Abstract
Seaweeds can play an essential role in controlling antibiotic resistance for bacterial diseases. Three extracts of two brown seaweeds Padina tetrastromatica and Sargassum ilicifolium were investigated as growth promoters and to provide immune protection against Vibrio parahaemolyticus. Extracts of the two seaweeds were administered at 2.5 and 5 g kg−1 with food for 45 days to Penaeus monodon juveniles. The maximum weight gain (6.28 and 7.08 g), specific growth rate (3.39 and 3.32%), and survival rate (78.45 and 82.45%) of the juvenile tiger shrimp were obtained at a dose of 5 g kg−1 with P. tetrastromatica extracted by methanol and ethanol, respectively; which was significantly higher than the other solvents and control group (p < .01). On the other hand, methanol extract of both seaweeds had maximal antibacterial activity against V. parahaemolyticus with zones of inhibition ranging between 20.25 ± 1.00 and 22.00 ± 0.50 mm. The minimal time required for haemolymph clotting was with methanol extracted of P. tetrastromatica at 5 g kg−1, which was significantly lower than the other groups (p < .05). After challenging with V. parahaemolyticus, the total haemocyte count (THC) in P. monodon treated with methanol-extract of P. tetrastromatica diet at 5 g kg−1 was higher (5.42 × 105 cells mL−1) but not significantly different than the other diets (p > .05). However, the administration of 5 g kg−1 methanol and ethanol extract of P. tetrastromatica improved growth performance and the immune capacity of P. monodon by increasing their phenoloxidase activity, superoxide anion concentration, and resistance against V. parahaemolyticus.
The maximum growth performance and survival of juvenile tiger shrimps obtained with Padina tetrastromatica extract at a dose of 5 g kg−1.
The methanol extract of P. tetrastromatica and Sargassum ilicifolium had maximal antibacterial activity against V. parahaemolyticus.
The administration of 5 g kg−1 methanol and ethanol extract of P. tetrastromatica improved growth performance and the immune capacity of P. monodon.
HIGHLIGHTS
Introduction
Bangladesh ranked 12th in the world in culturing shrimp (FAO Citation2018); the giant tiger shrimp Penaeus monodon was the top species (AftabUddin et al. Citation2018). The global production of Penaeid shrimps has exceeded 4200 metric tonnes with a value of USD 4.8 billion in 2016 (GAA (Global Aquaculture Alliance) Citation2017). For P. monodon and Litopenaeus vannamei pathogenic diseases have been one of the significant challenges for culture, and bacterial infections are the leading cause of mortality in managed waters (Lightner Citation1993; Leobert et al. Citation2015; Dabu et al. Citation2017). Vibriosis is an infection in penaeid shrimp caused by the Vibrio bacteria (e.g. V. alginolyticus, V. harveyi, V. parahaemolyticus and V. vulnificus). All these bacteria can cause significant mortality in hatcheries and grow-out ponds (Ishimaru et al. Citation1995; Lavilla-Pitogo et al. Citation1998; Karunasagar et al. Citation2007). However, V. parahaemolyticus is one of the most virulent and prevalent pathogens (Joshi et al. Citation2014; Pui et al. Citation2014). In shrimp, V. parahaemolyticus is the leading causative agent of acute hepatopancreatic necrosis disease (AHPND), or early mortality syndrome (EMS) (Tran et al. Citation2013). The outbreak of AHPND was first reported in China in 2009, and then it spread to Malaysia in 2010, to Vietnam in 2011, to Thailand in 2012, and Mexico in 2013 (Lightner Citation2012; Pui et al. Citation2014). Mortality from AHPND occurs within 20–30 days after stocking in culture ponds resulting in total mortality of 40–100% (De Schryver et al. Citation2014; Chu et al. Citation2016). Cumulative economic losses have been estimated at $23.6 billion from AHPND infestations and other shrimp diseases worldwide. Further, these outbreaks have resulted in a loss of $7 billion in feed sales (Han et al. Citation2019). Therefore, pathogen control is essential to prevent vibriosis and other bacterial outbreaks in shrimp culture. Though, the strain of V. parahaemolyticus used in this study was not a strain causing AHPND.
Farmers commonly use antibiotics and/or chemicals to combat pathogenic organisms. However, the use of antibiotics and chemicals have been limited because they are expensive, non-biodegradable, highly biomagnified, and antibiotic resistance has increased. Other undesirable side-effects and impacts on human health have resulted, as well as affecting non-target organisms (Aftabuddin et al. Citation2009; Vaseeharan and Thaya Citation2014; Grenni et al. Citation2018). Indiscriminate use of chemicals and antibiotics has stimulated intense debate among environmentalists and government agencies as to whether to ban these products entirely or to permit them to have sustainable production. The concept of pathogen control in aquaculture, especially disease prevention using herbal and phytochemicals, has received widespread attention over the last decade (Bulfon et al. Citation2015; Reverter et al. Citation2017).
Shrimp have innate immunity to combat pathogenic infections, which is a first-line defense mechanism (Deris et al. Citation2020). This system eliminates invading microorganisms by humoral and cellular immune responses (Söderhäll and Cerenius Citation1998). The innate defense system of shrimp is closely related to its blood (haemolymph), as it contains haemocytes that are involved in cellular defense mechanisms such as phagocytosis, foreign substance lysis, and encapsulations (Deachamag et al. Citation2006; Li and Xiang Citation2013). Immunostimulants are substances that stimulate the animal defense system, making them more resistant to microbial infections (Raa Citation1996). Various substances such as B-glucan, chitin, algal derivatives, bacterial and fungal polysaccharides, and synthetic materials such as levamisole have been used to improve immunity and resistance to diseases in fish and shrimp (Sakai Citation1999; Sinurat et al. Citation2016). Among the compounds, B-glucan has been widely used in a variety of aquaculture species as an immunostimulant, complement activity, levels of lysozymes, phagocytic activity, and respiratory bursts (Caipang et al. Citation2011). Seaweeds are potentially excellent sources of highly bioactive secondary metabolites such as sterols, hormones, vitamins, and bio-membrane structural components that can help to develop new, functional aquaculture ingredients (Lavanya and Veerappan Citation2011; Esquer-Miranda et al. Citation2016). Some of these compounds have been considered for their anticarcinogenic, antibacterial, and anti-inflammatory properties (Pandey Citation2012). Many researchers have concentrated on testing seaweeds and other natural products by providing new bioactive agents to improve shrimp defense systems (Redo et al. Citation1989; Silver and Bostian Citation1993; Immanuel et al. Citation2004; Valgas et al. Citation2007; Esquer-Miranda et al. Citation2016; AftabUddin et al. Citation2017; Dashtiannasab and Yeganeh Citation2017).
