Abstract
Non-esterified fatty acids (NEFAs) and Insulin-like growth factor-I (IGF-1) concentrations are modified after the induction of a "male effect". The present study examined the effect of the introduction of males into a group of females that were previously isolated from males, during different phases of the sexual cycle, to determine the changes to the NEFAs and IGF-1 concentrations. Sixty-four does were divided into six groups. The males were introduced with the females at different points after sponge removal. Introduction was carried out 48 h (n = 10, Group 48H), 72 h (n = 12, Group 72H), 4 days (n = 10, Group 4 D), 13 days (n = 10, Group 13 D) or 18 days after sponge removal (n = 10, Group 18 D), and a control group was implemented that had no contact with males (n = 12, Control Group). Plasma samples were taken every day to determine daily progesterone (P4) and NEFAs concentrations, and every second day for IGF-1 determination. No effects from the experimental groups were observed on the mean NEFAs or IGF-1 concentrations (p > .05). No differences between the time before male introduction and after male introduction were observed on the NEFAs concentrations (p > .05) or IGF-1 concentrations (p > .05). On the whole, only differences were observed in the NEFAs concentrations between the follicular and the luteal phases (9.48 ± 0.38 vs 8.15 ± 0.15 mg/dL for follicular and luteal phases, respectively, p < .01). The results of the present experiment demonstrated that the introduction of sexually active males at different moments of the oestrous cycle does not modify the NEFAs or the IGF-1 concentrations.
Results shows that NEFAs or IGF-1 concentrations did not vary after the introduction of males.
Results shows that NEFAs concentrations increases during the follicular phase.
Results shows that IGF-1 concentrations did not vary during the oestrous cycle.
Highlights
Keywords:
Introduction
The "male effect", has been widely used in extensive and semi-extensive goat production systems in Mediterranean countries during the seasonal anoestrous period. Though males cannot be used to induce spontaneous ovulation in cyclic females, there is evidence that they can affect the distribution of oestrus in goats (Chemineau Citation1983). In sheep, the response to the introduction of rams during the breeding season appears to be driven by an increase in pulsatile LH secretion similar to that observed in anovulatory ewes (Hawken et al. Citation2007). In goats, Chemineau (Citation1983) suggest a possible luteolytic effect when introduced males to female goats during the breeding season.
The principal circulating blood metabolites used to assess the energy status of ruminants are plasma glucose and non-esterified fatty acids (NEFAs). The increased demand for energy during fasting, pregnancy and lactation, the adipose tissue is mobilised and produces an increase in the NEFAs concentration (Bowden Citation1971). Metabolic hormones, such as Insulin-like growth factor-I (IGF-I), have important roles in the control of follicle development and are likely to be mediators of the effects of dietary intake on ovulation rate (Muñoz-Gutierrez et al. Citation2002). In nonpregnant animals, IGF-I was shown to be synthesised in the ovaries (Adashi et al. Citation1991; Spicer and Echternkamp Citation1995), oviducts (Schmidt et al. Citation1994; Wathes et al. Citation1998), uterus (Murphy et al. Citation1987; Wathes et al. Citation1998), ovine granulosa cells and corpora lutea (Adashi et al. Citation1991; Perks et al. Citation1995; Spicer and Echternkamp Citation1995). These findings suggested that locally produced IGF-I might result in changes in plasma IGF-I concentrations during the oestrous cycle in ruminants. Fluctuations of plasma IGF-I during the oestrous cycle in ruminants were examined by only a few research groups (Spicer and Zavy Citation1992; Funston et al. Citation1995; Burns et al. Citation1997).
The nutritional status of domestic ruminants affects their reproductive capacity. In Mediterranean extensive and semi-extensive stock-raising systems, food availability can vary widely and animals may experience an increase or reduction in body weight (BW) and body condition (BC). These fluctuations can modify the reproductive response to some practices used for controlling the reproductive seasonality, such as the "male effect" (Gallego-Calvo et al. Citation2015). In later work, Zarazaga et al. (Citation2017) and Gallego-Calvo et al. (Citation2018), performed during the seasonal anoestrous, observed an increase in IGF-1 concentrations around 3–4 weeks after male introduction and a peak of NEFAs in females with an increased BW/BC just after the introduction of males, while females with a decreased BW/BC showed a reduction in plasma NEFAs. None of the other metabolic parameters analysed (leptin, insulin or glucose) were modified after the introduction of the males.
