Abstract
Dietary manipulations play an important role in improving the rabbit behaviour and performance and mitigating the negative effects of heat stress (HS) in rabbits. Thus, this study was designed to determine the modulatory role of thyme essential oil (TEO) in improving the blood metabolites, antioxidant status, immunological response, ovarian activity, reproductive traits and fecundity of rabbit does kept under high environmental stress. A total of 100 nulliparous does were used and randomly assigned into five treatments of 20 does each. The basal diet fed to the experimental groups contained TEO at levels of 0, 50, 100, 150 and 200 mg/kg diet for the 1st, 2nd, 3rd, 4th and 5th groups, respectively. The period of TEO treatment was one month during an experimental period of three months during the summer season. Results showed that TEO at a level of 100 mg/kg diet decreased (p ≤ .001) MDA, while increased (≤.001) total proteins (TPs), albumin (AL), globulin (GL), blood haemoglobin, total antioxidant capacity (TAC), haematocrit (Ht) and glutathione (GSH). Conversely, the TEO treatment did not affect GSH peroxidase and superoxide dismutase (SOD). Immunological variables (IgG and IgM), milk production, ovulation rate (OR) and normal embryos percentage were significantly improved by 100 mg TEO/kg diet supplementation. The reproductive variable of stressed rabbit does fed TEO were significantly higher than those in the control diet. Collectively, dietary TEO supplementation can alleviate the negative influences of heat stressed-female rabbits, via enhancing the antioxidant capacity and immunological parameters.
Dietary manipulations play an important role in improving the rabbit behaviour and performance.
Addition of TEO to rabbit diets improved rabbit performance and health as well as reduced the negative effects of heat stress (HS).
Immunological indices and milk production, ovulation rates (ORs) and normal embryos were significantly improved by TEO.
Thyme essential oil (TEO) decreased MDA and increased protein and its fraction, blood haemoglobin and total antioxidant capacity (TAC).
Highlights
Introduction
Heat stress (HS) has globally known as a significant environmental factor with antagonistic effect on the reproductive efficiency of mammalian. Rabbits are more vulnerable to high environmental temperature and less efficient to eliminate HS due to scarcer sweat glands (Marai et al. Citation2002). HS causes inferior sexual receptivity, pregnancy, embryonic survival and overall reproductive performance in rabbits, by increasing the levels of oxidative stress, lipid peroxidation (Marco-Jiménez et al. Citation2017; Lu et al. Citation2019; El-Ratel et al. Citation2020; Sharaf et al. Citation2021; Sirotkin et al. Citation2021), and reducing the antioxidant capacity that negatively affect the immune responses (Sejian et al. Citation2018). Dietary manipulation is faithfully approaches associated with the practical cost and enhancement performance for mitigating or eliminating the negative effects of HS in rabbits (Abdelnour et al. Citation2020; Sirotkin et al. Citation2021). In recent years there has been expanding attention in using phytogenic as natural safe antioxidants and frequently used for animal feeding owing to their defensive outcome against oxidative stress. Moreover, antioxidant capacity of those phytogenic makes them attractive, and they have become a prevalent topic of research as well as can affect numerous biological and physiological processes in the animal organism (El-Ratel et al. Citation2020; El-Essawy et al. Citation2021). Thymus vulgaris is the utmost employed medicinal plants in the Lamiaceae family for cosmetic, gastric and medicinal purposes global (Abd El-Hack et al. Citation2016; Alagawany et al. Citation2021). Fifteen components representing 99.91% of the total detected constituents were identified. The major components were p-cymene (8.41%), γ-terpinene (30.90%) and thymol (47.59%), which suggests that the thyme essential oil (TEO) analysed belongs to the thymol chemotype in agreement with those previously reported (as reviewed by Alagawany et al. (Citation2021)).
Due to superior contents of thymol and carvacrol in TEO, it appears many beneficial aspects such antimicrobial, antiseptic and antioxidant properties (Abd El-Hack et al. Citation2016). Many scientific articles have already demonstrated their ability for enhancing appetite and promoting growth performance (Abdel-Wareth et al. Citation2018; Placha et al. Citation2019).
