 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of the present study was to determine the dietary effect of different growth stages of rapeseed (Brassica rapa L.) on nutrient intake and digestibility, nitrogen balance, and rumen fermentation kinetics in sheep. Four dietary treatments were utilised. A basal control diet based on alfalfa hay, oat hay, soybean meal and corn grain. Then alfalfa hay was replaced with 300 g/kg DM of rapeseed forage harvested at three different growth stages: Vegetative, Flowering and Pod. In vitro gas production was determined using three rumen cannulated Suffolk sheep in a completely randomised design, and nutrients intake and digestibility of each diet were determined using four Suffolk sheep in a 4 × 4 Latin square design with 21 d periods consisting of 14 d for diet adaptation and 7 d for sample collection. Feed intake and excretion of faeces and urine were recorded. Dry matter intake was higher for control and Pod compared to Vegetative and Flowering. The digestibility of dry matter, organic matter, neutral detergent fibre and acid detergent fibre were similar among treatments. Nitrogen intake was higher for control and Pod and lower for Vegetative and Flowering. In vitro gas production was similar among treatments (P > .05). In vitro gas yield at 24 h was higher (P < .05) for control than the rest of the treatments. Overall, inclusion of 300 g/kg DM of rapeseed forage harvested at pod stage as a substitute for alfalfa hay is an alternative source of protein without affecting nutrient intake and digestibility.
Effect of different growth stages of rapeseed on nutrient intake and digestibility was determined
Nutrient digestibility was similar between growth stages of rapeseed
In vitro gas production was similar between growth stages of rapeseed
Highlights
Introduction
Sustainable animal diets (StAnD) is a concept based on three dimensions: planet, people and profit, which among other objectives, promotes the use of local feed resources (Makkar and Ankers Citation2014). In this regard, the use of Brassica species in temperate grazing systems (Seguel et al. Citation2020) can be an alternative forage source (Barry Citation2013). These crops can be from Brasicca rapa and Brassica napus which are characterised by their high contents of crude protein (around 30% CP), high contents of metabolisable energy (around 12 MJ/kg DM) and low contents of neutral detergent fibre (around 17% NDF) compared to more common forage crops for ruminant feed such as perennial ryegrass pastures (Kaur et al. Citation2009). In the case of Brassicas, its chemical composition changes depending on its growth stage, year season and cultivars (Cartea et al. Citation2008). For example, rapeseed follows dry matter (DM) and nitrogen (N) uptake patterns similar to wheat, where the higher contents of DM and N accumulation will be observed between the beginning of stem elongation and/or branching and the end of flowering (Koenig et al. Citation2011). Until now, feeding rapeseed forage at different stages of digestibility or maturity has not been fully described in ruminant nutrient partitioning.
It is important to note that Brassica species contain compounds known to cause erythrocyte oxidant damage, including methaemoglobin formation in ruminants (Smith Citation1978). This occurs as a result of sulphur-containing compounds in plant tissues and their conversion to toxic products by rumen microorganisms. Also, Brassica species by-products such as canola meal (7-25 µmol/g) and rapeseed meal (7–210 µmol/g) contain glucosinolates (Barry Citation2013; Cartea et al. Citation2008). Glucosinolates have been shown to reduce feed intake and amino acid digestibility in animals (Tripathi and Mishra Citation2007).
In temperate regions, feeding cows with forage Brassica species has been shown to be an effective alternative to the use of perennial grass pastures such as rye grass maintaining milk yield and without impairing milk components (Seguel et al. Citation2020). In Australia, feeding forage Brassica to sheep was reported (Williams et al. Citation2016) to be an alternative to the use of perennial ryegrass, as it promoted increases in milk yield, and increased contents of milk fat and milk protein. Also, from an environmental point of view, feeding sheep with forage rape could be an alternative to reduce methane emissions (Sun et al. Citation2016).
