?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was aimed to evaluate the effects of the substitution of oregano essential oil (OEO) for antibiotics in the plateau-broiler diet. Partridge Shank chicken is a broiler breed which has stronger adaptability and disease resistance compared with commercial broiler strain. It is generally slaughtered at 75 -d-old. Here, after a 12-d pre-experiment, 144 57-d-old healthy Partridge Shank chickens were randomly divided into three groups, and fed a basal diet supplemented with OEO (group OEO), a basal diet supplemented with aureomycin (group aureomycin), or a basal diet alone (group control) for 28 days. At the end of the experimental period, production performance, mortality ratio, carcass traits, nutrient digestibility, serum antioxidant performance, intestinal morphology, and intestinal flora structure were determined in each group of broilers. We found that the chickens in group OEO and group aureomycin were improved in average daily gain, average daily feed intake, thigh yield, breast yield, and digestibility of crude protein, reduced in mortality ratio compared with group control (p < 0.05), while there were no significant differences in these indices between these two groups (p > .05). Besides, dietary supplementation with OEO improved serum antioxidant activity and intestinal morphology in the plateau broilers, as well as the richness of beneficial bacteria in the intestine. Thus, OEO improved production performance, nutrient digestibility and reduced mortality ratio of Partridge Shank chickens in the plateau by improving intestinal flora structure. Conclusively, it is practicable that use of OEO to replace growth-promoting antibiotic additives in the plateau-broiler diet.
Oregano essential oil may improved production performance, nutrient digestibility, carcass characteristics and reduced mortality ratio.
Dietary supplementation with Oregano essential oil improved the serum antioxidant performance of plateau broilers.
Oregano essential oil may improved intestinal flora structure of plateau-broilers.
Highlights
Introduction
On the Tibetan Plateau, which has an unusual hypoxic alpine environment, natural materials are scarce, and local breeds of livestock have low growth and reproductive performance (Zhang et al. Citation2007). Thus, large-scale rapid breeding programs are difficult to develop and popularise, resulting in a shortage of meat products in this region (Jia et al. Citation2016). At present, most plateau broilers on the market are transported to Tibet after hatching in areas in the plains (Liu et al. Citation2013). However, commercial broiler strain has higher mortality ratio for its poor tolerance on the plateau although it has higher growth performance (Li et al. Citation2014). Meanwhile, it was clearly stated in No. 194 regulation of Ministry of Agriculture and Rural Affairs of the People’s Republic of China that all varieties of growth-promoting feed additives except traditional Chinese medicine should be drop out, in order to regulate and limit the use of antibiotics. Thus, it is necessary to select a broiler breed with stronger adaptability and disease resistance, and non-antibiotic feed additives as growth-promoting antibiotic additives for improving production performance and reduced mortality ratio of broilers in the plateau.
Oregano essential oil (OEO), an extract of plant oregano, has been used as a feed additive for livestock and poultry (Zhou et al. Citation2020). OEO functions similarly to antibiotics by inhibiting or killing most zoonotic bacteria and plant pathogens; OEO may also improve disease resistance and promote growth in animals (Dragland et al. Citation2003). OEO as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers (Zhang et al. Citation2021). OEO improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota (Hall et al. Citation2021). The intestinal lumen is the main site colonised by bacteria and other microorganisms (Yadav et al. Citation2018). The symbiotic bacteria in the animal gut are essential for many physiological processes, including growth (Pickard et al. Citation2017). Many studies aiming to improve the efficiency of poultry production have investigated the effects of the gut flora on poultry growth and health, as well as the effects of non-antibiotic additives on these flora (Hosseindoust et al. Citation2017). Several studies have shown that OEO alters the structure of the intestinal flora in the animal host, increasing the relative abundance of Lactobacillus, improving feed palatability and conversion rate, and, consequently, enhancing host production performance (Cheng et al. Citation2018). However, few studies have investigated its effects as a dietary supplement for chickens in plateau areas. And Partridge Shank chicken, a native poultry strain in China, not only provide exquisite and nutrient-rich meat for Chinese consumers, but also exhibit strong adaptability and disease resistance compared with commercial broiler strain (Qu et al. Citation2019). It is generally slaughtered at 75 day-old. Aureomycin, which is an effective broad-spectrum antibiotic, was widely used as a feed additive for disease resistance and growth promotion in livestock and poultry before. Here, we compared the effects of OEO and aureomycin on Partridge Shank chickens grown in the plateau region, with respect to production performance, mortality ratio, carcass traits, nutrient digestibility, serum antioxidant properties, and the structure of the intestinal flora. We aimed to explore whether the effects of OEO on the plateau were similar to those of growth-promoting antibiotics. That would provide a basis for the use of OEO instead of antibiotics in the Tibetan poultry industry.