In these previous experiments, when the "male effect" was performed, the females probably were on different phases of the oestrous cycle and an effect of nutrition was included on the experimental design. Therefore, it is necessary to design an experiment in which the females are synchronised and under non-fluctuating nutritional conditions.
The present work aimed to determine if the introduction of males ("male effect") at different moments of the oestrous cycle was able to modify some metabolic parameters (NEFAs and IGF-1) in females previously isolated from males in non-fluctuating nutritional conditions.
Materials and methods
Animals and experimental design
The study was conducted at the experimental farm of the University of Huelva (latitude 37º 20’ N and longitude 6º 54' W). Sixty-four adult Blanca Andaluza, multiparous non lactating does were used in this study (4–5 years old at the start of the experiment). The main objective of the Blanca Andaluza breed is the meat production where kids must weigh 8-9 kg at slaughter. The breeding season of this breed last form August to February/March (Gallego-Calvo et al., 2014). These females had previously delivered between the end August and onset September and were completely dried before the onset of the experiment.
To ensure that the does were at the correct stage of their cycle on the day of male exposure (Day 0, 3rd November), the females were synchronised using intravaginal progesterone sponges impregnated with 20 mg of fluorogestone acetate (FGA, Chronogest®, Intervet S.A., Salamanca, Spain). These were inserted and remained in place for 11 days. Two days before sponge removal, 250 U of eCG (Intervet SA, Salamanca, Spain) and 6 mg of luprostiol (0.8 mL Prosolvin®, Virbac, Spain) were i.m-injected into each animal. Pessary insertion and withdrawal were staggered to produce the six groups on Day 0 of the experiment.
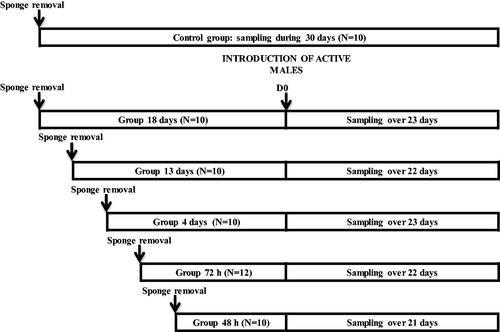
Females were divided into these six groups according to the introduction of males in relation to the time the sponge removal was done as follows: Group 48H (onset of the follicular phase), where males were introduced 48 h after sponge removal (n = 10). Group 72H (end of the follicular phase), where males were introduced 72 h after sponge removal (n = 12). Group 4 D (onset of the luteal phase), where males were introduced four days after sponge removal (n = 10). Group 13 D (middle of the luteal phase), where males were introduced 13 days after sponge removal (n = 10). Group 18 D (end of the luteal phase), where males were introduced 18 days after sponge removal (n = 10). Control Group, which was the group without any contact with males (n = 12). This group was maintained completely isolated from bucks during whole experiment. Figure shows the experimental design.
Figure 1. Experimental design of females synchronised using intravaginal progestagen sponges without any contact with males (Control Group), and females synchronised using the same treatment and submitted to the "male effect" during the breeding season at 48 hours (48H, very early follicular phase), 72 hours (72H, early follicular phase), 4 days (4 D, early luteal phase), 13 days (13 D, luteal phase), and 18 days (18 D, late luteal phase) after sponge removal.
Management and measurements
Management
All animals were fed daily with lucerne hay, barley straw (ad libitum) and commercial concentrate to maintain their weight in agreement with the INRA maintenance requirements for a goat of 50 kg live weight (Morand-Fehr and Sauvant Citation1988). All animals had free access to water and mineral supplements.
The “male effect”
Females were isolated from males for a period of three months before the start of the experiment and maintained with them after D0 (the day of male introduction) for the following 21 to 23 days, depending on the group. At this moment, they were housed in open barns completely isolated from the other groups. Two males were used for each group. All bucks were 2–3 years old at the beginning of the study. The bucks were exposed to the natural photoperiod during the whole experiment. Bucks before the start of the experiment were housed together and completely isolated from the does.