Recently, various novel extraction techniques have been applied to collect active ingredients from different seaweeds; these include microwave or ultrasound extraction, subcritical water extraction, supercritical or pressure-assisted fluid extraction, and rapid solvent extraction (Herrero et al. Citation2006; Ibáñez et al. Citation2012). Organic solvents tend to be more effective than water when extracting active substances (Abu-Ghannam and Rajauria Citation2013). Previous works have used different organic solvents, such as N-hexene, ethanol, and methanol, to extract bioactive compounds from seaweeds (Huang et al. Citation2006; Cabrita et al. Citation2010). Moreover, increasing demand for more environmentally friendly disease control schemes has stimulated researchers to examine alternative approaches. Thus, the main objective of this study was to investigate the effects of different solvent extracts of two brown seaweeds Padina tetrastromatica and Sargassum ilicifolium on the growth promotion, survival, and immune protection in tiger shrimp Penaeus monodon juveniles against V. parahaemolyticus.
Materials and methods
Ethics
This study was carried out in strict accordance with the recommendations of the Ministry of Fisheries and Livestock, Government of the People’s Republic of Bangladesh. The protocol was approved by the Local Ethical Committee for Experiments on Animals of the University of Chittagong, Bangladesh.
Collection and preparation of seaweed extract
Seaweeds samples (P. tetrastromatica and S. ilicifolium) were collected from the shallow intertidal zone of St. Martin’s Island, Bangladesh. These were washed with clean seawater to remove debris and other extraneous materials, then placed in plastic bags and transported to the laboratory on ice. In the laboratory, samples were cut into small pieces, dried, separately crushed, and powdered using a mortar (Immanuel et al. Citation2004). The extract preparations followed the method of Abu-Ghannam and Rajauria (Citation2013) and Thanigaivel et al. (Citation2014) with slight modification. The biological activity of extracted compounds depends significantly on the type of solvent used in the extraction procedure (Abu-Ghannam and Rajauria Citation2013). Most of the antimicrobial active compounds are not water soluble. Therefore, organic solvent extracts are more potent and has been used frequently. In the extraction of antimicrobial compounds from the seaweeds, the most commonly used solvents are methanol, ethanol, acetone, ethyl acetate, and chloroform. In the Present study, powdered seaweed samples were solubilised with different organic solvents to increase the polarity (i.e. ethyl acetate, 0.228; ethanol, 0.654, and methanol, 0.762 polarities, respectively). Powdered seaweed samples (100 g) were mixed with 1000 mL of each solvent (1:10, w/v) using separate conical flasks (1 L) and kept on a shaker for 24 to 48 hours. During extraction, solvents disperse from the seaweed material, and the polarity was increased to ensure that the extraction had a wide variety of compounds (Green Citation2004). Samples were filtered using a cheesecloth and centrifuged at 4000 rpm for 15 min. The supernatant was collected and evaporated under reduced pressure at 50–55 °C in a vacuum evaporator (RE, 200, Bibby Sterling Ltd.). The dried residues were stored in a refrigerator at 4 °C until further use.
Test diets preparation
A total of 12 test diets (6 × 2 seaweed species), e.g. ethyl acetate (EA2.5, EA5), ethanol (E2.5, E5) and methanol extract (M2.5, M5), were prepared by incorporating the extracts of the two brown seaweeds, P. tetrastromatica, and S. ilicifolium; they were mixed with feed ingredients at a concentration of 2.5 and 5 g kg−1. A control diet (C) was prepared without seaweed. The properties of the experimental diets are summarised in Table . Feed ingredients were purchased from a local market, dried and mixed, powdered using a mini-pulverizer, and then strained through a 180-μm sieve. The ingredients were then mixed with warm water (38 °C) to make it dough, and extracts from the seaweeds were added. Each feed was extruded using a mini-pelletizer and then sun-dried. The dry pellets of each type of feed were packed separately in airtight plastic containers and kept in a refrigerator until use.
Table 1. Composition of the formulated shrimp diets (g kg−1) supplemented with two brown seaweeds extracts.
Experimental feeding regime of Penaeus monodon post-larvae
Healthy, disease-free, P. monodon post-larvae weighing 2.03 ± 0.27 g were collected from a local shrimp trader. For this purpose, 120–150 PLs were examined using a glass beaker and a dissecting (stereo) microscope. Animals with a complete and well-developed rostrum, transparent body and tail muscle (not curved or cramped) with few pigmentation spots; well-formed eyes and eyestalks; active swimming with straight bodies and responding quickly to external stimuli; no reddish or pale discolouration, no epibiotic fouling; no luminescence under a dark room observation, and overall good physical appearance were chosen. As more than 90% of the examined PLs passed the score, it was determined that all shrimp PLs were apparently healthy. The shrimps were acclimatised with sterilised and continuously aerated seawater for three days in an FRP (fibre reinforced plastic) container (1000 L) and fed three times a day on a commercial shrimp feed (CP Ltd.). After acclimatisation, uniform-size shrimps were selected from the stock and transferred to individual experimental transparent FRP tanks (500-L capacity). Twelve experimental groups and a control group (C) were stocked in triplicate at a stocking density of 100 individuals/tank (n = 1300 × 3 = 3900). Water parameters were recorded during the 45-day experimental period; these included temperature (27 ± 2 °C), salinity (16 ± 2 ppt), and pH (7.6 ± 0.3). The shrimps were fed at 8–5% of their body weight (at 06:00, 14:00, and 22:00 h). About 20% of the water was exchanged daily, and unconsumed feed, faeces, moulted shells were removed daily before water. Survival and growth performance parameters of shrimp at the end of the experimental feeding included the number of live shrimps, average body weight, weight gain, specific growth rate (SGR), and survivability (%) for each experimental and control group was calculated according to AftabUddin et al. (Citation2017).
Isolation of Vibrio parahaemolyticus
Juveniles of P. monodon (body weight: 5–10 g) that have symptoms of bacterial diseases were collected from Cox’s Bazar shrimp culture pond and processed according to AftabUddin et al. (Citation2017). Moribund shrimps weighing 5–10 g, displaying clinical signs of bacterial disease, i.e. red coloured localised pits in the cuticle, infections of the gut or hepatopancreas, were taken from an unhealthy pond, sealed individually in polythene bags, placed in an icebox and brought to the laboratory. Then, the sample was surface sterilised with 10 ppm of sodium hypochlorite for 2 min before rinsed in filtered seawater. One gram of sample was taken separately, placed in individual sterile test tubes, and homogenised in 10 mL of filtered seawater using a sterile glass rod. This homogenate sample was made to give 1:10 dilution. The homogenate was then serially diluted up to 103 in filtered seawater. Bacterial samples were plated on Thiosulphate Citrate Bile Salt Sucrose (TCBS, Oxoid, UK) agar supplemented with 2.5% NaCl and incubated at 27 °C for 48 hours. A detailed bacterial examination was performed, according to Alsina and Blanch et al. (Citation1994a, 1994b). Fresh V. parahaemolyticus was grown for 24 hours at 35 °C on a shaker in brain heart infusion broth (BHI) in 200-mL conical flasks with 100 mL of 50% sterilised seawater (Immanuel et al. Citation2004). The bacterial strains were stored on a medium containing 20% glycerol at −20 °C.
