Abstract
The objective of the present study is to examine the effects of probiotics, organic acids, and their combination on growth performance, carcase characteristics, blood biochemical parameters, antioxidative status, immune responsiveness, and bacterial count in broilers. A total of 1,200 1-d-old unsexed broiler chicks (Ross 308) were randomly assigned in a completely randomised design according to a 2 × 3 factorial arrangement, with 2 levels of dietary probiotics (0% and 0.2%) possessing 4 × 109 cfu/g of Bacillus subtilis and Bacillus licheniformis and 3 levels (0, 0.03, and 0.06%) of organic acids blend of acetic and propionic acids for a total of 6 experimental treatments with 10 replicates each (20 birds/replicate). Dietary 0.02% probiotics increased final body weight. Addition of probiotics or organic acids increased plasma total protein concentration significantly. Dietary 0.02% probiotics increased plasma glutathione peroxidase (GSH-Px) concentration and enhanced humoral- and cell-mediated immune response. Supplementation of 0.03% and 0.06% organic acids improved cell-mediated immune response significantly. Interestingly, addition of probiotics and/or organic acids reduced total bacterial count in breast meat, and Salmonella was not detected in meat. A significant interaction between probiotics and organic acids was observed in plasma globulin concentration, plasma GSH-Px concentration, cell-mediated immune response at 24 h post-phytohaemagglutinin-P injection, intestinal Salmonella count, and total bacterial count in meat. It could be concluded that the inclusion of probiotics and/or organic acids enhanced humoral- and cell-mediated immunity and decreased the counts of E. coli and Salmonella in the gut, and reduced total bacterial count in breast meat.
Probiotics and/or organic acids enhanced immunity.
Probiotics and/or organic acids improved meat safety.
Probiotics and/or organic acids could be used as growth promoters.
HIGHLIGHTS
Introduction
For many years, antibiotics were used as growth promoters in poultry and livestock production. Nonetheless, the excessive using of antibiotics has led to the establishment of resistance by pathogenic bacteria and the presence of antibiotics residues in poultry products, which pose a risk to human health (Gadde et al. Citation2018). Therefore, since 2006 in European Union, the use of antibiotics for the purpose of stimulating growth has been prohibited, and their use is restricted to only necessary therapeutic purposes. Attention has been shifted to the natural growth promoters as alternatives to antibiotics, which include probiotics, prebiotics, organic acids, phytobiotics, mannanoligosaccharides, and enzymes (Khan and Iqbal Citation2016; Jha et al. Citation2020). Within these alternatives, probiotics and organic acids (acidifiers) have received global interest for enhancing the health and performance in poultry sector because of the increased require for antibiotic-free poultry products.
Probiotics are defined as ‘live micro-organisms, when supplemented in sufficient quantities, grant health benefits to the host’ (FAO/WHO Citation2001). Bacillus-based probiotics became a popular bacterium used in poultry industry because they are steady and active during feed processing, handling, and transition within the gastrointestinal tract (GIT, Chaucheyras-Durand and Durand Citation2010). Probiotics feed supplementation improved growth, feed efficiency, and intestinal health (Gong et al. Citation2018; Abdel-Moneim et al. Citation2020). Also, probiotics activate the immune response and strengthen the resistance to pathogenic bacteria colonisation such as Salmonella and E. coli (Fathi et al. Citation2017; Ebeid et al. Citation2019). These improvements are accomplished by lowering the intestinal pH, modulating the intestinal microbiota, and enhancing the digestibility. Likewise, probiotics are involved in stimulation of endogenous enzymes, reduction of toxic substance produced by metabolic reactions, and production of vitamins or antimicrobials such as bacteriocins that prohibit toxins' production and adhesion of pathogens (Vieco-Saiz et al. Citation2019; Ebeid et al., Citation2021).
Organic acids or acidifiers could be defined as weak carboxylic acids (R-COOH) such as acetic, propionic, formic, fumaric, lactic, and sorbic acids. It was reported that the inclusion of organic acids had a positive impact on growth performance, feed efficiency, and digestibility of nutrients (Nguyen et al. Citation2018; Yang et al. Citation2018). Also, organic acids might be involved in activating the immune response and suppressing the pathogenic bacteria colonisation (Ebeid and Al-Homidan Citation2021). These improvements might be accomplished by: (1) decreasing the GIT pH, (2) improving the nutrient utilisation in diets, (3) suppressing the pathogens' growth and proliferation, and (4) enhancing the immune responsiveness in poultry (Khan and Iqbal Citation2016).