Body weight and body condition
The body weight (BW) and body condition (BC) were recorded at sponge removal and at the end of the experiment. The BC was examined by lumbar palpation (always by the same handler) and recorded on a scale of 0–5, where 0 = emaciated and 5 = very fat, with increments of 0.25 (Hervieu et al. Citation1991).
Detection of oestrous behaviour
Oestrous activity was recorded every day from the introduction of the males by direct visual observation of the marks left by the marking harnesses.
Plasma samples and analysis
Blood samples were taken daily from the day of the sponge removal and for at least 21 days after sponge removal for each group by jugular venipuncture and using tubes containing 10 µl heparin. They were immediately centrifuged at 2300 x g for 30 min at 4 °C, and the resultant plasma was stored at −20 °C until analysis.
The occurrence of ovulation was monitored through the progesterone concentration of blood samples. Plasma progesterone was determined daily using a commercial enzyme-linked immunoassay (ELISA) kit (Ridgeway Science Ltd., Gloucester, UK), following the manufacturer’s instructions (Andueza et al. Citation2014). The sensitivity of the assay was 0.2 ng/mL. Intra- and inter-assay coefficients of variation for sample pools of 0.5 ng/mL and 1 ng/mL were 5.2% and 5.6%, and 7.7% and 8.1%, respectively. The plasma progesterone concentration and the detection of oestrous behaviour were used to determine the percentages of females showing oestrus with or without ovulation. NEFAs were determined daily by spectrophotometric assay using the NEFA-HR2 kit (Wako, Chemicals GMBH, Germany) (sensitivity 0.028 mg/dL, intra- and inter-assay coefficients of variation were 6.3% and 6.9%, respectively). Plasma IGF-1 was measured every two days using the commercial Goat Insulin-Like Growth Factor Kit (Finetest, Wuhan, Hubei, China) (sensitivity 1.0 ng/mL, intra- and inter-assay coefficients of variation were 4.4% and 4.8%, respectively).
Definitions of the oestrous cycle
For each goat, the duration of the follicular phase was defined as the time between the day of sponge removal and the last sample with progesterone concentrations below baseline (1.0 ng/mL). The date of the first plasma progesterone value above baseline, which was followed by the three or four consecutive plasma samples, was taken as the onset of the luteal phase. The cessation of the luteal phase was deemed to have occurred when two or three consecutive plasma samples showed progesterone concentrations below baseline. The date of the last plasma progesterone value above baseline was considered the day of the end of the luteal phase. The difference between the onset of the luteal phase and the cessation of the luteal phase was considered as the duration of the luteal phase. The sum of the duration of the follicular phase and the duration of the luteal phase was defined as the duration of the oestrous cycle.
Statistical analyses
Two females from the 72H, 4 D, 13 D groups and one female from the control group were discarded because they did not respond to the hormonal treatment. Three females from the 18 D group that responded with a short cycle were discarded as well.
The BW and BC at sponge removal and at the end of the experiment were analysed using a one-way ANOVA test on all groups (control, 48H, 72H, 4 D, 13 D and 18 D) as the main factor. The Duncan test was used to determine the differences between the groups.
The daily values for progesterone (P4) and NEFAs, and twice-daily values for IGF-1, were examined using an ANOVA test with time as a repeated measure and the experimental groups as main factors including the interaction between them. The Tukey test was used to determine the differences between the groups at each point. To determine whether differences in P4, NEFAs and IGF-1 occurred as a consequence of the male introduction, an ANOVA test with time before male introduction and time after male introduction within and between groups as main factors was performed. The samples obtained during the follicular or luteal phases to determine NEFAs and IGF-1 concentrations were analysed and studied using the ANOVA test with these periods and the experimental groups as main factors. The Duncan test was used to determine the differences between the groups. Differences in each daily sample between the control group and each experimental group were subjected to the ANOVA test.
Data were expressed as the mean ± SEM, and differences were considered significant at p ≤ .05. All calculations were performed using the SPSS-25 software package.
