Abstract
We aimed to investigate the effects of dietary supplementation with gallus epidermal growth factor (gEGF) from chicken embryos on growth performance, immunity, and intestinal morphology in broilers. 480 1-day-old AA broilers were randomly divided into 5 groups with 6 replicates of 16 chicks each. The control group was fed basal diet and other treatment diets were supplemented with 4, 6, 8, 12 ng/kg gEGF, respectively. The whole experiment lasted for 42 d. Broilers were harvested at the end of the experiment, and spleen, thymus, bursa, serum samples and small intestine were collected. Results showed that average daily growth (ADG) of 1–21d at the 4, 6 and 12 ng/kg groups were significantly increased (p < .05); ADG (22–42d, 1–42d) and ADFI of 1–42d at the 4 ng/kg group were also increased (p < .05). Dietary gEGF at 4 and 6 ng/kg groups improved catalase (CAT) activity, and total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-PX) activity were significantly increased (p < .05) while malondialdehyde (MDA) concentration significantly decreased (p < .05) at 12 ng/kg group. Compared with the control group, the thymus index of the 8 ng/kg group was significantly increased (p < .05). Dietary gEGF at less than or equal to 8 ng/kg level improved Bursa of Fabricius (BF) index (p < .05). Moreover, at the 8 ng/kg group, serum immunoglobulin A (IgA) and immunoglobulin M (IgM) concentrations were significantly higher (p < .05) than in the control group. Intestinal development was enhanced by gEGF inclusion. These findings demonstrated that dietary gEGF supplementation improved growth performance, antioxidant capacity, immunity and the development of the intestine in broilers. And the suitable dosage of gEGF in broiler diets is 8 ng/kg. The gEGF has the potential to be used as a feed additive in broilers.
4 ng/kg gEGF dietary supplementation had a positive effect (P < 0.05) on growth performance of broilers.
Antioxidant capacity (T-AOC, GSH-PX, CAT) of serum significantly increased, and MDA decreased at 12 ng/kg group.
Dietary gEGF exhibited positive influence on immunity of broilers.
Dietary gEGF improved the development of small intestinal by increasing the villus height and reducing the crypt depth of small intestine in broilers.
Highlights
Introduction
The epidermal growth factor (EGF) was first isolated from the mouse submandibular gland more than half a century ago (S.). EGF is a single-chain polypeptide composed of 53 amino acids with a molecular weight of 6000 Da. Studies have shown that EGF has high stability to acids, alkalis and proteases, and remains stable for a long time at −20 °C (Cohen and Savage Citation1974), which allows its delivery to the gastrointestinal tract to exert trophic effects (Clark et al. Citation2009). Previous studies have indicated that EGF can promote the growth, proliferation and differentiation of epidermal cells (Cohen Citation1965). It is also beneficial to skin wound healing (Gibbs et al. Citation2000) and repairing the cornea (Yan et al. Citation2013). EGF is one of the most abundant growth factors found in milk (Odle et al. Citation1996). In human colostrum, the concentration of EGF is 500 times higher than other growth factors, such as amphiregulin and transforming growth factor-alpha (TGF-α) (Nojiri et al. Citation2012), indicating that EGF plays a key role in the early intestinal development. Further studies have found that the concentration of EGF in the digestive tract is much higher than that in the circulation, and it has a good therapeutic effect on many gastrointestinal diseases such as necrotising colitis and gastrointestinal ulcers (Guglietta and Sullivan Citation1995). The dietary recombinant EGF (rEGF) addition was beneficial to intestinal morphology and immunity of jejunum in piglets including the villus height (VH) (Duh et al. Citation2000; Kitchen et al. Citation2005). The content of interleukin-3(IL-3), the number of goblet cells and the level of mucin-2 (MUC2) with dietary rEGF supplementation were increased (Warner and Warner Citation2005; Huai Citation2016; Wang et al. Citation2020). The diarrhoea rate and feed conversion ratio (FCR) of weaned piglets fed with rEGF decreased obviously (Wang, Xu, et al. Citation2014). In addition, EGF ameliorated alcohol-induced intestinal injury and promoted intestinal integrity and permeability in rats via injecting EGF. In general, mammal EGF showed benefits to growth and intestine health of animals.
Recently, modern intensive poultry production has obviously been achieved. With the increase of food safety awareness, the introduction of relevant laws and regulations to control the use of antibiotics, many studies for antibiotic alternatives have started in the poultry industry. In Europe, the ban of antibiotic growth promoters in 2006 increased the incidence of certain animal infectious diseases (Santovito et al. Citation2018; Cheng et al. Citation2021). Therefore, maintaining a healthy status of the poultry gut to ensure the high efficiency is not compromised (Rhayat et al. Citation2017).