Moreover, TEO can have a valuable influence on animal health, welfare and overall, the performance under elevated environmental situations, however, little information is presented on the precise machinery of their action (Placha et al. Citation2019; Büyükkılıç Beyzi et al. Citation2020). Consequently, dietary inclusion with 0.5 g/kg TEO enhanced intestinal integrity, immunological response and health status of rabbits (Placha et al. Citation2019), possible mediated via natural antioxidants that can improve meat criteria and preservation mainly by inhibiting lipid oxidation (Toschi et al. Citation2020). Using TEO in animal feeds have relied on their capabilities to prevent bacterial growth, reduce oxidative stress and enhance the antioxidant capacity as well as regulate innate immunity of animals (Nazar et al. Citation2019; Toschi et al. Citation2020). Dietary supplementation of TEO (Abdel-Wareth et al. Citation2018) or thymol leaves (Ahmed et al. Citation2020) improved significantly feed intake, growth performance, feed efficiency and meat quality in heat-stressed growing rabbit (Abdel-Wareth et al. Citation2018; Ahmed et al. Citation2020), however, the effects of TEO on the reproductive performance of rabbits does have been undiscovered, especially under hot environmental conditions. Furthermore, thyme leaves supplemented to the rabbit diets (16 g/kg of the diet) enhanced the liver and kidney functions, reduced faecal ammonia and testosterone levels and semen characteristics (Ahmed et al. Citation2020). Thymol supplementation (2 g/kg) increased blood biochemical constituents, inflammatory responses and decreased antibody titres in quail exposed to chronic HS (Nazar et al. Citation2019). Additionally, TEO administration in rats reduced the oxidative damage and augmented the antioxidant capacity and enhanced the sperm quality variables (Güvenç et al. Citation2019). In prior research, the effects of TEO on growth performance, feed efficiency, meat quality and blood parameters were investigated in rabbit and poultry species (Abdel-Wareth et al. Citation2018; Ahmed et al. Citation2020). However, to our knowledge, the impact of TEO on the blood haematological, biochemical, immunity, lipid production, antioxidant capacity and reproductive outcomes of rabbit does under HS conditions have not been explored. Therefore, the intent of this work was to discovery the potentiality effect of TEO for mitigating the negative impacts of HS in rabbit does.
Materials and methods
This work was carried out at a private commercial rabbit farm, Mansoura (Zagazig, N, 42′, 30′; E, 48′, 13′; East Delta), Dakahlia Province, Egypt in cooperation with the Physiology and Biotechnology Laboratory, Animal Production Department, Faculty of Agriculture, Mansoura University, Egypt. All procedures and experimental protocols were performed according to the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 legislations on the protection of animals used for scientific purposes. In agreement with the Directive 2010/63/EU, the experimental procedures were accepted by the Scientific Research Ethics Committee, Mansoura University, Egypt.
Tested compound
Thyme oil (Thymus vulgaris L) was identified, purchased and produced by the Horticulture Research Institute, Agricultural Research Centre (ARC), Egypt. The total phenolic contents (TPC) were determined according to Bettaieb et al. (Citation2010) (equivalent to Gallic acid; while total flavonoid content (TFC) was assayed according to the method of Riahi et al. (Citation2013). The radical scavenging activity was determined as quercetin equivalent according to Lu et al. (Citation2007) (Table ).
Meteorological parameters
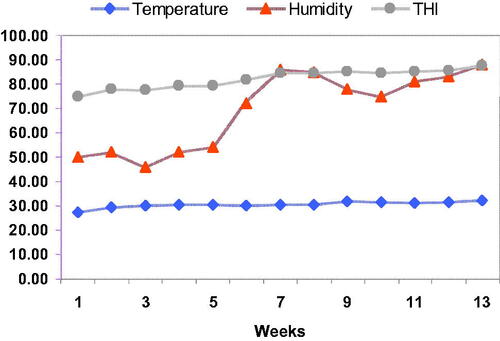
The data of ambient temperature (TM) and relative humidity (RH) were recorded (at 1400 h) daily during the full study period by an automatic Thermo hygrometer (Dostmann GmbH and Co. KG, Wertheim, Germany) set in the rabbitry established on a natural climate of HS in Dakahlia Province, Egypt. Based on the records of RH and TM, the temperature-humidity index (THI) was considered according to Marai et al. (Citation2002) as the following equation: THI = dbF ((0.55–0.55 RH) (dbF − 58)), where RH is the RH as a percentage, and dbF is dry bulb temperature in Fahrenheit degrees. To affirm the exposure of rabbits does to natural HS, the evaluation of TM, RH and THI were recorded inside the farm based on a natural climate of HS in Dakahlia Province, Egypt according to Marai et al. (Citation2002).
Animals, experimental design and diets
A total of one hundred healthy mature APRI rabbit does (Egyptian line, selected for litter weight at weaning (Abou Khadiga et al. Citation2010), weighing 3024 ± 52.89 g and six months of age were included in this research. Does were kept individually in galvanised wire cages with standard dimensions (60 × 55 × 40 cm3) and were equipped with kindling nest-box (43 × 26 × 26 cm3). The rabbit cages were supplied with manual feeders and an automatic system of nipple drinkers to offer fresh water ad libitum. The experimental groups were as follows: the 1st was fed a basal diet (TEOZ) served as the control group, while the 2nd, 3rd, 4th and 5th groups were fed with basal diet supplemented with TEO at doses of 50 (TEO50), 100 (TEO100), 150 (TEO150) and 200 (TEO200) g/kg diet, respectively. The period of TEO treatment was one month during three months as an experimental period under the Egyptian hot conditions (June–August). Does were received a basal diet formulated to provide the nutrient requirements of nulliparous does rabbits (De Blas and Mateos Citation2010). Chemical analysis and ingredients of the basal diet of nulliparous do rabbits are presented in Table . Chemical analyses of feeds were performed according to Horwitz et al. (Citation1970). Does were kept under unique hygienic, managerial and management conditions. During the experimental period, animals were imperilled to regular examinations for body condition and health status. The evaluations of body condition were conducted via touching the pelvis, spine and ribs of the rabbits.
Table 1. Composition of antioxidant activity of thyme essential oil.