Materials and methods
Animal care
All of the methods used in this study comply with the standards of the institutional guideline for ethics in animal experimentation (Rule number 86/609/EEC-24/11/86), and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Tibet Agricultural and Animal Husbandry University (Linzhi, China). The institutional certification number is 12540000MB0P013721.
Animals and housing
All of the methods used in this study comply with the standards of the institutional guideline for ethics in animal experimentation (Rule number 86/609/EEC-24/11/86), and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Tibet Agricultural and Animal Husbandry University (Linzhi, China). The institutional certification number is 12540000MB0P013721.
The research was conducted in the Tibet Agricultural and Animal Husbandry University. All of the feeding experiments took place on its practice farm (Linzhi city, average altitude 2,986 m above sea level). 1-d-old Partridge Shank chickens were obtained from a commercial hatchery at Nanning (average altitude 79 m above sea level) and transported to Xialong farm (average altitude 2,950 m above sea level) by air. Then 45-d-old Partridge Shank chickens used in the experiment, were obtained from Xizang Xialong farm. After a 12-d pre-test, One hundred forty-four 57-d-old healthy chickens of similar weights were divided into three groups using a completely randomised design. Chickens were fed in cages, with each cage representing a replicate. Each group included six replicates, and each replicate included four cocks and four hens. So each group had a total of 48 chickens. During the pre-test, the chickens were fed a basal diet (Supplementary material, Table S1). During the experiment, the three groups were fed a basal diet supplemented with OEO (group OEO, NG), a basal diet supplemented with aureomycin (group aureomycin, JG), or a basal diet alone (group control, KG) for 28 days. OEO was purchased from Jiangxi Tianjia Biotechnology Co., LtD. (Nanchang, Jiangxi, China), and aureomycin was purchased from Jinhe Biotechnology Co., Ltd. (Hohhot, Inner Mongolia, China).
Table 1. Production performance and mortality of plateau broilers among the three groups.
Production performance and mortality measurements
The feed was weighed every day, and the daily feed intake per cage was recorded. Each chicken was weighed after fasting every Sunday morning; initial weight and final weight were recorded. Various production performance indexes, including the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR), were recorded for each group using production performance calculation formulas.
During the experiment, the incidence and death of chickens were recorded every day. All dead birds were necropsied individually to determine the cause of death. The dead chicken would be considered ascites death if it has these symptoms such as a light yellow or yellow gelatinous fluid appears in the abdominal cavity, body and pericardium hydrocephalus, right ventricular hypertrophy (right heart index >0.29) and so on. When none of the symptoms above occurred in a dead bird, the death was categorised as a non-ascites induced death (Druyan et al. Citation2007).
Carcass traits measurements
At the end of experiment, two chickens per case (one male, one female), close to the average pen weight, were selected for processing. Each bird was weighed and leg-banded for identification. Feed was removed 12 h with ad libitum provision of water before processing. Each bird was exsanguinated by cutting the jugular vein. Carcass traits (including carcass yield, eviscerated yield with giblet, eviscerated yield, thigh yield and breast yield) were calculated after dissection.