Previous studies showed that dietary combination of probiotics and organic acids hold several promises in improving intestinal morphology, energy and protein utilisation, and gut microflora and consequently growth performance in broiler chickens (Agboola et al. Citation2016; Nari et al. Citation2020). It could be hypothesised that there may be a synergism between probiotics and organic acids to protect against pathogenic bacteria and enhance beneficial bacteria in the GIT. To our knowledge, studies examining the combined effect of dietary probiotics and organic acids are still scarce. Therefore, the objective of the present study is to examine the impacts of probiotics, organic acids, and their combination on growth performance, carcase traits, blood biochemical parameters, antioxidative status, immune responsiveness, and bacterial count in broiler chickens.
Material and methods
Birds, housing, and dietary treatments
A total of 1,200 1-d-old unsexed broiler chicks (Ross 308) were bought from a commercial hatchery. The broiler chicks were randomly allocated in a completely randomised design according to a 2 × 3 factorial arrangement, with 2 levels of dietary probiotics (0% and 0.02%) possessing 4 × 109 cfu/g of Bacillus subtilis and Bacillus licheniformis (Bioplus® VC, Biochem© Zusatzstoffe Handels-und Produktionsges, 49393 Lohne, Germany) and 3 levels (0, 0.03, and 0.06%) of organic acids blend of acetic and propionic acids (Galliacid®S, Vetagro, Italy) for a total of 6 experimental treatments. Probiotics were added to diets and organic acids blend was added to drinking water. Each treatment (n = 200) comprised 10 floor pens (replicates) of 20 birds/pen. Chicks were placed in floor pens covered with 5–6 cm wood shavings. Each pen size was 220 cm in width, 320 cm in length, and 400 cm in height. Chicks were fed a mash starter-grower diet for 4 wk and mash finisher feed for 2 wk. The experimental diets primarily consisted of ground yellow corn and soybean meal (Table ). Birds were kept in a semi-closed building. Room temperature was 25–29 °C with proportional moisture between 50% and 70% during the trial. Broilers were given unlimited access to the experimental diets and water. Light was offered for 23 h daily. Chicks were reared according to the Ross Broiler Commercial Management Guide's recommendations. The Animal Ethics Committee of Qassim University in Saudi Arabia approved all of the procedures used in the present study. All precautions were taken to reduce pain during the experimental period.
Table 1. Ingredients and composition of the starter-grower and finisher diets (as-fed basis).
Growth performance
All chicks were individually weighed when they arrived to assess their starting weight. Subsequently, body weight (BW) in grams was weekly measured until 6 wk of age. From feed intake (FI) and body weight gain (BWG), feed conversion ratio (FCR) was determined for each replicate within each treatment (0–42 d). The FI and FCR were balanced for mortality when necessary.
Carcase characteristics
At 6 wk of age, 10 broiler chickens from each treatment (1 broiler chicken/pen) were randomly taken and sacrificed to evaluate carcase traits. Live BW, blood, feathers, dressing carcase, whole leg, thigh, drumstick, breast muscles (Pectoralis major and Pectoralis minor) from the right side, giblets (liver, heart, and gizzard), and lymphoid organs (bursa of Fabricius and spleen) were separated, weighed, and calculated as a percentage of live BW.
Blood plasma biochemical parameters
At 6 wk of age, 10 blood samples from each treatment (1 blood sample/pen) were collected into heparinised test tubes, centrifuged at 6,000 × g for 10 minutes at 4 °C to separate the plasma and stored at −20 °C until analysis. Plasma total protein, albumin, total cholesterol, and triglycerides concentrations were spectrophotometrically measured using commercial kits (Stanbio Laboratory, Boerne, TX). The globulin concentration was calculated as the difference between the total protein and albumin. Plasma triiodothyronine (T3) and thyroxine (T4) concentrations were evaluated using commercial ELISA kits (BioCheck®, Foster City, USA).
Antioxidant status and lipid peroxidation
The concentrations of glutathione peroxidase (GSH-Px) and total antioxidant capacity (TAC), and malondialdehyde (MDA) in blood serum were evaluated using commercial kits of Bio-Diagnostic, Giza, Egypt. The tests were performed in accordance with the manufacturer's guidelines.