Table 2. Ingredients and chemical analysis of the diet used for feeding rabbit does in different experimental treatments.
Blood haematology and biochemical assessments
At the end of the study, six does (9 months of age) in each treatment were carefully chosen randomly for blood collection. Blood samples were collected from the marginal ear-vein of the does after topical anaesthetised by Xylocaine 4% anaesthetic through heparinised pipes and separated into two subsamples. The first one was employed to assess some haematological parameters in terms of haemoglobin concentration (Hb, mg/dL), and count of red blood cells (RBCs, 106/mm3), white blood cells (WBCs, 103/mm3), platelets and haematocrit (Ht, %) using blood haematology analyser (HB 7021). Another subsample was subjected to centrifugation at (at 700 × g) for 20 min (T32c; Janetzki, Wallhausen, Germany), and plasma sections were detached and stayed in 1.5 mL Eppendorf tubes at 20 °C for further assessment.
Blood constituents
Several plasma metabolites comprising total protein (TP), albumin (AL), glucose, total cholesterol (TC), triglycerides (TG), urea and creatinine (CR) contents were determined in the blood plasma using colorimetric technique by commercial kits (BioSystem S.A., Barcelona, Spain). Globulin (GL) levels were assessed by subtracting the values of AL from the corresponding values of TP. The activities of alanine transaminase (ALT) and aspartate transaminase (AST) were measured using commercial kits (BioSystems, Barcelona, Spain).
Redox status and immunity assessments
For redox status and immunity valuations, the activities of glutathione (GSH), total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx) and malondialdehyde (MDA) in plasma were assessed via commercial kits and a spectrophotometer (Shimadzu, Kyoto, Japan) according to manufacturer’s instructions. Immunoglobulins levels (IgG and IgM) in blood plasma were evaluated by using ELISA kits.
Reproductive performance
At the end of treatment period (one month, July), all animals do were naturally mated with adult APRI rabbit bucks (sexual ratio was 1:5). Female animals were admitted to the bucks’ cage twice with 30-min intervals (Boiti et al. Citation2005). At the time of mating, doe was scored receptive when its vulva was red–violet and turgid (Theau-Clément et al. Citation2005; Eiben et al. Citation2007). Sexual receptivity rate was measured as the proportion of does that accepted mating/total number of detected does at the first mating test. Does were presented to the bucks after the 10–12 d post-parturition, which is considered the best receptivity time for the mating in this breed. Through abdominal palpation, does were palpated for validation the pregnancy after 12 d of mating (Theau-Clément et al. Citation2005; El-Ratel and Gabr Citation2019). The conception rate (CR) was calculated as follows: (number of delivered rabbit does/number of pregnant rabbit does) × 100. Total litter size (TLS) and live litter size at birth (LSB) were totalled 12 h after kindling. Kits were weaned at 28 d of age and then litter size at weaning (LSW) was determined. Also, viability rates at birth (VRB) and weaning (VRW) were documented. For the time of sucking, the cages of kids were opened for nursing litters once daily in the morning (for a maximum of 5 min) at a fixed time (7 am). Milk yield (MY, g) was measured by weighing the rabbit does immediately before and after suckling (Volek et al. Citation2018; El-Ratel and Gabr Citation2019).
Ovulatory response
For assessment the ovarian activity of does rabbits received TEO during HS, subsequent the end of suckling period (after weaning), receptive does were naturally mated, then five conceived does from each treatment were taken, transported to laboratory and slaughtered 60–46 h post-mating (El-Ratel and Gabr Citation2019). Instantly after slaughtering, ovaries were removed and then number of large follicles (LH, > 2 mm in diameter), haemorrhagic follicles (HFs), total follicles (LH + HF) and corpora lutea (CLs) on the ovarian superficial tissues were documented for all selected dose in each treatment. Ovulation rate (OR) was calculated as the following equation: OR = CLs (n)/TF (n) × 100 (El-Ratel et al. Citation2020).
Statistical model and analysis procedure
A MIXED procedure (SAS Citation2002) was used for assessing haemato-biochemical parameters, redox status, immunity indicators, fertility traits and milk production. Thyme oil levels were introduced in the statistical model as a fixed factor whereas doe individual was introduced as a random factor. Multiple comparisons among means were carried out by the Duncan’s Multiple Range Test (DMRT; Steel et al. Citation1980). Chi-square test used to determine the effect of TEO levels on CRs. The statistical significance was accepted at probability less than .05. Shapiro–Wilk test was conducted in order to check for normality (Razali and Wah Citation2011).
Results
Temperature humidity index (THI) values
Data in Figure display that through the first six weeks of the research, the estimated THI values ranged between 74.78 and 81.69, revealing slight HS. For the duration from the six weeks to the end of experiment, the expected THI values fluctuated from 84.39 to 87.51 demonstrating a considerable degree of HS on rabbit does.
Haematological attributes and blood metabolites
The highest values of TP, AL and GL contents and the lowest contents of glucose, TG, CR, AST and ALT were recorded in group treated with 100 mg TEO/kg diet when compared with the heat-stressed group (Table ). All levels of TEO decreased significantly both blood serum of TC (p ≤ .0001) and TG (p ≤ .0001) levels, being the lowest with TEO at a level of 150 mg/kg. Comparable to the TEOZ group, animals received TEO exhibited significant reduction in the levels of AST and ALT. In connected to the haematological attributes, the dietary TEO inclusion also caused an increase in the concentrations of Hb (p < .0001) and Ht (p = .0460) contents. Conversely, the TEO supplementation did not affect blood platelets and the counts of RBCs and WBCs in rabbit does.
Table 3. Effect of adding different levels of thyme essential oil on blood metabolites and haematological attributes of rabbit does exposed to heat stress conditions (mean ± SEM).
Redox status and immunity
As shown in Table , rabbit does receive TEO at any level had significantly lowered the GSH (p ≤ .0001) and MDA (p ≤ .0001) contents compared with the TEOZ group. Dietary supplementation with TEO significantly enhanced the TAC levels (p < .0001). Supplementing of TEO to the diets had no affects the contents of GPx and SOD of plasma compared with the control group (Table ). The minimum values were observed at 100 mg/kg diet for MDA, while the highest values of TAC and GSH were recorded at 100 mg/kg diet, respectively. For immunity indicators, all treated groups had higher (p < .05) contents of blood IgG and IgM when compared with untreated groups. Moreover, the highest values were observed in rabbit does received 100 mg/kg diet during HS conditions. The greatest values of both of IgG and IgM were recorded at 100 mg/kg diet. Overall, TEO supplementation improve the heat tolerance of rabbits does via augmented immunological (IgG and IgM) and antioxidant capacity such GSH and TAC levels.
Table 4. Effect of different levels of thyme essential oil on redox status and immunity indicators of rabbit does rabbit does exposed to heat stress conditions (mean ± SEM).
Reproductive performance
Rabbit does were given TEO at any level had higher CR (p ≤ .05) compared with control group (Table ). Inclusion of TEO into the diets of heat-stressed rabbit does enhanced significantly TLS (p = .0005), LSB (p ≤ .0001), LSW (p ≤ .0001) and VRW (p = .0420) and VRB aspects (p ≤ .0001) (Table ). The greatest value of TLS and LSB was recorded at 100 mg/kg diet, while it was at 150 mg/kg diet for VRW and LSW. Dietary TEO significantly improved the milk production of rabbit does exposed to HS conditions. The greatest values were observed at 100 mg/kg diet for milk production at 1–4 weeks.
Table 5. Effect of different levels of thyme essential oil on reproductive traits of rabbit does rabbit does exposed to heat stress conditions (mean ± SEM).
Ovarian activity and embryo quality
Heat-stressed rabbit does receive TEO at various levels did not demonstrate statistical differences (p > .05) in the number of large follicles and the percentage of embryo recovery in comparison with the control (TOZ; Table ). Supplementation of TEO to the diets of the heat-stressed rabbit does induced significant decrease in the number of haemorrhagic and total follicles as well abnormal embryos (%) compared with the control (TEOZ group). Ovulation, and the percentages of normal embryos were significantly (p < .05) increased by dietary TEO supplementation compared to the control. Overall, dietary inclusion of TEO significantly improved the embryo quality of heat-stressed rabbits.
Table 6. Effect of adding different levels of thyme essential oil on ovarian activity and the quality of embryos of rabbit does rabbit does exposed to heat stress conditions (mean ± SEM).
Discussion
In this study, it was found that the supplement of TEO at level of 100–150 mg/kg diet facilitated to enhance the haemato-biochemicals constitutes, immunologic status, antioxidant capacity, reduce the lipid peroxidation by enhancing the heat tolerance, which induced enhancements to female rabbit fertility during high environmental temperatures. The beneficial feature of TEO supplementation improved blood metabolites molecules with thermoregulatory agent and activated the antioxidant agent as well as stimulate the synthesis of immunological bodies such IgM and IgG under HS conditions. Additionally, TEO supplementation could effectively decreased MDA levels and augmented the contents of antioxidant enzymes which resulted in a decreased of lipid peroxidation and ROS synthesis mainly induced by HS. These results suggest that the protective action of TEO was due to the supportive heat tolerance and antioxidant effect, hence resulting in enhance the reproductive efficiency of female rabbit under HS.
Rabbit females have a damaging reproductive function through summer months due to the Previous studies reported that the exposure of female rabbit to HS could evoke negative effects by disturbing the immune status, suppress rabbit viability (Sirotkin et al. Citation2021), destruction of ovarian cells (Naseer et al. Citation2017), reducing the antioxidant capacity (Madkour et al. Citation2020, Citation2021) and pregnancy failure and finally reduce the reproductive capability. Several reports on reproductive aspects indicated that the potentiality role of natural phytochemicals compounds, which have facilitated the research on the development of therapeutics including CoQ10, carnitine, quercetin, ascorbic acid and vitamin E (Lu et al. Citation2019; Sharaf et al. Citation2021). The results showed that the increasing level of TEO up to 150 mg/kg diet improves the TP, AL and GL levels. This could be mediated by the proteinogenic and chaperone properties of TEO (Nazar et al. Citation2019; Alagawany et al. Citation2021). This property is crucial under environmental HS conditions since it regulates water balance and facilitates the maintenance of protein and enzyme stability.