Determination of antimicrobial activities
An antibacterial test of the two brown seaweeds was performed by the disc diffusion method, as described by Ismail et al. (Citation2016); 0.1 mL of the test organism of V. parahaemolyticus from stock BHI broth was spread on Muller-Hinton Agar plates by using a sterilised cotton swab. Different extracts (250 µg) were dissolved separately in 20 µL of dichloromethane solvent, and the solution with extract (20 µL) was added to sterile filter paper discs (6 mm) and dried at room temperature; each disc was impregnated with 250 µg of seaweed extract residue. Negative control was prepared by using the respective solvents, while ciprofloxacin 5 µg/disc (Oxoid, Hampshire, UK, Code no. CT0425B) was used as a positive control. The plates were incubated at 37 °C for 24 h, and the inhibition zones that formed around the discs were measured by a hand ruler (diameter, mm) with an average of the triplicates of each test.
Vibrio parahaemolyticus challenge on Penaeus monodon juveniles
Shrimp treated with the seaweed concentration of 5 g kg−1 were chosen to perform bacterial challenge as they showed good growth and survival rates. Two groups were done for each treatment. The first group of each treatment (ten shrimps) was injected intramuscularly (in the ventral sinus of the cephalothorax) with pathogenic V. parahaemolyticus (1 mL of 106 cells) using individual 1-mL tuberculin syringes. For this, P. tetrastromatica experimental group was denoted as EA5, E5, M5, and S. ilicifolium were EA5, E5, and M5. The second group was divided into three groups; group-1 comprised the positive control that was fed only a basal diet (without seaweed) and injected with V. parahaemolytiucus; group-2 injected with 1.0 mL of phosphate-buffered saline (blank control) without V. parahaemolyticus; and group-3 as a negative control included juvenile shrimp that were not challenged with bacteria. All were done in 3 replicates. After injection, both subclasses were placed into separate plastic tanks (100-L capacity and 15 ppt salinity) with continuous aeration. The number of dead shrimps was recorded for 10 days following the challenge test.
Assessing haematological and immunostimulatory activity
Haemolymph collection
At the 1st, 4th, 7th, and 10th days following the challenge with V. parahaemolyticus bacteria, three shrimp were randomly selected from each tank (both control and experimental) for the collection of haemolymph. After collection of the sample, haemolymph clotting time, total haemocytes, phenoloxidase (PO) activity, and superoxide anion (SO) concentration were determined. Samples of 100 µL of haemolymph were taken using a 25-gauge needle with a 1-mL syringe containing 0.9 mL of precooled (4 °C) anticoagulant solution (trisodium citrate 30 mM, NaCl 338 mM, glucose 115 mM, EDTA 10 mM, pH 7·5).
Total haemocyte count
The blood clotting time of the haemolymph was determined by the capillary method of Sachdev (Citation1983). Total haemocyte count (THC) was determined by using a Neubaeur’s haemocytometer (Yeh et al. Citation2006). A drop of an anticoagulant–haemolymph mixture (100 µL) was placed on the haemocytometer and haemocytes were counted in all four squares by observing under a compound microscope and expressed as cells mL−1 haemolymph. The rest of the haemoplymph mixture was used for subsequent tests.
Phenol oxidase activity
The phenoloxidase (PO) activity of the haemocytes was measured spectrophotometrically (in triplicate) by recording the formation of dopachrome produced from l-dihydroxyphenylalanine (L-DOPA) as described by Gigi (Citation2011). Briefly, 50 µL of haemolymph was taken and mixed with 50 µL of 10% SDS and 1.0 mL l-dihydroxyphenylalanine (0.19% L-DOPA in Tris-HCl buffer) and incubated for 30 min at 25 °C in 96-microliter plates (flat bottomed). The formation of dopachrome was measured every 30 s for 3 min in a spectrophotometer at 490 nm. The PO activity was expressed as dopachrome formation per 50 µL of haemolymph.
Superoxide anion (NBT reductase assay)
The superoxide anion (SO) activity was determined following the method of Gigi (Citation2011) by using nitro blue tetrazolium (NBT) (Song and Hsieh Citation1994) and the optical density was read at 630 nm. For this, 100 µL haemolymph was incubated for 30 min at room temperature (28 °C) with 100 µL of 0.2% NBT for adherence of the haemocytes, then centrifuged (13,000 × g, 4 °C, 10 min.) and finally fixed in 100% methanol. Samples were held 10 min at room temperature and then subjected to centrifugation (4000 × g, 4 °C, 10 min). The supernatant was removed after centrifugation, and the cells were dried, then rinsed in 50% methanol, and finally solubilised in 140-µL DMSO and 120-µL 2 M KOH. The absorbance at 630 nm was recorded, and the activity expressed as O.D. 100 µL−1 haemolymph.
Data analysis
All the statistical analyses were performed using STATISTICA v 13.2 (Statsoft Inc., Tulsa, OK, USA). For both seaweed species, growth parameters among different treatments were compared using a two-way ANOVA, followed by a Tukey’s test. Alpha was set at 0.05 for all the analyses. A series of two-way ANOVA was also performed among different treatment groups to test the differences in blood clotting time, total haemocyte count, Phenol oxidase activities, and superoxide anion activities at different exposure periods (1, 4, 7, and 10 days). All these analyses were performed separately for both seaweed species.
Results
Growth parameters
The weight gain, specific growth rate, and survival of juvenile tiger shrimp P. monodon after 45 days of treatment with P. tetrastromatica or S. ilicifolium extracts are presented in Table . Among the two concentrations (e.g. 2.5 and 5 g kg−1) of three solvent extracts (e.g. ethyl acetate, ethanol, and methanol) of two seaweeds P. tetrastromatica and S. Ilicifolium, the test diet of 5 g kg−1 showed promising results. The maximum weight gain, specific growth rate, and survival rate of the juvenile tiger shrimp were obtained in P. tetrastromatica extracted by ethanol and methanol at a dose of 5 g kg−1; this regimen was higher than the ethyl acetate solvents and control group (p < .01). Similarly, the mean weight gain and specific growth rate of juveniles in treatments with S. ilicifolium extracted by ethanol, and methanol at a dose of 5 g kg−1 were higher than the treatments with ethyl acetate solvents and control group (p < .01). The survival rate of juvenile tiger shrimp was higher in S. ilicifolium extracted by ethanol and methanol treatments at a dose of 5 g kg−1 compared to the ethyl acetate solvents (p < .01) but was not significantly different from those in the control group (p > .05).
Table 2. Mean (±SE) weight gain, specific growth rate and survival rate of P. monodon juveniles fed on brown seaweeds extract incorporated diets and control group (C).