Cell-mediated immunity assay
The cellular immune response was determined by cutaneous basophilic hypersensitivity test using phytohaemagglutinin-P (PHA-P, lectin from Phaseolus vulgaris) following the toe-web injection technique. At 5 wk of age, 1 broiler/pen was randomly taken (10 from each treatment). Each broiler was intradermally injected in the toe web with 100 μg PHA-P (Sigma Chemical Co., St. Louis, MO, USA) in 0.1 mL sterile saline. The thickness of the web was assessed with a constant tension calliper pre-injection and at 24, 48, and 72 h post-injection of PHA-P. The web swelling was determined as the difference between the thickness of the web before and after injection.
Assay of serum newcastle disease virus (NDV) antibody titre
Birds were vaccinated against Newcastle disease virus (NDV) vaccine (LaSota strain) via drinking water 7 days before serum collection. Serum antibody titres against NDV were evaluated by ELISA using an NDV antibody commercial kit (Bio-Chek B.V., Reeuwijk, Netherlands). The test was performed according to Fathi et al. (Citation2020) using the manufacturer’s instructions.
Concentrations of blood immunoglobulins (IgY, IgA, and IgM)
At 6 wk of age, 10 blood samples from each treatment (1 blood sample/pen) were randomly collected and used in determining plasma concentrations of IgY, IgA, and IgM by a sandwich ELISA using microtiter plates and chicken-specific IgY, IgA, and IgM ELISA quantitation kits (Bethyl Laboratories, Montgomery, TX, USA).
Bacterial count
At 6 wk of age, 10 broiler chickens per treatment (1 broiler chickens/pen) were slaughtered to collect the caecal contents and breast meat samples, which were carefully hand-stripped into sterile containers. Total aerobic bacteria, Salmonella spp. Escherichia coli and Bacillus subtilis were analysed in the caecal digesta and breast meat samples according to the procedures described by McDonald et al. (Citation1983), Horn et al. (Citation1996) and Hossain et al. (Citation2016). Briefly, 1/10 serial dilutions of digesta were prepared in buffered peptone water (1 g/L peptone, 8 g/L NaCl, and 0.5 g/L L-cysteine hydrochloride) for the enumeration of total aerobic bacteria, E. coli, B. subtilis and Salmonella. Each dilution was cultured on selective media for each bacterial strain to be counted or detected. Nutrient agar was used for the total aerobic bacteria count, and MacConkey agar was used for the E. coli count and Mannitol Egg Yolk Polymyxin agar was used for B. subtilis. Detection of Salmonella spp. was achieved by multiplying the dilutions in Tetrathionate broth for 24 h and culturing onto modified brilliant green agar supplemented with 20 mg/mL novobiocin. The culture plates for total aerobic bacteria, E. coli, B. subtilis, and Salmonella spp. were incubated at 37 °C in an aerobic environment. The colony-forming units (cfu) in log10 per gram for total aerobic bacteria, E. coli and B. subtilis within digesta was counted on the basis of the colony morphology and characteristics. Salmonella spp. was expressed as a percentage of detection.
Statistical analysis
Data were analysed by two-way ANOVA using JMP Ver. 11, Cary, NC, USA (SAS Institute, Citation2013), with the probiotics and organic acids as fixed effects. The significance of difference among the experimental treatments was determined using Tukey’s test. Statistical significance was considered when p < .05.
Results
Growth performance and carcase characteristics
Results presented in Table show the effect of adding probiotics to feed and organic acids blend to water on growth performance measurements in broiler chickens. Results clarified that broiler chickens fed 0.02% probiotics in their diets have significantly heavier BW at 6 wk of age compared to those fed the control diet. There is no significant effect of adding either probiotics or organic acids on FI, FCR, and mortality percentage of broiler chickens. The influence of adding different levels of probiotics and organic acids on carcase and carcase parts percentages was summarised in Table . No significant differences were noticed among experimental treatments in the percentages of whole carcase and carcase parts (whole leg, thigh, drumstick, major pectoral, minor pectoral, heart, liver, gizzard, bursa of Fabricus, and spleen).
Table 2. Effect of probiotic and organic acids supplementation on growth performance and mortality rate of broiler chickens.
Table 3. Effect of probiotic and organic acids supplementation on carcase traits of broiler chickens.
Blood plasma biochemical parameters
Blood plasma concentrations of total protein, albumin, globulin, triglycerides, total cholesterol, T3, and T4 are shown in Table . Data exposed a significant impact of probiotics and organic acids on plasma total protein concentration, where adding 0.02% probiotics to broiler feed increased plasma total protein concentration compared to control (p = .002). Also, administration of 0.03% organic acids increased plasma total protein level in comparison with control (p = .04). Dietary 0.02% probiotics increased plasma albumin concentration (p = .007), however, the organic acids treatments did not differ. Administration of 0.03% organic acids increased plasma globulin concentration in broilers (p = .04). Blood plasma globulin concentration was influenced by the interaction between probiotics and organic acids (p = .003, Figure ). No significant differences were observed in plasma total cholesterol, triglycerides, T3, and T4 concentrations among the experimental treatments.