Ethical note
The present study was performed in accordance with the Spanish Animal Protection Policy described in Real Decreto 53/2013, which conforms to the European Union Directive 2010/63 regarding the protection of animals used in scientific experiments. The procedures of the present experiment were evaluated by the ethical committee for animal experimentation (CEEA-OH) from the University of Granada and authorised with the reference number 297-CEEA-OH-2018, and by the Andalusia Regional Government under the authorisation number 22/05/2019/094. The study was conducted at the experimental farm of the University of Huelva (latitude 37° 20′ N and longitude 6° 54′ W), which meets the requirements of the European Community Commission for Scientific Procedure Establishments (2010/63).
Results
Body weight and body condition
No differences (p > .05) were observed between groups in the live weight and body condition score at sponge removal (44.5 ± 1.2 kg and 2.64 ± 0.03, respectively) and at the end of the experiment (44.4 ± 1.3 kg and 2.68 ± 0.04, respectively).
Progesterone concentrations
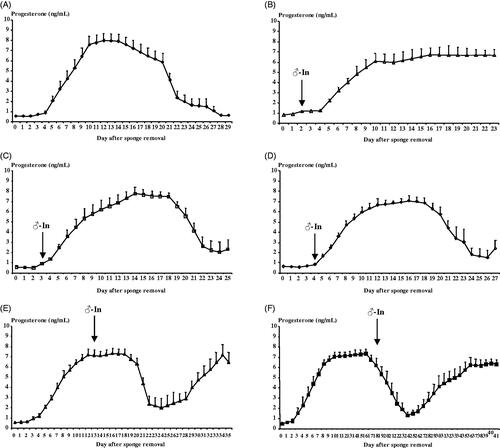
Figure shows the evolution of the progesterone concentrations of each experimental group. The evolution of the progesterone concentrations confirms that the moments selected for the introduction of the males were chosen correctly. A clear effect of time (p < .001) but no difference between groups (p > .05) was observed. The results showed low progesterone concentrations in the 48H and 72H groups, increasing progesterone concentrations in the 4 D group, and high progesterone concentrations in the 13 D and 18 D groups, but decreasing in this last group because the luteal phases is finishing. Interaction between time and experimental group was observed on progesterone concentrations (p < .01) but was determined by the group 48H (when this group was discarded from the statistical analysis this interaction was not significant) that showed higher progesterone concentrations at the end of the experiment because a high number of the does became pregnant.
Figure 2. Evolution of progesterone concentrations (ng/mL) in females synchronised using intravaginal progestagen sponges without any contact with males (Control Group, A), and females synchronised using the same treatment and submitted to the "male effect" during the breeding season at 48H (very early follicular phase, B), 72H (early follicular phase, C), 4 D (early luteal phase, D), 13 D (luteal phase, E), and 18 D (late luteal phase, F) after sponge removal. The arrow indicates the moment of the introduction of males to the groups.
NEFAs concentrations
No effects were observed on the mean NEFAs concentrations (p > .05) in the experimental groups. In comparing the control group and each experimental group, in general, no differences between the daily samples were observed except for some isolated points (Figure ).
Figure 3. Evolution of Non-esterified fatty acids (NEFAs) concentrations (mg/dL) in females synchronised using intravaginal progestagen sponges without any contact with males (Control Group, ◆), and females synchronised using the same treatment and submitted to the "male effect" during the breeding season at 48H (very early follicular phase, △; (A)), 72H (early follicular phase, □; (B)), 4 D (early luteal phase, ⋄; (C)), 13 D (luteal phase, ▲; (D)), and 18 D (late luteal phase, ■; (E)) after sponge removal. The arrow indicates the moment of the introduction of males to the groups. *p<.05; **p<.01; ***p<.001.