Antibacterial sensitivity test
The crude extracts from the three different solvents (e.g. ethyl acetate, ethanol, and methanol) of P. tetrastromatica and S. ilicifolium were tested antibacterial activity against V. parahaemolyticus. Of these three extracts, methanol extracts of the two brown seaweed exhibited maximal antibacterial activity against V. parahaemolyticus bacteria. The inhibition zone of P. tetrastromatica and S. ilicifolium methanolic extracts were ranged between 22.65 ± 0.57 and 20.25 ± 1.0 mm, respectively, which was significantly different (p < .05) compared to other solvents (Table ).
Table 3. Anti V. parahaemolyticus activity of two brown seaweeds extracted by three different solvents – ethyl acetate, ethanol and methanol.
Challenge test
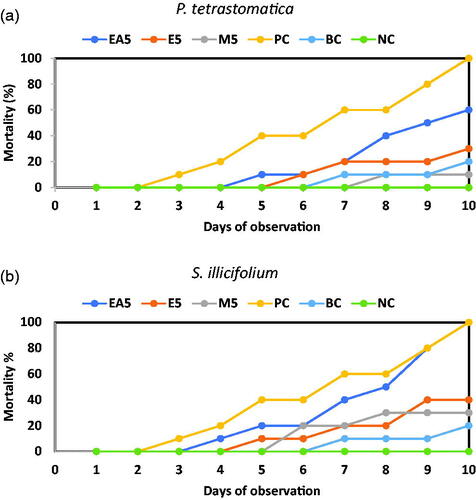
The mortality of the positive control (PC) and the three different solvents (e.g. ethyl acetate, ethanol, and methanol) of P. tetrastromatica and S. ilicifolium extract incorporated diets fed shrimps is presented in Figure . Results indicated that all the unchallenged shrimp (negative control) survived, but the challenged group died within three days. Two-way ANOVA revealed that variation in the survival rate of P. monodon fed with control and seaweed extract diets was statistically significant (p < .01). The positive control group of shrimps fed on a diet devoid of seaweed extract acceded to death (60%) within seven days after challenging with V. parahaemolyticus. Penaeus monodon juveniles fed with ethanol and methanol-extract diets (5 g kg−1) of P. tetrastromatica had lower mortality (30% and 10%, respectively) after 10 days of the challenge. The survival rate (90%) of shrimps was observed when they fed on P. tetrastromatica methanolic extract diet (M5). The challenge test revealed that V. parahaemolyticus could cause 100% mortality in the positive control (PC) and ethyl acetate (EA5) of S. ilicifolium extract diets within 10 days.
Figure 1. Percentages of mortality of P. monodon juveniles fed (a) P. tetrastromatica and (b) S. ilicifolium extracted diets and control diets after challenged with V. parahaemolyticus. EA5: ethyl acetate 5 g kg−1; E5: ethanol 5 g kg−1; M5: methanol 5 g kg−1; PC: positive control; BC: blank control; NC: negative control.
Haematological and immunostimulatory activity of Penaeus monodon
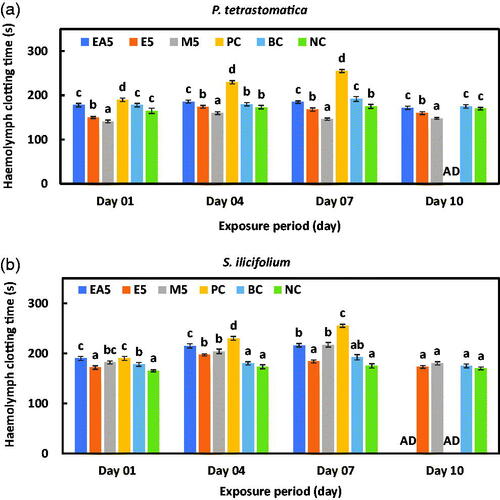
The haemolymph clotting time in P. monodon treated with ethyl acetate, ethanol, and methanol extracted diets of P. tetrastromatica and S. ilicifolium at different exposure periods are presented in Figure , respectively. The positive control group for both seaweeds died after 10 days of exposure. The minimal time required for haemolymph clotting with 5 g kg−1 methanol-extracted diets after 10 days of exposure was significantly lower (148 ± 2.1 s) for P. tetrastromatica than for other solvents and control groups (p < .05). The ethyl-acetate solvent group and the positive control group of S. ilicifolium died after 10 days of exposure, and there were no significant differences among the other solvents and control groups (p > .05).
Figure 2. Mean haemolymph clotting time of P. monodon with (a) P. tetrastromatica and (b) S. ilicifolium incorporated diet after challenge. Data at the same exposure time with different letters are significantly different (p < .05) among different treatment groups. EA5: ethyl acetate 5 g kg−1; E5: ethanol 5 g kg−1; methanol 5 g kg−1; PC: positive control; BC: blank control; NC: negative control; AD: all died.
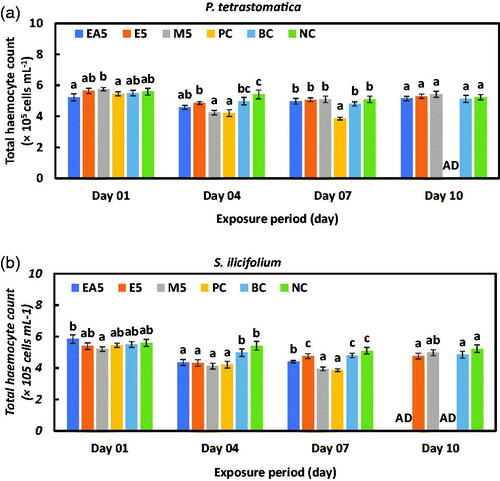
The total haemocyte of P. monodon juveniles treated with ethyl acetate, ethanol, and methanol-extract diets of P. tetrastromatica and S. ilicifolium at different exposure periods are presented in Figure , respectively. All the juveniles from the positive control group of P. tetrastromatica and S. ilicifolium died after 10 days of exposure. No significant difference was observed (p > .05) among the solvents and negative control groups after 10 days of exposure for both seaweed species.
Figure 3. Total haemocyte count (THC) of P. monodon with (a) P. tetrastromatica and (b) S. ilicifolium incorporated diet after challenging with V. parahaemolyticus bacteria (1.0 mL of 106 cells) days onward. Data at the same exposure time with different letters are significantly different (p < .05) among different treatment groups. EA5: ethyl acetate 5 g kg−1; E5: ethanol 5 g kg−1; methanol 5 g kg−1; PC: positive control; BC: blank control; NC: negative control; AD: all died.