However, knowledge concerning EGF in broilers is limited despite the availability of EGF in mammals. EGF derived from Lactobacillus lactis (EGF-LL) significantly improved the growth performance and immune function of broilers by reducing the feed conversion ratio (FCR) and increasing the value of thymus index, spleen index, immunoglobulin A (IgA), and immunoglobulin G (IgG) concentrations in serum and secretory immunoglobulin A (slgA) concentrations of the duodenum (Zhou et al. Citation2021), respectively. In this study, we made a preliminary analysis of the feasibility of gEGF as a feed additive in broilers and its effects on growth performance, serum biochemical and antioxidant indices, immune function and small intestinal development.
Materials and methods
gEGF extract preparation
The experimental procedures were conducted with reference to the methods in a previously published patent (Lu et al. Citation2017). According to the preliminary results of our research team, the isolation experiments were carried out on 5-day-old chicken embryos with the highest EGF concentration. Chicken embryos were shelled and weighed, 0.05 mol/L acetic acid (4 °C) was added to homogenate the tissues. The prepared homogenate was centrifuged at 10,000 × g at 4 °C for 30 min, and the supernatant was collected. The precipitation was further homogenised with 0.05 mol/L acetic acid (4 °C), centrifuging at 10,000 × g for 30 min and then combining two supernatant and removing the supernatant surface fat. Then, the sodium benzoate (25 g/L) was added to the supernatant obtained and stirred until completely dissolved. The solution was adjusted pH to 6.5 by adding acetic acid (4 °C), then stirred for half an hour and filtered under reduced pressure. The precipitates were then dried at room temperature, and 20 mL acetone (4 °C) was added per gram of precipitation. After standing overnight, decompression and filtration were performed. Repeating the above steps several times, the crude extract of powdered gEGF with a purity of about 80% was obtained. The purity is calculated by the percentage of EGF content in the total organic matter. The total carbon content of the accumulated organic matter was determined using total organic carbon (TOC) analyser (TOC-VCPH, Shimadzu, Japan). Finally, gEGF extract was dissolved in PBS buffer, the concentration of gEGF was determined with an Enzyme-linked immunoassay (ELISA) kit (purchased from Shanghai Enzyme Biotechnology Co., Ltd.).
gEGF products used in breeding experiments
The gEGF extract prepared above was dissolved and diluted with PBS and sprayed evenly on corncob powder in a certain proportion (the value of gEGF extract/corncob powder was 7:3.). The gEGF products for feeding experiments was gained after drying naturally off. The content of gEGF in products was 8 µg/kg as measured by ELISA kits.
In vitro digestion test of gEGF
Artificial gastric juice
Sodium chloride (2 g) was dissolved in 7 mL of hydrochloric acid and Distilled water (DW) (Kimura et al. Citation2016). Pepsin (activity 1:10,000; Wako Pure Chemical Industries, Ltd., Osaka, Japan) was added at a concentration of 0.1%, and DW was added to adjust the volume to 1 L (pH 2.0). One millilitre of gEGF solution was added to 24 mL of artificial gastric juice, which was then incubated at 37 °C for 0, 0.5, 1.5, 2.5, 3.5 and 4.5 h, Sodium hydroxide (1 M) was added to adjust pH of the solution to 6.5. ELISA was used to determine the concentration of gEGF.
Artificial intestinal juice
Artificial intestinal juices were adjusted by mixing 250 mL of disodium hydrogen orthophosphate (0.2 M) with 118 mL of sodium hydroxide (0.2 M) and pancreatin (Wako Pure Chemical Industries, Ltd.) to a concentration of 0.1%, and DW was added to adjust the volume to 1 L (pH 8.0) (Kimura et al. Citation2016). One millilitre of gEGF solution was added to 24 mL of artificial intestinal juices, which were then incubated at 37 °C for 0, 0.5, 1, 2, 3 h. Then the concentration of EGF was determined using an ELISA kit.
Experimental design, animals, and diets
A total of 480 one-day-old AA broilers of similar weight were randomly divided into 5 groups with 6 replicates and 16 birds of each replicate (per cage), fed with gEGF products at the following different levels in a feeding trial: 0 g/t, 500 g/t, 750 g/t, 1 000 g/t and 1 500 g/t respectively, and calculated as 0, 4, 6, 8 and 12 ng/kg gEGF (content in feed), respectively. The basal diet was formulated according to the American NRC (1994) broiler feeding standard. The composition and nutrient content of the basal diet (starter diet and grower diet) are presented in Table .
Table 1. Composition and nutrient levels of the basal diet (air-dry basis).
Throughout the 42-day experiment, all birds were raised in cages and free to water and feed. The room temperature inside the cages was 34 °C in the first week and reduced gradually to 2 °C each week. At the completion of the study, the temperature was about 26 °C. The humidity of the whole period inside the room was maintained between 40 and 60%, natural ventilation was maintained, and illumination was maintained for 18 h every day. The broilers were vaccinated with the Newcastle disease vaccine and the infectious bursal vaccine on days 7 and 14 of the experiment, respectively. Generally, the housing and care of birds was in accordance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching (third edition, 2010). All birds in each replicate were weighed individually after a 12 h feed deprivation on the mornings of days 21 and 42. The consumption of the diet by birds was recorded on a replicate basis to calculate average daily feed intake (ADFI), average daily gain (ADG), and feed conversion rate (FCR) during the starter (1–21 day), grower (22–42 day), and overall (1–42 day) period.
