Abstract
A total of 120 growing rabbits (7-weeks old) reared under high ambient temperature were divided into four equal groups, 30 rabbits each. The first group was received a basal diet without Phycocyanin (PC) (0 g/kg diet) and served as a control (PC0). The other three groups were received diets containing PC (50 (PC50), 100 (PC100), 150 (PC150) mg/kg diet, respectively). Live body weight (LBW) and feed conversion ratio (FCR) were enhanced significantly by 11.83% (p = .028) and 18.23% (p = .002) in PC50 group, and; respectively, compared with the PC0 group. Red blood cells, platelet, and haemoglobin values were significantly increased, while white blood cells were reduced (p = .033) in rabbits received diets containing PC (50, 100 and 150 mg/kg) compared to the PC0 group. Plasma urea, total bilirubin, and the gamma gamma-glutamyl transferase in the group PC100 were significantly higher than those in the PC group. Compared with the PC150 and control groups, treatment with 50 or 100 mg/kg decreased plasma interleukin-4 (p < .0001) and interferon γ (p < .0001) levels, but increased the levels of nitric oxide (p < .0001). Activities of antioxidants indices were improved (p < .0001) in the group treated with PC (50 and 100 mg/kg). However, lipid peroxidation (malondialdehyde) was decreased (p = .001) in PC50 compared with the other groups. The caecal bacterial populations were lowered in the groups treated with (50, 100 and 150 mg/kg) compared with those in the PC0 group. Conclusively, dietary inclusion of PC (100 mg/kg) could be effectively used to enhance the performance, antioxidants indices, decrease inflammatory responses and intestinal pathogens and hence enhance the health status of growing rabbits during the summer season.
Phycocyanin (PC) is one of the most bioactive compounds of spirulina platensis.
PC improved rabbit’s growth, immune, and antioxidants under heat stress.
HIGHLIGHTS
Introduction
Along with global warming, heat stress becomes one of the most central issues harmfully affecting overall livestock in the world, especially in the tropical climate. Rabbits are more susceptible to heat stress owing to the decreasing number of sweat glands and they are underdeveloped (Marai et al. Citation2002; El-Desoky et al. Citation2017). Heat stress has been presented to cause substantial harmful effects in rabbits such as reduced growth performance, reduction of antioxidants capacity, compromised immune function, alteration in intestinal injury and population, and inflammatory responses (Abdelnour et al. Citation2019a; Sheiha et al. Citation2020). The response pathways of heat stress are somewhat accredited from the upregulation synthesis of pro-inflammatory cytokines, which may lead to intestinal injury and inflammation (Sejian et al. Citation2018). Cumulative evidence also revealed that the augmented cytokines such as interleukins (ILs) and interferon- γ (IFN- γ), regulate perturbation of tight intercellular junctions hot summer months, leading to improved intestinal permeability and augmented exposure of intestine to exotic (Passos and Moraes-Filho Citation2017).
Reducing the negative influences of heat stress on rabbits involves a multidisciplinary tactic with importance on animal nutrition, welfare, and health. Nutritional intervention is a vital strategy to ameliorate the negative impacts of heat stress in livestock (Al-Sagheer et al. Citation2017; Abd El-Hack et al. Citation2020). Many studies reported that dietary manipulations, such as vitamin E, or C and microalgae (Farag et al. Citation2016; Mirzaie et al. Citation2018), phytogenic feed additives (El-Desoky et al. Citation2017), and nano selenium (Sheiha et al. Citation2020), are employed in rabbits to boost their capacity against inflammation injury persuaded by exposure to the hot environment. Thus, the documentation of competent antioxidants and anti-inflammatory features for application versus heat stress is indispensable. Spirulina platensis (SP) is blue-green algae, usually employed as a food complement and a good source of vitamins and proteins (Farag et al. Citation2016). Several reports have explored the biological activities of SP constituents and described some remarkable results. However, the main active components of SP, such as phycocyanin (PC), have been pay attention to the application in several scientific fields. Phycocyanin can ameliorate lipid peroxidation and inhibits the generation of pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) and the activities of inducible nitric oxide synthase (iNOS) as well as cyclooxygenase 2 (COX-2) enzymes (Hwang et al. Citation2011). Xie et al. (Citation2019) indicated that PC might enhance gut health by stimulating the synthesis of short-chain fatty acids and decrease intestinal pathogens in mice. The activities of these bioactive constituents in SP may elucidate the broadly informed health benefits of SP, including anti-inflammatory, antioxidant, immunomodulatory, and chemoprotective properties (Sitohy et al. Citation2015; El-Shall et al. Citation2019). Phycocyanin is a primary photosynthetic accessory pigment among the phycobiliprotein pigments in cyanobacteria and is of incredible significance due to its various biological properties. However, limited evidence is available about the influences of PC on the hematological blood variables, antioxidants indices, and inflammatory, intestinal microbiota response of rabbits under heat stress exposure. Some earlier studies demonstrated the effects of SP as a feed additive for rabbits, but surveys on the usage of its bioactive component as PC are very scarce. It is hypothesised that the dietary inclusion of PC is anticipated to exert favourable impacts on the growing rabbits exposed to heat stress. Hence, the present research was planned to explore the impacts of dietary incorporation of PC at various concentrations (0, 50, 100 and 150 mg/kg diet) on the performance, hematological variables, blood constituents, antioxidant indices, pro-inflammatory responses and caecal microbiota of the growing rabbits reared under summer season.