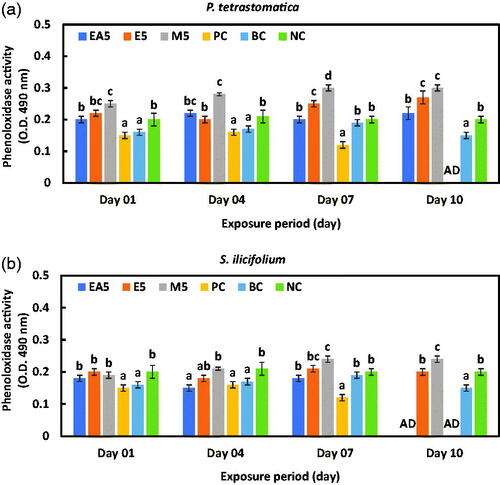
The maximum phenoloxidase activity (PO) was found in the methanol extract group of P. tetrastromatica and S. ilicifolium on the 10th day of the experimental trial (Figure ). However, the positive control group died within seven days. One-way ANOVA revealed that the variation between the PO activities of P. monodon fed with control and seaweed extract-incorporated diets was significant (p < .01).
Figure 4. Phenol oxidase (PO) activity of haemocytes of P. monodon fed (a) P. tetrastromatica and (b) S. ilicifolium extracted immunostimulants diets and control diets after challenged with V. parahaemolyticus. Data at the same exposure time with different letters are significantly different (p < .05) among different treatment groups. EA5: ethyl acetate 5 g kg−1; E5: ethanol 5 g kg−1; methanol 5 g kg−1; PC: positive control; BC: blank control; NC: negative control; AD: all died.
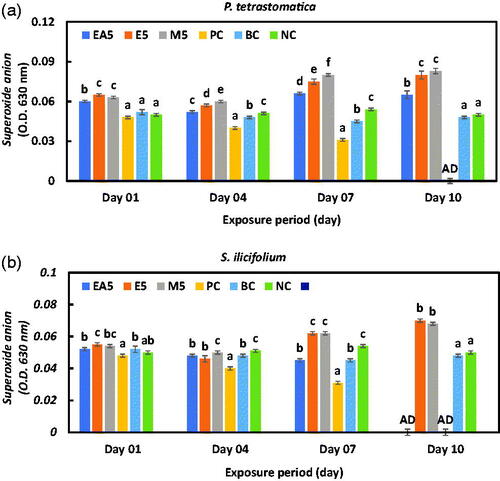
The superoxide (SO) anion concentrations gradually decreased in all the seaweed extracts incorporated groups within four days of exposure and then sharply increased at the end of the experimental trial (Figure ). The creation of SO in the ethanol and methanol group was markedly higher after 10 days of exposure compared to the other groups (p < .01), while it was a bit lower in the blank and negative control groups.
Figure 5. Intra-cellular superoxide (SO) anion production of haemocytes of P. monodon fed (a) P. tetrastromatica and (b) S. ilicifolium extracted immunostimulants diets and control diets after challenged with V. parahaemolyticus. Data at the same exposure time with different letters are significantly different (p < .05) among different treatment groups. EA5: ethyl acetate 5 g kg−1; E5: ethanol 5 g kg−1; methanol 5 g kg−1; PC: positive control; BC: blank control; NC: negative control; AD: all died.
Discussion
Growth parameters
After 45 days of the experimental trial, juvenile tiger shrimps treated by P. tetrastromatica and S. ilicifolium extract with different solvent showed significant differences in mean weight gain (g), SGR (%), and survival rate (%) (p < .05). In the present study, isoenergetic diets were not employed. Therefore, higher growth performance can also be a consequence of using non- isoenergetic diets for the juveniles. Immanuel et al. (Citation2004) showed that P. indicus reared in V. paraheamolyticus inoculated systems fed Artemia with butanolic extracts of Ulva lactuca and Sargassum wightii, the mean survival rate was 51.10 ± 6.93 and 45.55 ± 5.09% for Ulva lactuca and Sargassum wightii, respectively; which is very close to the results of our present study. Shrimps fed on 5 g kg−1 methanolic extract of P. tetrastromatica had a maximum mean weight gain (7.08 g), which was very close to the results reported by Huxley and Lipton (Citation2009) achieved in P. monodon shrimp fed with a diet containing 3 g kg−1 feed of S. wightii methanolic extract. Feeding ethanol extract of different red seaweeds like Laurencia snyderiae, Hypnea cervicornis, and Crypto nemia showed positive effects on the growth of juvenile L. vannamei (Da Silva and Barbosa Citation2009; Dashtiannasab and Yeganeh Citation2017). Contrary, P. indicus juveniles fed on Artemia, enriched with butanolic extracts of U. lactuca and S. wightii had shown lower mean weight gain (3.07 ± 0.32 and 2.81 ± 0.16 g, respectively) and SGR (1.95 ± 0.03 and 1.54 ± 0.03, respectively) (Immanuel et al. Citation2004). It has not been clear how the active compounds of different seaweeds enhance the growth of shrimp. However, the growth of shrimps could be attributed to the vitamin and mineral contents, lipid mobilisation, and improved absorption and assimilation efficiency ratios of the seaweeds (Cruz‐Suárez et al. Citation2009).
Antibacterial sensitivity and challenge test
Bacterial diseases are quite common in P. monodon cultures and cause substantial mortality. Seaweeds contain many antimicrobial compounds that have the potential for providing new drugs against microbial infections, cancer, and inflammations (Selvin and Lipton Citation2004). In our present study, the methanolic extracts of P. tetrastromatica and S. ilicifolium significantly inhibited the growth of V. parahaemolyticus bacteria compared to ethanol and ethyl-acetate extracts. The antimicrobial properties of the methanolic extracts of green seaweed Caulerpa sertularioides against V. parahaemolyticus were studied and significantly inhibited the growth of V. parahaemolyticus (Esquer-Miranda et al. Citation2016). Antibacterial activity of butanolic extracts of U. lactuca (17.5 ± 0.81 mm) and S. wightii (16.3 ± 0.47 mm) was also effective against V. parahaemolyticus (Immanuel et al. Citation2004). Therefore, our findings suggest that methanolic extracts of P. tetrastromatica and S. ilicifolium can control the growth of V. parahaemolyticus bacteria for farm-shrimp, and therefore might have potential as an antibacterial agent.
The challenge test against V. parahaemolyticus revealed the ability of P. tetrastromatica and S. ilicifolium ethanolic and methanolic extracts (at 5 g kg−1) to protect P. monodon from V. parahaemolyticus. The previous study has shown that after V. alginolyticus infection, the methanolic extract of Caulerpa sertularioides at 300 mg L−1 significantly reduced the mortality of L. vannamei (Esquer-Miranda et al. Citation2016). Yeh et al. (Citation2006) have observed the lowest mortality levels using 300 and 500 mg L−1 Sargassum duplicatum extracts exposed to juvenile L. vannamei shrimp infected with V. alginolyticus. Besides, higher concentrations of Gracilaria tenuistipitata extracts (600 mg L−1) were found to be associated with lower mortality levels for L. vannamei (Yeh and Chen Citation2009). In the present study, we have used much higher (10-fold) concentrations of P. tetrastromatica and S. ilicifolium ethanolic and methanolic extracts, which results in lower mortality for P. monodon juveniles.