Until now, no reports have been published on the use of Brassica species and their different vegetative stages, more specifically forage rapeseed in central Mexico, where this forage material is readily available and can be a low-cost and viable alternative for sheep feeding. In temperate regions of Mexico, farmers traditionally produce Brassica species (Vieyra-Odilon and Vibrans Citation2001), which are harvested at the beginning of flowering for collection of tender leaves, tender stems and flowers (Bye and Linares Citation2000). Moreover, effects on rumen fermentation kinetics and nutrient utilisation from sheep fed with rapeseed at different growth stages remains unclear. Thus, the objective of the present study was to determine the effect of different growth stages of rapeseed (Brassica rapa L.) as hay on nutrient intake, nutrient digestibility, nitrogen balance and rumen fermentation kinetics in sheep diets. This objective was achieved by replacing part of the forage in the diets with forage rapeseed at different growth stages. We hypothesised that early vegetative stages of forage rapeseed will result in higher nutrient intake and higher digestibility compared to late stages of growth.
Materials and methods
Rapeseed crop and sampling conditions
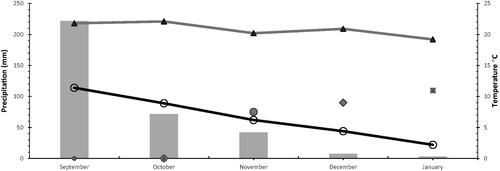
Forage samples of Brassica rapa L. (rapeseed) were collected from San Pedro Tlanixco, municipality of Tenango del Valle, State of Mexico. The location is 2840 m above sea level, between the coordinates 19°04′ north latitude and 99°32′ west longitude. According to the Köppen climate classification, the climate is humid temperate, Cb (w2) (w) (i') (g), with summer rains and little thermal oscillation (Figure ) (CONAGUA Citation2015).
Figure 1. Temperature (min ○, max ▴) and precipitation (grey bars) in San Pedro Tlanixco, Mexico, during the growing season of forage rapeseed collected at Vegetative (•), Flowering (♦) or Pod (▪) growth stages.
The soil from the study site had a clay soil texture with 62% sand, 10% silt and 28% clay. The land had a slope of 2 to 6%. The dominant rocks were of volcanic and clastic type. Soils were pelic vertisol type and haplic phaeozem (WRB Citation2006), and they were characterised by being very compact and clayish; similarly, they exhibited wide and deep cracks in the epoch of drought and showed a layer of tepetate (interstratified volcanic banks) between 10 and 50 cm of depth. It had a pH of 5.8, 23.8 cmol+/kg of capacity of cationic interchange, 0.31% of total nitrogen, 7.56% of organic matter and 0.8 dS/m of electrical conductivity (Vaca García et al. Citation2014).
The seeds used for rapeseed were harvested in May 2013 from maize and potato fields, where the plant grew as native forage (Vieyra-Odilon and Vibrans Citation2001). Rapeseed seeds were planted in October 2013. Sowing was done manually using 6 kg/ha of rapeseed seed, for a dry matter (DM) yield of 8132 kg/ha, with a DM concentration in the forage of 320 g/kg and an estimated germination percentage between 70 and 80%. A total area of 530 m2 was divided into three plots previously fertilised with sheep manure. For the study, the vegetative stage (Vegetative) refers to the first flower buds and tender stems harvested at 70 d after cropping. The flower (Flowering) was harvested with 80% plants flowering at 90 d, and pods (Pod) were harvested when the plants contained 80% of their pods reaching their final size at 110 d. Samples from Vegetative, Flowering and Pod stages (100 kg of each stage) were collected from the whole plot and dried for 5 days at room temperature. Then, samples were ground in a hammer mill (5 mm Ø) for later use.
Experimental procedures
In vivo trial: animals and diets
Animal care and procedures were carried out according to the guidelines of the Animal Care and Use Committee of Universidad Autónoma del Estado de México (project code UAEM 4335/2017). Four Suffolk sheep were used, with a mean age of 10 months and 47.8 ± 4 kg of body weight. Animals were arranged in a 4 × 4 Latin square design with 21 d periods consisting of 14 d for diet adaptation and 7 d for sample collection. Animals were weighed at the beginning of the study and at the end of each experimental period.