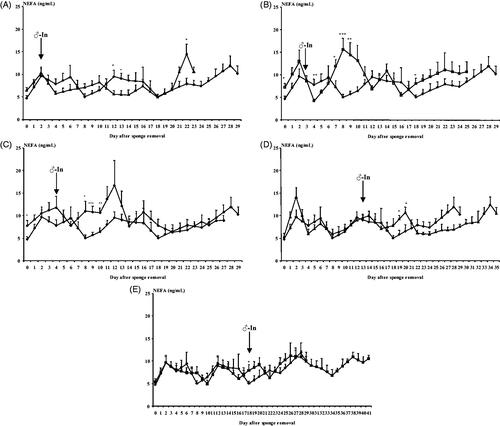
For each group, a clear effect of time on NEFAs concentrations was observed (at least, p < .05) but no difference between groups (p > .05) was observed (Figure ). Interaction between time and experimental group was observed on NEFAs concentrations (p < .01). This interaction was determined by the groups 48H, 72H and 4 D that showed variations on their NEFAs concentrations but not during the days of male introduction. The group 48H showed lower NEFAs concentrations on days 12 and 13 after male introduction. The group 72H showed high NEFAs concentrations on days 9 to 12 after male introduction. The group 4 D increased their NEFAs concentrations between 9 to 13 days after D0 (Figure ).
No differences were observed between the time before male introduction and after male introduction (8.49 ± 0.16 mg/dL, p > .05). Possible differences in NEFAs concentrations between the follicular phase and the luteal phase of the oestrous cycle induced by the progestagen treatment were studied. Differences in NEFAs concentrations were observed between the follicular and the luteal phases (9.48 ± 0.38 mg/dL vs 8.15 ± 0.15 mg/dL for the follicular and luteal phases, respectively, p < .01).
IGF-1 concentrations
No effect of time, no effect of group nor interaction between them was observed on IGF-1 concentrations (p > .05). No effects were observed on the mean IGF-1 concentrations (p > .05) in the experimental groups. In comparing the control group and each experimental group, in general, no differences were observed between the daily samples except for some isolated points (Figure ).
Figure 4. Evolution of Insulin-like growth factor-I (IGF-1) concentrations (ng/mL) in females synchronised using intravaginal progestagen sponges without any contact with males (Control Group, ◆), and females synchronised using the same treatment and submitted to the "male effect" during the breeding season at 48H (very early follicular phase, △; (A)), 72H (early follicular phase, □; (B)), 4 D (early luteal phase, ⋄; (C)), 13 D (luteal phase, ▲; (D)), and 18 D (late luteal phase, ■; (E)) after sponge removal. The arrow indicates the moment of the introduction of males to the groups. *p<.05; **p <.01; ***p<.001.
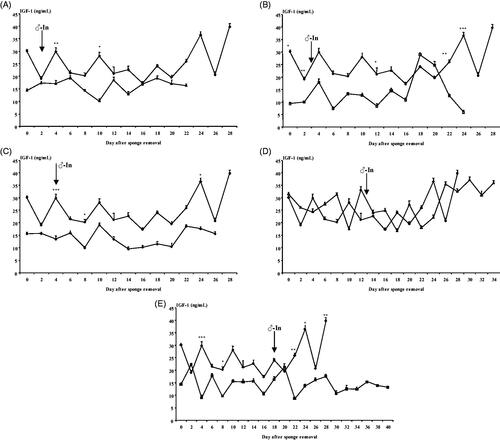
For each group, no effect of time of sampling on IGF-1 concentrations was observed (Figure ). No differences were observed between the time before male introduction and after male introduction (16.53 ± 0.71 ng/mL, p > .05). As no differences between the period before male introduction and after male introduction were observed, the IGF-1 concentrations during the follicular phase and the luteal phase of the induced oestrous cycle were studied. No differences were observed between the follicular and luteal phases (17.52 ± 0.64 ng/mL for the mean IGF-1 concentrations of all experimental groups, p > .05).
Discussion
The results of the present experiment demonstrate that, at Mediterranean latitudes, the introduction of active males (‘male effect’) does not modify the metabolic parameters, such as NEFAs and IGF-1 concentrations, under non-fluctuating nutritional conditions. Moreover, of these metabolic parameters, only the NEFAs concentrations were different during the oestrous cycle, being higher during the follicular phase than during the luteal phase.
The response to the hormonal treatment was adequate and similar to those reported by other authors using sponges containing 20 mg of progestagen (Leboeuf et al. Citation2003; Zarazaga et al. Citation2014).