Sample collection
On day 21 and 42, two birds of similar weight were randomly picked from each replicate. Blood samples were collected from the underwing vein. After standing for several hours, the serum was separated by centrifugation at 3000 rpm and stored at −80 °C for further analysis.
After broilers are euthanized by cutting the carotid arteries following cervical dislocation and necropsied immediately, thymus (from the left and right side of the neck), spleen and bursa of Fabricius (BF) were removed and weighed to calculate the immune organ index of each group. Organ weight was expressed in percentage relative to the individual broiler’s body weight. The immune organ index calculation formula is immune organ weight (g)/pre-slaughter live weight (kg).
After removing the intestinal contents with pre-cooled saline, 1 cm intestinal segment from the middle of the duodenum, jejunum, and ileum were collected and fixed in 4% paraformaldehyde solution. For histological analysis, samples were dehydrated, fixed, sliced and haematoxylin and then eosin stained and sheet sealed. The duodenum, jejunum, and ileum tissues were sliced, and the VH, CD, and (VH/CD) of each intestinal segment were calculated, and the slices were measured and counted using the MShot Image Analysis System software.
Serum biochemical and antioxidant indices, and enzyme-linked immunoassay
The serum lipid metabolites of malondialdehyde concentrations (MDA), the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), total antioxidant capacity (T-AOC), and catalase (CAT), total protein levels (TP), albumin (ALB), glucose (GLU), urea acid (UA), glutamate pyruvic transaminase (GPT), glutamate oxalacetic transaminase (GOT), diamine oxidase (DAO) in serum, were measured by the corresponding commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China), following the manufacturer's instructions. ELISA kit was used to determine the level of serum IgA, IgM and IgG.
Statistical analysis
Statistical analysis was performed with one-way ANOVA followed by Turkey multiple comparison tests with SPSS 19.0 (SPSS, Chicago, IL, USA). Linear, quadratic and cubic effects were tested by SPSS 19.0 and considered significant at p < .05. Data are presented as means and SEM and are considered significant at p < .05.
Results
The stability of gEGF in vitro digestion test
The gEGF degradation in artificial gastric juices and artificial intestinal juices, by digestive enzymes, was examined. After 0.5, 1.5, 2.5, 3.5, and 4.5 h of digestion of gEGF in the artificial gastric juice, the change of concentration as shown in Figure . The concentration of gEGF remained basically stable.
Figure 1. Changes of gEGF concentration after digestion with artificial gastric juice and artificial intestinal juice. (a) artificial gastric juice. (b) artificial intestinal juice. values are mean ± SEM. n = 3. a–b–c Means with different superscripts in the same row indicate significantly difference (p < .05). Abbreviations: gEGF: gallus epidermal growth factor; SEM: Standard error of means.
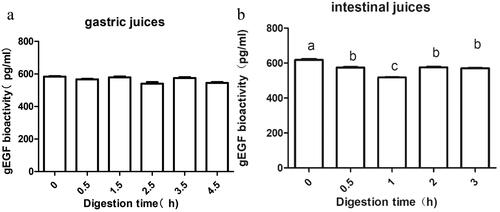
After 0.5, 1, 2, and 3 h of digestion of gEGF in the artificial intestinal juice, the change of gEGF concentration was shown in Figure . The concentration of gEGF first decreased significantly and then increased. The results showed that gEGF was rapidly degraded within the first hour, then rose to a certain extent and remain stable in the artificial intestinal juices.
Growth performance
The effect of dietary gEGF on growth performance is shown in Table . ADG (starter period) of 4, 6 and 12 ng/kg gEGF group was significantly increased (p < .05) than that of the control group. ADG (grower period, overall period) and ADFI (overall period) were significantly increased (p < .05) at 4 ng/kg gEGF in comparison with the control group. No significant influence of gEGF on FCR (starter period, grower period, overall period) was observed (p > .05).
Table 2. Effect of dietary gEGF on the growth performance of broilers.
Biochemical indices in serum
The effect of dietary gEGF on serum biochemical indicators is showed in Table . DAO concentration was significantly decreased (p = .001) at 8 ng/kg dietary gEGF. No significant influence of serum GOT, GPT, GLU, UA, TP and ALB concentrations were observed among all groups.
Table 3. Effect of dietary gEGF on the serum biochemical indices of broilers.
Antioxidant capacity in serum
The effect of dietary gEGF on the antioxidant capacity of serum is shown in Table . CAT activity of dietary gEGF at 4 and 6 ng/kg groups had a higher (p < .05) performance than that of the control group, and T-AOC, GSH-PX activity were significantly increased (p < .05) while MDA concentration significantly decreased (p < .05) at 12 ng/kg group.
Table 4. Effect of dietary gEGF on serum antioxidant capacity of broilers.