Nutrient digestibility measurements
Faeces were collected daily during the 28-day experimental period (500 g/d), dried at 65 °C, allowed to regain moisture at room temperature for 24 h, ground, blended, and stored at −20 °C. Sample collection was performed following standard GB/T 14699.1-2005; crude protein (CP) was measured following GBT 6432-1994; crude fibre (CF) was measured following GB/T 6434-2006; crude ash (CA) was measured following GB/T 6438-2007; and ether extract (EE) was measured following GB/T 6433-2006. All the measurement procedures were performed strictly in accordance with the standard instructions. The apparent nutrient digestibility for each group was calculated using the nutrient digestibility formula as follows:
(1)
(1)
Antioxidant properties
All of the birds were slaughtered by intravenous bleeding at 85-d-old. After slaughter, 5 mL blood samples were collected from the wing veins of all chickens. For each sample, the blood serum was separated, and total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity, and malondialdehyde (MDA) activity were determined using kits (Shanghai Optimisation Company), following the manufacturer’s instructions exactly.
Intestinal morphology assessment
The intestines of the slaughtered chickens were rinsed with cold physiological saline to remove contents. The duodenum, jejunum, and ileum were removed and fixed in a solution of 4% formaldehyde. Paraffin sections were prepared, stained with haematoxylin and eosin, and sealed with rhamsan gum. Intestinal images were collected using an Olympus camera microscope, and three typical visual fields were selected for analysis with Image-Pro Plus 5.02 (Media Cybernetics Imaging Technology Inc.). The 10 longest villi and crypts that were reasonably straight and stretchy were chosen for measurements of villus height (VH), crypt depth (CD), and villus height-to-crypt depth ratio (V/C). VH was equal to the vertical distance from the apex of the villus to the opening of the crypt, and CD was equal to the vertical distance from the crypt opening to the base of the crypt. V/C was calculated as VH/CD.
Intestinal flora identification
The contents of large and small intestines of the slaughtered chickens were collected separately, snap-frozen in liquid nitrogen, and sent to Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) for the 16S rRNA genome sequencing of the intestinal flora.
Statistical analysis
All the data were recorded and analysed in Excel, and one-way analyses of variance (ANOVAs) were performed using SPSS 19.0 (IBM Corporation). The results were expressed as mean ± standard deviation (SD). Differences among groups were examined using the Tukey–Kramer’s multiple range tests. We considered p < .05 to indicate a significant difference, and p > .05 to indicate no significant difference.
Results
Production performance and mortality
As shown in Table . During 57- to 85-d-old, the chickens of group OEO (NG) and group aureomycin (JG) were improved in average daily gain and feed intake, reduced in mortality ratio compared with group control (KG) (p < .05), while there were no significant differences in these indexs between these two groups (p > .05). Besides, there were no significant differences in FCR and ascites mortality among the three groups (p > .05).
Carcass traits
As shown in Table . During 57- to 85-d-old, the chickens of group OEO (NG) and group aureomycin (JG) were improved in thigh yield and breast yield compared with group control (KG) (p < .05), whereas there were no significant differences in these indices between these two groups (p > .05). Besides, there were no significant differences in carcass yield, eviscerated yield with giblet and eviscerated yield among the three groups (p > .05).
Table 2. Carcass traits of Partridge Shank chickens among the three groups.
Apparent digestibility of nutrients
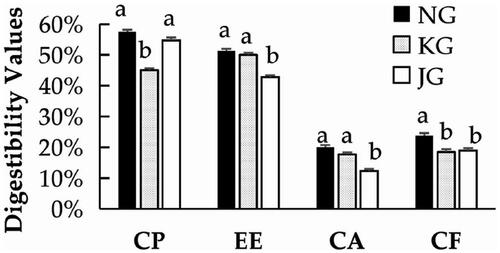
The effects of OEO on nutrient digestibility in the plateau broilers are shown in Figure . In the NG group, the apparent digestibility of CP, CF, EE, and CA were all higher than in the KG and JG groups; of these, the digestibility of EE and CA were significantly greater in group NG than in group JG (p < .05). CP digestibility was significantly greater in the KG group (p < .05). CF digestibility was significantly greater in groups KG and JG than in group NG (p < .05); there was no significant difference in CP digestibility between KG and JG (p > .05).
Serum antioxidant properties
The effects of OEO on the serum antioxidant indexes of plateau broilers are shown in Table . SOD activity in the NG broilers was significantly greater than that in the KG and JG broilers (p < .05). While MDA activity was the lowest in group NG and T-AOC was highest, there were no significant differences in MDA activity and T-AOC among groups (p > .05). These results suggested that dietary supplementation with OEO improved the serum antioxidant performance of plateau broilers.