The haematological and immunostimulatory activity of Penaeus monodon
Crustaceans have no adaptive defense system, and they rely solely on their innate immunity against pathogenic infection. An innate defense system or in-born immunity system is an evolutionarily older defense strategy, and the shrimp defense system eliminates invading microorganisms through humoral and cellular responses (Söderhäll and Cerenius 1998; Kimbrell and Beutler Citation2001; Loof et al. Citation2011). The humoral response index includes clotting, antimicrobial peptides (AMPs) stimulation, superoxide dismutase (SOD) generation, transmembrane glycoproteins, and other stress-responsive proteins and molecules (Maningas et al. Citation2013). The first baseline defense mechanism of shrimp humoral immune response is the clotting system, which is vital for the prevention of haemolymph damage during injury and wound healing and prevents the entry of opportunistic pathogens (Maningas et al. Citation2013). On the other hand, P. monodon cellular immune response involves different types of cells, including haemocytes, which are initiated by a cascade of phenoloxidase (PO) activating processes leading to phagocytosis, encapsulation, coagulation, and melanisation of invading pathogens (Deris et al. Citation2020). In the present study, methanolic-extract of P. tetrastromatica incorporated into diets and fed to shrimp, lowered haemolymph clotting time after 10 days of exposure with V. parahaemolyticus bacteria, thus indicating good immune effect in the treated group. However, the haemolymph clotting time increased in the positive control (injected V. parahaemolyticus) groups of shrimps increased with increasing exposure period, and finally, all died. The increase of haemolymph clotting time for the positive control group was due to the substantial bacterial load in the haemolymph by infection, which had prolonged the clotting time.
In decapods, there are three major types of blood cells (haemocytes), and each has distinctive morphological characteristics and physiological functions (Johansson et al. Citation2000). Haemocytes are responsible for clotting, hardening of shells, and removal of foreign particles, i.e. antigens (Song and Hsieh Citation1994). In the present study, after challenging with V. parahaemolyticus bacteria, the THC levels of all groups were sharply decreased on the 4th day of the experimental trial, and after that, gradually increased. Juvenile P. monodon that was administrated on 5 g kg−1 P. tetrastromatica methanolic-extract incorporated diet showed significantly higher (5.42 × 105 cells mL−1) THC than that of shrimp fed on S. ilicifolium extract (4.98 × 105 cells mL−1) after 10 days of exposure. Citarasu et al. (Citation2006) found that P. monodon fed on a herbal immunostimulant diet for 25 days and then infected with WSSV, the THCs of the immunostimulant groups were drastically decreased for the first few days. In their experiment, the amount of haemocyte was 61.33 ± 1.5 × 106 cells mL−1 on the 1st day at 0.8 g kg−1 immunostimulant incorporated diet group. On the 6th day, the count decreased to 54.3 ± 1.50 × 106 cells mL−1 and then increased up to the average level (63.0 ± 1.0 × 106 cells mL−1) after 20 days of exposure. The THC of L. vannamei injected with hot-water extract of 20 mg g−1 and 10 mg g−1 brown seaweed S. duplicatum was significantly higher than that of shrimp injected with 6 mg g−1 and 2 mg g−1 saline water (Yeh et al. Citation2006). The THC of L. vannamei was increased with the application of S. hemiphyllum in seawater. Red seaweed Gracilaria fisheri fed on ethanolic extract of the P. monodon post-larvae and juveniles showed an increase in THC while challenged with V. harveyi (Kanjana et al. Citation2011). However, haemocytes play a crucial role in cellular defense, while a low circulating haemocyte in crustaceans correlated to a higher sensitivity to pathogens (Le Moullac et al. Citation1998. One of the effective immune defences of invertebrates is the phenoloxidase system. If phenol oxidase enzyme activity is decreased, the phagocytosis activity may fail, and that is the mutual process in the cellular defense of crustaceans. The activated haemocytes correspondingly produce other bactericidal substances, like H2O2 and superoxide anion (O2−), which may increase disease resistance (Song and Hsieh Citation1994).
Furthermore, in the synthesis of melanin, the phenol oxidase (PO) is an essential enzyme in haemolymph as an inactive pro-enzyme like prophenoloxidase (ProPO). The ProPO is activated and transforms to PO when it reacts with zymosan (carbohydrates from yeast cell walls), bacterial lipopolysaccharide (LPS), urea, calcium ion, and trypsin (Chang et al. Citation2003). Although the exact mechanism by which the Vibrio bacteria affects the phenoloxidase enzyme system in P. monodon is not clear, some studies suggest that infection mediated oxidative stress is responsible for the reduction of phenoloxidase activity (Le Moullac et al. Citation1998). In the present study, shrimps fed on methanolic extract of P. tetratromatica showed significantly higher PO concentrations compared to the other groups (p < .05). The immunological parameters revealed a gradual increase of PO activity with the methanolic extract of both seaweed groups, indicating that the defense systems of the juveniles were enhanced. An increase in PO activity was reported in P. monodon (with WSSV challenge) by administering the beta-glucan (Chang et al. Citation2003) immunostimulant herbs (Citarasu et al. Citation2006), and DNA vaccination (Kumar et al. Citation2008). Extract from a brown algae Laminaria digitata also increased the ProPO activity in brown shrimp Farfantepenaeus californiensis (Hernández-López et al. Citation1996).
Administration of methanolic extracts of green seaweed Caulerpa sertularioides (Esquer-Miranda et al. Citation2016) and ethanolic extract of red seaweed G. fisheri (Kanjana et al. Citation2011) increased the THC, PO activity and superoxide anions of the white shrimp L. vannamei and P. monodon. These extracts improved their immunity against V. parahaemolyticus and V. harveyi, respectively (Kanjana et al. Citation2011; Esquer-Miranda et al. Citation2016). In the present study, increasing of phenol oxidase (PO) activity and superoxide (SO) anions production for an ethanolic and methanolic extract of these two seaweeds incorporated diets seems to act as a promoter for shrimp defense system against the V. parahaemolyticus infection. The methanolic extract of P. tetrastromatica for P. monodon juveniles was correlated with increased total haemocyte counts, PO, and superoxide anion production. All these immune response indices are the components of the antibacterial defense mechanism of crustaceans (Soderhall Citation1999). During bacterial infection, haemocytes ingest pathogens by phagocytosis and eliminate them from the cytoplasm by the production of superoxide anions, hydrogen peroxide, and hydroxyl radicals (Chien et al. Citation2003). Additionally, haemocytes produce superoxide dismutase (SOD) that catalyses superoxide anions to H2O2 to produce the common antibacterial ingredient, hypochlorous acids (Beutler Citation2004). All these improved activities and the increased haemocyte could have improved the antimicrobial activity of the methanol extract and accounted for the ability of the shrimps treated with the extracts to better defend against V. parahaemolyticus.
