C, and total protein quantified by the BSA method was extracted. Further, moderate proteins were subjected to 10% sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred to the nitrocellulose membrane according to the corresponding molecular weight. The membrane was blocked with 5% skim milk at room temperature for 1 h, and then incubated overnight at 4 °C with the following primary antibodies from Cell Signalling Technology, Inc.: rabbit anti-Beclin-1 (1:1000); rabbit anti-Dynein (1:1000); rabbit anti-ATG-5 (1:1000); rabbit anti-microtubule-associated protein 1 A/1B-light chain 3 (LC3-B, 1:1000); anti-GAPDH (1:1000). Subsequently the membrane was washed and probed with secondary antibody (1:10000 dilution) for 1 h at 37 °C. Blots were developed with an enhanced chemiluminescent (ECL) assay (Hangzhou Fude Biological Technology Co., Ltd.). Image J software for quantifying the scanned greyscale was applied to measure the relative greyscale. All experiments were performed in triplicate.
Statistical analysis
All data and histogram were performed using GraphPad Prism software (version 7; Graph Pad Prism software Inc., San Diego). The normality of all variables was examined with the Kolmogorov–Smirnov test. The significance of between-group differences was evaluated with an independent sample t test. Differences between means were assessed by Unpaired t test. The data are expressed as the mean ± standard error of mean. Differences were considered to be significant at p < .05.
Results
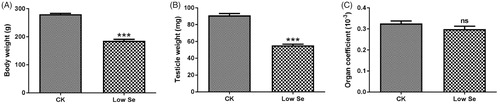
Effects of Se-deficiency on the weight and male organ coefficient of chicks
As shown in Figure , the body and testicle weights of the chicks were significantly reduced in the Low-Se group compared with the CK group (p < .001). Compared with the normal reared chicks, the organ coefficient of chicks fed with Se deficiency was also decreased to a certain extent, but no significant difference between the two groups was found (p > .05, Figure ).
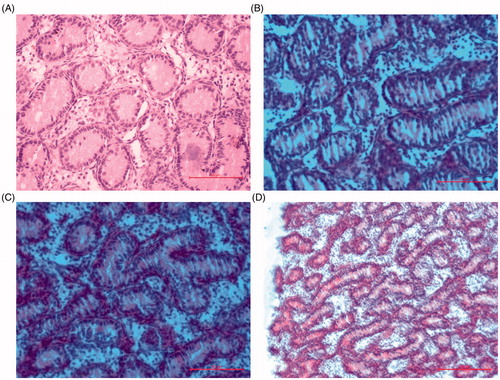
Se-deficiency leads to pathological changes in the chick testis
Histological examination showed that chicks in the CK group displayed a complete morphological structure in testes characterised by well-organised and structured tissue, well developed seminiferous tubules with regular shape and intact basement membrane, closely arranged spermatogonia with clear layers, clear structure and circular shape closely adhered to the basement membrane in the seminiferous tubules, intact interstitial structure, as well as abundant testicular mesenchymal cells (Figure ). On the contrary, chicks in the Low-Se group exhibited various morphological changes, including swelling of the seminiferous tubules, partly damaged seminiferous tubules, reduced spermatogonia in size, increased spacing between the cells, and disorganised structure. Moreover, we observed a significant decrease in the number of testicular mesenchymal cells in the chicks (Figure ).
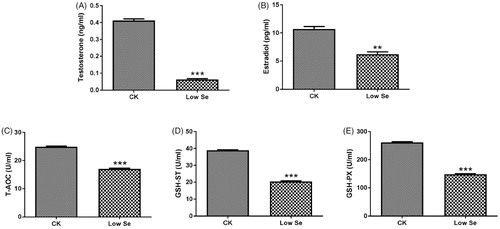
Effects of Se deficiency on the concentration of serum sex hormones and antioxidant enzyme activity
The level of serum sex hormones in chicks was determined by ELISA assay. The analysis showed that the levels of testosterone and oestradiol in the Low-Se group were significantly lower than those in the CK group (p < .01, p < .001, Figure ). Meanwhile, antioxidant enzyme assay revealed that the activities of T-AOC, GSH-ST and GSH-PX were significantly decreased in the Low-Se group compared with the CK group (p < .001, Figure ).