Table 3. Serum antioxidant indexes for plateau broilers of the three groups.
Intestinal morphology
The effects of OEO on the mucosal morphology of the small intestine are shown in Table . Mean duodenum, jejunum, and ileum VH were significantly greater in group NG than in groups KG and JG (p < .05). In all of the three intestinal segments, the CD in group JG were longer than those of group of KG, but this difference was not significant (p > .05). The V/C was the highest in group NG and lowest in group KG, but there were no significant differences among groups (p > .05).
Table 4. Morphological characteristics of the intestines for plateau broilers of the three groups.
Intestinal flora
Species annotations
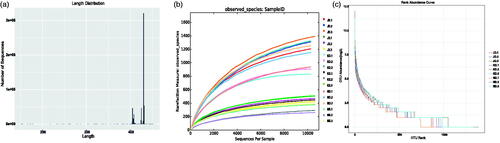
In this study, 18 samples from the contents of the small and large intestines of three groups of plateau broilers were sequenced. After data separation, splicing, filtering, and chimaera identification, we obtained 701,922 effective tags. The average number of effective tags per sample in group NG was 43,132.83 (38,044–55,568); the average number of effective tags per sample in group KG was 35,946.17 (29,142–40,669); and the average number of effective tags per sample in group JG was 37,908 (31,470–41,447). The high-quality sequences were between 410 and 440 bp long; most sequences were 420 bp long (Figure ). The rarefaction curve showed that the existing data volume essentially covered all microbial communities in all samples (Figure ). The rank abundance curve, which reflects the distribution of species across samples, showed that the abundance distributions among samples were fairly uniform and that the samples met the quality control requirements (Figure ).
Figure 2. Intestinal flora. (a) The length distribution of the sequences; (b) rarefaction curve under 97% similarity in all samples. ND: large intestine of the oregano oil group; JD: large intestine of the aureomycin group; KD: large intestine of control group. NX: small intestine of the oregano oil group; JX: small intestine of the aureomycin group; KX: small intestine of the control group; (c) Rank abundance curve of species.
Operational taxonomic unit (OTU) statistics
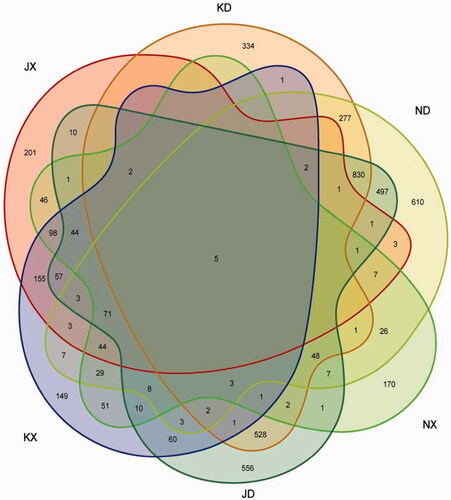
The Venn diagram showed that six OTUs were shared among NG, KG, and JG (Figure ). We identified 149, 170, 201, 334, 610, and 556 unique OTUs in the small intestine of the control group (KX), the small intestine of the OEO group (NX), the small intestine of the aureomycin group (JX), the large intestine of the control group (KD), the large intestine of the OEO group (ND), and the large intestine of the aureomycin group (JD), respectively.
Figure 3. OTU distribution from a Venn plot. ND: large intestine of the oregano oil group; JD: large intestine of the aureomycin group; KD: large intestine of control group. NX: small intestine of the oregano oil group; JX: small intestine of the aureomycin group; KX: small intestine of the control group; OTU: operational taxonomic unit.