Individual ingredients (Table ) and four dietary treatments were evaluated (Table ). A basal or control diet was based on alfalfa hay, oat hay, soybean meal, and corn grain (Table ). Then, alfalfa hay was replaced with 300 g/kg of dried forage rapeseed at three different growth stages and fed as Vegetative, Flowering and Pod. Control diet was formulated to contain 150 g/kg CP and 2.7 Mcal ME/kg DM according to the nutritional requirements of growing sheep (NRC Citation2007) (Table ). Animals were housed in individual metabolic cages (1.20 × 0.80 m), fed individually twice a day (08h00 and 15h00), with free access to water.
Table 1. Chemical composition (g/kg DM) of the ingredients used in the diets with inclusion of rapeseed (Brassica rapa) at different growing stages.
Table 2. Contents of ingredients and chemical composition (g/kg DM) of dietary treatments from sheep fed with 300 g/kg DM of forage rapeseed at vegetative, flowering or pod growth stages.
Feed intake, refusals and water intake was measured daily but only data from the last 7 days of each period were accounted for statistical analysis. During the last 7 d of each period, samples of faeces and urine were collected daily and 10% of the total samples for faeces and urine were frozen at −20 °C for further analysis.
In vitro trial: gas production and fermentation kinetics
Animal care and procedures for extraction of rumen inoculum were approved by the Ethics Committee for Animal Experimentation (UAEM 4335/2017). Care and management of animals was according to UAEMex Policy for Animal protection of animals used for experimental and scientific purposes.
Three fistulated sheep (40 ± 3 kg of body weight) were used as donors of rumen fluid and fed with the same experimental basal diet (Table ) with free access to water. Ruminal contents (500 mL of liquid and semisolid phases) were placed in thermos flasks and immediately transferred to the laboratory for incubation. To determine the kinetics of ruminal fermentation, three incubation runs (96 h) were carried out, in a water bath at 39°, according to Theodorou et al. (Citation1994).
After rumen fluid extraction, samples were mixed and filtered through a triple layer of gauze, and homogenised with CO2 for five minutes. A total of 16 bottles per run were incubated, with three bottles per treatment, two bottles without substrate (considered as blanks of inoculum), and two bottles with known substrate (barley hay) (considered as standard for correction purposes). Incubation data where differences between the mean gas production of standard obtained and the standard value were higher than 10% were not included in the analysis. Then 800 g/DM of each diet mixture were incubated in glass bottles of 125 mL, and incubation runs were performed. In each incubation run, the bottles were filled under anaerobic conditions with 10 mL of rumen inoculum and 90 mL of a buffer solution (Theodorou et al. Citation1994). In 1 L, this solution consisted of 238 mL/L of buffer solution (14 g NaHCO3 and 1.5 g (NH4)HCO3 per L), 238 mL/L of a macro mineral solution (5.7 g Na2HPO4, 6.2 g KH2PO4 and 0.6 g MgSO4.7H2O per L), 474 mL/L of distilled water, 0.1 mL/L of micro minerals (13.2 g CuCl22H2O, 10.0 g MnCl24H2O, 1.0 g CoCl26H2O, 8.0 g FeCl26H2O and made up to 100 mL with H2O) and 50 mL/L of a reduction solution (47.5 mL distilled water, 2 mL of 1 N NaOH and 313 mg HCl-cysteine), and resazurin (phenoxazine dye).
Bottles were filled with the incubation solution under a CO2 stream, sealed and incubated for 96 h in a water bath at 39 °C. The gas volume was recorded at 3, 6, 9, 12, 24, 36, 48, 72 and 96 h of incubation in three different runs for incubations. The pressure produced in each bottle was measured with a HD8804 manometer provided with a TP804 pressure gauge (Delta OHM, Caselle di Selvazzano, Italy). Readings corrected for atmospheric pressure were converted to volume (mL) using a pre-established linear regression developed in our laboratory [mL gas = (2.7384x) − 0.0243, n = 45, R2 = 0.994, where x = psi hour] and expressed as a unit of incubated dry matter.