Effects of Se-deficiency on mRNA and protein expression of autophagy-related factors
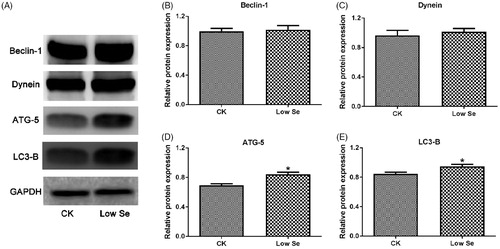
Differential mRNA expression of Beclin-1, Dynein, ATG-4B, ATG-5, LC3-A and LC3-B in testis tissue in two groups was detected by qPCR assay (Figure ). The assay showed that the mRNA levels of the above factors in the Low-Se group were significantly higher than those in the CK group (p < .05, p < .01). Meanwhile, western blot analysis was used to detect the protein expression of Beclin-1, Dynein, ATG-5 and LC3-B in testis tissue in two groups. As shown in Figure , the protein expression of ATG-5 and LC3-B was significantly increased in the Low-Se group compared with the CK group (p < .05). By contrast, the protein expression of Beclin-1 and Dynein in the Low-Se group was increased to some extent, while no significant difference in the protein levels was observed between the two groups (p > .05).
Figure 4. Effects of Se-deficiency on autophagy-related factors mRNA expression in chick testis. The mRNA expression of (A) Beclin1, (B) Dynein, (C) ATG-4B, (D) ATG5, (E) LC3-A and (F) LC3-B genes, respectively. In the experiment, the relative mRNA expression of the autophagy genes was detected by qPCR. *, **Significant differences (p < .05, p < .01) between the CK group and the low-Se group.
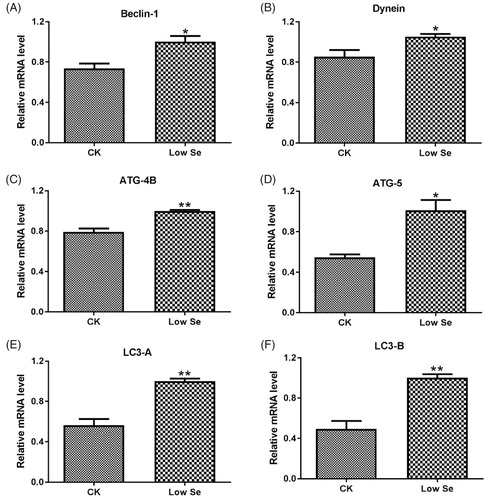
Figure 5. Effects of Se-deficiency on autophagy-related factors protein expression in chick testis. The protein expression of (B) Beclin1, (C) Dynein, (D) ATG5 and (E) LC3-B genes, respectively. In the experiment, the relative protein expression of autophagy genes was detected by western blot. *Significant differences (p < .05) between the CK group and the low Se group.
Discussion
In the present research, we found that a Se deficiency diet for chicks reduced the body and testis weights of chicks. Combined with the pathological morphology image, this study revealed that Se deficiency exerts adverse effects on the male reproductive system. Moreover, we observed a significant decrease in the levels of sex hormones and antioxidant enzymes activity, as well as a marked increase in the levels of autophagy in the Low-Se group, providing more evidence indicative of the negative effects of Se deficiency on the testis development in chicks.
The testis is the most important reproductive gland for male animals, and testis development is critical to male fertility. Testicle weight is a traditional quantitative indicator of the organ and is closely related to the quantity of germ cells. Body weight and organ coefficient are important non-specific observation indicators in animal experiments. In this study, we found that chicks fed with Se-deficiency developed slowly. Meanwhile, the body and testicle weights as well as the testicular organ coefficient were significantly decreased in the Low-Se group compared with the CK group, suggesting that the testis of chicks with a Se-deficiency diet may be damaged to a certain extent. In addition, while histopathological assessments of testis show a relatively great predictive value for fertility, sections of organs and tissues can be used to determine the structure status of organs and tissues. In this study, HE staining revealed that in the Low-Se group, a damage in histomorphological structures occurred in a number of tissues ranging from the blood vessels to the seminiferous tubules in the chick testis. The status of testicle structure and function plays a role in determining the sperm quality, and histopathological alterations in chick testis in the Low-Se group could easily lead to reproductive system dysfunction. Khalid et al. (Citation2016) have investigated the effects of dietary Se supplementation on the development of chicken testis, and the results were showed that sufficient Se in the diet could improve the quantity of germ cells and the survivability of supporting cells in seminiferous tubules. These results indicated that Se deficiency could damage testis tissue of chicks, and supplement with an appropriate amount of Se could promote the growth and development of the body and testes to a certain extent.