The absence of effect of male introduction at different moments of the oestrous cycle on NEFAs and IGF-1 concentrations was not supported by our previous results (Gallego-Calvo et al. Citation2015, Citation2018; Zarazaga et al. Citation2017). In all cases, we observed a peak of NEFAs in females with increased body weight/body condition after the introduction of the males, while females with decreased BW/BC showed a reduction in plasma NEFAs and an increase in IGF-1 concentrations around 3–4 weeks after male introduction. There are different possibilities for explaining these discrepancies. Firstly, in previous experiments, the goats were overfed or undernourished females. In the present experiment, however, the females were submitted to a constant level of nutrition adapted to their maintenance requirements, which could induce a different response regarding NEFAs and IGF-1 concentrations. Secondly, in this experiment, we introduced the bucks at different moments of the oestrous cycle. In former experiments, however, females were submitted to the "male effect" during the seasonal anoestrous period and, consequently, there was a reproductive response (‘male effect’) that was not observed in this experiment because the females were cycling. Thirdly, in previous experiments, we considered the reason for these variations to be due to changes in the synthesis of hormones, especially progesterone, placental lactogen or those related to pregnancy. The absence of differences during the oestrous cycle in the present work, however, suggests that NEFAs and/or IGF-1 are not related to the synthesis of progesterone but perhaps to the synthesis of other hormones, such as placental lactogen, as well as the pregnancy status. In this way, the development of embryos during the preimplantation period is affected by various hormones, such as plasma NEFAs, insulin, IGF-I and leptin (Kaye Citation1997). In our previous experiments, we observed an increase in NEFAs concentrations in females that did not show parturition. Moreover, IGF-I is produced locally in many organs of the body, including the reproductive tract of cyclic and pregnant ewes (Stevenson et al. Citation1994). In the uterus, it stimulates cell proliferation and differentiation of the early embryo and endometrial cells (Kaye Citation1997). For this reason, in former experiments, we observed an increase in IGF-1 concentrations precisely in females that showed parturition. In the present experiment, however, most of the animals did not become pregnant.
The absence of effect of male introduction on NEFAs concentrations is not supported by other results on the bibliography. Firstly, when males are introduced with females, more agonistic interactions among the individuals could be observed (Miranda de Lama and Mattiello Citation2010); secondly, when does are in oestrous and a buck is housed nearby, the doe will attempt to reach him. Eating and resting time will be reduced, and overall walking will increase (Hurnik Citation1987). In all cases a great mobilisation of fat reserves can be observed and an increase of NEFAs concentrations. The reason for this discrepancy could be the social structure on our farm. In stable groups, goats develop affinity and affiliative relationships, which increase the cohesion of the group and decrease the frequency of agonistic interactions (Miranda de Lama and Mattiello Citation2010). Moreover, in the present experiment, the "male effect" did not induce a oestrous response, as consequence the percentage of females in oestrous was low except for the groups 48H and 72H.
As no change to the oestrous cycle after the introduction of the males and no differences between the period before male introduction and after male introduction occurred, we were able to study the metabolic parameters (NEFAs and IGF-1 concentrations) as a whole. The statistical analysis for the NEFAs concentrations indicated a clear effect of time. There could be various explanations for this effect. Firstly, it has been demonstrated (Diskin et al. Citation1999) that concentrations of NEFAs in pre-feed blood samples are significantly greater than in post-feed samples but, within a particular sample time, the concentration of NEFAs in serum decreased in proportion to the level of energy feeding. This explanation, however, must be discarded because, in the present experiment, all samples were obtained at the same hour, before nutrition distribution, and all females were nourished at a similar level. Secondly, in the present experiment, for all groups as a whole, the NEFAs concentrations during the period of the follicular phase were higher than during the luteal phase of the oestrous cycle induced by the hormonal treatment. Besides the reason exposed previously, perhaps, other hormones as cortisol, can explain this increase of NEFAs concentrations during the follicular phase as induces with the glycaemia in ewes (Pinto-Santini and Ungerfeld Citation2019). Nevertheless, Rondina et al. (Citation2005), working with goats submitted to two different feeding levels, produced results that had higher NEFAs concentrations at the onset of the oestrous cycle than during the luteal phase of the high-level group, although the authors did not study those differences. The lower level of NEFAs concentrations during the luteal phase could be linked to progesterone concentrations. A possible explanation could perhaps be that NEFAs concentrations are inhibited by progesterone concentrations. Kaye (Citation1997) observed reduced NEFAs concentrations during the preimplantation period—a period with high progesterone concentrations.