Index of immune organs and immunoglobulins in serum
The effect of dietary gEGF on index immune organs indices is showed in Table . Thymus index increased at 8 ng/kg gEGF supplementation (p < .05) in comparison with that of the control group, BF index significantly increased (p < .05) at 4, 6, 8 ng/kg. No significant difference in spleen index was observed between gEGF treatments and the control group (p > .05).
Table 5. Effect of dietary gEGF on the index of immune organs of broilers.
The effect of dietary gEGF on serum immunoglobulins is showed in Table . There were significantly higher serum IgA and IgM concentrations with gEGF at 8 ng/kg compared with those of the control group (p < .05). However, there was no significant difference in serum IgG concentrations (p > .05).
Table 6. Effect of dietary gEGF on serum immunoglobulins of broilers.
Intestinal morphology
The effect of dietary gEGF on morphometric traits of the small intestine is showed in Table . CD (duodenum) of gEGF supplementation at 6 and 8 ng/kg significantly decreased (p < .05), and V/C (duodenum) was significantly increased at 8 ng/kg group (p < .05).
Table 7. Effect of dietary gEGF on mucosal morphological traits of small intestine of broilers.
VH (jejunum) at 4, 8 and 12 ng/kg groups were significantly higher (p < .05) than that of the control group while CD (jejunum) at 6 and 8 ng/kg groups was lower (p < .05) than that of the control group. In addition, CD (jejunum) was increased (p < .05) at gEGF of 8 ng/kg in comparison with the control group.
The value of V/C (ileum) was significantly increased (p < .05) of gEGF supplementation at 4, 6 and 8 ng/kg, and CD (ileum) was sharply lower (p < .01) at 4, 6 and 12 ng/kg groups as compared with the control group.
Discussion
The stability of gEGF in vitro digestion test
EGF is an important biologically active substance in the organism. It can promote the growth of the gastrointestinal tract, improve the secretion of gastric acid and the activity level of intestinal enzymes, and protect gut mucosa. The biological activity of gEGF was very important. EGF is usually completely absorbed and utilised through pinocytosis in the gastrointestinal tract of young animals. A study reported that EGF of goat milk decreased rapidly within 1 h of digestion in artificial gastrointestinal juice, and was basically stable after 1 h of digestion, and was more stable than recombinant pure EGF (Yun et al. Citation2016). Some studies had shown that EGF existed stably in the gastric juice of mice and humans, and degraded to a variable range in the intestinal juice (Britton et al. Citation1988). These findings were consistent with previous findings (Cohen and Savage Citation1974).
Growth performance
EGF is a multifunctional polypeptide that regulates cell proliferation, differentiation, metabolism, survival and apoptosis, which could be used to solve problems in the poultry industry such as increasing the growth performance of commercial broilers or enhancing resistance to diseases under adverse conditions. Very few studies have focussed on gEGF despite the availability of numerous studies on mammalian EGF. Wang, Duan, et al. Citation2014 reported that ADG and ADFI of early-weaned piglets feeding with rpEGF-LL (Recombinant Porcine Epidermal Growth Factor by Lactobacillus lactis) were significantly increased, and had no significant effect on FCR as compared with the control group. Levesque (Levesque et al. Citation2018) found that feeding early-weaned piglets with diets containing EGF-PP (P. pastoris fermentation supernatant with EGF) increased (p < .05) ADG and BW. On the contrary, Wang et al. Citation2019 has been demonstrated that dietary supplementation of EGF in early-weaned piglets has no significant effect on ADG, ADFI or FCR. However, broilers fed with gEGF-LL (Recombinant Gallus Epidermal Growth Factor by Lactobacillus lactis), whose BW, ADG and ADFI were significantly increased, and FCR was decreased as compared with the control group (Zhou et al. Citation2021).
In the current study, results showed that dietary gEGF increased ADG of the starter period and ADFI of the grower period of broilers. These findings were basically consistent with previous studies. In addition, gEGF had no significant effect on FCR, which may be related to the amount and source of EGF, and further experiments are needed to draw more accurate conclusions.
Biochemical indices in serum
The content of serum TP and ALB reflect the nutritional status and protein metabolism level of the body. The increase of serum TP and ALB content can promote the utilisation of feed, improve the absorption of nutrients in the body, and reduce feed consumption (Zheng Citation2019). Serum GOT and GPT are indicators of liver function. When the liver or muscles are damaged, serum GOT and GPT activity will increase. Dietary supplemented with 400 μg/kg EGF significantly reduced serum GOT and GPT (Zhu Citation2018). In contrast, our results found that gEGF had no significant effect on the value of GOT or GPT, although GPT decreased by 40% and 30% when adding 8 and 12 ng/kg EGF, respectively. UA is the final product of purine metabolism. Our results showed that dietary gEGF supplementation tended to increase serum UA of broilers. EGF cannot be directly absorbed, but mainly acts on the intestinal mucosa of animals. Therefore, dietary EGF may affect purine metabolism by regulating intestinal digestion, absorption or metabolic function. There are few studies of the effect of EGF on UA production in the body. It is necessary to further clarify the internal mechanism of the effect of dietary EGF on uric acid production in subsequent studies. DAO is an intracellular enzyme, mainly produced in the mucosa of the small intestine and exists in the cytoplasm (Thompson et al. Citation1987). When the intestinal barrier is damaged, the permeability of the intestinal barrier is increased and large amounts of DAOs are released into the blood (Cheng YF et al. Citation2019). Therefore, serum DAO activity can be used as a marker to monitor intestinal permeability and barrier injury. In this study, we observed that gEGF could reduce the activity of DAO, especially under 8 ng/kg. These results suggested that dietary gEGF supplementation had no negative effect on internal environment homeostasis and can alleviate intestinal barrier function damage by decreasing intestinal permeability and maintaining intestinal morphology in broilers external stress.