Material and methods
Compliance with ethical standards
All procedures by this study were following the international ethical standards. The research involved no human participants.
Isolation and characterisation of phycocyanin
The indigenous cyanobacterial strain (Spirulina platensis SOS11; SP) was isolated and characterised by the Department of Agricultural Microbiology, Faculty of Agriculture- Zagazig University, Egypt. Spirulina platensis was routinely grown in the Zarrouk medium at 28 °C for ten days. After ultimate growth, Phycocyanin was extracted from the fresh SP strain biomass using the adjusted procedures of as previously described (Sitohy et al. Citation2015). The levels of the phycocyanins (CP) were performed using spectrophotometrically technique. The CP was dried and added into the diets according to the study procedures.
Animals, experimental design and diets
A total of one hundred and twenty growing weaned New Zeeland White rabbits (males, seven weeks of age) with average body weight (BW) 804.6 g ± 9.5 g were included in the present research. Animals were randomly divided into four treatment groups, each of which (10 cages/group and three rabbits/cage) for seven consecutive weeks during the summer season (July and August) in Egypt (Zagazig, N, 42°, 30°; E, 48°,13°; East Delta). The animals were kept in galvanised wire battery cages (50 × 45 × 40 cm3) in a well-ventilated rabbitry with freshwater and feed provided ad libitum. The treatment groups as follows: (1) basal diet without addition (0 mg of phycocyanin (PC)/kg diet), (2), (3), and (4) basal diet + 50, 100 and 150 mg of PC/kg diet, respectively. Animals were retained under the same management, hygienic, and environmental conditions. The basal diet was expressed for covering the nutrient requirements of growing rabbits according to de Blas and Mateos (Citation2010); (Table ).
Table 1. Ingredients and nutrient contents of the basal diet of growing rabbits (% as fed).
Meteorological parameters
Environmental temperature (°C) and relative humidity were assessed (at 1400 h) daily throughout the entire experimental period by an automatic Thermo hygrometer (Dostmann GmbH and Co. KG, Wertheim, Germany) set in the rabbitry. The temperature-humidity index (THI) was measured according to LPHSI (1990) as the following equation: THI = db°F − ((0.55–0.55RH) (db°F − 58)), where RH is the relative humidity as a percentage, and db°F is dry bulb temperature in Fahrenheit degrees. The environmental temperature and RH values were utilised to evaluate the daily mean of each parameter (Marai et al. Citation2002).
Growth performance
Live body weight (LBW) and feed intake (FI) for each rabbit in each cage were recorded weekly through the study period. Average daily weight gain (ADGW) was estimated based on LBW, and feed conversion ratio also was referred depending on FI and ADGW.
Blood haematology
At the termination of the research, five rabbits in each treatment were selected randomly for blood collection. Each rabbit was belonged to different cage (experimental unit). The blood samples were collected from the marginal ear vein of the rabbit using heparinised tubes and distributed into two subsamples. The first one was utilised to evaluate the haematology profile by an automated haematology analyser (Hospitex Hema Screen 18, Sesto Fiorentino, Italy). The second subsample was centrifuged at (G force rate = 2 146.56 × g) for 20 min by a centrifuge (T32c; Janetzki, Wallhausen, Germany), and plasma samples were separated and kept at −20 °C for further analysis.
Blood constituents
Blood plasma metabolites including creatinine, urea, total bilirubin (TB), direct bilirubin (DB), triglycerides (TG), gamma-glutamyl transferase (GGT) were determined spectrophotometrically using commercial kits from Biodiagnostic Company (Giza, Egypt). For antioxidant indices and oxidative stress assessments, the activities of the enzymes of catalase (CAT), reduced glutathione (GSH) and superoxide dismutase (SOD), malondialdehyde (MDA) levels and the total antioxidant capacity (TAC) in plasma were determined using commercial kits and a spectrophotometer (Shimadzu, Kyoto, Japan).
Inflammatory estimation
By the turbidimetric method, the lysozyme activity was assayed (Ellis Citation1990). The levels of interleukin four and interferon-gamma in rabbit plasma were quantitated using Elisa kit assay conferring to the producer's instructions (MyBioSource, San Diego, CA, USA). The nitric oxide (NO) was assayed in the plasma by Griess reagent, as described previously (Rajaraman et al. Citation1998).