Figure 1. Interaction between probiotics (0% and 0.2%) and organic acids (0, 0.03, and 0.06%) supplementation on plasma concentrations of glutathione peroxidase (GSH-Px) and globulin, and cell-mediated immune response at 24 h post-phytohaemagglutinin-P injection (toe web swelling difference). Different superscripts in a column differ significantly (p < .05). Abbreviations: P, probiotics; OA, organic acids.
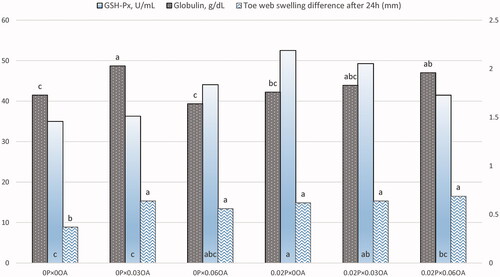
Table 4. Effect of probiotic and organic acids supplementation on blood biochemical constituents and antioxidative status of broiler chickens.
Antioxidative status
Dietary 0.02% probiotics increased plasma GSH-Px concentration (p = .003), however, this difference was not existed in organic acids groups. There is an interaction between probiotics and organic acids was observed in plasma GSH-Px activity (p = .02, Figure ). Supplementation of 0.02% probiotics plus 0.03% organic acids improved plasma GSH-Px concentration. Plasma concentration of TAC and MDA didn’t significantly differ among the experimental groups in broiler chickens.
Immune response
The effects of adding probiotics and/or organic acids blend in humoral- and cell-mediated immunity were summarised in Table . Dietary 0.02% probiotics supplementation enhanced cell-mediated immune response (PHA-P induced proliferative response) in comparison with control at 24 and 48 h post injection (p = .01 and p = .04; respectively). Inclusion of organic acids improved cell-mediated immune response at 24 and 48 h post injection of PHA-P (p = .01 and p = .05; respectively). As graphically illustrated in Figure , an interaction between probiotics and organic acids was observed in cell-mediated immune response at 24 h post injection of PHA-P (p = .03). Inclusion of 0.02% probiotics plus 0.06% organic acids improved cell-mediated immune response at 24 h post injection of PHA-P. As shown in Table , dietary 0.02% probiotics increased serum concentration of IgY in broiler chickens at 6 wk of age (p = .03), while the increase in IgA and IgM immunoglobulins values was not significant. It is noteworthy to mention that dietary 0.02% probiotics supplementation improved NDV titre comparing with control group (p = .01).
Table 5. Effect of probiotic and organic acids supplementation on cellular and humoral immune response of broiler chickens.
Bacterial count
The influence of supplementation with probiotics (B. subtilis and B. licheniformis) and organic acids blend (acetic and propionic acids) on the total aerobic bacteria, E. coli, Salmonella, and B. subtilis counts in gut and breast meat of broilers are presented in Table . In gut, dietary supplementation of 0.02% probiotics significantly reduced the counts of total aerobic bacteria and Salmonella, while B. subtilis count was increased (p = .001). Administration of 0.06% organic acids reduced intestinal E. coli and Salmonella count in comparison with control (p = .001). There is an interaction between probiotics and organic acids in intestinal Salmonella count (Figure ). Supplementation of probiotics or organic acids alone or in combination reduced intestinal Salmonella count significantly. Interestingly, in meat, addition of probiotics and/or organic acids to broilers reduced the pathogenic bacterial count (Table ). Dietary supplementation of probiotics reduced total bacterial count in breast meat in comparison with that in control (p = .001). Addition of 0.03% organic acids increased B. subtilis count in breast meat in comparison with control. Also, supplementation of 0.06% organic acids reduced the total bacterial count and E. coli count in breast meat compared with control (p = .001 and p = .01; respectively). There is a significant interaction between probiotics and organic acids in reducing total bacterial count in meat (p = .01) and increasing B. subtilis count in meat (p = .01, Figure ). Supplementation of 0.02% probiotics plus organic acids (0.03 or 0.06%) reduced total bacterial count in meat.