In terms of IGF-1, concentrations were not altered by the time and moments of the oestrous cycle (follicular and luteal phases) and showed no difference between the experimental groups. In cattle, Funston et al. (Citation1995) reported no modifications of the IGF-1 concentrations during the oestrous cycle. This result, however, is in contrast with previous results in goats, obtained by Hashizume et al. (Citation2000), who observed elevated IGF-1 concentrations around the preovulatory LH surge. One reason for this discrepancy could be that in the former, only 2–5 goats were used. Moreover, the concentrations shown in the females in the former were around 300 ng/mL, and in the present experiment were around 20 ng/mL, which is perhaps dependent on the kind of animals used.
Conclusion
In conclusion, the results of the present experiment demonstrate that the introduction of sexually active males at different moments of the oestrous cycle is not able to modify the studied nutritional/metabolic parameters (NEFAs and IGF-1). The NEFAs were higher during the follicular phase than during the luteal phase. The IGF-1 concentrations were not modified during the oestrous cycle.
Author contributions
L.A.Z., C.G. and J.L.G.: Conceptualisation; L.A.Z., C.G. and J.L.G.: Data Curation; L.A.Z. and J.L.G.: Formal Analysis, Investigation and Methodology; L.A.Z. and J.L.G.: Funding Acquisition, Project Administration, Supervision; L.A.Z., C.G. and J.L.G.: Roles/Writing—original draft; Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors wish to thank the farm staff of Huelva University for their technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Adashi EY, Resnick CE, Hurwitz A, Ricciarelli E, Hernandez ER, Roberts CT, Leroith D, Rosenfeld R. 1991. Insulin-like growth factors: the ovarian connection. Hum Reprod. 6:1213–1219.
- Andueza D, Alabart JL, Lahoz B, Muñoz F, Folch J. 2014. Early pregnancy diagnosis in sheep using near-infrared spectroscopy on blood plasma. Theriogenology. 81:509–513.
- Bowden DM. 1971. Nonesterified fatty acids and ketone bodies in blood as indicators of nutritional status in ruminants. A review. Can J Anim Sci. 51:1–13.
- Burns PD, Spitzer JC, Henricks DM. 1997. Effect of dietary energy restriction on follicular development and luteal function in nonlactating beef cows. J Anim Sci. 75:1078–1086.
- Chemineau P. 1983. Effect on oestrus and ovulation of exposing creole goats to the male at three times of the year. J Reprod Fertil. 67:65–72.
- Diskin MG, Stagg K, Mackey DR, Roche JF, Sreenan JM. 1999. Nutrition and oestrus and ovarian cycles in cattle. Galway (Ireland): Athenry Research Centre, Athenry, Co.
- Funston RN, Moss GE, Roberts AJ. 1995. Insulin-like growth factor-I (IGF-I) and IGF-binding proteins in bovine sera and pituitaries at different stages of the estrous cycle. Endocrinology. 136:62–68.
- Gallego-Calvo L, Gatica MC, Santiago-Moreno J, Guzmán JL, Zarazaga LA. 2015. Reproductive performance response to the male effect in goats is improved when doe live weight/body condition score is increasing. Anim Reprod Sci. 157:24–32.
- Gallego-Calvo L, Gatica MC, Guzmán JL, Zarazaga LA. 2018. Reproductive responses to sexually active buck of does treated with melatonin when body weight/body condition is increasing or decreasing. Anim Reprod Sci. 190:75–84.
- Hashizume T, Ohtsuki K, Matsumoto N. 2000. Plasma insulin-like growth factor-I concentrations increase during the estrous phase in goats. Domest Anim Endocrinol. 18:253–263.
- Hawken PAR, Beard AP, Esmaili T, Kadokawa H, Evans ACO, Blache D, Martin G. 2007. The introduction of rams induces an increase in pulsatile LH secretion in cyclic ewes during the breeding season. Theriogenology. 68:56–66.
- Hervieu J, Morand-Fehr P, Schmidely P, Fedele V, Delfa R. 1991. Measures anatomiques permettant d’expliquer les variations desnotes sternales, lombaires et caudales utilisées pour estimer l’étatcorporel des chèvres laitières. Opt Méditerr. 13:43–56.
- Hurnik JF. 1987. Sexual behavior of female domestic mammals. Vet Clin N Am: Food Animal Practice. 3:423–461.
- Kaye PL. 1997. Preimplantation growth factor physiology. Rev Reprod. 2:121–127.
- Leboeuf B, Forgerit Y, Bernelas D, Pougnard JL, Senty E, Driancourt MA. 2003. Efficacy of two types of vaginal sponges to control onset of oestrus, time of preovulatory LH peak and kidding rate in goats inseminated with variable numbers of spermatozoa. Theriogenology. 60:1371–1378.
- Miranda de Lama GC, Mattiello S. 2010. The importance of social behaviour for goat welfare in livestock farming. Small Rumin Res. 90:1–10.
- Morand-Fehr P, Sauvant D. 1988. Goat nutrition. In: Jarrige R, editor. Alimentation des Bovins, Ovins et Caprins. Paris (France): Institut National de la Recherche Agronomique, p. 281–304.
- Muñoz-Gutierrez M, Blache D, Martin GB, Scaramuzzi RJ. 2002. Folliculogenesis and ovarian expression of mRNA encoding aromatase in anoestrous sheep after 5 days of glucose or glucosamine infusion or supplementary lupin feeding. Reproduction. 124:721–731.
- Murphy LJ, Murphy LC, Friesen HG. 1987. Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol Endocrinol. 1:445–450.
- Perks CM, Denning-Kendall PA, Gilmour RS, Wathes DC. 1995. Localization of messenger ribonucleic acids for insulin-like growth factor I (IGF-I), IGF-II, and the type 1 IGF receptor in the ovine ovary throughout the estrous cycle. Endocrinology. 136:5266–5273.
- Pinto-Santini L, Ungerfeld R. 2019. The phase of the estrous cycle modifies the endocrine, metabolic and behavior rhythms in ewes. Physiol Behav. 204:324–335.
- Rondina D, Freitas VJF, Spinaci M, Galeati G. 2005. Effect of nutrition on plasma progesterone levels, metabolic parameters and small follicles development in unstimulated goats reared under constant photoperiod regimen. Reprod Domest Anim. 40:548–552.
- Schmidt A, Einspanier R, Amselgruber W, Sinowatz F, Schams D. 1994. Expression of insulin-like growth factor 1 (IGF-1) in the bovine oviduct during the oestrous cycle. Exp Clin Endocrinol. 102:364–369.
- Spicer LJ, Echternkamp SE. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest Anim Endocrinol. 12:223–245.
- Spicer LJ, Zavy MT. 1992. Concentrations of insulin-like growth factor-I in serum of sheep with different ovulation rates: changes during the estrous cycle. Theriogenology. 37:395–405.
- Stevenson KR, Gilmour RS, Wathes DC. 1994. Localization of insulin-like growth factor-I (IGF-I) and –II messenger ribonucleic acid and type 1 IGF receptors in the ovine uterus during the estrous cycle and early pregnancy. Endocrinology. 134:1655–1664.
- Wathes DC, Reynolds TS, Robinson RS, Stevenson KR. 1998. Role of the insulin-like growth factor system in uterine function and placental development in ruminants. J Dairy Sci. 81:1778–1789.
- Zarazaga LA, Gatica MC, Gallego-Calvo L, Celi I, Guzmán JL. 2014. The timing of oestrus, the preovulatory LH surge and ovulation in Blanca Andaluza goats synchronised by intravaginal progestagen sponge treatment is modified by season but not by body condition score. Anim Reprod Sci. 146:170–175.
- Zarazaga LA, Gatica MC, Gallego-Calvo L, Guzmán JL. 2017. The reproductive performance of female goats treated with melatonin is not improved after introduction to bucks displaying springtime sexual activity if these does are experiencing decreasing body weight/condition score. Anim Reprod Sci. 179:57–66.