Beta diversity analysis
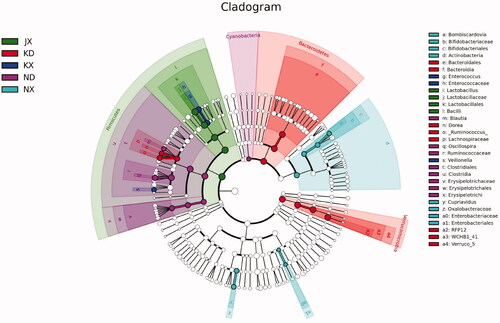
Differences in microbial flora among groups at different levels of classification are shown in Figure . The phylum Firmicutes was most abundant in NX, whereas Cyanobacteria was more abundant in ND. The phyla Bacteroidetes and Verrucomicrobia were most abundant in KD. The genera Veillonella and Enterococcus were most abundant in KX; Oscillospira and Blautia were most abundant in ND; Lactobacillus was most abundant in NX; Bombiscardovia and Cupriavidus were most abundant in JX; and Ruminococcus and Dorea were most abundant in KD.
Discussion
OEO is an environmentally friendly functional feed additive that does not leave drug residue (Abouelezz et al. Citation2019). The unique aroma of OEO can stimulate the receptors on the alimentary canal mucous of poultry, and activate the activity of digestive enzymes, thus promote digestion and absorption and increase weight (Du et al. Citation2016). OEO improves broiler and pigs production performance (Tan et al. Citation2015; Zhai et al. Citation2018). Mexican oregano essential oils at 400 mg/L can serve as natural alternative additives in drinking water to improve broiler production and meat quality (Hernandez-Coronado et al. Citation2019). Supplementation with Rhodiola might reduce the effects of hypoxia on broilers and consequently decrease mortality rate, but it did not effect on ascites induced mortality at high altitude in Tibet (Li et al. Citation2014). Above results were basically coincide with ours. We found that the chickens of group OEO and group auromycin were improved in average daily gain, average daily feed intake, thigh yield, breast yield, and digestibility of crude protein, reduced in mortality ratio compared with group control, while there were no significant differences in these indices between these two groups. Besides, there were no significant differences in FCR, ascites mortality, carcass yield, eviscerated yield with giblet and eviscerated yield among the three groups. Some of our results were not quite consistent with the findings of the previous study. The reasons that maybe caused by the differences of the testing time, kinds or the dosage of additives, etc. The specific reasons need to be further study.
The active ingredient of OEO has strong lipid solubility which can quickly penetrate the cell membrane of pathogenic microorganisms and change the permeability of them, then makes the content loss, also OEO can prevent the oxidation process of mitochondria effectively which can makes pathogenic microorganism lack of energy and die (Mitsch et al. Citation2004). In the ingredients of OEO, phenolic acids play an anti-oxidation role, the phenolic hydroxyl of the benzene ring involved in antioxidant activity as active group, which can clear the oxyalkoxide radical through dehydrogenation and produce relatively stable phenoxy radicals relatively generated to terminate the chain reaction (Bun et al. Citation2011). The oxidative metabolism, which detoxifies and sterilises, has an important effect on broiler growth (Su et al. Citation2018). The oxidative metabolism also produces an abundance of MDA (Tsikas Citation2017). SOD reduces MDA-induced cellular damage and protects cellular enzymes (Deng et al. Citation2000). Serum MDA levels reflect the degree of free radical-mediated lipid peroxidation (Ookawara et al. Citation2003). In sheep, dietary supplementation with OEO improved meat quality, muscle antioxidant capacity, antioxidant capacity, and immune function (Clemmons et al. Citation2019). Increases in serum SOD activity in broilers corresponded to increases in antioxidant capacity (Hashemipour et al. Citation2013). Here, serum T-AOC and SOD activity were significantly higher in the NG group compared with the KG and JG groups (p < .05), and MDA content was the lowest in the NG group. Thus, dietary supplementation with OEO might improve the serum antioxidant properties, which would promote the disease resistance of the organism and reduce mortality ratio of broilers on plateau.