Testosterone is the most active component of androgens, which is important for spermatogenesis, maturation, growth and secretory activity of accessory sexual organs, and various metabolic processes. Previous studies have found that Se (0.4 mg/kg BW) could increase testosterone synthesis in male mice (Ren et al. Citation2012) and enhance testosterone production in sheep Leydig cells (Shi et al. Citation2017), suggesting that Se may play an important role in regulating male fertility and spermatogenesis. Here, we showed that Se deficiency causes testicular damage and reduced testosterone synthesis, which may be one of the causes of developmental disability in the testis. In addition, oestradiol can regulate the spermatogenic process and sperm development by affecting the secretion of male gonadotropin and androgens. In this study, we found significantly reduced levels of oestrogen in the Low-Se group, which were consistent with the previous reports that Se stimulates the synthesis of oestradiol and maintains oestrogen levels (Basini and Tamanini Citation2000). Under normal physiological conditions, chick testes contain various antioxidants and antioxidant enzymes for protecting the organs from oxidative attack. It has been reported that Se-deficiency can cause oxidative stress damage in chick testes (Zhang et al. Citation2012). T-AOC, GSH-ST and GSH-PX constitute the main enzyme system for scavenging free radicals. Here, we provided the first demonstration that Se-deficiency significantly decreased the activity of antioxidant enzymes in chick serum. All the above observations lead us to propose that in chicks with a Se-deficiency diet, decreased activity of antioxidant enzymes may be attributed to excessive production of free radicals, which needs excessive consumption of antioxidant enzymes, or a disturbance of antioxidant enzyme synthesis. The detailed underlying mechanism remains to be further investigated.
Testis damage occurs concurrently with the increase in autophagosomes, lysosomes, and autophagy levels. Se can restore the balance between mitochondrial fission and fusion and prevent the activation of autophagy (Kumari et al. Citation2012). The autophagy-related genes play essential roles at different stages of the autophagic process. As a specific autophagosome marker, LC3 is essential for the formation of autophagosome, and the ATG proteins are crucial for autophagosome assembly. LC3 and ATG5 have been shown to participate in the formation of the autophagosome (Bansal et al. Citation2018). Dyneins are large multi-component microtubule-based molecular motors involved in many fundamental cellular processes, and may act as transporters critically functioning in the combination of autophagosomes with lysosomes induced by Se deficiency (Wenzhong et al. Citation2017). Beclin1 is involved in the early stages of autophagosome formation, promoting autophagosome nucleation (Aguilera et al. Citation2012). Consistent with the above observations, this study revealed that the mRNA expression of Beclin-1, Dynein, ATG-4B, ATG-5, LC3-A and LC3-B in the Low-Se group were significantly higher than that in the CK group. Moreover, the Low-Se group displayed a significant increase in the protein expression of ATG-5 and LC3-B, indicating that Se-deficiency up-regulated the autophagy levels. Notably, no significant difference in the protein expression of Beclin-1 and Dynein was detected between the two groups; this observation may be attributed to the different time of action, or the absence of marked change in the protein levels after protein resynthesis. We speculate that Se-deficiency could lead to one or more continuous destructive factors generated in testis tissues, such as high local testicular temperature, hypoxia, oxidative stress, immune abnormalities, and aberrant hormone secretion, which destroy spermatogenic epithelial cells, and subsequently activate autophagy. Autophagy may serve as a protective pathway to remove those damaged organelles or proteins in a timely manner, thereby reducing the secondary damage caused by damaged spermatogenic epithelial cells.
Conclusions
In conclusion, dietary Se-deprived chicks display poor testicular development, impaired sex hormone synthesis, reduced activity of antioxidant enzymes, and increased mRNA and protein levels of autophagy-related factors. Low concentrations of Se can promote rapid expansion of chick testes and improve reproductive function as well as the body development, whereas excessively high concentrations of Se exert markedly toxic effects on the testes. Therefore, while special attention should be paid to the use of an appropriate dose of Se for a trace element additive, the load should be mixed evenly to prevent poisoning. To our knowledge, this study is the first one to investigate whether antioxidant enzyme activity and autophagy are involved in the regulation of the occurrence and development of chicks testicle degeneration induced by Se deficiency. This study may provide new directions for elucidating Se-deficiency-caused decline in male reproductive performance as well as facilitating clinical infertility treatment.
Ethical approval
All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University under the approved protocol number SRM-06.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper.
Additional information
Funding
References
- Domoslawskia A, Zdunczk S, Janowski T. 2019. Improvement of sperm motility within one month under selenium and vitamin E supplementation in four infertile dogs with low selenium status. J Vet Res. 63(2):293.
- Aguilera MO, Beron W, Colombo MI. 2012. The actin cytoskeleton participates in the early events of autophagosome formation upon starvation induced autophagy. Autophagy. 8(11):1590–1603.
- Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz M.H, Iqbal Z. 2014. Role of selenium in male reproduction—a review. Anim Reprod Sci. 146(1-2):55–62.
- Bansal M, Moharir SC, Sailasree SP, Sirohi K, Sudhakar C, Sarathi DP, Lakshmi BJ, Buono M, Kumar S, Swarup G. 2018. Optineurin promotes autophagosome formation by recruiting the autophagy-related Atg12-5-16L1 complex to phagophores containing the Wipi2 protein. J Biol Chem. 293(1):132–147.
- Basini G, Tamanini C. 2000. Selenium stimulates estradiol production in bovine granulosa cells: possible involvement of nitric oxide. Domest Anim Endocrinol. 18(1):1–17.