Calculations
The accumulated gas volume of each of the samples (diets) was adjusted to the model proposed by France et al. (Citation1993):
where: "Y" is the cumulative gas production (mL), "t" is the incubation time (hours), A is the asymptote curve (total gas produced, mL), B (h−1) and C (h-½) are the gas production constants, T is the time of delay (hours) for microbial colonisation in order to begin fermentation.
After in vitro incubation periods, these bottles were opened, and the whole incubation content was filtered and dried (48 h, 60 °C) to measure the dry matter disappearance (DMD96h), and gas yield production at 24 h (GY24) was determined. The volume of gas (mL gas/g DM) produced after 24 h of incubation was estimated by dividing the amount of DMD (g): Gas production (GY24) = mL gas 24 h/g DMD (González Ronquillo et al. Citation1998).
The partitioning factor at 96 h of incubation (PF96; a measure of fermentation efficiency) was estimated as the ratio of in vitro DMD (DMD, mg) to the volume (mL) of GP at 96 h (i.e. DMD/total gas production (GP96)) according to Blümmel et al. (Citation1997).
Analytical procedures
All chemical analysis were performed in triplicate. Feed orts, faecal samples, individual feedstuffs and diets were dried in a forced air oven (60 °C, 48 h) an analysed for dry matter (DM), then ground in a mill (Wiley Mill, 2 mm Ø Arthur H. Thomas Philadelphia, PA) to determine organic matter (OM; 942.05) AOAC (Citation1990). Total nitrogen (N; 954.01) was determined by the Kjeldahl method (AOAC Citation1990) multiplied by 6.25 for the crude protein content, acid detergent fibre (ADF) and lignin were determined according to Van Soest et al. (Citation1991). Neutral detergent fibre (aNDF) content was determined as described by Van Soest et al. (Citation1991) using thermostable alpha-amylase without sodium sulphite and corrected for residual ash (Mertens Citation2002) using an Ankom 200 Fibre Analyser (Ankom Technology, New York). Ingredients and total mixed rations components were analysed for ether extract (method 920.39, AOAC Citation1997), while non-structural carbohydrates (NSC) were calculated as follows: NSC = [100-(NDF + CP + Ash + Fat)]. Chemical composition of diets is shown in Table .
Faecal and urine samples were used to determine nitrogen excretion. Faecal and urine samples were subjected to nitrogen (N; 991.20) determination (AOAC Citation1990). Nutrient digestibility coefficients (Wiseman Citation2018) were determined as: Digestibility = (nutrient intake – nutrient excreted)/(nutrient intake)
Statistical analysis
For the in vivo experiment, a 4 × 4 Latin square design (SAS, Statistical Analysis System Institute Citation1999) was used with the following model:
where Yij = is each observation, μ is the general mean; Ai is the effect due to the animal, Txi (i = 4) is the treatment effect; Pk is the effect due to the period and εij is experimental error. A P < 0.05 value was considered as a significant difference between treatments. The Tukey test was used when significant differences were observed between treatments p < .05.
A completely randomised design was used to measure chemical composition, in vitro gas production parameters and in vitro fermentation. Diet (n = 4) was considered as factor and the incubation series (n = 3) as a block. For total gas production (96 h) and DMD, the experimental unit was the average of the three bottles per treatment incubated for 96 h within the same run.
where Yij = is each observation of treatments it; μ is the general mean; Tx (i = 4) is the treatment effect; and εij is the experimental error. Tukey test was used when significant differences were observed between treatments p < .05.
Results
Intake, digestibility, and nitrogen balance
Nutrient intake and digestibility are shown in Table . Live metabolic weight were marginal (P = .09) for Vegetative and Pod, compared with the rest of the treatments. Water intake was similar between treatments (P > .05). Intakes of dry matter, organic matter and neutral detergent fibre were higher P < .05) for control and Pod, compared with Flowering and Vegetative. Ether extract intake was greater (P <.05) in Pod than the rest of the treatments. Intake values of non-structural carbohydrates, neutral detergent fibre, acid detergent fibre and metabolisable energy were higher (P < .05) in control and Pod. Digestibility coefficients of dry matter, organic matter, acid detergent fibre and neutral detergent fibre were similar among treatments. Nitrogen intake was higher (P < .001) for control. Nitrogen excretion in faeces and urine and N balance was similar between treatments (Table ).