Figure 2. Interaction between probiotics (0% and 0.2%) and organic acids (0, 0.03, and 0.06%) supplementation on bacterial count in meat and gut. Different superscripts in a column differ significantly (p < .05). Abbreviations: P, probiotics; OA, organic acids.
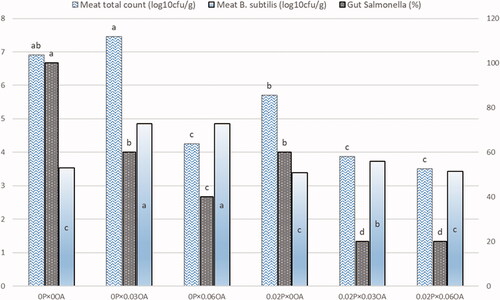
Table 6. Effect of probiotic and organic acids supplementation on bacterial count in gut and breast meat of broiler chickens.
Discussion
Growth performance and carcase measurements
Results presented in Table clarified that dietary 0.02% probiotics increased BW at 6 wk of age in broiler chickens. These findings are in agreement with previous studies which confirmed that dietary Bacillus strains improved growth performance in broiler chickens (Bai et al. Citation2017; Gong et al. Citation2018). Probiotics promote growth by modulating the gut microbiota (Ebeid et al. Citation2019), enhancing gut's microstructure and physiological function (Ebeid et al. Citation2019; Abdel-Moneim et al. Citation2020), increasing serum T3 and T4 concentrations (Abdel-Moneim et al. Citation2020), and activating of the immune system (Fathi et al. Citation2017). Moreover, probiotics supplementation is involved in activating secretion of several digestive enzymes such as protease, amylase, and lipase, which help in enhancing digestion and absorption of nutrients leading to promote growth performance in broiler chickens (Gong et al. Citation2018; Abdel-Moneim et al. Citation2020). Results of the current study showed a slight increase in serum concentrations of T3 and T4 but this increase might have in vivo physiological impact in activating growth in broiler chickens.
As shown in Table , addition of 0.03% and 0.06% organic acids blend had a numerical increase in BW (p = .07). This slight improvement might be attributed to an enhancement in serum concentrations of T3 and T4 (Table ) and reducing the intestinal microbial pathogenic load (Table ) in the present study. Previous studies confirmed that organic acids might be involved in enhancing growth performance via their role in reducing intestinal microbial pathogenic load leading to increase the availability of dietary energy and nutrients to the host animal (Kaczmarek et al. Citation2016), enhancing the digestibility and utilisation of nutrients (Yang et al. Citation2018), increasing thyroid hormones (T3 and T4) concentrations (Abdelrazek et al. Citation2016), and improving intestinal morphology characteristics (Emami et al. Citation2017; Sikandar et al. Citation2017).
Addition of probiotics and/or organic acids had no significant effect on carcase percentage and percentage of different parts of the carcase (Table ). These findings are in agreement with previous studies (Cengiz et al. Citation2015; Ebeid et al. Citation2019), which revealed that carcase percentage and the relative weights of breast meat, abdominal fat, liver, spleen, and gizzard were not influenced by feeding dietary probiotics (B. subtilis). Also, the inclusion of organic acids had no significant impact on carcase characteristics (Isabel and Santos Citation2009).
Blood plasma biochemical parameters
With respect to the effect of supplementation of probiotics and/or organic acids on blood plasma constituents in growing broilers, plasma total protein was significantly increased when probiotics and/or organic acids were supplemented (Table ). Also, dietary 0.02% probiotics increased plasma albumin concentrations. These results are in agreement with those of Abdel-Moneim et al. (Citation2020) who found that dietary B. subtilis elevated serum concentrations of total proteins and albumin. Similarly, Yazhini et al. (Citation2018) observed that dietary probiotics supplementation increases serum total proteins. This increase in plasma concentrations of total protein and albumin could be demonstrated by enhancing dietary protein utilisation via activation of digestive enzymes such as protease and consequently increasing dietary protein efficiency as well as enhancing the surface area for nutrients absorption in form of increasing villus height, and villus: crypt ratio in the small intestine (Ebeid et al. Citation2019; He et al. Citation2019; Abdel-Moneim et al. Citation2020). As presented in Table , inclusion of 0.03 and 0.06% organic acids increased plasma total protein concentrations. These results are in accordance with Natsir et al. (Citation2017) who noted that dietary acidifiers or herb-acidifiers blend increased serum total protein and albumin. Blood plasma globulin concentration was significantly influenced by the interaction between probiotics and organic acids (Figure ). This increase in plasma globulin concentration could support the results of cell-mediated immune response at 24 h post-PHA-P injection in the present study.