The small intestine, which is the primary site of nutrient digestion and absorption, must maintain normal structure and function to guarantee full nutrient digestion and absorption (Grant et al. Citation2015). OEO can immunologically regulate the inflammatory response of broilers induced by Lipopolysaccharide, and reduce intestinal damage, so as to improve the tissue morphology of the small intestine. Studies have shown that OEO can enhance the function of intestinal mucosal barrier and maintain intestinal integrity (Placha et al. Citation2014). The normal structure and function of villi and microvilli in small intestine are the basic guarantee of digestion and absorption in animals. Villous shrinkage means fewer mature cells and decreased absorption function. The shallow crypt indicated increased cell maturation rate and increased secretion function (Wlodarska et al. Citation2015). The height of the intestinal villi reflects the nutrient absorption capacity of the intestine (Lang et al. Citation2019). Shallow crypts indicate that intestinal absorption function is enhanced, whereas lower V/C ratios in the small intestine indicate that digestion ability is weak (Yang et al. Citation2020). Studies have shown that the absorption of nutrients in the intestinal lumen primarily depends on various carrier transport systems in the brush edges and basement membranes of epithelial cells (Suthongsa et al. Citation2017). In this study, duodenum, jejunum, and ileum VHs were significantly greater in the NG group than in the KG and JG groups (p < .05). In addition, the V/C ratio was highest in the NG group, and lowest in the KG group. Thus, the fixed point release of OEO in the intestine might improve nutrient absorption in the small intestines, which would result in higher production performance of broilers on plateau.
OEO significantly increased the abundance of Lactobacillus and Bifidobacteria in broiler guts (Betancourt et al. Citation2014). OEO has antibacterial and bactericidal effects through its active ingredients carvyl and thymol, which have antibacterial effects mainly through degeneration and coagulation of bacterial cell wall structure proteins, and then change the permeability of cytoplasmic membrane to K+ and H+, so that phenolic substances interact with each other, which can make cell components leak and lose balance, as a result leading bacterial to death. In addition, OEO significantly reduced the incidence of diarrhoea in piglets; OEO also increased Lactobacillus abundance in the piglet intestine while decreasing Escherichia coli abundance (Wang et al. Citation2013). Several studies have shown that antibacterial compounds in oregano kill harmful microorganisms and maintain beneficial flora in the gastrointestinal tract, thus improving feed conversion efficiency and growth in livestock and poultry (Katsoulos et al. Citation2017). This was consistent with our experimental results: We found that the abundance of Lactobacillus in the large and small intestines was significantly greater in broilers fed OEO or aureomycin as compared to the control group. In addition to breaking down carbohydrates to improve digestive function, Lactobacillus also secretes large amounts of lactic acid, which inhibits the growth and reproduction of harmful bacteria, and improves host immunity (O’Callaghan and O’Toole Citation2013). Here, we found that the richness of an undefined genus of Verrucomicrobiae decreased significantly in the large intestine; this genus was similar to Chlamydia. In addition, the richness of an undefined genus of Clostridiales increased in the large intestine. The main function of this genus is to ferment sugars, which improves the digestion and absorption of saccharide feeds (Munir et al. Citation2016). After being fed OEO or aureomycin, the richness of the Desulfovibrionaceae in the broiler small intestine decreased significantly. As bacteria in the Desulfovibrionaceae restore sulphate, increases in the abundance of this family are characteristic of intestinal polyps and ulcerative colitis (Chen et al. Citation2016). Our results indicated that dietary supplementation with OEO improved the richness and diversity of beneficial bacteria in the intestines of plateau broilers while reducing the abundance of harmful bacteria. Thus, OEO improves the structure of the intestinal flora and promotes nutrient digestion, which would also result in higher production performance and lower mortality ratio of broilers on plateau.
Conclusions
In conclusion, in plateau broilers, dietary supplementation with OEO changed the morphology of the small intestine, improved the structure of the intestinal flora, increased nutrient digestion and absorption in the intestinal tract, and enhanced SOD in serum. These factors led to increase in growth performance and reduce in mortality ratio. Thus, OEO is an ideal substitute for growth-promoting antibiotics in the plateau-broiler diet.
Supplemental Material
Download MS Word (41.5 KB)Acknowledgements
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abouelezz K, Abou-Hadied M, Yuan J, Elokil AA, Wang G, Wang S, Wang J, Bian G. 2019. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal. 13(10):2216–2222.