Table 3. Intake (g/LW0.75/day) and digestibility coefficient from sheep fed with 300 g/kg DM of forage rapeseed at vegetative, flowering or pod growth stages.
Table 4. Nitrogen retention (g N/day, %) from sheep fed with 300 g/kg DM of forage rapeseed at vegetative, flowering or pod growth stages.
In vitro gas production and fermentation kinetics
With regard to in vitro fermentation parameters for dietary treatments (Table ), fermentation rates B (h−1) and C (h-½), and lag time were similar between treatments. Dry matter disappearance at 96 hours was lower (p < .05) for control compared with Flowering and Pod. Gas production at 48 hours (GP48) tended (P = .08) to be higher for control and Flowering, whereas gas yield at 24 h (GY24h) was higher (p < .05) for control (297 ml gas/g DMD) than the rest of the treatments (218 ± 9.0 ml gas/g DMD).
Table 5. In vitro rumen gas kinetics (mL gas/ g DM) and fermentation profile from sheep fed with 300 g/kg DM of forage rapeseed at vegetative, flowering or pod growth stages.
Discussion
With regard to dietary treatments chemical composition, Pod had the lowest crude protein content compared to Vegetative and Flowering, and this agrees with previous research (Liu et al. Citation2016; Espinoza-Canales et al. Citation2017) showing that rapeseed seeds and husk increases fibre content and decreases the concentration of crude protein and amino acids. Also, ether extract contents in Pods were higher, which was expected as with flowering, the formation and grain filling begins. It is important to consider that the decline in nutritional quality is linked to advance growth where the leaf:stems ratio decreases and lignin and cell wall in stems increases in Brassica napus (Kaur et al. Citation2011).
Crude protein contents were similar in Vegetative and Flowering; however, in Pod, CP contents were slightly lower. Although, control diet was theoretically formulated to fulfil the nutritional requirements of growing sheep with an average daily gain of 250 g/d (NRC, Citation2007), replacing alfalfa hay with 300 g/kg of forage rapeseed in the treatment diets was not enough to balance CP in Pod treatment, with less protein (38gPC/kg DM) than the control diet. It is known that CP varies depending on the growth stage of forage rapeseed (Koenig et al. Citation2011). The CP content of rapeseed at their different vegetative stages decreases across maturity stage, compared with alfalfa or other leguminous plants this could range from 160 to 190 g/kg DM (NRC Citation2007), except for rapeseed pod with CP ranged in 119 g/kg DM, similar to other forages as ryegrass or barley hay at maturity stage (NRC Citation2007). Therefore, our data demonstrated that rapeseed forage could be used to supply adequate CP to meet maintenance or growth requirements in sheep diets (NRC Citation2007).
In vivo trial: intake, digestibility, and nitrogen balance
Previous studies have reported and observed reduced growth in sheep fed with high contents of glucosinolates in sheep fed with mustard oil (Tripathi et al. Citation2004), rapeseed and safflower meals (Thomas et al. Citation1984). The content of glucosinolates varies according to the development of the plant parts, such as roots, flowers, leaves, pods (Suzuki et al. Citation2006; Mohn et al. Citation2007, Barry Citation2013). In early vegetative states, more glucosinolates are retained in the reproductive organs, including seeds, flowers and pods, which are expected to have the highest concentrations of these defense compounds (Brown et al. Citation2003). Newkirk et al. (Citation2003) found that in addition to the toxic effect of glucosinolates, these are found in greater quantity in wild plants of this genus and their bitter taste causes a reduction in feed intake. This was also noted in the present study; however, DM intake was higher than that reported by Bastida Gacia et al. (Citation2011), who used other alternative forage sources such as field pea hay or barley hay. This may be partly explained by the fact that in the present study, the diets contained more than 12% CP, which allowed a higher ingestion of N and a greater synthesis of microbial protein production, and thereby improving nutrient utilisation and intake.