Conclusions
Successful shrimp production requires effective disease prevention strategies and a good understanding of the primary immune functions. The shrimp defense system involves actions and reactions against its pathogens. The present study documented that the administration of methanolic extract of P. tetrastromatica through enrichment technique increased the defense capacity of P. monodon by increasing THC, phenoloxidase activity (PO), superoxide anion, and resistance against V. parahaemolyticus. It gave a better survival rate and production of shrimps. However, more research is required to determine which compounds are present in the methanolic extracts and which one exactly causes this response.
Acknowledgments
The authors thank William L. Shelton, Biology Department, University of Oklahoma, Norman, OK 73019, United States, for his valuable comments and criticisms on the early draft of this manuscript.
Disclosure statement
The authors declare that they have no conflict of interest. All persons listed as authors have read, contributed, and attested to the validity and legitimacy of the data and agreed to this submission.
Additional information
Funding
References
- Abu-Ghannam N, Rajauria G. 2013. Antimicrobial activity of compounds isolated from algae. In: Dominguez H, editor. Functional ingredients from algae for foods and nutraceuticals. Cambridge (UK): Woodhead Publishing; p. 287–306.
- Aftabuddin S, Kader A, Kamal AM, Zafar M. 2009. Present status on the use of antibiotics and chemicals in shrimp hatcheries and grow-out ponds and their environmental implications in Bangladesh. Aquac Aquar Conserv Legis. 2:369–379.
- AftabUddin S, Roman WU, Hasan CK, Ahmed M, Rahman H, Siddique MAM. 2018. First incidence of loose-shell syndrome disease in the giant tiger shrimp Penaeus monodon from the brackish water ponds in Bangladesh. J Appl Anim Res. 46(1):210–217.
- AftabUddin S, Siddique MAM, Romkey SS, Shelton WL. 2017. Antibacterial function of herbal extracts on growth, survival and immunoprotection in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 65:52–58.
- Alsina M, Blanch AR. 1994a. A set of keys for biochemical identification of environmental Vibrio species. J Appl Bacteriol. 76(1):79–85.
- Alsina M, Blanch AR. 1994b. Improvement and update of a set of keys for biochemical identification of Vibrio species. J Appl Bacteriol. 77(6):719–721.
- Beutler B. 2004. Innate immunity: an overview. Mol Immunol. 40(12):845–859.
- Bulfon C, Volpatti D, Galeotti M. 2015. Current research on the use of plant derived products in farmed fish. Aquac Res. 46(3):513–551.
- Cabrita MT, Vale C, Rauter AP. 2010. Halogenated compounds from marine algae. Mar Drugs. 8(8):2301–2317.
- Caipang CMA, Lazado CC, Berg I, Brinchmann MF, Kiron V. 2011. Influence of alginic acid and fucoidan on the immune responses of head kidney leukocytes in cod. Fish Physiol Biochem. 37(3):603–612.
- Chang CF, Su MS, Chen HY, Liao IC. 2003. Dietary β-1, 3-glucan effectively improves immunity and survival of Penaeus monodon challenged with white spot syndrome virus. Fish Shellfish Immunol. 15(4):297–310.
- Chien YH, Pan CH, Hunter B. 2003. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture. 216(1-4):177–191.
- Chu KB, Ahmad I, SitiZahrah A, Irene J, Norazila J, NikHaiha N, Teoh TP. 2016. Current status of acute hepatopancreatic necrosis disease (AHPND) of farmed shrimp in Malaysia. Proceedings of the ASEAN Regional Technical Consultation on EMS/AHPND and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia; 2016 Feb 22–24; Makati City, Philippines. p. 55–59.
- Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V. 2006. Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol. 21(4):372–384.
- Cruz‐Suárez L, Tapia‐Salazar M, Nieto‐López M, Guajardo‐Barbosa C, Ricque‐Marie D. 2009. Comparison of Ulva clathrata and the kelps Macrocystis pyrifera and Ascophyllum nodosum as ingredients in shrimp feeds. Aqua Nutri. 15:421–430.
- Da Silva RL, Barbosa JM. 2009. Seaweed meal as a protein source for the white shrimp Litopenaeus vannamei. J Appl Phycol. 21(2):193–197.
- Dabu IM, Lim JJ, Arabit PMT, Joi Ann S, Orense B, Tabardillo JA Jr, Corre Mary Beth VL Jr, Maningas B. 2017. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquac Res. 48(3):792–799.
- Dashtiannasab A, Yeganeh V. 2017. The effect of ethanol extract of a macroalgae Laurencia snyderia on growth parameters and vibriosis resistance in shrimp Litopenaeus vannamei. Iranian J Fish Sci. 16:210–221.
- De Schryver P, Defoirdt T, Sorgeloos P. 2014. Early mortality syndrome outbreaks: a microbial management issue in shrimp farming? PLoS Pathog. 10(4):e1003919.
- Deachamag P, Intaraphad U, Phongdara A, Chotigeat W. 2006. Expression of a phagocytosis activating protein (PAP) gene in immunized black tiger shrimp. Aquaculture. 255(1-4):165–172.
- Deris ZM, Iehata S, Ikhwanuddin M, Sahimi MBMK, Do TD, Sorgeloos P, Sung YY, Wong LL. 2020. Immune and bacterial toxin genes expression in different giant tiger prawn, Penaeus monodon post-larvae stages following AHPND-causing strain of Vibrio parahaemolyticus challenge. Aquac Reports. 16:100248.
- Esquer-Miranda E, Nieves-Soto M, Rivas-Vega ME, Miranda-Baeza A, Pi P. 2016. Effects of methanolic macroalgae extracts from Caulerpa sertularioides and Ulva lactuca on Litopenaeus vannamei survival in the presence of Vibrio bacteria. Fish Shellfish Immunol. 51:346–350.
- FAO. 2018. The State of World Fisheries and Aquaculture: meeting the sustainable development goals. Rome (Italy): FAO Fisheries and Aquaculture Department; p. 227.
- GAA (Global Aquaculture Alliance). 2017. Shrimp Production Review. [accessed 2020 May 15]. https://www.aquaculturealliance.org
- Gigi P. 2011. Immune response of Penaeus monodon to the inactivated white spot syndrome virus preparation [dissertation]. Cochin University of Science and Technology.
- Green RJ. 2004. Antioxidant activity of peanut plant tissues [master’s thesis]. North Carolina State University.
- Grenni P, Ancona V, Caracciolo AB. 2018. Ecological effects of antibiotics on natural ecosystems: a review. Microchem. J. 136:25–39.
- Han JE, Kim JE, Jo H, Eun JS, Lee C, Kim JH, Lee KJ, Kim JW. 2019. Increased susceptibility of white spot syndrome virus-exposed Penaeus vannamei to Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease. Aquaculture. 512:734333.
- Hernández-López J, Gollas-Galván T, Vargas-Albores F. 1996. Activation of the prophenoloxidase system of the brown shrimp Penaeus californiensis Holmes. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 113(1):61–66.
- Herrero M, Jaime L, Martín-Álvarez PJ, Cifuentes A, Ibáñez E. 2006. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J Agric Food Chem. 54(15):5597–5603.