- Betancourt L, Rodriguez F, Phandanouvong V, Ariza-Nieto C, Hume M, Nisbet D, Afanador-Tellez G, Van Kley AM, Nalian A. 2014. Effect of Origanum chemotypes on broiler intestinal bacteria. Poultr Sci. 93(10):2526–2535.
- Bun SD, Guo YM, Guo FC, Ji FJ, Cao H. 2011. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poultr Sci. 90(6):1220–1226.
- Chen L, Brar MS, Leung FC, Hsiao WL. 2016. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget. 7(21):31226–31242.
- Cheng C, Xia M, Zhang X, Wang C, Jiang S, Peng J. 2018. Supplementing oregano essential oil in a reduced-protein diet improves growth performance and nutrient digestibility by modulating intestinal bacteria, intestinal morphology, and antioxidative capacity of growing-finishing pigs. Animals. 8(9):159.
- Clemmons BA, Voy BH, Myer PR. 2019. Altering the gut microbiome of cattle: considerations of host-microbiome interactions for persistent microbiome manipulation. Microb Ecol. 77(2):523–536.
- Deng XL, Qian ZY, Liu NF, Ma XY, Wang HF, Hou ZJ. 2000. Antagonistic effect of 3,6-dimethamidodibenzopyriodonium gluconate on lipid peroxidation in cerebral cortical neuronal cultures and rat brains during focal cerebral ischemia reperfusion. Acta Pharmacol Sin. 21(5):460–462.
- Dragland S, Senoo H, Wake K, Holte K, Blomhoff R. 2003. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr. 133(5):1286–1290.
- Druyan S, Shlosberg A, Cahaner A. 2007. Evaluation of growth rate, body weight, heart rate, and blood parameters as potential indicators for selection against susceptibility to the ascites syndrome in young broilers. Poult Sci. 86(4):621–629.
- Du E, Wang W, Gan L, Li Z, Guo S, Guo Y. 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 7:19.
- Grant CN, Mojica SG, Sala FG, Hill JR, Levin DE, Speer AL, Barthel ER, Shimada H, Zachos NC, Grikscheit TC. 2015. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am J Physiol Gastrointest Liver Physiol. 308(8):G664–677.
- Hall HN, Wilkinson DJ, Le Bon M. 2021. Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim Microbiome. 3(1):2.
- Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. 2013. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poultr Sci. 92(8):2059–2069.
- Hernandez-Coronado AC, Silva-Vazquez R, Rangel-Nava ZE, Hernandez-Martinez CA, Kawas-Garza JR, Hume ME, Mendez-Zamora G. 2019. Mexican oregano essential oils given in drinking water on performance, carcass traits, and meat quality of broilers. Poultr Sci. 98(7):3050–3058.
- Hosseindoust AR, Lee SH, Kim JS, Choi YH, Noh HS, Lee JH, Jha PK, Kwon IK, Chae BJ. 2017. Dietary bacteriophages as an alternative for zinc oxide or organic acids to control diarrhoea and improve the performance of weanling piglets. Veterinarni Medicina. 62(No. 2):53–61.
- Jia CL, He LJ, Li PC, Liu HY, Wei ZH. 2016. Effect of egg composition and oxidoreductase on adaptation of Tibetan chicken to high altitude. Poult Sci. 95(7):1660–1665.
- Katsoulos PD, Karatzia MA, Dovas CI, Filioussis G, Papadopoulos E, Kiossis E, Arsenopoulos K, Papadopoulos T, Boscos C, Karatzias H. 2017. Evaluation of the in-field efficacy of oregano essential oil administration on the control of neonatal diarrhea syndrome in calves. Res Vet Sci. 115:478–483.
- Lang W, Hong P, Li R, Zhang H, Huang Y, Zheng X. 2019. Growth performance and intestinal morphology of Hyline chickens fed diets with different diet particle sizes. J Anim Physiol Anim Nutr. 103(2):518–524.
- Li L, Wang H, Zhao X. 2014. Effects of Rhodiola on production, health and gut development of broilers reared at high altitude in Tibet. Sci Rep. 4:7166.
- Liu C, Zhang LF, Li N. 2013. The specific expression pattern of globin mRNAs in Tibetan chicken during late embryonic stage under hypoxia. Comp Biochem Physiol A Mol Integr Physiol. 164(4):638–644.