In this study, rapeseed forage increased concentrations of NDF, ADF and lignin, and this was related to advanced growth stages from vegetative to pod. This is because NDF digestibility is a function of the potentially digestible fraction of dietary fibre and its rate of digestion and rate of passage (Oba and Allen Citation1999). The increase in DMI in Pod enhanced NDF digestibility, because forages with high NDF digestibility have shorter rumen retention times, allowing greater DMI at the expense of NDF digestibility. Although ADF and NDF are indicators of fibre contents in forages, they do not directly indicate how digestible the dietary fibre is. In general, an increased NDF digestibility will result in higher digestible energy and forage intake. In this regard, it has been suggested that a one-unit increase in NDF digestibility will be associated with a 0.17 kg increase in DMI in dairy cows (Oba and Allen Citation1999).
Our data showed that compared to control Vegetative, Flowering and Pod decreased N intake while Pod had the highest content of microbial protein. According to Nousiainen et al. (Citation2004), the increase of dietary protein is the most important nutritional factor influencing milk urea N efficiency and this could be used as a diagnostic of protein feeding in ruminants and used to predict urinary N excretion. In this study, based on N balance, rapeseed at Pod and Flowering stage could be used to supply dietary crude protein, which later is used for microbial protein production and secreted as milk protein or growing performance.
In vitro gas production and fermentation kinetics
In vitro digestibility parameters were similar to Kaur et al. (Citation2011) who reported that the age of brassicas such as Brassica napus, is a factor that affects rumen degradation due to lignification. Similarly, Chapoutot and Sauvant (Citation1997), who determined the nutritional value of raw and extruded pea-rapeseed blends in ruminants, reported decreases in organic matter digestibility with extrusion. Sun et al. (Citation2012) obtained lower values for apparent total tract nutrient’s digestibility, when sheep were fed fresh brassicas (Brassica spp.) compared to perennial ryegrass. They reported that there was a functional alteration of the rumen, which led to high concentrations of propionate, affecting acetate:propionate ratio, and thereby reducing available hydrogen for the methane production. In the present study, compared to the control group, the different vegetative stages of forage rapeseed yielded lower gas production (GY24h) and therefore, one promising research line is to analyse at which growth stage of rapeseed will be best for reducing methane production without disturbing overall animal performance.
Glucosinolates are present in all brassicas and are responsible for the decrease in feed intake (Barry Citation2013) which coincides with the vegetative and flowering diets in the present study. Vincent et al. (Citation1990) did not find an effect on dry matter intake in brassica-fed sheep, but observed an increase in thyroid weight, which is a symptom of iodine deficiency caused by glucosinolates (Tripathi and Mishra Citation2007). These effects could be due to a longer exposure time in the consumption of these compounds and the amount ingested, especially at vegetative and flowering stages, thus, results from the present study should be taken with caution and it is suggested to further investigate their long-term effects.
Conclusions
Based on the digestibility and estimated nutrient supply, the inclusion (up to 300 g/kg DM) of forage rapeseed at the pod stage was a suitable replacement for alfalfa hay in sheep diets. Therefore, inclusion of rapeseed pods in sheep diets can be a viable alternative for small-scale farmers and thereby optimise the use of fodder resources since rapeseed pods have no negative effects on nutrient intake and digestibility. Data from this study will be useful for farmers looking for the precise growth stage of forage rapeseed for sheep feeding.
Ethical approval
The protocol used in this experiment approved by the Animal Care and Use Committee of Universidad Autónoma del Estado de México (project code UAEM 4335/2017).
Acknowledgments
Amelia Zetina Sánchez thanked the National Council for Science and Technology (Conacyt, Mexico) for the scholarship for her studies at the Universidad Nacional Autónoma de México. Lizbeth Esmeralda Robles Jimenez was awarded a Conacyt-Mexico scholarship during her PhD program at the Agricultural Sciences and Natural Resources at the Universidad Autónoma del Estado de México. During the study, Dr. Einar Vargas-Bello-Pérez was a visiting scholar also supported by project number 4974/2020CIB from Universidad Autónoma del Estado de México.
Disclosure statement
The authors declare there are no conflicts of interest.