Antioxidant capacity in serum
With the industrialisation development of broilers breeding, broilers are exposed to various external pressures and caused excessive production of reactive oxygen species (ROS) and disturbing the redox balance in the chicken body, leading to oxidative stress (Lee et al. Citation2019). The antioxidant system maintains a balance between the generation and elimination of ROS through the activity of antioxidant enzymes (including GSH, SOD and CAT) (Li et al. Citation2016). T-AOC is the overall evaluation index of the antioxidant function of the animal body; T-SOD is considered to be the first line of defense of the antioxidant system. It can catalyse the conversion of superoxide anion radicals (O2–) into hydrogen peroxide. The latter reacting with CAT produces water and oxygen, which is very important to the body’s scavenging free radicals; GPX can specifically catalyse glutathione to reduce hydrogen peroxide oxidative damage (Ramay and Yalçın Citation2020). The MDA content reflects the degree of lipid peroxidation and indirectly reflects the degree of body damage (Wei et al. Citation2005). Our current study found that the basal diet supplemented with EGF had an increasing trend on the serum CAT, T-SOD, T-AOC and GSH-PX activity, meanwhile having a decreasing trend on the serum MDA concentrations of broilers. Compared with the control group, at 4 and 6 ng/kg groups, dietary gEGF significantly increased CAT activity; at 12 ng/kg group, gEGF significantly increased T-AOC and GSH-PX activity and decreased the MDA concentrations.
The antioxidant properties of EGF in broilers are rarely studied. EGF significantly decreased the LPS-induced induction of apoptosis, dehydrogenase (LDH) release and MDA production; upregulated antioxidant enzyme secretion and genes expression of T-AOC, SOD, CAT and GSH-PX to alleviate oxidative injury (Tang et al. Citation2018). EGF prevented hydrogen peroxide-induced tight junctions and adhesive junctions in the bile duct epithelium (Guntaka et al. Citation2011). Under high-density feeding conditions, the antioxidant capacity of poultry cells is weakened, and they are more susceptible to oxidative attack and damage (Liu Citation2020). Therefore, we can preliminarily infer that gEGF can improve the oxidative damage caused by external conditions and scavenge excess free radicals to promote the health and growth of broilers. The specific regulation mechanism needs further researches.
Index of immune organs and immunoglobulins in serum
The thymus, bursa of Fabricius and spleen are important immune organs for broilers. Generally, cell growth, development and division promote the weight of animal immune organs, and the weight of immune organs reflects the level of immune function (Guo et al. Citation2015). The elevation of the immune organ indexes indicated the maturation of the immune system (Shi Citation2020). We found that compared with the control group, dietary supplementation with gEGF significantly increased the index of BF; the thymus index was higher at 8 ng/kg EGF group than that of the control group; gEGF exhibited no significant difference on the index of the spleen. Yu’s research showed that the thymus index and spleen index increased significantly after feeding broilers with EGF for two weeks (Zhou et al. Citation2021). The finding was consistent with our results. This suggested that EGF can promote the development of immune organs in broilers. IgG, IgA and IgM are considered important components of the animal humoral immune system, which can directly reflect the immune status of the body (Song Citation2021). IgG participates in humoral immunity, phagocytosis, agglutination and precipitation. IgA binds to the antigens and clears them without developing an inflammatory response. IgM can regulate and sterilise in the early stage of pathogen infection. Furthermore, birds being stimulated by antigens first produced IgM and then produce IgG and IgA by helper T cells and cytokines mediated conversion (Yang Citation2007). In our study, gEGF supplementation significantly increased the contents of serum IgA and IgM at the 8 ng/kg group. Similarly, Wang found that serum IgA, IgG and IgM levels increased significantly when feeding early-weaned piglets with rpEGF expressed by Saccharomyces cerevisiae (Wang, Zhou et al. Citation2015). This suggested that gEGF can improve the immune function of the body and enhance the immunity of the body to a certain extent. In addition, our previous results showed that gEGF supplementation increased growth performance, immune index and antioxidant capacity significantly. These results suggested that gEGF may improve the growth performance of broilers by improving the immune function and antioxidant capacity.