- Bharti S, Misro MM, Mathur A, Rai U. 2011. Role of estrogen in the regulation of spermatogenesis in the Indian wall lizard Hemidactylus flaviviridis. Gen Comp Endocrinol. 172(2):225–233.
- Graupner A, Instanes C, Andersen JM, Brandt-Kjelsen A, Dertinger SD, Salbu B, Brunborg G, Olsen AK. 2015. Genotoxic effects of two-generational selenium deficiency in mouse somatic and testicular cells. Mutagenesis. 30(2):217–225.
- Chen F, Gao J, Wu D, Xu L, Han W, Zhang D, Bi X, He M, Pan Y. 2017. Clinical and pathologic features of a suspected selenium deficiency in captive plains zebras. Biol Trace Elem Res. 176(1):114–119.
- Hu X, Tan S, Yin H, Khoso PA, Xu Z, Li S. 2020. Selenium-mediated gga-miR-29a-3p regulates LMH cell proliferation, invasion, and migration by targeting COL4A2. Metallomics. 12(3):449–459.
- Huang H, Wang Y, An Y, Jiao W, Xu Y, Han Q, Teng X, Teng X. 2019. Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology. 131:146–152.
- Huang Y, Li W, Xu D, Li B, Tian Y, Zan L. 2016. Effect of dietary selenium deficiency on the cell apoptosis and the level of thyroid hormones in chicken. Biol Trace Elem Res. 171(2):445–452.
- Kaftanovskaya EM, Huang Z, Barbara AM, De Gendt K, Verhoeven G, Gorlov IP, Agoulnik AI. 2012. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol Endocrinol. 26(4):598–607.
- Khalid A, Khudhair N, He H, Peng Z, Yaguang T, Guixue Z. 2016. Effects of dietary selenium supplementation on seminiferous tubules and SelW, GPx4, LHCGR, and ACE expression in chicken testis. Biol Trace Elem Res. 173(1):202–209.
- Kumari S, Mehta SL, Li PA. 2012. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: preventive effects of selenium. PLoS One. 7(6):e39382.
- Oropeza-Moe M, Wisloff H, Bernhoft A. 2015. Selenium deficiency associated porcine and human cardiomyopathies. J Trace Elem Med Biol. 31:148–156.
- Popp G, Walcher W. 2015. Immunolocalization of sex steroid receptors in the epididymis and ductus deferens of immature and mature Japanese Quail, Coturnix Japonica. Anim Sci J. 73(5):339–346.
- Qazi IH, Angel C, Yang H, Zoidis E, Pan B, Wu Z, Ming Z, Zeng C-J, Meng Q, Han H, et al. 2019. Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences. Antioxidants (Basel). 8(8):268.
- Ren X-m, Wang G-g, Xu D-q, Luo K, Liu Y-x, Zhong Y-h, Cai Y-q. 2012. The protection of selenium on cadmium-induced inhibition of spermatogenesis via activating testosterone synthesis in mice. Food Chem Toxicol. 50(10):3521–3529.
- Roberta C, Glorimar R, Grazielle H, Ronir , 2015. The association of selenium status with thyroid hormones and anthropometric values in dyslipidemic patients. Nutricion Hospitalaria. 31(3):1832–1838.
- Schwarz K, Mertz W. 1957. A glucose tolerance factor and its differentiation from factor 3. Arch Biochem Biophys. 72(2):515–518.
- Shi L, Song R, Yao X, Ren Y. 2017. Effects of selenium on the proliferation, apoptosis and testosterone production of sheep Leydig cells in vitro. Theriogenology. 93:24–32.
- Sun D, Li C, Gao J, Li S, Wang H. 2015. Effects of selenium deficiency on principal indexes of chicken kidney function. Biol Trace Elem Res. 164(1):58–63.
- Varlamova E. G. 2016. The role of selenium and selenocysteine-containing proteins in the mammalian male reproductive system. Biophysics. 61(4):580–584.
- Wang XY, Yang H, Wang MG, Yang DB, Wang ZY, Lin W. 2017. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis. 8(10):e3099.
- Wang Y, Zhao H, Guo M, Fei D, Zhang L, Xing M. 2020. Targeting the miR-122/PKM2 autophagy axis relieves arsenic stress. J Hazard Mater. 383:121217.
- Wenzhong W, Tong Z, Hongjin L, Ying C, Jun X. 2017. Role of hydrogen sulfide on autophagy in liver injuries induced by selenium deficiency in chickens. Biol Trace Elem Res. 175(1):194–203.
- Zhang Z, Liu Q, Yang J, Yao H, Fan R, Cao C, Liu C, Zhang S, Lei X, Xu S. 2020. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct. 11(2):1312–1321.
- Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW. 2012. Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res. 149(3):352–361.