Additional information
Funding
References
- AOAC 1990. Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed. Washington, DC, USA: Association of Official Analytic Chemist.
- AOAC 1997. Association of Official Analytical Chemists. Official Methods of Analysis. 16th ed.Arlington, VA: Association of Official Analytical Chemists.
- Barry TN. 2013. The feeding value of forage brassica plants for grazing ruminant livestock. Anim Feed Sci Tech. 181(1-4):15–25.
- Bastida Garcia JL, González-Ronquillo M, Domínguez Vara IA, Romero-Bernal J, Castelán Ortega O. 2011. Effect of field pea (Pisum sativum L.) level on intake, digestion, ruminal fermentation and in vitro gas production in sheep fed maintenance diets. Anim Sci J. 82(5):654–662.
- Blümmel M, Steingaβ H, Bec Ker K. 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br J Nutr. 77(6):911–921.
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochem. 62(3):471–481.
- Bye R, Linares E. 2000. Los quelites, plantas comestibles de México: una reflexión sobre el intercambio cultural. CONABIO. Biodiversitas. 31:11–14.
- Cartea ME, Velasco P, Obregón S, Padilla G, de Haro A. 2008. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry. 69(2):403–410.
- Chapoutot P, Sauvant D. 1997. Nutritive value of raw and extruded pea-rapeseed blends for ruminants. Anim Feed Sci Tech. 65(1-4):59–77.
- CONAGUA 2015. Comisión nacional del agua. Informe (2015) Sistema Nacional de Información del Agua (SINA). http://www.conagua.gob.mx/Contenido.aspx?n1=3&n2=60&n3=60. [Accessed on 5th of august 2020]
- Espinoza-Canales A, Gutiérrez-Bañuelos H, Sánchez-Gutiérrez RA, Muro-Reyes A, Gutiérrez-Piña FJ, Corral-Luna A. 2017. Calidad de forraje de canola (Brassica napus L.) en floraciones temprana y tardía bajo condiciones de temporal en Zacatecas. RMCP. 8(3):243–248.
- France J, Dhanoa MS, Theodorou MK, Lister SJ, Davies DR, Isac DA. 1993. Model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J Theo Biol. 163(1):99–111.
- González Ronquillo M, Fondevila M, Barrios Urdaneta A, Newman Y. 1998. In vitro gas production from buffel grass (Cenchrus ciliaris L.) fermentation in relation to the cutting interval, the level of nitrogen fertilisation and the season of growth. Anim Feed Sci Tech. 72(1-2):19–32.
- Kaur R, Garcia SC, Fulkerson WJ. 2009. Feeding time and sequence of forage rape and maize silage does not affect digestibility and rumen parameters in sheep. Anim Prod Sci. 49(4):318–325.
- Kaur R, Garcia SC, Fulkerson WJ, Barchia IM. 2011. Degradation kinetics of leaves, petioles and stems of forage rape (Brassica napus) as affected by maturity. Anim Feed Sci Tech. 168(3-4):165–178.
- Koenig RT, Hammac WA, Pan WL. 2011. Canola growth, development, and fertility. Washington State University Extension. Pullman, W.A. USA. 8pp.
- Liu Y, Jaworski N, Rojas O, Stein H-H. 2016. Energy concentration and amino acid digestibility in high protein canola meal, conventional canola meal, and in soybean meal fed to growing pigs. Anim Feed Sci Tech. 212:52–62.
- Makkar HP, Ankers P. 2014. Towards sustainable animal diets: a survey-based study. Anim Feed Sci Tech. 198:309–322.
- Mertens DR. 2002. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 85:1217–1240.
- Mohn T, Cutting B, Ernst B, Hamburger M. 2007. Extraction and analysis of intact glucosinolates-a validated pressurized liquid extraction/liquid chromatography-mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. J Chromatogr A. 1166(1-2):142–151.
- Newkirk RW, Classen HL, Scott TA, Edney MJ. 2003. The digestibility and content of amino acids in toasted and non-toasted canola meals. Can J Anim Sci. 83(1):131–139.
- NRC. 2007. National Research Council. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. The National Academies Press. Washington, D.C. USA. 392 pp.