Caecal microbiota
For microbial analysis in the caecum, five rabbits at 14 weeks from each experimental group were randomly chosen and slaughtered. Each rabbit was belonged to different cage (experimental unit). The fresh digesta (1 g/rabbit) of caecal were collected in sterilised bottles provided by a stream of CO2 and transported directly to the microbiology lab. Total bacterial count (TBC) was verified from plate count agar (Merck, 1.05463, Darmstadt, Germany) at 30 °C for 48 h. After incubation for 24 h at 37 °C, Violet Red Bile Agar (Biolife) and Eosin Methylene Blue agar plates (Merck) were used for counting coliform and Escherichia coli, respectively. Using biochemical examines of Voges Proskauer, indole, methyl red, and citrate reactions, the identification of E. coli has been made. Additionally, after incubation for 24 h at 37 °C, Salmonella enterica was counted on xylose lysine deoxycholate agar plates (Merck). Using the Rose Bengal Chloramphenicol agar method, total yeasts and moulds count (TYMC) were determined at 25◦C for 5d. Bacterial counts (TBC, TYMC, lactobacilli count, Salmonella spp., coliform, and E. coli) were recorded, conferring to (Xia et al. Citation2004; Ismail et al. Citation2019).
Statistical analysis
All the results were performed using the SPSS (version 16.0, SPSS Inc., Chicago, IL, USA). For the growth performance traits, LBW, FI, BWG, and FCR, the statistical repetitions were ten cages with three rabbits in cages for each treatment. For the hematological, blood plasma constituents, and caecal microbiota analysis, the statistical repeats were used 5 rabbits for each treatment. All data were analysed with a one-way ANOVA (with the diet as the fixed factor) using the post-hoc Tukey’s test. The statistical significance was considered at p < .05. All results were expressed as mean (±SEM).
Results
Meteorological parameters
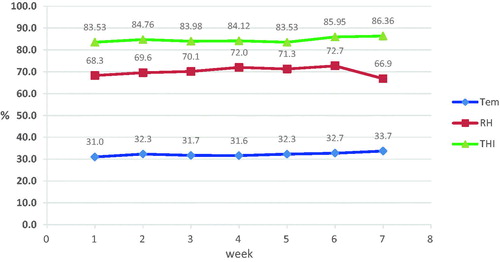
In the present study, the temperature-humidity index (THI) was high and ranged from 83.53 to 86.36 (Figure ). The relative humidity was around 70% (66.9–72.7%) during the whole experimental period. Additionally, the environmental temperature was ranged from 31 to 33.7 °C.
Growth performance
Based on the obtained consequences, in Table , it can be decided that the inclusion of PC in the diet of heat-stressed growing rabbits led to induce significant differences in growth variables. The addition of PC at level 50 mg/kg diet enhanced (p < .05) LBW at 14 weeks (age) compared with the non-treated group. At the age (10 weeks), the highest ADGW was recorded in all PC treated diets compared with the untreated group. Rabbits that given diets 50 or 100 mg PC showed significantly increased ADGW compared with those in the other groups at fourteen weeks of age. Feed intake (FI) was significantly increased at ten-week of age in all treated groups, while at the end of the experiment (14 weeks of the age), PC50 and P100 showed higher FI compared with both PC150 and control groups. Rabbits received 50 or 100 mg PC in the diets had better (p < .05) FCR than the PC150 and control at 14 weeks of age. Overall, the addition of 50 of PC into the diets of heat-stressed rabbits enhanced LBW and FCR significantly compared with those in the control and PC150 groups.
Table 2. Effect of different levels of dietary supplemental phycocyanin on growth performance of growing rabbit PC150(nPC150 PC150=PC150 30/ treatmentPC150) exposed to high ambient temperature PC150(MeanPC150 PC150±PC150 SEMPC150)
Hematological variables
The hematological variables of heat-stressed growing rabbits are revealed in Table . The supplementation of PC in growing rabbit diets exposed to heat stress enhanced the erythrocytes counts (RBCs, platelet, haemoglobin). It decreased leucocytes counts (WBCs, and monocytes) compared with that in control. Different levels of PC did not affect haematocrit, MCV, MCH, MCHC, and differential WBCs counts. The groups that received PC (50 or100 mg/kg) had significantly upper RBCs (p < .0001) compared to PC (150 mg/kg) and the control. The platelet counts were markedly increased in the PC50 treatment, while the intermediates’ values were recorded in PC100 and PC150 groups. Dietary supplementation of PC (50, 100, or 150 mg/kg) during heat stress period in rabbits, increased haemoglobin (p = .002) values. White blood cells (WBCs) counts were reduced (p < .05) in all PC treated groups compared with those in control, but monocytes were not influenced by the treatments.
Table 3. Effect of different levels of dietary supplemental phycocyanin on hematological variables of growing rabbit (n = 5/treatment) exposed to high ambient temperature (Mean ± SEM).
Blood constituents
The results in Table shown significant influences of dietary inclusion of PC on the urea, gamma-glutamyl transferase (GGT), total bilirubin (TB), and direct bilirubin (DB). However, there were no significant variances in the levels of creatinine and triglycerides with the inclusion of PC to the rabbit diets at any level (p > .05). Urea levels in the groups (PC100 and PC50) were lower (p = .015) than those in the PC150 and control groups. Liver function (TB; p = .018; DB; p = .003 and GGT; p = .002) values in the plasma were significantly decreased in 50, and 100 mg/kg treated groups compared to those in the other groups (control and PC150). While the intermediate values of TB and DB were recorded in the PC150 group.
Table 4. Effect of different levels of dietary supplemental phycocyanin on kidney and liver functions of growing rabbit (n = 5/treatment) exposed to high ambient temperature (Mean ± SEM).
As shown in Table , the inclusion of PC (50 or 100 mg/kg) declined the levels of interleukin 4 (IL-4) and gamma interferon (INF-γ) (p < .0001) in the plasma, but increased significantly the activity of lysosome (LYZ) and nitric acid (NO) level (p = .011 and p < .0001, respectively) in those groups. For pro-inflammatory variables, there was no significant difference between the control and PC150 groups. The results of antioxidants parameters in the plasma of heat-stressed rabbits showed significant differences (except SOD). The lowest values of MDA levels in the plasma were recorded in the PC100, without significant difference compared to the PC50 group. Both control and PC150 groups exhibited higher levels of MDA compared to that in the others. GSH values were significantly increased (p < .0001) in PC50 following by the PC100 group, while no statistical differences were recorded between PC150 and the control group. The groups that received PC (50 or 100 mg/kg) had significantly upper TAC (p < .0001) compared to the PC (150 mg/kg) and the control groups.
Table 5. Effect of different levels of dietary supplemental phycocyanin on inflammatory responses and antioxidants indices of growing rabbit (n = 5 rabbits treatment) exposed to high ambient temperature (Mean ± SEM).
Microbiological analysis
The intestinal microbiota of growing rabbits affected by heat stress and treated with PC is presented in Table . Heat stressed rabbits fed diets incorporated with PC (50, 100, or 150mgPC/kg) showed lower (p < .0001) TBC, and the accounts of Escherichia coli, Enterococci, Salmonella, and Enterococci colonisation in the caecum than those the control group. On the other hand, the lactic acid bacteria in the caecum of rabbits received PC was increased (p < .0001) compared to that of the non-treated group. Feeding heat-stressed rabbit with PC at different levels had no effects (p = .99) on yeasts and moulds colonisation in rabbit caecum.
Discussions
Mitigation of adverse environmental stress on the livestock industry is a primary challenge in hot semi-arid environments. Hence, nutritional efforts must be given to mitigate the negative influences of heats stress in animals, mainly when rabbits reared in tropical and subtropical zones (El-Desoky et al. Citation2017; Sheiha et al. Citation2020). In the present study, the temperature-humidity index (THI) indicated that growing rabbits were suffered from severe heat stress (Figure , 83.53–86.36; Marai et al. Citation2002). Physiologically, rabbits do not have enough functional sweat glands for adapting to excess heat load by increasing the sweating rates (Marai et al. Citation2002; Hashem et al. Citation2013). Recently, enhancing the immune system in animals during adverse environmental stressors, it became an effective novel strategy (El-Desoky et al. Citation2017; Abd El-Hack et al. Citation2020; Sheiha et al. Citation2020).
Several published works provided evidence that phycocyanin (PC) has a significant activity such as antioxidants, anti-inflammatory, or immune-modulatory properties (Riss et al. Citation2007; Farag et al. Citation2016; Osman et al., Citation2019; Xie et al. Citation2019). Our research indicates that the supplementation of PC has a valuable influence on the growth performance, inflammatory, and antioxidants responses of growing rabbits exposed to hot environmental temperatures. Likewise, results show enhancing the caecum microbiota in rabbits exposed to heat stress that given PC (50, 100, or 150 mg) by decreasing pathogenic bacteria and increase lactic acid bacteria. Together, PC reduced the adverse effects of heat stress via their potentiality antioxidant and anti-inflammatory properties (Figure ).
Figure 2. Effect of different levels of dietary supplemental phycocyanin on weekly growth performance of growing rabbit (n = 30/treatment) exposed to high ambient temperature.
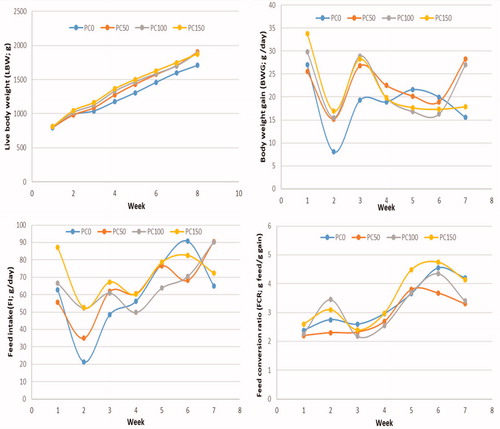
Rabbits supplemented with PC had better LBW, ADGW, and FCR compared with the control group (Figure ). This enhancement could be associated with the capability of PC to enhance antioxidants status and inhibits the production of pro-inflammatory cytokines (IL-4 and TNF-γ). Moreover, PC is a hydrophilic protein, suggesting that it is responsible for vascular colloidal osmotic pressure and a significant issue in keeping equilibrium with body fluids (Wu et al. Citation2016; Aladaileh et al. Citation2020). The inclusion of SP (0.5%) in the weaned piglet’s diet improved the BWG, ADGW, and decreased FCR (Zhang et al. Citation2019). This effect could be related to perfecting animals’ immunity and their ability to fight stress. Herein, the increase in FI and BWG in heat-stressed rabbits received PC (50 or 100 mg/kg) may attribute to the ability of PC to enhance the immunity status and stimulate the appetite of rabbits to consumed more feed. PC is a rich source of amino acids which play an essential function in enhancing the health status as well as coping with the deleterious impacts of heat stress and decreasing health disorders (Osman et al. Citation2019). Conversely, Mirzaie et al. (Citation2018) revealed that no statistical impact of dietary SP (0.5, 1, or 2%) on BWG, ADGW, and FCR of broiler reared under ambient temperature.
The chemical analysis of PC isolated from SP showed that PC contained 18.45, 16.02, and 13.46% of aspartic, proline, and glutamic acids, respectively (Osman et al. Citation2019). These amino acids could be absorbed quickly and is a component of collagen and many proteins for growth stimulation, and also playing an essential role in nutrition or wound healing. Additionally, Bownik et al. (Citation2019) reported that supplementation of L-proline at level 10, 20, or 50 mg/L into the culture medium might protect crustaceans against high environmental temperatures. In the present study, the enhancement of rabbit growth during exposure to heat stress should be associated with the thermo-protective activity of L-proline characteristic to most osmolytes. Additionally, an aspartic amino acid (which is found in PC) is used for protein biosynthesis and releasing hormones in the body (Ruth and Field Citation2013). Also, it has been reported that aspartic acid plays a significant role in neurotransmission in the body function and contributes to homeostasis and equilibrium in body fluids (Ruth and Field Citation2013).
The hematological variables are primary indicators for the health and immunity status of animals. In this respect, the present results revealed that erythrocytes were increased significantly as a response to PC addition, which may be attributed to its bioactive contents. According to its antioxidative capacity, PC is a proficient agent to assist in the regeneration of RBCs in bone narrows (Aladaileh et al. Citation2020). Furthermore, it has been indicated that heat stress could decrease levels of RBCs and platelet counts and haemoglobin concentration in the blood (Hashem et al. Citation2013). The vital role of PC can be accredited from the constructive impacts on blood picture through regulatory oxygen transportation. This response may be associated with amino acids present in PC, which reveal play vital roles in physiological homeostasis, containing in protein synthesis, decreasing muscle impairment, and lipid or glucose metabolic activity (Osman et al. Citation2019). However, the appropriate level at which to manage PC in heat stress conditions to develop thermoregulatory functions in animals is still unidentified. The present study indicates that PC can be able to reduce the counts of WBCs in rabbits exposed to heat stress. It was accepted that heat stress disrupts the immune responses through the elevation of WBCs counts (Hashem et al. Citation2013; Abdelnour et al. Citation2019a). Using natural antioxidants such as PC is making the body to reduce the WBCs with the normal range as reported in the present study. Also, PC regulates the generation of WBCs synthesis in the blood, particularly during the adverse environmental conditions. This pattern might be related to the anti-inflammatory and antioxidant roles of PC.
The plasma urea level was decreased significantly in the PC100-treated group compared with those in the PC150 and control groups. While there were no significant differences between PC50 and PC100, where PC50 showed intermediate values, on similar ground, (Wu et al. Citation2016) reported that the administration of PC (100, 150 and 200 mg/Kg BW) could play an essential role in lessening the level of urea and creatinine in rats exposed to carbon tetrachloride. We did not find any significant alterations in the creatinine contents of rabbits among the experimental groups and the control group. Urea level in the plasma is used for studying the renal function, and its elevation represents a failure of kidney function (Abdelnour et al. Citation2019a). This effect could be associated with the antioxidant capacity of PC for enhancing and protecting the renal cells (Farag et al. Citation2016), leading to the normal function of the kidney during a tropical environment.
Application natural antioxidants, for instance, SP and its derivatives in the livestock sector, are essential for enhancing the activity of liver and kidney function. Mirzaie et al. (Citation2018) point out that the inclusion of SP (2%) in the heat-stressed broiler diets reduced (p < .05) the total lipid, cholesterol, TG, and HDL concentrations in the serum. However, the present study shows that no effects of PC on TG levels, but the TB and DB were reduced significantly by the PC inclusion in the diets of rabbits exposed to heat stress. The activity of the GGT enzyme represents appropriate indicators of liver impairment. It has been reported that heat stress elevates the GGT levels, which might be a consequence of liver damage, disturbance of enzyme synthesis, and change in membrane permeability of hepatic cells, resulting in leakage of enzymes into the bloodstream (Abdelnour et al. Citation2019a). Conversely, El-Ratel and Gabr (Citation2019) clarified that the addition of 300 mg/kg BW to the rabbit does reduce significantly (p < .05) total glycerides. Our findings indicated that PC has a healthy defensive possible against heat stress hepatotoxicity by lessening the mentioned enzymatic activity.
Studies have reported that L-proline (as a central component of PC) could provide energy for cellular metabolism without further heat production (Bownik et al. Citation2019). Additionally, the considerable amount of glutamine in PC plays a strategic role in cytokine production and lymphocyte function in healthy weaning piglets (Ruth and Field Citation2013). These amino acids are considered a source of energy for lymphocyte generation and modulate pro- and anti-inflammatory cytokine synthesis in intestinal epithelial cells (Wu et al. Citation2011). Other chemical compounds with antioxidants, anti-inflammatory, and antibacterial effects such as arginine, phenylalanine, lysine, and serine were detected in PC.
The attendance of these amino acids may service the competence of animals for maintaining cellular integrity through providing endogenous cellular defense types of machinery to manage with inflammation and oxidative stress persuaded by heat stress (Marai et al. Citation2008). In light of our study, rabbits that given PC had better oxidative stability as represented by augmented the levels of antioxidants indices (TAC and GSH) and reduced the pro-inflammatory responses in the plasma (Table ). The marks of the current study in MDA, GSH and SOD values in heat-stressed growing rabbits were in agreement with Mirzaie et al. (Citation2018). They described that the addition of 2% SP to the diet reduced the level of MDA significantly and augmented significantly the defense system representing SOD and GSH in broilers. Additionally, PC acts as a free-radical scavenger that could protect the hepatocyte membrane from oxidative stress, thus falling the leaking of cellular function (Nagaraj et al. Citation2012).
Table 6. Effect of different levels of dietary supplemental phycocyanin on caecal microbiota in growing rabbit (n = 5 rabbits/treatment) exposed to high ambient temperature (Mean ± SEM).
Additionally, promoting the endogenous defense pattern by reducing the lipid oxidation (MDA as an indicator of lipid peroxidation) and enhancing the synthesis of lysosome activity may be a useful policy for optimising the animal growth under high environmental temperature (Guo et al. Citation2015; Sejian et al. Citation2018). It is interesting to observe a high level of nitric oxide (NO) in the plasma of treated rabbits, which is associated with increases in the antioxidant indices (GSH and TAC). NO is a free radical gaseous molecule that is synthesised by many cell types in the body. Recently, studies on animals have indicated that NO could play a crucial role in animal defense during adverse environmental conditions or pathogen attacks (Sheiha et al. Citation2020) by enhancing non-specific immunity (Guo et al. Citation2015). Mounting evidence suggests NO plays an essential role in immune regulation, neurotransmission, vascular homeostasis, and host defense. Following the present results, Guo et al. (Citation2015) clarified that diet inclusion with suitable arginine levels enhanced the NO synthesis in serum and lymphocyte of broilers (at 21 old).
Elevated ambient temperature-induced upper contents of pro-inflammatory cytokines such as IL-4 and IFN- γ in the plasma leading to increased intestinal permeability and augmented exposure of intestine to exotic in poultry (Abd El-Hack et al. Citation2020). In this research, PC resulted in a noteworthy decrease in pro-inflammatory cytokines such as IL-4 and IFN- γ in the plasma. This may be because PC enhances the immune status through reduces the synthesis of IFN- γ and IL-4 or the augmented the TAC and GSH, leading to growth stimulation. Generally, a previous study suggested that SP in which source of PC, with antioxidants activity, could prevent the development of atherosclerosis in hamsters (Riss et al. Citation2007), and enhances the subchronic toxicities of lead in rabbits (Aladaileh et al. Citation2020) and aspirin-induced gastric ulcer in mice (Mahmoud and Abd El-Ghffar Citation2019) via anti-oxidative, anti-inflammatory, and immune-stimulatory properties.
The ecosystem of microbiota in mammalian intestinal is hugely complex and in dynamic equilibrium (Passos and Moraes-Filho Citation2017), and the balance of the intestinal microbiota is indispensable to sustaining the host's health. Phytogenic feed additives or natural antioxidants may affect gut ecosystem and functions as they alter the pH of intestinal level and boost intestinal secretion of mucus in rabbits, which prejudice adhesion of pathogens (Abdelnour et al. Citation2019b). Phycocyanin, as a constituent of SP, presented a moderate antibacterial activity at low values of pH, representing that the efficiency of PC could be independent of pH (Xie et al. Citation2019). Accordingly, the capability of PC to lower the pH values in intestinal, leading to a decline of the pathogens bacteria in the intestine, which boosts the intestinal health. Moreover, Xie et al. (Citation2019) reported that PC intervention reduced intestinal permeability and increased the intestinal barrier function in mice.
Studies suggested that the ability of microalgae and its bioactive compounds to border the activities of several pathogenic bacterial in the animals’ intestinal. It was rich from the present investigation that rise PC concentrations reduced the caecal microbiota of growing rabbits exposed to heat stress. Phycocyanin has numerous biological properties, including an anti-inflammatory, antitumor, and immunity‐improving agent (Abd El-Hack et al. Citation2019) and also antimicrobial function contra pathogenic bacteria, including Enterococci, Salmonella enteritidis, and E. coli (Xie et al. Citation2019). The beneficial impact of PC on caecum microbiota through regulatory pathogens and this may be attributed to different amino acids presented in PC, which show antimicrobial activities against ailment triggered by bacteria (Wu et al. Citation2016).
Heat stress increased the secretion of pro-inflammatory factors (IL-4 and IFN- γ), and this was significantly decreased in the PC-treated groups. Levels of NO, LYZ, and antioxidants indices (SOD, MDA, and TAC) were increased upon heat stress. Additionally, heat stress alters the diversity of the intestinal microbiota, but PC intervention decreased the pathogen bacteria and raised the family Lactobacillaceae, which exerts a bifidogenic effect (Xie et al. Citation2019). PC inhibited these increases from managing the harmful influences of heat stress in animals. After that, results proposed that PC that natural antioxidant and inflammatory supplements could be beneficial in the future to enhance the health status of rabbits during the adverse environmental temperature.
Conclusions
According to the results above, dietary phycocyanin in the diet of naturally heat-stressed rabbits can overcome the adverse impacts of heat stress on growth performance, antioxidants criteria, reduce the inflammatory response and decline intestinal pathogens, therefore enhancing the health status of growing rabbits reared under heat stress conditions. Upcoming explorations are needed to distinguish the major biologically features in spirulina and hence to support the commercial production of microalgae-derived growth promoters with definite biological effects.
Ethical approval
All procedures of the current study were followed according to the Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010, on the safety of animals used for scientific purposes. The experimental protocol regarding the care and handling of animals had been approved by the Ethics Committee of Department of Animal Production, Faculty of Agriculture, Zagazig University, Egypt.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper. The authors alone are responsible for the content and writing of this article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abd El-Hack ME, Abdelnour SA, Taha AE, Khafaga AF, Arif M, Ayasan T, Swelum AA, Abukhalil MH, Alkahtani S, Aleya L, et al. 2020. Herbs as thermoregulatory agents in poultry: an overview. Sci Total Environ. 703:134399.
- Abd El-Hack ME, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, Mahgoub SA, Elnesr SS, Gebriel MG. 2019. Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother. 111:42–50.
- Abdelnour SA, Abd El-Hack ME, Khafaga AF, Arif M, Taha AE, Noreldin AE. 2019a. Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J Therm Biol. 79:120–134.
- Abdelnour SA, Abd El-Hack ME, Alagawany M, Farag MR, Elnesr SS. 2019b. Beneficial impacts of bee pollen in animal production, reproduction and health. J Anim Physiol Anim Nutr. 103(2):477–484.
- Aladaileh SH, Khafaga AF, Abd El-Hack ME, Al-Gabri NA, Abukhalil MH, Alfwuaires MA, Bin-Jumah M, Alkahtani S, Abdel-Daim MM, Aleya L, et al. 2020. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti- inflammatory, and immune stimulatory properties. Sci Total Environ. 701:134879.
- Al-Sagheer AA, Daader AH, Gabr HA, Abd El-Moniem EA. 2017. Abd El-Moniem, E.A. Palliative effects of extra virgin olive oil, gallic acid, and lemongrass oil dietary supplementation on growth performance, digestibility, carcass traits, and antioxidant status of heat-stressed growing New Zealand White rabbits. Environ Sci Pollut Res Int. 24(7):6807–6818.
- Bownik A, Szabelak A, Kulińska M, Wałęka M. 2019. Effects of L-proline on swimming parameters of Daphnia magna subjected to heat stress. J Therm Biol. 84:154–163.
- de Blas, C., Mateos, G.G. 2010. Feed formulation. In: de Blas, C., Wiseman, J. editors, Nutrition of the rabbit, (2nd ed.). Wallingford: CAB International, UK.
- El-Desoky NI, Hashem NM, Elkomy A, Abo-Elezz ZR. 2017. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal. 11(9):1549–1557.
- Ellis AE. 1990. Lysozyme assays. In: Techniques in fish immunology. Stolen, J.S., Fletcher, D.P., Anderson, B.S., Roberson, B.S., editors, Fair Haven: SOS Publications, USA. p. 101–103.
- El-Ratel IT, Gabr AA-W. 2019. Effect of spirulina and vitamin E on reproduction and in vitro embryo production in heat-stressed rabbits. Pakistan J Biolog Sci. 22(11):545–553.
- El-Shall NA, Awad AM, El-Hack MEA, Naiel MAE, Othman SI, Allam AA, Sedeik ME. 2019. The simultaneous administration of a probiotic or prebiotic with live salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in salmonella-challenged broilers. Animals. 10(1):70.
- Farag MR, Alagawany M, Abd El-Hac ME, Dhama K. 2016. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int J of Pharmacol. 12(1):36–51.
- Guo Y, Shi B, Yan S, Xu Y, Li J, Li T. 2015. Effects of arginine on cytokines and nitric oxide synthesis in broilers. J Anim Plant Sci. 25:366–371.
- Hashem NM, El-Hady AA, Hassan O. 2013. Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livestock Science. 157(2–3):520–526.
- Hwang J-H, Chen J-C, Yang S-Y, Wang M-F, Liu T-C, Chan Y-C. 2011. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. 121(2):361–364.
- Ismail IE, Abdelnour SA, Shehata SA, Abd El-Hack ME, El-Edel MA, Taha AE, Schiavitto M, Tufarelli V. 2019. Effect of Dietary Boswellia serrata Resin on growth performance, blood biochemistry, and cecal microbiota of growing rabbits. Front Vet Sci. 6:471.
- LPHSI. 1990. Livestock and poultry heat stress indices: the heat stress indices for poultry, Cattle, Sheep and Goats. The agriculture engineering technology guide, Clemson (SC): Clemson University.
- Mahmoud YI, Abd El-Ghffar EA. 2019. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother. 109:314–321.
- Marai IFM, Habeeb AAM, Gad AE. 2008. Performance of New Zealand White and Californian male weaned rabbits in the subtropical environment of Egypt. Anim Sci J. 79(4):472–480.
- Marai IFM, Habeeb AAM, Gad AE. 2002. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci. 78(2):71–90.
- Mirzaie S, Zirak-Khattab F, Hosseini SA, Donyaei-Darian H. 2018. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas J Anim Sci. 31(4):556–563.
- Nagaraj S, Arulmurugan P, Rajaram MG, Karuppasamy K, Jayappriyan KR, Sundararaj R, Vijayanand N, Rengasamy R. 2012. Hepatoprotective and antioxidative effects of C-phycocyanin from Arthrospira maxima SAG 25780 in CCl4-induced hepatic damage rats. Biomed Prev Nutr. 2(2):81–85.
- NRC. 1977. Nutrient requirements of rabbits: 1977. Washington, DC: National Academies Press.
- Osman A, Abd-Elaziz S, Salama A, Eita AA, Sitohy M. 2019. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. EurAsia J BioSci. 13:105–112.
- Passos M. d C F, Moraes-Filho JP. 2017. Intestinal microbiota in digestive diseases. Arq Gastroenterol. 54(3):255–262.
- Rajaraman V, Nonnecke B, Franklin S, Hammell D, Horst R. 1998. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J Dairy Sci. 81(12):3278–3285.
- Riss J, Décordé K, Sutra T, Delage M, Baccou J-C, Jouy N, Brune J-P, Oréal H, Cristol J-P, Rouanet J-M. 2007. Phycobiliprotein C-Phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem. 55(19):7962–7967.
- Ruth MR, Field CJ. 2013. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J Anim Sci Biotechnol. 4(1):27.
- Sejian V, Bhatta R, Gaughan JB, Dunshea FR, Lacetera N. 2018. Review: adaptation of animals to heat stress. Animal. 12(s2):s431–s444.
- Sheiha AM, Abdelnour SA, Abd El-Hack ME, Khafaga AF, Metwally KA, Ajarem JS, Maodaa SN, Allam AA, El-Saadony MT. 2020. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 10(3):430.
- Sitohy MO, Ghany A, Salama AGA. 2015. A. Antibacterial phycocyanin from Anabaena oryzae SOS13. Int J Appl Res Nat Prod. 8:27–36.
- Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. 2011. Triennial growth symposium: important roles for L-glutamine in swine nutrition and production1, 2. J Anim Sci. 89(7):2017–2030.
- Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. 2016. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 90(8):1817–1840.
- Xia MS, Hu CH, Xu ZR. 2004. Effects of copper-bearing montmorillonite on growth performance, digestive enzyme activities, and intestinal microflora and morphology of male broilers. Poult Sci. 83(11):1868–1875.
- Xie Y, Li W, Zhu L, Zhai S, Qin S, Du Z. 2019. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen. 8(9):e00825.
- Zhang L, Jin P, Qin S, Liu J, Yang Z, Zhao H, Sheng Q. 2019. Effects of dietary supplementation with S. platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp. and E. coli contents of weaned piglets. Livest Sci. 225:32–38.