- Huang X, Zhou H, Zhang H. 2006. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 20(5):750–757.
- Huxley V, Lipton A. 2009. Immunomodulatory effect of Sargassum wightii on Penaeus monodon (Fab.). Asian J Anim Sci. 4:192–196.
- Ibáñez E, Herrero M, Mendiola JA, Castro-Puyana M. 2012. Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M, editor. Marine bioactive compounds. Boston (MA): Springer; p. 55–98.
- Immanuel G, Vincybai V, Sivaram V, Palavesam A, Marian MP. 2004. Effect of butanolic extracts from terrestrial herbs and seaweeds on the survival, growth and pathogen (Vibrio parahaemolyticus) load on shrimp Penaeus indicus juveniles. Aquaculture. 236(1-4):53–65.
- Ishimaru K, Akagawa-Matsushita M, Muroga K. 1995. Vibrio penaeicida sp. nov., a pathogen of Kuruma prawns (Penaeus japonicus). Int J Syst Bacteriol. 45(1):134–138.
- Ismail A, Ktari L, Ahmed M, Bolhuis H, Boudabbous A, Stal LJ, Cretoiu MS, Bour M. 2016. Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Frontiers Microbiol. 7:1072.
- Johansson MW, Keyser P, Sritunyalucksana K, Söderhäll K. 2000. Crustacean haemocytes and haematopoiesis. Aquaculture. 191(1-3):45–52.
- Joshi J, Srisala J, Truong VH, Chen I-T, Nuangsaeng B, Suthienkul O, Lo CF, Flegel TW, Sritunyalucksana K, Thitamadee S. 2014. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture. 428-429:297–302.
- Kanjana K, Radtanatip T, Asuvapongpatana S, Withyachumnarnkul B, Wongprasert K. 2011. Solvent extracts of the red seaweed Gracilaria fisheri prevent Vibrio harveyi infections in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 30(1):389–396.
- Karunasagar I, Shivu MM, Girisha SK, Krohne G, Karunasagar I. 2007. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture. 268(1-4):288–292.
- Kimbrell DA, Beutler B. 2001. The evolution and genetics of innate immunity. Nat Rev Genet. 2(4):256–267.
- Kumar SR, Ahamed VI, Sarathi M, Basha AN, Hameed AS. 2008. Immunological responses of Penaeus monodon to DNA vaccine and its efficacy to protect shrimp against white spot syndrome virus (WSSV). Fish Shellfish Immunol. 24(4):467–478.
- Lavanya R, Veerappan N. 2011. Antibacterial potential of six seaweeds collected from gulf of Myanmar of southeast coast of India. Adv Biol Res. 5:38–44.
- Lavilla-Pitogo CR, Leaño EM, Paner MG. 1998. Mortalities of pond cultured juvenile shrimp Penaeus monodon associated with dominance of luminescent Vibrios in the rearing environment. Aquaculture. 164(1-4):337–349.
- Le Moullac G, Soyez C, Saulnier D, Ansquer D, Avarre JC, Levy P. 1998. Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol. 8(8):621–629.
- Leobert D, Cabillon NA, Catedral DD, Amar EC, Usero RC, Monotilla WD, Calpe AT, Fernandez DD, Saloma CP. 2015. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis Aqua Organisms. 116(3):251–254.
- Li F, Xiang J. 2013. Recent advances in researches on the innate immunity of shrimp in China. Dev Comp Immunol. 39(1-2):11–26.
- Lightner DV. 1993. Disease of cultured penaeid shrimp. In: McVey JP, editor. CRC handbook of mariculture. 2nd ed., Vol. 1, Crustacean aquaculture. Boca Raton (FL): CRC Press; p. 393–486.
- Lightner DV. 2012. Early mortality syndrome affects shrimp in Asia. Global Aquaculture Advocate.
- Loof TG, Schmidt O, Herwald H, Theopold U. 2011. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: the same side of two coins? J Innate Immun. 3(1):34–40.
- Maningas MBB, Kondo H, Hirono I. 2013. Molecular mechanisms of the shrimp clotting system. Fish Shellfish Immunol. 34(4):968–972.
- Pandey K. 2012. Potent bioactive compounds from the ocean: some interesting aspects and applications. Pharmacognosy Journal. 4(27):1–5.
- Pui CF, Bilung LM, Zin NBM, Abidin NNBZ, Vincent M, Apun K. 2014. Risk of Acquiring Vibrio parahaemolyticus in Water and Shrimp from an. Aquaculture Farm. Kuroshio Science. 8(1):59–62.
- Raa J. 1996. The use of immunostimulatory substances in fish and shellfish farming. Rev Fish Sci. 4(3):229–288.
- Redo M, Rios J, Villar A. 1989. A review of some antimicrobial compounds isolated from medicinal plants reported in the literature 1978–1988. Phytother Res. 3(4):117–125.
- Reverter M, Bontemps NT, Sasal P, Saulnier D. 2017. Use of medicinal plants in aquaculture. In: Austin B, Newaj-Fyzul A, editors. Diagnosis and control of diseases of fish and shellfish. Hoboken (NJ): Wiley; p. 223–261.
- Sachdev K. 1983. Clinical pathology and clinical bacteriology. New Delhi (India): Jaypee Brother; p. 133.
- Sakai M. 1999. Current research status of fish immunostimulants. Aquaculture. 172(1-2):63–92.
- Selvin J, Lipton AP. 2004. Biopotentials of Ulva fasciata and Hypnea musciformis collected from the peninsular coast of India. J Mar Sci Tech. 12:1–6.
- Silver LL, Bostian K. 1993. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicrob Agents Chemother. 37(3):377–383.
- Sinurat E, Saefudin E, Peranginangin R, Pws SH. 2016. Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vannamei. J Appl Phar Sci. 6(10):82–91.
- Soderhall K. 1999. Invertebrate immunity-editorial. Dev Comp Immunol. 23:263–266.
- Söderhäll K, Cerenius L. 1998. Role of the prophenoloxidase-activating system in invertebrate immunity. Current Opinion in Immunology, 10(1):23-28.
- Song YL, Hsieh YT. 1994. Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol. 18(3):201–209.
- Thanigaivel S, Vijayakumar S, Mukherjee A, Chandrasekaran N, Thomas J. 2014. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture. 433:467–475.
- Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV. 2013. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Org. 105(1):45–55.
- Valgas C, Souza SMd, Smânia EF, Smânia A. Jr 2007. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 38(2):369–380.
- Vaseeharan B, Thaya R. 2014. Medicinal plant derivatives as immunostimulants: an alternative to chemotherapeutics and antibiotics in aquaculture. Aquacult Int. 22(3):1079–1091.
- Yeh ST, Chen JC. 2009. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed earlier recovery in immunity after a Vibrio alginolyticus injection. Fish Shellfish Immunol. 26(5):724–730.
- Yeh ST, Lee CS, Chen JC. 2006. Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 20(3):332–345.