- Nousiainen J, Shingfield KJ, Huhtanen P. 2004. Evaluation of milk urea nitrogen as a diagnostic of protein feeding. J Dairy Sci. 87(2):386–398.
- Oba M, Allen MS. 1999. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: effects on dry matter intake and milk yield of dairy cows. J Dairy Sci. 82(3):589–596.
- SAS, Statistical Analysis System Institute 1999. Statistical Analysis System Institute Inc. SAS/STAT User’s Guide, Cary, North Carolina, USA.
- Seguel G, Keim JP, Vargas-Bello-Pérez E, Geldsetzer-Mendoza C, Ibáñez RA, Alvarado-Gilis C. 2020. Effect of forage brassicas in dairy cow diets on the fatty acid profile and sensory characteristics of Chanco and Ricotta cheeses. J Dairy Sci. 103(1):228–241.
- Smith RH. 1978. S-methylcysteine sulphoxide, the brassica anaemia factor (a valuable dietary factor for man?). Vet Res Commun. 2(1):47–61.
- Sun X, Pacheco D, Luo D. 2016. Forage brassica: a feed to mitigate enteric methane emissions? Anim Prod Sci. 56(3):451–456.
- Sun XZ, Waghorn GC, Hoskin SO, Harrison SJ, Muetzel S, Pacheco D. 2012. Methane emissions from sheep fed fresh brassicas (Brassica spp.) compared to perennial ryegrass (Lolium perenne). Anim Feed Sci Tech. 176(1-4):107–116.
- Suzuki C, Ohnishi-Kameyama M, Sasaki K, Murata T, Yoshida M. 2006. Behavior of glucosinolates in pickling cruciferous vegetables. J Agric Food Chem. 54(25):9430–9436.
- Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Tech. 48(3-4):185–197.
- Thomas VM, Katz RJ, Auld DL, Peterson CL. 1984. Value of mechanically extracted rape and safflower oilseed meals as protein supplements for growing lambs. Anim Feed Sci Tech. 11(4):269–277.
- Tripathi MK, Mishra AS. 2007. Glucosinolates in animal nutrition: A review. Anim Feed Sci Tech. 132(1-2):1–27.
- Tripathi MK, Santra A, Chaturvedi OH, Karim SA. 2004. Effect of sodium bicarbonate supplementation on ruminal fluid pH, feed intake, nutrient utilization and growth of lambs fed high concentrate diets. Anim Feed Sci Tech. 111(1-4):27–39.
- Vaca García VM, Martínez Villanueva JJ, González Huerta A, Morales Rosales EJ, Zamudio González B, Gutiérrez Rodríguez F. 2014. Compactación de un vertisol bajo tres sistemas de labranza en maíz (Zea mays L.). Rev Mex Cienc Agríc. 5(8):1495–1507.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods of dietary, neutral detergent fiber and non starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74(10):3583–3597.
- Vieyra-Odilon L, Vibrans H. 2001. Weeds as crops: The value of maize field weeds in the valley of Toluca. Econ Bot. 55(3):426–443.
- Vincent IC, Thompson J, Hill R. 1990. The voluntary feed intake and weight gain of lambs given compound concentrate feeds containing rapeseed meal with a range of glucosinolate contents. Proc Br Soc Anim Prod (1972)). 1990:142–142.
- Williams SRO, Moate PJ, Deighton MH, Hannah MC, Wales WJ, Jacobs JL. 2016. Milk production and composition, and methane emissions from dairy cows fed lucerne hay with forage brassica or chicory. Anim Prod Sci. 56(3):304–311.
- Wiseman J. 2018. Editorial: Digestibility and degradability in animal nutrition studies. J Agric Sci. 156(10):1161–1162.
- WRB 2006. International Union of Soil Science – International Soil Reference and Information Centre and Food and Agriculture Organization of the United Nations (IUSS Working Group WRB. IUSS-ISRIC and FAO). 2006. World reference base for soil resources. A framework for international classification, correlation and communication. 2nd edition. World Soil Res Rep No. 103. Rome, Italy. 114 p.