On the other hand, increasing TEO inclusion in the heat-stressed does rabbit reduced blood plasma of liver function such (ALT and AST activities), kidney function such uric and creatine levels as well as TG and CH concentrations. The consequences are in accordance with several previous works in growing rabbit. It has been demonstrated that HS have altered lipid and protein metabolism and absorption in the liver. Superior extracellular of AST and ALT levels is thought a deleterious sign of liver function, hence TEO might have a hepatic-protective impact (Abdelnour et al. Citation2020). Accumulated researches reported that animals exposed to high temperatures have reduced ATP production, higher free radical, fluctuations in the precursor source for gluconeogenesis and accumulation of lipids in the hepatic tissues that may contribute to fatty accumulation in the liver (Sejian et al. Citation2018).
Our study demonstrates the capability of TEO in reducing the fatty accumulation in the liver with potential protective role to counteract several metabolic disorders associated with blood metabolites and female reproductive disorder (Lu et al. Citation2019; Nazar et al. Citation2019). In line with our results, Saravanan and Pari (Citation2015) reported that TEO supplementation had ability to reduce the levels of plasma TG, TC, free fatty acids (FFAs), low-density lipoprotein (LDL) in high-fat diet-induced type 2 diabetes in mice. Based on this previous feature of TO, it has anti-hyperglycaemic and anti-hyperlipidaemic activities (Saravanan and Pari Citation2015). Several studies suggested that the fatty accumulation in the liver resulted in reproductive disorder (Wu et al. Citation2018) and other metabolic syndrome (Tarantino et al. Citation2019). Furthermore, rabbit female treated with TEO have more constancy of the liver function by declining the plasma levels of TG TC, CR, uric acid and the activities of AST and ALT activities (Table ).
The detected boost in the liver function of treated HS rabbits could be accredited to the TEO has a pharmacological profile of a new antilipolytic mediator. As for thymol, positive effects have been described when this natural compound was included in the diet of poultry species (Abd El-Hack et al. Citation2016) and fish (Alagawany et al. Citation2021), improving the heat tolerance via increasing the RBCs and haemoglobin as noted in this study. These boosts are also essential adaptive mechanisms during high environmental temperatures to enhance oxygen circulation during panting process, enhancing the panting process efficiency and thus heat loss (Abdelnour et al. Citation2021). Similarity Nazar et al. (Citation2019) found that the addition of 2 g of thymol improved blood metabolites, such as TP, AL and glucose in Japanese quail after exposure to chronic HS.
Using thymol and its derivatives (leaves or oil) as a potential natural antioxidant in livestock diets are effective factor for enhancing the reproductive, productive traits, meat quality under hot conditions (Abdel-Wareth et al. Citation2018; Placha et al. Citation2019; Ahmed et al. Citation2020). To our knowledge, for the first time to use TEO to frustration the undesirable influences of a tropical elevated environment in female rabbit. In the sequence of this antioxidant capability of TEO, and in respect of their ability to distraction oxidative stress that could damage other molecules, health-promoting effects are described in this research (Table ).
TEO supplementation enhanced the redox status of naturally heat-stressed rabbit does by increasing the activity of GSH and TAC in blood plasma. The TAC and GSH are valuable indictors for the availability of the lowering OS in blood plasma, and hence the capacity of plasma TAC and GSH to neutralise OS formed from oxidation pathways induced by HS (Abdelnour et al. Citation2020; Sheiha et al. Citation2020). Enhancing the redox status of rabbit does by TEO administration could improve the reproductive outcomes which were observed in this study. Authors suggested that higher OS is involved in pathophysiology of subfertility in female rabbits reared under hot conditions. Antioxidants are supposed to neutralise the damage which are formed during the initial step of lipid oxidation caused by HS. Thymol oil has oxygen radical scavenging activities that may contribute to these observations towing to its antioxidant properties (Abdollahzadeh et al. Citation2014). The phenolic OH group in thymol is responsible for its antioxidant capabilities, whereby it serves as a hydrogen donor and can neutralise the peroxyl radicals (Güvenç et al. Citation2019). The decline levels of MDA in does treated with TEO indicate that it is an adequate antioxidant capable of removing OS in the plasma. Rats received thymol at 10 or 20 mg/kg diet exhibited significant reduction in the MDA levels in kidney, liver and testicles tissues compared to the control group (Güvenç et al. Citation2019). Moreover, the same authors reported that an increase of antioxidant levels and enhanced the sperm variables were observed in rats fed with thymol molecules (Güvenç et al. Citation2019). The antioxidant activities of TEO have a great defensive impact in protecting intracellular lipids against peroxidation, as well as on MDA levels as an indicator of lipid peroxidation (Bacova et al. Citation2020) and ovarian tissue against oxidative stress triggered by HS and to enhance reproduction aspects in HS conditions (El-Ratel et al. Citation2020). Recently, El-Zaher et al. (Citation2021) found that ewes received a mixture of thyme (10 g/head/d) and celery (10 g/head/d) boosted (p < .001) antioxidant capacity in the luteal phase compared with the follicular stage in ewes. These results suggest that supplementation with this phytogenic product in does diets stimulated antioxidant responses and therefore augmented weight gain in its offspring’s and improve the reproductive outcomes.
HS results in a reduce the immune capacity in female rabbits via reducing the synthesis of immunological variables (IgG and IgM) (El-Ratel et al. Citation2020). This alteration in the immune capacity could make animal more sensitivity to diseases (Abdelnour et al. Citation2020), and thus any reduction in the immunity with HS may also impede the reproductive efficiency in rabbit (El-Ratel et al. Citation2020). In this outcome, dietary TEO supplementation offers an avenue to overcome the negative effects of HS in rabbit does through boosting the synthesis of immunological variables. Several natural antioxidants used to overcome the negative impacts of HS in rabbits (Abdelnour et al. Citation2020; El-Ratel et al. Citation2020; Sharaf et al. Citation2021). Immunoglobulins levels such IgG and IgM were increased significantly by 18 and 21%, respectively, in rabbit does receive TEO a level 100 mg/kg. In line with this data, we previously found that the addition of allicin (10 mg/kg diet) enhanced the immunological variables in rabbit does (El-Ratel et al. Citation2020).
A growing form of evidence highlights the negative effects of HS on the reproductive capacity of rabbit does (Sejian et al. Citation2018; Sirotkin et al. Citation2021), through reducing the ovarian activity, hormonal alterations and sexual receptivity (Abdelnour et al. Citation2020; Sirotkin et al. Citation2021). Reproductive criteria are significantly affected by environmental issues, such as temperature, humidity and light length. HS during pregnancy and lactation can affect not only foetal development but also postnatal growth of kits (Sirotkin et al. Citation2021). Enhancement of reproduction and fertility of rabbits during exposing to HS conditions are important topics in livestock production particularly in developing countries (Abdelnour et al. Citation2020). Several in vitro and in vivo studies have been applied to overcome the negative impacts of HS to sustain the reproductive competence of rabbit farms in summer months. Therefore, it is effective to expand applicable therapies that normalise fertility capacity and overall reproduction efficiency in HS by phytogenic supplementation.
Goats were nourished a basal diet TEO at a daily dose of 2 mL/head/d had helpful impacts on digestion, absorption, rumen fermentation variables and milk composition (El-Essawy et al. Citation2021). In this study, dietary TEO supplementation significantly enhanced the sexual receptivity and CR in heat-stressed rabbit does (Table ). In addition, litter size at birth and weaning as well as viability rates in both at birth and weaning were improved by dietary TEO supplementation under HS conditions.
Our results demonstrated that the milk production was enhanced by dietary TEO supplementation at level 100 and 150 mg/kg diet. This increase in milk production reflects the improvement of litter size and viability at weaning. As reported by El-Essawy et al. (Citation2021), the dietary inclusion of TEO in dairy goats could enhance the milk composition and milk fatty acid profile. However, in our study, we have not estimated the milk fatty acid profile in neither heat-stressed rabbit nor supplemented groups and further exploration are needed in this topic. So, we will focus on the upcoming research on the beneficial impacts of phytogenic on milk profile and other components in the rabbit milk as well the alteration of HS on the previous variables. For the ovarian activity, the dietary TEO supplementation to the diets of heat-stressed does significantly (p ≤ .001) reduced the number of HFs and the percentage of abnormal embryos compared with the control group. The percentages of normal embryo and ovulation were improved in all treated group. According to our results, TEO inclusion in the does rabbit exposure to HS could improve the reproductive capacity of rabbits by improving immune response and antioxidant activity, whereas TEO molecules may act as a potential feed additive that can be applied in rabbit production and preventing the negative impacts of HS. Thus, these effects of dietary TEO supplementation may be related to sexual neurotransmitter signalling pathways and blood flow throughout the genital tract, leading to improvements in the overall sexual performance of rabbit females. Besides, TEO contributes to multiple signalling pathways that are involved in the molecular regulation of folliculogenesis and embryo development in rabbit. In goats, it was found that TEO enhanced the synthesis of estradiol-17β (E2) during the follicular phase of the oestrous cycle and increase in progesterone (P4) during the luteal phase (El-Essawy et al. Citation2021). In accordance with our results, Tafesh et al. (Citation2021) found an increase of the relative uterus and placenta weights as well as the litter size and weight in rat exposed to moderate does of TEO. This improvement could associate with the capability of TEO crossing the placental barrier in rats and improved the resorption index during pregnancy period. A superior number of apoptotic cells were detected in antral and primary follicles of rabbit does exposed to HS (Naseer et al. Citation2017).
Overall, the supplement of TEO at level 100–150 mg/kg diet enhanced the heat tolerance and female rabbit fertility during high environmental temperatures by improving the immunologic status, antioxidant capacity, reduce the lipid peroxidation. The impact of thymol oil on oocyte competence and further embryonic progress needs to be discovered. Moreover, an elaborative study about the potential role of TEO in follicular and embryonic development which could be supportive to comprehend strategy for mitigating HS in rabbits.
Conclusion
Generally, dietary inclusion of TEO may help in mitigating the harmful impacts of HS in rabbit does through modifying the haematological, metabolites, reduces lipid peroxidation, enhancing the antioxidant capacity and immunological parameters as well as improve the ovarian activity and embryo grading. These consequences were epitomised by enhancing the fertility outcomes and heat tolerance of rabbit does received TEO supplementation (100–150 mg/kg diet). Further exploration is needed to examine the transgenerational long-time effects of TEO and determine the optimum dose for effective thermoregulatory agent in rabbit farms.
Ethical approval
Animal care and maintenance were performed in accordance with the guidelines of the Egyptian Research Ethics Committee and the guidelines specified in the Guide for the Care and Use of Laboratory Animals (2011).
Acknowledgements
The authors extend thanks to their respected institutes and universities.
Disclosure statement
No potential conflicts of interest declared.
References
- Abdel-Wareth AAA, Taha EMM, Südekum KH, Lohakare J. 2018. Thyme oil inclusion levels in a rabbit ration: evaluation of productive performance, carcass criteria and meat quality under hot environmental conditions. Anim Nutr. 4(4):410–416.
- Abd El-Hack ME, Alagawany M, Farag MR, Tiwari R, Karthik K, Dhama K, Zorriehzahra J, Adel M. 2016. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J Essential Oil Res. 28(5):365–382.
- Abdelnour SA, Al-Gabri NA, Hashem NM, Gonzalez-Bulnes A. 2021. Supplementation with proline improves haemato-biochemical and reproductive indicators in male rabbits affected by environmental heat-stress. Animals. 11(2):373.
- Abdelnour SA, El-Saadony MT, Saghir SAM, Abd El-Hack ME, Al-Shargi OYA, Al-Gabri N, Salama A. 2020. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest Sci. 240:104220.
- Abdollahzadeh E, Rezaei M, Hosseini H. 2014. Antibacterial activity of plant essential oils and extracts: the role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control. 35(1):177–183.
- Ahmed AE, Alkahtani MA, Wareth AAA. 2020. Thyme leaves as an eco-friendly feed additive improves both the productive and reproductive performance of rabbits under hot climatic conditions. Vet Med. 65(12):553–563.
- Abou Khadiga G, Youssef YMK, Saleh K, Nofal RY, Baselga M. 2010. Genetic trend in selection for litter weight in two maternal lines of rabbits in Egypt. World Rabbit Sci. 18(1):27–32.
- Alagawany M, Farag MR, Abdelnour SA, Elnesr SS. 2021. A review on the beneficial effect of thymol on health and production of fish. Rev Aquacult. 13(1):632–641.
- Bacova K, Zitterl-Eglseer K, Chrastinova L, Laukova A, Madarova M, Gancarcikova S, Sopkova D, Andrejcakova Z, Placha I. 2020. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals. 10(8):1248.
- Bettaieb I, Bourgou S, Wannes WA, Hamrouni I, Limam F, Marzouk B. 2010. Essential oils, Phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J Agric Food Chem. 58(19):10410–10418.
- Boiti C, Castellini MTM, Besenfelder U, Liguori L, Renieri T, Pizzi F. 2005. Guidelines for the handling of rabbit bucks and semen. World Rabbit Sci. 13:71–91.
- Büyükkılıç Beyzi S, Konca Y, Kaliber M, Sarıözkan S, Kocaoğlu Güçlü B, Aktuğ E, Şentürk M. 2020. Effects of thyme essential oil and A, C, and E vitamin combinations to diets on performance, egg quality, MDA, and 8-OHdG of laying hens under heat stress. J Appl Anim Res. 48(1):126–132.
- De Blas C, Mateos GG. 2010. Feed formulation. In: De Blas C, Wiseman J, editors. Nutrition of the rabbit. Wallingford: CABI Publishing; p. 222–232.
- Eiben C, Tóbiás G, Kustos K, Gódor-Surmann K, Kotány S, Gulyás B, Szira G. 2007. The change of nursing for oestrus induction (biostimulation): effect of contact between rabbit doe and its young. Livest Sci. 111(3):193–203.
- El-Essawy AM, Anele UY, Abdel-Wahed AM, Abdou AR, Khattab IM. 2021. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Anim Feed Sci Technol. 271(114760):114760.
- El-Ratel IT, Abdel-Khalek AKE, Gabr SA, Hammad ME, El-Morsy HI. 2020. Influence of allicin administration on reproductive efficiency, immunity and lipid peroxidation of rabbit does under high ambient temperature. J Anim Physiol Anim Nutr. 104(2):539–548.
- El-Ratel IT, Gabr AA-W. 2019. Effect of spirulina and vitamin e on reproduction and in vitro embryo production in heat-stressed rabbits. Pak J Biol Sci. 22 (11):545–553.
- El-Zaher HM, Eid SY, Shaaban MM, Ahmed-Farid OA, Abd El Tawab AM, Khattab MSA. 2021. Ovarian activity and antioxidant indices during estrous cycle of Barki ewes under effect of thyme, celery and salinomycin as feed additives. Zygote. 29(2):155–160.
- Güvenç M, Cellat M, Gökçek İ, Yavaş İ, Yurdagül Özsoy Ş. 2019. Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats. Arch Physiol Biochem. 125(5):396–403.
- Horwitz W, Chichilo P, Reynolds H. 1970. Official methods of analysis. Rockville (MD): AOAC.
- Lu J, Zhao H, Chen J, Fan W, Dong J, Kong W, Sun J, Cao Y, Cai G. 2007. Evolution of phenolic compounds and antioxidant activity during malting. J Agric Food Chem. 55(26):10994–11001.
- Lu Z, He X, Ma B, Zhang L, Li J, Jiang Y, Zhou G, Gao F. 2019. Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor α pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J Sci Food Agric. 99(13):5631–5637.
- Madkour M, Aboelenin MM, Aboelazab O, Elolimy AA, El-Azeem NA, El-Kholy MS, Alagawany M, Shourrap M. 2021. Hepatic expression responses of DNA methyltransferases, heat shock proteins, antioxidant enzymes, and NADPH 4 to early life thermal conditioning in broiler chickens. Ital J Anim Sci. 20(1):433–446.
- Madkour M, Aboelenin MM, Younis E, Mohamed MA, Hassan H, Alagawany M, Shourrap M. 2020. Hepatic acute-phase response, antioxidant biomarkers and DNA fragmentation of two rabbit breeds subjected to acute heat stress. Ital J Anim Sci. 19(1):1558–1566.
- Marai IFM, Habeeb AAM, Gad AE. 2002. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci. 78(2):71–90.
- Marco-Jiménez F, García-Diego FJ, Vicente JS. 2017. Effect of gestational and lactational exposure to heat stress on performance in rabbits. World Rabbit Sci. 25(1):17–25.
- Naseer Z, Ahmad E, Epikmen ET, Uçan U, Boyacioğlu M, İpek E, Akosy M. 2017. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology. 96:136–141.
- Nazar FN, Videla EA, Marin RH. 2019. Thymol supplementation effects on adrenocortical, immune and biochemical variables recovery in Japanese quail after exposure to chronic heat stress. Animal. 13(2):318–325.
- Placha I, Ocelova V, Chizzola R, Battelli G, Gai F, Bacova K, Faix S. 2019. Effect of thymol on the broiler chicken antioxidative defence system after sustained dietary thyme oil application. Br Poult Sci. 60(5):589–596.
- Razali NM, Wah YB. 2011. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Modeling Anal. 2(2):21–33.
- Riahi L, Chograni H, Elferchichi M, Zaouali Y, Zoghlami N, Mliki A. 2013. Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind Crops Prod. 46:290–296.
- SAS. 2002. SAS user’s guide, statistics. Cary (NC): SAS Institute.
- Saravanan S, Pari L. 2015. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. Eur J Pharmacol. 761:279–287.
- Sejian V, Bhatta R, Gaughan JB, Dunshea FR, Lacetera N. 2018. Review: adaptation of animals to heat stress. Animal. 12:s431–444.
- Sharaf AK, El-Darawany AA, Nasr AS, Habeeb AAM. 2021. Alleviation the negative effects of summer heat stress by adding selenium with vitamin E or AD3E vitamins mixture in drinking water of female rabbits. Biolo Rhythm Res. 52(4):535–548.
- Sheiha AM, Abdelnour SA, Abd El-Hack ME, Khafaga AF, Metwally KA, Ajarem JS, Maodaa SN, Allam AA, El-Saadony MT. 2020. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 10(3):430.
- Sirotkin AV, Parkanyi V, Pivko J. 2021. High temperature impairs rabbit viability, feed consumption, growth and fecundity: examination of endocrine mechanisms. Domest Anim Endocrinol. 74:106478.
- Steel RGD, Torrie JH, Dickey DA. 1980. Principles and procedures of statistics. McGraw-Hill, New York. Principles and procedures of statistics. 2nd ed. New York (NY): McGraw-Hill.
- Tafesh ZQ, Mansoor KA, Qinna NA, El-Hajji FD, Arafat TA, Abu-Qatouseh LF. 2021. Reproductive safety assessment of Thymus vulgaris L. extract and quantification of thymol sulfate in pregnant rats and fetuses using a validated LC/MS method of analysis. J Appl Pharm Sci. 11:144–151.
- Tarantino G, Citro V, Capone D. 2019. Nonalcoholic fatty liver disease: a challenge from mechanisms to therapy. J Clin Med. 9(1):15.
- Theau-Clément MML, Castellini C, Besenfelder U, Boiti C. 2005. Recommendations and guidelines for applied reproduction trials with rabbit does. World Rabbit Sci. 13:147–164.
- Toschi A, Tugnoli B, Rossi B, Piva A, Grilli E. 2020. Thymol modulates the endocannabinoid system and gut chemosensing of weaning pigs. BMC Vet Res. 16(1):289.
- Volek Z, Ebeid TA, Uhlířová L. 2018. The impact of substituting soybean meal and sunflower meal with a mixture of white lupine seeds and rapeseed meal on rabbit doe milk yield and composition, and the growth performance and carcass traits of their litters. Anim Feed Sci Technol. 236:187–195.
- Wu J, Yao XY, Shi RX, Liu SF, Wang XY. 2018. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod Health. 15(1):77.