Intestinal morphology
The small intestine is important for nutrient absorption, the VH and CD are the key parameters for evaluating intestinal morphology (Missotten et al. Citation2013). EGF is a mitogen, promotes cell proliferation and differentiation, and plays an important role in the development of the gastrointestinal tract of young animals (Cheung et al. Citation2009). The VH of the duodenum and jejunum was significantly increased fed with rpEGF in weaned piglets, but EGF had no significant effect on CD and VH/CD (Bedford et al. Citation2015). The VH/CD of PEDV (porcine epidemic diarrhoea virus) + EGF piglets was significantly higher than only PEDV piglets (Jung et al. Citation2008). Compared with the control group, VH of the duodenum was significantly higher in the EGF-LL group, the EGF-LL, and rEGF group than that of the control group; no significant influence on CD was observed among all groups; the EGF-LL treatment tended to increase the weight of the intestine (Kang et al. Citation2010). Furthermore, the number of intestinal crypt epithelial cells in PEDV + EGF piglets was more than in PEDV only piglets and promoted recovery from atrophic enteritis in PEDV-infected piglets (Jung et al. Citation2008). Exogenous EGF increased the number of proliferating cell nuclear antigen-positive cells and their mRNA expression, which indicated that the production of pig intestinal crypt cells was increased (Cheung et al. Citation2009; Kang et al. Citation2010). Moreover, the number of apoptotic bodies in crypt and villi was reduced with systemic EGF treatment (O’Brien et al. Citation2002). Our study showed that the CD was decreased and the VH/CD and jejunal VH of the small intestine was increased with dietary gEGF in broilers. The results were consistent with previous studies. Furthermore, EGF was mediated by EGF receptor (EGFR), which was located on the intestinal enterocytes microvilli and basolateral membranes (Tang et al. Citation2016). The relative content of PCNA in the villi of small intestine with EGF-LL was lower than the control group (Huai Citation2016).
In addition, our study showed that the overall feeding effect of the 12 ng/kg group is not as good as that of the 8 ng/kg group. This is consistent with previous research results (Lee DN et al. Citation2008; Wang LX et al. Citation2020). We confirmed that dietary EGF stimulated immunity, intestinal morphology and growth performance partly through EGFR and Wnt/β-catenin signalling. The study has shown that EGFR had different fates through different internalisation pathways under different concentrations of EGF (von Zastrow and Sorkin Citation2007; Sigismund et al. Citation2008), one was circulated to the cell surface to continue to play a role (clathrin-mediated endocytosis, CME); the other was further transported to the late endosomes and lysosomes for degradation (non-clathrin endocytosis, NCE) (Mukhopadhyay and Riezman Citation2007). Compared with a low EGF concentration (1.5 ng/ml, when CME was predominant and 30% of the internalised ligand was degraded), at a high EGF concentration (100 ng/ml), 55% of the ligand was degraded (60% and 40% EGF enters through the CME and NCE, respectively) (Wei et al. Citation2021).
So, we speculated that dietary supplementation with suitable gEGF can promote intestinal morphology and improve the function of the damaged intestine by stimulating crypt and villi cell proliferation.
Conclusion
In summary, the current study suggested that dietary gEGF was stable in feeds and was beneficial to growth performance, immune function and antioxidant capacity of broilers. In addition, gEGF was great of benefit to improve intestinal morphology by increasing VH and reducing CD of broilers. Our findings supported the application of gEGF in broilers’ diet, especially at the starter period, and provided a scientific basis for the use of gEGF as feed additives in the future. In the current study, the suitable dosage of gEGF in broiler diet is 8 ng/kg.
Ethical approval
The present study was approved by the Animal Care and Welfare Committee and the Scientific Ethical Committee of the Zhejiang University (No. ZJU2013105002).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Bedford A, Chen T, Huynh E, Zhu C, Medeiros S, Wey D, de Lange C, Li J. 2015. Epidermal growth factor containing culture supernatant enhances intestine development of early-weaned pigs in vivo: potential mechanisms involved. J Biotechnol. 196–197:9–19.
- Britton JR, George-Nascimento C, Koldovsky O. 1988. Luminal hydrolysis of recombinant human epidermal growth factor in the rat gastrointestinal tract: segmental and developmental differences. Life Sci. 43(17):1339–1347.
- Cheng YF, Chen YP, Chen R, Su Y, Zhang RQ, He QF, Wang K, Wen C, Zhou YM. 2019. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poultr Sci. 98(10):4767–4776.
- Cheng YH, Horng YB, Dybus A, Yu YH. 2021. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J Poult Sci. 58(1):30–39.
- Cheung QC, Yuan Z, Dyce PW, Wu D, DeLange K, Li J. 2009. Generation of epidermal growth factor-expressing Lactococcus lactis and its enhancement on intestinal development and growth of early-weaned mice. Am J Clin Nutr. 89(3):871–879.
- Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. 2009. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 297(3):G471–479.
- Cohen S. 1962. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and and eyelid opening in the new-born animal. J Biol Chem. 237(5):1555–1562.
- Cohen S. 1965. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 12(3):394–407.
- Cohen S, Savage CR Jr. 1974. Recent studies on the chemistry and biology of epidermal growth factor. Proceedings of the 1973 Laurentian Hormone Conference. 30:551–574.