- Mitsch P, Zitterl-Eglseer K, Kohler B, Gabler C, Losa R, Zimpernik I. 2004. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poultr Sci. 83(4):669–675.
- Munir RI, Spicer V, Krokhin OV, Shamshurin D, Zhang X, Taillefer M, Blunt W, Cicek N, Sparling R, Levin DB. 2016. Transcriptomic and proteomic analyses of core metabolism in Clostridium termitidis CT1112 during growth on α-cellulose, xylan, cellobiose and xylose. BMC Microbiol. 16:91.
- O'Callaghan J, O'Toole PW. 2013. Lactobacillus: host-microbe relationships. Curr Top Microbiol Immunol. 358:119–154.
- Ookawara T, Eguchi H, Kizaki T, Nakao C, Sato Y, Imazeki N, Matsubara O, Ohno H, Suzuki K. 2003. An inter-subunit disulfide bond affects affinity of human lung extracellular superoxide dismutase to heparin. Free Radic Res. 37(8):823–827.
- Pickard JM, Zeng MY, Caruso R, Nunez G. 2017. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 279(1):70–89.
- Placha I, Takacova J, Ryzner M, Cobanova K, Laukova A, Strompfova V, Venglovska K, Faix S. 2014. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci. 55(1):105–114.
- Qu H, Cheng Y, Chen Y, Zhao Y, Li J, Wen C, Zhou Y. 2019. Dietary tea tree (Melaleuca alternifolia) oil supplementation improves growth performance, cecal microflora, immunity, and antioxidant capacity of Partridge Shank chickens. J Poult Sci. 56(3):212–219.
- Su Y, Chen Y, Chen L, Xu Q, Kang Y, Wang W, Wang A, Wen C, Zhou Y. 2018. Effects of different levels of modified palygorskite supplementation on the growth performance, immunity, oxidative status and intestinal integrity and barrier function of broilers. J Anim Physiol Anim Nutr. 102(6):1574–1584.
- Suthongsa S, Pichyangkura R, Kalandakanond-Thongsong S, Thongsong B. 2017. Effects of dietary levels of chito-oligosaccharide on ileal digestibility of nutrients, small intestinal morphology and crypt cell proliferation in weaned pigs. Livestock Science. 198:37–44.
- Tan C, Wei H, Sun H, Ao J, Long G, Jiang S, Peng J. 2015. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed Res Int. 2015:1–9.
- Tsikas D. 2017. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 524:13–30.
- Wang HF, Gao K, Wang C, Zhang WM, Liu JX. 2013. Effects of feeding bamboo vinegar and acidifier as an antibiotic substitute on the growth performance and intestinal bacterial communities of weaned piglets. Acta Agriculturae Scandinavica. 63(3):143–150.
- Wlodarska M, Willing BP, Bravo DM, Finlay BB. 2015. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci Rep. 5:9253.
- Yadav M, Verma MK, Chauhan NS. 2018. A review of metabolic potential of human gut microbiome in human nutrition. Arch Microbiol. 200(2):203–217.
- Yang J, Zhan K, Zhang M. 2020. Effects of the use of a combination of two bacillus species on performance, egg quality, small intestinal mucosal morphology, and cecal microbiota profile in aging laying hens. Probiotics Antimicrob Proteins. 12(1):204–213.
- Zhai H, Liu H, Wang S, Wu J, Kluenter AM. 2018. Potential of essential oils for poultry and pigs. Anim Nutr. 4(2):179–186.
- Zhang H, Wu CX, Chamba Y, Ling Y. 2007. Blood characteristics for high altitude adaptation in Tibetan chickens. Poult Sci. 86(7):1384–1389.
- Zhang LY, Peng QY, Liu YR, Ma QG, Zhang JY, Guo YP, Xue Z, Zhao LH. 2021. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poultr Sci. 100(7):101163.
- Zhou R, Wu J, Lang X, Liu L, Casper DP, Wang C, Zhang L, Wei S. 2020. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J Dairy Sci. 103(3):2303–2314.