Antioxidative status
Dietary probiotics are able to activate the antioxidative enzymes involved in scavenging reactive oxygen species (including GSH-Px, superoxide dismutase, and catalase) and reducing MDA concentration as indicator of lipid peroxidation (Wu et al. Citation2019; Abdel-Moneim et al. Citation2020; Ebeid et al., Citation2021). As presented in Table , dietary 0.02% probiotics increased plasma GSH-Px concentration significantly. As shown in Figure , addition of 0.02% probiotics plus 0.03% organic acids improved plasma GSH-Px concentration. These results are in correspondence with Bai et al. (Citation2017) who illustrated that dietary inclusion of Bacillus subtilis enhanced GSH-Px, superoxide dismutase, and glutathione reductase activities in serum of broiler chickens. Also, He et al. (Citation2020) declared that supplementation of sodium butyrate in drinking water increased serum concentrations of GSH-Px in broilers. However, plasma concentration of TAC and MDA didn’t significantly differ among the experimental groups in broiler chickens (Table ). Also, the both additives did not show any synergistic effect on plasma concentration of TAC and MDA. These results are in harmony with Abudabos et al. (Citation2017) who indicated that dietary organic acids blend and B. subtilis had no significant effect on plasma TAC and thiobarbituric acid reactive substances (as indicator of lipid peroxidation) in broiler chickens.
Immune response
One of the main results of the current study was that the dietary Bacillus-based probiotics of diets and/or organic acids blend in drinking water for growing broilers enhanced the humoral- and cell-mediated immunity (Table ). Dietary 0.02% probiotics supplementation improved cell-mediated immune response (PHA-P induced proliferative response), serum IgY, and serum NDV titre significantly. Similarly, Fathi et al. (Citation2017) illustrated that dietary B. subtilis improved humoral- and cell-mediated immunity in broilers under heat stress conditions. It was illustrated that dietary probiotics supplementation increased serum concentration of immunoglobulins in broiler chickens (Fathi et al. Citation2017) and laying hens (Fathi et al. Citation2018). Furthermore, probiotics strains stimulated monocytes and macrophages, regulated the concentrations of the pro- and anti-inflammatory interleukins IL-6, IL-8, and IL-10 (Vieco-Saiz et al. Citation2019) and increased gene expression of interferon-gamma (IFN-γ), IL-6, and IL-10 (Wu et al. Citation2019, Ebeid et al., Citation2021). Regarding the influence of organic acids on immune responsiveness, it could be observed in Table that supplementation of 0.03% and 0.06% organic acids improved cell-mediated response significantly at 24 and 48 h post injection of PHA-P and numerically increased serum concentration of IgY, IgA, and IgM immunoglobulins. These results are in accordance with that of Sikandar et al. (Citation2017) who proved that dietary sodium butyrate improved cell-mediated immune response at 48 h post injection of PHA-P. Also, Emami et al. (Citation2017) proved that dietary organic acids (formic and propionic acids) mixture supplementation improved humoral- and cell-mediated immunity in form of increasing of cutaneous basophil hypersensitivity response, IgG and IgM concentrations, and CD4-expressing. Furthermore, organic acids blocked nitric oxide synthesis, and minimised the cytokines production, including interleukins (IL-6, IL-10, IL-1β) and IFNγ, which regulating the inflammation and immune homeostasis (Zhou et al. Citation2014). Interestingly, as graphically illustrated in Figure , an interaction between probiotics and organic acids was observed in cell-mediated immune response at 24 h post-PHA-P injection. It is possible to suggest that a synergism between probiotics and organic acids is probably to be participated in improving humoral- and cell-mediated immunity. This assumption is confirmed by Nari et al. (Citation2020) who illustrated that dietary butyric acid + Saccharomyces boulardii enhanced cell-mediated immune response (cutaneous basophil hypersensitivity to PHA-P). Taken together, administration of probiotics and/or organic acids are able to stimulate the humoral- and cell-mediated immunity and regulate pro- and anti-inflammatory cytokines in the host. Getting such benefits is a necessity in antibiotic-free poultry.
Bacterial count
Probiotics and organic acids possess a significant positive impact on gut health via controlling the composition and function of the gut microbiome. Results illustrated in Table proved that in gut, dietary supplementation of 0.02% probiotics significantly reduced the total bacterial count and Salmonella, while B. subtilis count was increased (Table ). