Abstract
Pain alleviation associated with surgical castration of piglets is a debated welfare issue. The present study compares the effect of different protocols involving analgesia and/or anaesthesia or sedation suitable under field conditions, with the aim to alleviate pain due to castration in piglets. A randomised within-litter design, with 50 replicates, compared 7 treatments applied 10 min before castration: HAND: positive control, handling only; CTRL: negative control, physiological saline, i.m.; MEL: meloxicam, i.m.; AZA: azaperone, i.m.; PROC: local anaesthesia with procaine and adrenaline, subcutaneously; AZA-MEL: joint administration of azaperone and meloxicam; PROC-MEL: procaine and meloxicam. Efficacy of pain relief was assessed during a 180 min period after castration by serum cortisol and glycaemia, algometry and behaviour. CTRL, AZA, PROC and AZA-MEL piglets showed an increase in cortisol concentration 60 min after castration compared to HAND. Both groups with azaperone (AZA and AZA-MEL) developed concentrations even higher than CTRL (p < .001). HAND treatment showed cortisol levels comparable only to MEL and PROC-MEL (p > .05). CRTL and PROC piglets reacted to the algometer at an average lower pressure than HAND (p = .03), differently to the other treatments that showed similar skin sensitivity to HAND (p > .05). No differences in glycaemia and behaviour were observed among treatments. The results suggest that using meloxicam alone might offer a promising option in reducing the expression of pain-related parameters in piglets after surgical castration, however, it appears more efficient when used alone than in association with the anaesthetic agents tested. Procaine administered alone and azaperone seems unsuited to the purpose considered.
Meloxicam used alone is suggested for reducing the expression of pain-related parameters in piglets after surgical castration;
When a protocol using procaine is used on the farm during piglets castration, the association with meloxicam reduces some pain-related indicators;
Procaine administered alone and azaperone seems unsuited to manage pain after surgical castration of piglets.
HIGHLIGHTS
Keywords:
Introduction
Public opinion considers surgical castration of male piglets without pain relief to be a violation of the animal’s wellbeing and integrity, even if it is permitted by the EU regulation in force when the animal is less than 7-days-old (Council Directive 2008/120/EC). Partly for such reason, the issue of castrating young boars has grown in importance in recent years. The European Commission succeeded in securing a voluntary agreement (European declaration on alternatives to surgical castration of pigs Citation2010) with major stakeholders in the pig industry that required analgesia and/or anaesthesia for surgical castration at any age as of 1 January 2012, specifying that surgical castration must cease entirely by 1 January 2018. However, a derogation for pig meat registered under guaranteed traditional specialities, with geographical indications, and pig meat produced for traditional high-quality products were introduced to meet current quality standards, so these productions should continue to castrate piglets even after the 2018 deadline. The complexity of the subject poses an enormous challenge to all concerned: public opinion, pig producers, animal welfare organisations, and consumers. Considering that pig meat taste and odour is a very important aspect that consumers take into account when buying pork and that meat from entire males might have an unpleasant boar taint, castration becomes a drastic market-driven choice, not a producer’s decision. In addition, to involve many different parties in the pork supply chain, the practical aspects related to surgical castration management are complex and multi-faceted.
As reviewed by Dzikamunhenga et al. (Citation2014), most previous research has considered one or two analgesic or anaesthetic treatments whereas a more recent study compares the effects, benefits, and limitations of different approaches and products farmers can use to alleviate the pain experienced by piglets in parallel and under the same conditions (Gottardo et al. Citation2016). Investigations on the effects of analgesia and anaesthesia administered together however are limited in the literature (Hansson et al. Citation2011; Bonastre et al. Citation2016; Burkemper et al. Citation2020).
The aim of this study was to assess the efficacy in pain relief of different analgesic, anaesthetic and sedative agents administered alone or in association in piglets undergoing castration within the first week of age by evaluating behaviour, algometry, serum cortisol, and glycaemia. The analgesics, anaesthetics and sedatives tested were selected on the basis of the efficacy reported in the literature and real applicability under field conditions (see below in the discussion).
Materials and methods
The present study was conducted under field conditions during routine animal management and procedures observing the EU Directive 2008/120 laying down minimum standards for the protection of pigs. The protocol was, submitted and tacitly approved by the ethical committee of the University of Padova. The study took place between February and April at a commercial farm in Italy. The study was conducted on a commercial pig farm rearing 400 sows located in northeast Italy (Carmignano di Brenta, Padova, Italy). The piglets were of commercial hybrid genotype (75% Large White, 25% Belgian Landrace). The farrowing barn consisted of eight identical rooms; each room held 12 farrowing pens, with two rows of six crates separated by a corridor. Ventilation and temperature were automatically controlled by fans and air heating to keep the room temperature constant at 21 ± 2 °C; the light/dark cycle was 8/16 h. The farrowing crates (1.5 × 2.0 m) had fully-slatted floors of wire mesh covered with rubber. Piglets were allowed access to a creep area heated by a 150 W radiant infra-red heat lamp (Philips, Milan, Italy) with shredded paper as bedding material. Immediately after farrowing, fostering was performed between litters according to litter size and piglet BW. At the time of data collection, the piglets were four days old and had not been exposed to any previous husbandry procedures such as tail docking or tooth resection.
Experimental design and treatments
A randomised block design was used with 7 treatments applied in parallel within the litter. These comprised of: HAND: positive control of handling only that simulated a surgical castration procedure with restraint and manipulation of the scrotum; CTRL: negative control of castration without pain relief but with a physiological saline i.m. injection; MEL: castration was carried out after an intramuscular injection of meloxicam (Metacam® 5 mg/mL, Boehringer Ingelheim Vetmedica GmBH, Ingelheim, Germany); AZA: castration was carried out after intramuscular injection of azaperone (Stresnil® 40 mg/mL, Elanco, Sesto Fiorentino, Italy); PROC: castration was carried out after pain relief using local anaesthesia administered subcutaneously (a solution containing procaine and adrenaline: Aticain®, 40 mg/mL, 0.036 mg/mL, Ati, Bologna, Italy); AZA-MEL: castration was carried out after two intramuscular injections, one of meloxicam, the other of azaperone; PROC-MEL: castration was carried out after intramuscular injection of meloxicam and a subcutaneous injection of procaine. The nonsteroidal anti-inflammatory i.m. injection (MEL), local anaesthesia s.c. injection (PROC), sedative i.m. injection (AZA), and their combination (AZA-MEL; PROC-MEL) were administered 10 min before castration. The treatments are detailed in Table .
Table 1. Summary description of the experimental treatments, timing and type of application, type and amount of products used (HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam).
Meloxicam and azaperone injections were given intramuscularly in the neck (21 G × 12.7 mm needle), procaine injection was made subcutaneously in the scrotal area with a double-needle syringe overlaying each testicle. After injection, the piglets were returned to a box under the heat lamp in their nest until gathered for castration.
The study involved a total of 50 litters. Each was randomly selected from among litters containing at least 7 healthy male piglets (not cross-fostered) at 4 days post-partum for a total of 350 piglets. Data collection was performed on 12 different castration days, during which a maximum number of 5 litters was evaluated. In order to avoid any influence by the castration session or to the litter, all 7 treatments were contemporarily tested in each litter (one piglet per treatment in each litter). Piglets were individually identified by numbers on their back with a non-toxic marker 20 min before their surgical or sham castration simultaneously to the first data collection time-point where required (see below). Surgical or sham castration of each piglet was always performed by the same experienced veterinarian (blind to the treatment group excluding HAND) in less than 30 s. The castration procedure began with the restraint of the piglet in a head-down position and immobilising it between the legs of the operator. A 1 cm-long incision was made over the first testicle using a scalpel, the testicle was pulled from the scrotum and exteriorised. The spermatic cord was severed by cutting. The same steps were then repeated for the second testis. Chlorhexidine antiseptic was then applied to the wound site. Piglets were castrated according to the sequence of administration of the experimental treatments and after castration were all accommodated together in a plastic box with sawdust bedding in the corridor of the farrowing room. They were all then returned to the farrowing crate under the heat lamp at the same time after litter processing.
Pain evaluation was assessed using a multidisciplinary set of measures including serum cortisol, skin pressure sensitivity, and behaviour. In order to avoid any influence by the different manipulation of the piglets required for each different parameter, the 50 litters were divided into three groups of evaluation: details and timing of these assessments are summarised in Table and in the next sub-paragraphs.
Table 2. Summary of the timing of different experimental measures made to evaluate pain in piglets.
Blood collection
Three blood samples were taken from each piglet of the 18 litters by puncture of the cranial vena cava. Blood samples were associated with routine Porcine Reproductive and Respiratory Virus testing and Porcine Circovirus type 2 on the farm, therefore no additional sampling for each piglet was required for the study. At the time of first sampling, 20 min before castration (T-20), serum cortisol level and glycaemia were evaluated to provide a baseline value. Blood samples were also taken from all the piglets at 60 (T60) and 180 (T180) min post-surgery. The samples were taken alternately from the left and right sides of the neck to minimise tissue damage.
Approximately 1.5 mL of blood was drawn into a 2.5 mL syringe and then immediately and gently placed into a glass tube without anticoagulant. The numbered tubes were placed in a refrigerator at a temperature of 4 °C. At the end of the sampling day, the samples were transferred to the Istituto Zooprofilattivo Sperimentale delle Venezie laboratory (Legnaro, Italy) where they were centrifuged at 2500 g for 10 min and the serum was extracted. The serum cortisol was quantified by an immunologic chemiluminescent kit (LKC01, Medical System, Genoa, Italy). Glucose concentration (glycaemia) was determined using the hexokinase method with a dedicated commercial kit applied to the automated clinical chemistry analyser Cobas C501 (Roche Diagnostics, Mannheim, Germany). Intra-assay and inter-assay coefficient of variation was 0.7 and 1.2%, respectively.
Evaluation of skin pressure sensitivity (algometry)
This assessment measured the response of the piglet to stimulation of the wound site at controlled pressure and was measured in 14 litters (14 piglets per treatment). The algometer used in the test was the Pressure Rate Onset Device (PRODPLUS model, Topcat Metrology Ltd., Ely UK) equipped with a probe with a 4-mm diameter tip (slightly blunt) manually set to small animal mode with an indicated pressure range of between 0.1–13 kg/cm2 in order to reduce the risk of lesions of the skin with the application of higher pressures.
The measurement procedures used in this study and collection details have been described in detail by Gottardo et al. (Citation2016). The operator applied increasing pressure to the skin of the scrotum in three different points besides the wounds (on the right, on the left, and between the two wounds) until the piglet showed a clear behavioural reaction (sudden movement of a limb, an acute vocalisation, sudden agitation which decreased instantaneously when the pressure was removed, or instantaneous contraction of the muscles of the trunk or neck followed by excitement). In order to avoid operator variability, measurements were always taken by the same person. Measurement was repeated four times on each piglet: the first immediately before drug treatment and identification (T-20) in order to obtain a baseline value; the second 15–20 min after castration (T20), and the third and the fourth respectively 60 min (T60) and 180 min (T180) after castration.
Behavioural observations
Behavioural observations were made by a one trained observer. The observer was blind to the type of pain relief treatment the piglets received. Observations began immediately after the castrated piglets were returned from the plastic box to the farrowing crate and continued for 60 min. A scan sampling methodology (Martin and Bateson Citation1993) was used in which observations were made on each individual piglet at 3 min. intervals. The ethogram of behaviours considered was modified from Gottardo et al. (Citation2016). In addition, latency until movement (time required by the piglet to start walking after being placed in the farrowing crate after treatment) and suckling (time required by the piglet to start suckling the udder after placement in the farrowing crate following castration) were considered (Table ).
Table 3. Ethogram of behaviours used in the study (modified from Gottardo et al. Citation2016).
Statistical analysis
All analyses were conducted using SAS (SAS Inst. Inc., Cary, NC). Data were analysed based on their distribution (Shapiro–Wilk test for normality). Normally distributed variables were analysed using a parametric approach (ANOVA), others with a non-parametric approach (Kruskal–Wallis test). For both approaches, post-hoc pairwise comparisons were performed using Bonferroni correction. For algometer data analysis, the mean of values collected from the three areas of the scrotum was used. Serum data and those related to response following stimulation by algometry were processed by repeated measures mixed analysis with the baseline value used as a covariate. The model considered the effect of treatment (7 levels), time (60 min vs 180 min), and their interaction. Piglet within litter was considered as a repeated effect. The sow was included in the model as a random effect.
Behaviour data were analysed as the percentage of scans in which the piglet was seen to exhibit certain behaviour. Some behaviours (standing, lying down, suckling, isolated) were transformed into minutes (considering that each scan lasted 3 min) and therefore into the time required to perform a certain behaviour. The ANOVA mixed model included the fixed effect of treatment and sow as a random effect.
Results
Blood collection
A significant effect of time (p < .001), treatment (p < .001), and treatment x time interaction (p < .001; Figure ) was found for serum cortisol. CTRL group piglets showed an increase in cortisol concentration at T60 compared to HAND piglets (200.7 vs 74.0 mmol/l; p = .0054). The same increase was also showed by treatment groups AZA (395.8 mmol/l; p < .001), PROC (305.8 mmol/l; p < .001) and AZA-MEL (396.9 mmol/l; p < .001). The increase in serum cortisol level showed by MEL and PROC-MEL groups was more contained, and no difference was showed when compared to HAND piglets. However, no difference emerged also from the same treatment groups vs CTRL group comparison, showing intermediate concentrations. Both groups with azaperone (AZA and AZA-MEL) developed concentrations even higher than CTRL group (p = .0019 and p = .0026). No difference in cortisol concentration was found 180 min after castration. Considering glycaemia, no difference emerged among HAND (7.16 mmol/l) and all the other groups (mean = 7.25 mmol/l).
Figure 1. The effect of treatment on serum cortisol concentration in piglets at different times (T60 and T180) after castration. Different letters (a, b, c, d) mean significant differences between values. [HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam].
![Figure 1. The effect of treatment on serum cortisol concentration in piglets at different times (T60 and T180) after castration. Different letters (a, b, c, d) mean significant differences between values. [HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam].](/cms/asset/e10740fb-0255-4db0-9348-3efcaed66b48/tjas_a_1873707_f0001_b.jpg)
Evaluation of skin pressure sensitivity (algometry)
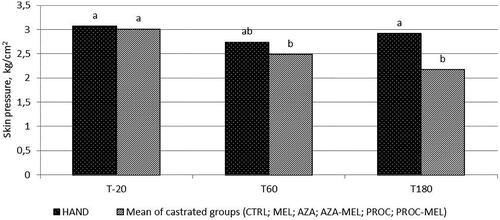
Significant effects of time (p < .001) and treatment (p < .001) were observed. For all groups subjected to castration (i.e. except HAND), the mean skin pressure eliciting a piglet reaction was higher 20 min after surgery than those recorded at T60 and T180 (3.01 vs 2.52 and 2.27 kg/cm2 respectively; p < .001). Differently, HAND piglets showed the same level of tolerance to pressure at all time-points (T20 = 3.07 kg/cm2; T60 = 2.73 kg/cm2; T180 = 2.91 kg/cm2; p > .05; Figure ). As regards average skin pressure levels, HAND piglets reacted to the algometer at a higher pressure than CTRL piglets (2.90 vs 2.19 kg/cm2; p = .03) and PROC group piglets (2.20 kg/cm2; p = .03). The other treatments showed a mid-level tolerance to skin pressure, without differences between HAND nor CTRL groups, except AZA and AZA-MEL, which increased the skin pressure leading to a reaction to HAND group level (2.91 and 3.18 kg/cm2; p > .05). The average skin pressure levels for each group are shown in Figure .
Figure 2. The effect of time on the average maximal pressure applied by algometer to the scrotum and eliciting a response in piglets after castration (mean of the castrated groups: CTRL; MEL; AZA; AZA-MEL; PROC; PROC-MEL treatments) or handling. Different letters mean significant differences between values. (HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam).
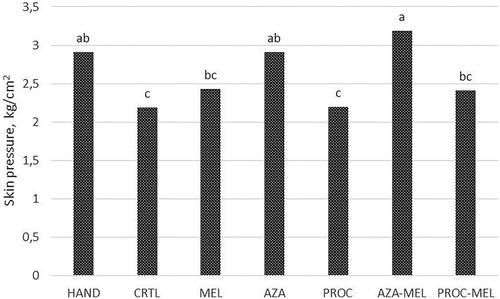
Figure 3. The effect of treatment on the average maximal pressure applied by algometer to the scrotum and eliciting a response in piglets after castration or handling. Different letters mean significant differences between values. (HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam).
Behavioural observations
None of the behaviours recorded showed a significant difference between HAND and CRTL groups. Differences emerged only among other treatments; in particular, groups treated with azaperone (AZA and AZA-MEL) showed a generally reduced activity expressed by a higher percentage of time spent lying (50.33 and 54.50% respectively vs 37.83% of HAND group) and isolated (7.33 and 9.83 vs 1.67%), and a lower percentage of time spent standing (8.50 and 5.17 vs 21.33%) and suckling (2.33 and 1.83 vs 12.50%). A greater latency was also shown both before movement (6.61 and 11.72 vs 0.00%) and before suckling (47.78 and 50.33 vs 20.06%). Differently, groups locally treated with procaine (PROC and PROC-MEL) showed a higher percentage of time spent expressing pain-related behavioural signs (5.23 and 3.23 respectively vs 0.00% of HAND group). MEL group showed similar lying behaviour compared to HAND piglets (34.24 vs 37.83%), and different from CRTL group (43.33%). Details are reported in Table .
Table 4. Mean percentage of time spent by piglets performing each behaviour and relative p-value among experimental groups (HAND = Handling; CRTL = Negative control; MEL = Meloxicam; AZA = Azaperone; AZA-MEL = Azaperone + Meloxicam; PROC = Procaine; PROC-MEL = Procaine + Meloxicam).
Discussion
Starting from the animal welfare implications associated with surgical castration, alternative methods to avoid surgical castration such as raising intact boars, immunocastration, sexed semen, and genetic modifications have been evaluated as appropriate alternatives (AVMA Citation2013). However, implementing these strategies is difficult because some technologies are not fully commercially available at this time. Moreover, certain typical swine production systems (e.g. heavy pig production) exacerbate the risk of boar taint in the meat because the animals are always slaughtered after reaching sexual maturity (170 kg of live weight and at least 9 months of age). For these reasons, finding techniques that mitigate the pain associated with surgical castration in modern industry is still crucial. This research aims to preliminary assess the efficacy in pain relief of analgesic, anaesthetic and sedative agents administered alone or in association in piglets undergoing surgical castration, in order to identify effective protocols to test later under field conditions.
There are several classes of analgesic drug that can be used to control pain; nonsteroidal anti-inflammatory drugs (NSAIDs) seem the most promising for on-farm use due to their sustainable cost, easy administration and the availability of active principles authorised for swine (Tenbergen et al. Citation2014). Meloxicam, in particular, a member of the oxicam family, seems to be very effective in inhibiting the COX-2 pathway and therefore decreasing prostaglandin synthesis (Engelhardt Citation1996; Gottardo et al. Citation2016). Meloxicam is also the only NSAID currently licenced for post-operative castration pain of pigs in the European Union and Canada (EMA Citation2006; NFACC 2014). In the US, meloxicam can be used to ‘alleviate pain and suffering in pigs’ under the Animal Medicinal Drug Use Clarification Act (AMDUCA Citation1994).
Procaine hydrochloride was chosen as the only local anaesthetic specifically authorised for swine in Europe at present. The use of regional anaesthetic techniques seems to be more economical for handlers and safer for piglets than general anaesthetic techniques (O’Connor et al. Citation2014). This drug’s main effect is the blocking of voltage-dependent sodium channels and the induction of short-term anaesthesia (Willatts and Reynolds Citation1985). The administration of anaesthetics by local subcutaneous routes increases cortisol levels (Zöls et al. Citation2006), stress calls, and defense behaviour (Leidig et al. Citation2009) after intra-testicular injection, however, probably due to the stimulation of mechanical nociceptors by the needle and the volume injected. Nociception is not blocked immediately (Haga and Ranheim Citation2005), and the pain caused by intra-testicular or funicular injection has been described by Waldmann et al. (Citation1994) and Zankl et al. (Citation2007).
Azaperone is one of the most widely used sedatives in swine (Moon and Smith 1996), and it can be combined with several anaesthetics for the immobilisation or premedication of pigs (Heinonen et al. Citation2009). None of the general anaesthetics legally authorised for swine in Europe is easily applicable on large scale in intensive farms, however, primarily due to the administration route (i.e. venous or inhalation). Considering the lack of alternative general anaesthetics, the authors decided to test azaperone alone or in combination with meloxicam to verify its use as a valid substitute, even if fully aware of the sedative nature of the drug.
In the present study, serum cortisol collected 60 min after castration and skin pressure sensitivity were used to successfully identify differing pain responses between the positive (only handled piglets – HAND) and the negative control (castrated without any pain relief – CRTL) groups in agreement with the literature (Prunier et al. Citation2005; Llamas Moya et al. Citation2008; von Borell et al. Citation2009; Di Giminiani et al. Citation2013; Gottardo et al. Citation2016). The stress and pain associated with surgical castration activate the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, stimulating the release of adrenocorticotropic hormone (ACTH) that induces secretion of cortisol in the blood. The disadvantage of the parameter is that the method of collection is, in itself, stressful for the animals and may obscure other more subtle pain indicators (Gottardo et al. Citation2016). Assessing pressure sensitivity using algometry is a promising alternative that avoids this inconvenience because it is a non-invasive method that reflects the inflammatory response associated with tissue damage capable of inducing later hyperalgesia (increased sensitivity to pain) or allodynia (the perception of pain from normally innocuous stimuli) (Di Giminiani et al. Citation2013). Considering both these parameters, meloxicam administered alone or in combination with procaine before castration was the only active principle able to maintain these pain indicators with no statistical difference with HAND group, confirming results previously reported by other authors (Keita et al. Citation2010; Tenbergen et al. Citation2014; Gottardo et al. Citation2016), even if the levels expressed by both the parameters are intermediate between HAND and CTRL (no statistical difference even with CTRL group). However, the use of procaine without meloxicam seems to be detrimental. In fact, algometric results suggest the presence of hyperalgesia in the scrotal area, and cortisol levels increased 60 min after castration. While the former might suggest the inability of procaine alone to fully block nociception for a sufficient period of time after surgical castration, the latter might be related to the burning perception experienced by piglets at the time of inoculation of the local anaesthetic due to the low pH of the solution, as previously reported by Waldmann et al. (Citation1994) and Zankl et al. (Citation2007). Moreover, because procaine hydrochloride is an anaesthetic drug in the group of esters known to induce possible local tissue reactions at the site of inoculation causing side effects such as skin irritation, hives, dermatitis, and skin allergies in predisposed subjects, combination with meloxicam might have prevented the activation of cyclooxygenases and therefore the entire cascade of inflammation mediators. Similar results were previously reported by Bonastre et al. (Citation2016), who observed a significant decrease in cortisol concentration 20 min after castration when preventively administering injectable local anaesthesia (i.e. lidocaine) with meloxicam, and Zankl et al. (Citation2007), who concluded that the intra-testicular or intra-scrotal injection of several local anaesthetics without any NSAIDs 15 min before castration in pigs did not result in a significant reduction of serum cortisol. Suggestion from the present study might be to always associate meloxicam when a protocol using injectable local anaesthesia with procaine is used on farm during piglets castration.
Unexpectedly, the use of azaperone alone or in association with meloxicam seems to be always detrimental for the piglets’ physiologic reaction at castration expressed by cortisol level. Although the authors were fully aware of the inability of this active principle to exert its effects on pain-sensitive pathways and of the way the adrenal response is produced without any impulse transmission alteration even during a state of sedation, some degree of synergy with meloxicam was expected, however. Yet, on the contrary, serum cortisol values 60 min after castration with azaperone and/or without meloxicam were clearly above not only those of the HAND group but also those of the CRTL group. This finding might suggest the possible involvement of factors other than those of painful stimuli perception alone. Donald et al. (Citation2011) reported that a low dosage of azaperone (1 mg/kg BW) induces positive psychological effects in pigs experiencing stressful conditions, suggesting the connection between the use of the active principle and the affective state of the animals and their cognitive perception of external stimuli. When used at a higher dosage to obtain sedative effects, azaperone should be administered leaving the pig undisturbed for 20 min for best effect (Clarke et al. Citation2014). Since pigs treated with azaperone are only sedated but conscious and sensitive to external stimuli, events such as the screams of roommate castrated piglets, the presence of other much more active littermates, and the subsequent manipulations for castration might have aroused a state of amplified fear and therefore further stress. Cortisolemia provides a general indication of an unpleasant experience, which includes both physical and emotional components (Mellor and Stafford Citation1999). Therefore, the use of azaperone not only showed no desirable effects in terms of physiological response, it even caused a worsening. No increased response was revealed by algometry, probably due to the after-effect of sedation in itself, with a state of the obtuseness of the sensory perception system that resulted in delayed and less determined movements and reactions.
Unfortunately, glycaemia and behavioural observation fail to show differences between positive and negative control groups. After a painful event, epinephrine stimulates the mobilisation of muscular glycogen and its consequent hepatic metabolism, resulting in an increment of glucose concentrations in blood (Mayes Citation1995). The parameter was therefore expected to vary between treatments and to be higher in piglets experiencing greater visceral pain. The lack of a significant treatment effect on glucose could be due to insufficient hepatic glycogen stores in 4-day-old piglets, as supposed by Prunier et al. (Citation2005), and the result is an agreement with Lonardi et al. (Citation2015). The absence of behavioural differences, more difficult to justify from a pain-related point of view, might be linked to the high number of treatment groups involved in the statistical analysis and their variability, thus reducing the probability of obtaining significant difference among levels. For hypothesis testing, the problem of comparing more than two means consists in the increase of Type I error that occurs when statistical tests are used repeatedly (Boole’s inequality). Most behaviours were largely affected by alterations to anaesthetic drugs, thus reducing the possibility to investigate smaller changes in groups without anaesthetics. The analysis showed only a general reduction of activity and more time spent in isolation in piglets treated with azaperone (alone or in combination with meloxicam), as well as a greater latency between castration and movement or suckling, and a higher percentage of time spent expressing pain-related behavioural signs in piglets treated with procaine when used alone. Regarding treatment with meloxicam, a result was the similar frequency of lying behaviour compared to handled piglets, differently from the increased frequency of this parameter in control ones. The reduced time spent lying of animals treated with meloxicam might suggest less pain during activity, confirming suggestions previously stated by McGlone et al. (Citation1993).
Conclusions
The study is far from finding the ideal protocol for surgical castration in piglets. The results suggest that using meloxicam alone might offer a promising option in reducing the expression of pain-related parameters in piglets after surgical castration, however, and surprisingly, it appears to be more efficient when used alone than in association with the anaesthetic agents tested. For this reason, and based on the European declaration on alternatives to surgical castration of pigs (Citation2010) that requires the use of analgesia ‘and/or’ anaesthesia, the final suggestion for farmers based on the present results is, therefore, to use meloxicam alone instead of the other tested treatments when surgical castration of piglets must be carried out. In the case of farms that are adopting a protocol with injectable local anaesthesia with procaine, the association with meloxicam is strongly suggested to reduce some pain-related indicators. The lack of anaesthetic agents specifically registered for swine and easily administered on large scale limited the investigation of several other protocols in the field; additionally, the results suggest that azaperone seems unsuited to the purpose considered.
Acknowledgements
The authors thank the Carolo Farm for participation in the research project and their kindness to host personnel of the University in charge of data collection. Thanks also to Società Cooperativa Agricola O.P.A.S. Coop. (Organizzazione di Produttori Allevatori di Suini, Carpi, Modena, Italy) for encouraging the data collection with the aim to improve welfare in its swine farms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- U.S. FDA: Animal Medicinal Drug Use Clarification Act (AMDUCA). 1994. Silver Spring (MD): Food and Drug Administration; [accessed 2020 Oct 10]. https://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/ActsRulesRegulations/ucm085377.htm
- AVMA: Literature Review on the Welfare Implications of Swine Castration. 2013. Schaumburg, (IL): American Veterinary Medical Association; [accessed 2020 Oct 10]. https://www.avma.org/KB/Resources/LiteratureReviews/Documents/swine_castration_bgnd.pdf
- Bonastre C, Mitjana O, Tejedor MT, Calavia M, Yuste AG, Úbeda JL, Falceto MV. 2016. Acute physiological responses to castration-related pain in piglets: the effect of two local anesthetics with or without meloxicam. Animal. 10(9):1474–1481.
- Burkemper MC, Pairis-Garcia MD, Moraes LE, Park RM, Moeller SJ. 2020. Effects of oral meloxicam and topical lidocaine on pain associated behaviors of piglets undergoing surgical castration. J Appl Anim Welf Sci. 23(2):209–218.
- Clarke KW, Trim CM, Hall LW. 2014. Anaesthesia of the pig. In: Veterinary anaesthesia. 11th Ed. Philadelphia (PA): W.B. Saunders; p. 385–403.
- Di Giminiani P, Petersen LJ, Herskin MS. 2013. Nociceptive responses to thermal and mechanical stimulations in awake pigs. Eur J Pain. 17(5):638–648.
- Donald RD, Healy SD, Lawrence AB, Rutherford KM. 2011. Emotionality in growing pigs: is the open field a valid test? Physiol Behav. 104(5):906–913.
- Dzikamunhenga RS, Anthony R, Coetzee J, Gould S, Johnson A, Karriker L, McKean J, Millman ST, Niekamp SR, O’Connor AM. 2014. Pain management in the neonatal piglet during routine management procedures. Part 1: a systematic review of randomized and non-randomized intervention studies. Anim Health Res Rev. 15(1):14–38.
- EMA: Metacam and why it is authorised in the EU. 2006. Amsterdam (NL): European Medicines Agency; [accessed 2020 Oct 10]. https://www.ema.europa.eu/en/documents/overview/metacam-epar-summary-public_en.pdf
- Engelhardt G. 1996. Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Rheumatology. 35(1):4–12.
- European Commission: European declaration on alternatives to surgical castration of pigs. 2010. Bruxelles (BE): [accessed 2020 Oct 03]. https://ec.europa.eu/food/animals/welfare/practice/farm/pigs/castration_alternatives_en
- Gottardo F, Scollo A, Contiero B, Ravagnani A, Tavella G, Bernardini D, De Benedictis GM, Edwards SA. 2016. Pain alleviation during castration of piglets: a comparative study of different farm options. J Anim Sci. 94(12):5077–5088.
- Haga HA, Ranheim B. 2005. Castration of piglets: the analgesic effects of intratesticular and intrafunicular lidocaine injection. Vet Anaesth Analg. 32(1):1–9.
- Hansson M, Lundeheim N, Nyman G, Johansson G. 2011. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet Scand. 53(1):34.
- Heinonen ML, Raekallio MR, Oliviero C, Ahokas S, Peltoniemi OA. 2009. Comparison of azaperone-detomidine-butorphanol-ketamine and azaperone-tiletamine-zolazepam for anaesthesia in piglets. Vet Anaesth Analg. 36(2):151–157.
- Keita A, Pagot E, Prunier A, Guidarini C. 2010. Preemptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet Anaesth Analg. 37(4):367–374.
- Leidig MS, Hertrampf B, Failing K, Schumann A, Reiner G. 2009. Pain and discomfort in male piglets during surgical castration with and without local anaesthesia as determined by vocalisation and defence behaviour. Appl Anim Behav Sci. 116(2–4):174–178.
- Llamas Moya S, Boyle LA, Lynch PB, Arkins S. 2008. Effect of surgical castration on the behavioural and acute phase responses of 5-day-old piglets. Appl Anim Behav Sci. 111(1–2):133–145.
- Lonardi C, Scollo A, Normando S, Brscic M, Gottardo F. 2015. Can novel methods be useful for pain assessment of castrated piglets? Animal. 9(5):871–877.
- Martin P, Bateson P. 1993. Measuring behaviour, an introductory guide. Cambridge (UK): Cambridge University Press.
- Mayes PA. 1995. Metabolisme du glycogene. In: Précis de biochimie de Harper. St-Foy (Canada): De Boeck Université; p. 207–216.
- McGlone JJ, Nicholson RI, Hellman JM, Herzog DN. 1993. The development of pain in young pigs associated with castration and attempts to prevent castration-induced behavioral changes. J Anim Sci. 71(6):1441–1446.
- Mellor D, Stafford K. 1999. Assessing and minimising the distress caused by painful husbandry procedures in ruminants. In Pract. 21(8):436–446.
- Moon PF, Smith LJ. 1996. General anesthetic techniques in swine. Vet Clin North Am Food Anim Pract. 12: 663–691.NFACC: Code of practice for the care and handling of pigs. 2014. Ottawa (ON): National Farm Animal Care Council; [accessed 2020 Oct 10]. http://www.nfacc.ca/pdfs/codes/pig_code_of_practice.pdf
- O’Connor A, Anthony R, Bergamasco L, Coetzee J, Gould S, Johnson AK, Dzikamunhenga RS. 2014. Pain management in the neonatal piglet during routine management procedures. Part 2: grading the quality of evidence and the strength of recommendations. Anim Health Res Rev. 15:39–62.
- Prunier A, Mounier AM, Hay M. 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J Anim Sci. 83(1):216–222.
- Tenbergen R, Friendship R, Cassar G, Amezcua MR, Haley D. 2014. Investigation of the use of meloxicam for reducing pain associated with castration and tail docking and improving performance in piglets. J Swine Health Prod. 22:64–70.
- von Borell E, Baumgartner J, Giersing M, Jäggin N, Prunier A, Tuyttens FAM, Edwards SA. 2009. Animal welfare implications of surgical castration and its alternatives in pigs. Animal. 3(11):1488–1496.
- Waldmann KH, Otto K, Bollwahn W. 1994. Ferkelkastration - Schmerzempfindung und Schmerzausschaltung. Dtsch Tierärztl Wschr. 101(1994):105–109.
- Willatts DG, Reynolds F. 1985. Comparison of the vasoactivity of amide and ester local anaesthetics. An intradermal study. Br J Anaesth. 57(10):1006–1011.
- Zankl A, Ritzmann M, Zols S, Heinritzi K. 2007. Untersuchungen zur Wirksamkeit von Lokalanaesthetica bei der Kastration von männlichen Saugferkeln. Dtsch tierarztl Wschr. 114(2007):418–422.
- Zöls S, Ritzmann M, Heinritzi K. 2006. Effect of analgesics on castration of male piglets. Berliner Und Mü Nchener Tierä rztlicheWochenschrift. 119:193–196.