- Duh G, Mouri N, Warburton D, Thomas DW. 2000. EGF regulates early embryonic mouse gut development in chemically defined organ culture. Pediatr Res. 48(6):794–802.
- Gibbs S, Silva Pinto AN, Murli S, Huber M, Hohl D, Ponec M. 2000. Epidermal growth factor and keratinocyte growth factor differentially regulate epidermal migration, growth, and differentiation. Wound Repair Regen. 8(3):192–203.
- Guglietta A, Sullivan PB. 1995. Clinical applications of epidermal growth factor. Eur J Gastroenterol Hepatol. 7(10):945–950.
- Guntaka SR, Samak G, Seth A, LaRusso NF, Rao R. 2011. Epidermal growth factor protects the apical junctional complexes from hydrogen peroxide in bile duct epithelium. Lab Invest. 91(9):1396–1409.
- Guo S, Fu S, Xu Q, Zhang Z, Wang Y, Shen Z. 2015. Immune function of Chinese formula Qingwen Baidu granule in broilers. Cent Eur J Immunol. 40(2):149–152.
- Huai YZ, Liang S. Ji 2016. Effects of recombinant epidermal growth factor on the proliferation of the intestinal epithelial cells in early-weaned pigs. Anim Husbandry Veterinary Med. 48(44):70–75.
- Jung K, Kang BK, Kim JY, Shin KS, Lee CS, Song DS. 2008. Effects of epidermal growth factor on atrophic enteritis in piglets induced by experimental porcine epidemic diarrhoea virus. Vet J. 177(2):231–235.
- Kang P, Toms D, Yin Y, Cheung Q, Gong J, De Lange K, Li J. 2010. Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs. J Nutr. 140(4):806–811.
- Kimura M, Maeshima T, Kubota T, Kurihara H, Masuda Y, Nomura Y. 2016. Absorption of orally administered hyaluronan. J Med Food. 19(12):1172–1179.
- Kitchen PA, Goodlad RA, FitzGerald AJ, Mandir N, Ghatei MA, Bloom SR, Berlanga-Acosta J, Playford RJ, Forbes A, Walters JR. 2005. Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor. JPEN J Parenter Enteral Nutr. 29(4):248–254.
- Lee DN, Chuang YS, Chiou HY, Wu FY, Yen HT, Weng CF. 2008. Oral administration recombinant porcine epidermal growth factor enhances the jejunal digestive enzyme genes expression and activity of early-weaned piglets. J Anim Physiol Anim Nutr. 92(4):463–470. eng.
- Lee MT, Lin WC, Lee TT. 2019. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals – a review. Asian-Australas J Anim Sci. 32(3):309–319.
- Levesque CL, Akhtar N, Huynh E, Walk C, Wilcock P, Zhang Z, Dyce PW, de Lange CFM, Khafipour E, Li J. 2018. The impact of epidermal growth factor supernatant on pig performance and ileal microbiota. Translational Anim Sci. 2(2):184–194.
- Li WH, Wang L, He HY, Chen J, Yu YR. 2016. Expression of neutrophil gelatinase-associated lipocalin in low osmolar contrast-induced nephropathy in rats and the effect of N-acetylcysteine. Exp Ther Med. 12(5):3175–3180.
- Liu HYY, SJ, Xi YM , et al. 2020. Effects of stocking density on growth performance. Serum Biochemical Indexes Antioxidant Capacity in Geese. 41(10):42–48.
- Lu J, Dong X, Yao J, Wang Y, Hu C, inventors; Univ Zhejiang (Uyzh-C) Univ Zhejiang (Uyzh-C), assignee. 2017. CN107252001-A 17 Oct A23K-050/30 201777 Pages: 6 Chinese CN107252001-B 29 Nov 2019 A23K-050/30 201994 Chinese. Separating and extracting chicken embryo EGF for preparing animal intestinal mucosal nutrition repair agent comprises e.g. harvesting hatching chicken embryo, removing shell by adding acetic acid solution, homogenizing and centrifuging. Patent CN107252001-A; CN107252001-B.
- Missotten JA, Michiels J, Dierick N, Ovyn A, Akbarian A, De Smet S. 2013. Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. Br Poult Sci. 54(5):627–634.
- Mukhopadhyay D, Riezman H. 2007. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 315(5809):201–205.
- Nojiri T, Yoshizato T, Fukami T, Obama H, Yagi H, Yotsumoto F, Miyamoto S. 2012. Clinical significance of amphiregulin and epidermal growth factor in colostrum. Arch Gynecol Obstet. 286(3):643–647.
- O’Brien DP, Nelson LA, Williams JL, Kemp CJ, Erwin CR, Warner BW. 2002. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J Surg Res. 105(1):25–30.
- Odle J, Zijlstra RT, Donovan SM. 1996. Intestinal effects of milkborne growth factors in neonates of agricultural importance. J Anim Sci. 74(10):2509–2522.
- Ramay MS, Yalçın S. 2020. Effects of supplemental pine needles powder (Pinus brutia) on growth performance, breast meat composition, and antioxidant status in broilers fed linseed oil-based diets. Poult Sci. 99(1):479–486.
- Rhayat L, Jacquier V, Brinch KS, Nielsen P, Nelson A, Geraert PA, Devillard E. 2017. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult Sci. 96(7):2274–2280.
- Santovito E, Greco D, Logrieco AF, Avantaggiato G. 2018. Eubiotics for food security at farm level: yeast cell wall products and their antimicrobial potential against pathogenic bacteria. Foodborne Pathog Dis. 15(9):531–537.
- Shi TX, Liu Q, Jing X, Wei P, Yan Y, Zhang C, Fu R. Liu 2020. Effects of dietary metabolizable energy and crude protein on growth performance, slaughter index and immune organ index of Wenshang luhua rooster aged 1 to 56 days. Feed Industr. 41(20):39–44.
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. 2008. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 15(2):209–219.
- Song XH, Peng C, Yu J, Chen L, Yang C, Hu Z. Zhang 2021. Effects of Lactobacillus plantarum on growth performance, slaughter performance, serum immune and antioxidant function of Dahen broilers. Chinese J Anim Nutr. 10.
- Tang X, Liu B, Wang X, Yu Q, Fang R. 2018. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int J Mol Sci. 19(3):848.
- Tang X, Liu H, Yang S, Li Z, Zhong J, Fang R. 2016. Epidermal growth factor and intestinal barrier function. Mediators Inflamm. 2016:1927348.
- Thompson JS, Vaughan WP, Forst CF, Jacobs DL, Weekly JS, Rikkers LF. 1987. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. JPEN J Parenter Enteral Nutr. 11(1):28–32.
- von Zastrow M, Sorkin A. 2007. Signaling on the endocytic pathway. Curr Opin Cell Biol. 19(4):436–445.
- Wang D, Xu S, Lin Y, Fang Z, Che L, Xue B, Wu D. 2014. Recombinant porcine epidermal growth factor-secreting Lactococcus lactis promotes the growth performance of early-weaned piglets. BMC Vet Res. 10(1):171.
- Wang L, Zhu F, Yang H, Li J, Li Y, Ding X, Xiong X, Yin Y. 2019. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J Anim Physiol Anim Nutr. 103(2):618–625.
- Wang LX, Zhu F, Li JZ, Li YL, Ding XQ, Yin J, Xiong X, Yang HS. 2020. Epidermal growth factor promotes intestinal secretory cell differentiation in weaning piglets via Wnt/β-catenin signalling. Animal. 14(4):790–798.
- Wang S, Zhou L, Chen H, Cao Y, Zhang Z, Yang J, Huang Y, Guo C. 2015. Analysis of the biological activities of Saccharomyces cerevisiae expressing intracellular EGF, extracellular EGF, and tagged EGF in early-weaned rats. Appl Microbiol Biotechnol. 99(5):2179–2189.
- Wang Y, Duan Y, Xia L, Chen F. 2014. Effects of recombinant porcine epidermal growth factor on growth performance of early-weaner piglets. Chinese J Anim Nutr. 26:3787–3792.
- Warner BW, Warner BB. 2005. Role of epidermal growth factor in the pathogenesis of neonatal necrotizing enterocolitis. Semin Pediatr Surg. 14(3):175–180.
- Wei S, Wang W, Li L, Meng HY, Feng CZ, Dong YY, Fang XC, Dong QQ, Jiang W, Xin HL, et al. 2021. Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs. Mil Med Res. 8(1):18eng.
- Wei X, Liu H, Sun X, Fu F, Zhang X, Wang J, An J, Ding H. 2005. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett. 386(1):58–62.
- Yan L, Wu W, Wang Z, Li C, Lu X, Duan H, Zhou J, Wang X, Wan P, Song Y, et al. 2013. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res. 49(3):150–160.
- Yang LH. 2007. Study on the development and the immune function genesis of B lymphocyte in the chicken bursa of Fabricius [D]. Jilin Agricultural University.
- Yun Dz F, Hou Y, Yu L, Lei F, Gao J, Wang Y, Xu L. 2016. Study on the stability of goat milk EGF in simulated gastrointestinal fluid. Food Fermentation Industr. 42(09):68–74.
- Zheng AJW, Pirzado ZK, Shoaib A, Cai HY, Chen ZM, Chang WH, Deng XJ, Liu GH. 2019. Effects of Clostridium butyricum on growth performance and serum biochemical indices of broilers. Chinese J Anim Nutr. 31(12):5519–5525.
- Zhou Y, Chen P, Shi S, Li X, Shi D, Zhou Z, Li Z, Xiao Y. 2021. Expression of gallus epidermal growth factor (gEGF) with food-grade Lactococcus lactis expression system and its biological effects on broiler chickens. Biomolecules. 11(1).
- Zhu F, Yin H, Yang Y. Yin 2018. Effects of dietary epidermal growth factor on serum biochemical indices, serum free amino acid and intestinal mucosal hydrolytic amino acid contents of weaned piglets. Chinese J Anim Nutr. 30:2519–2528.
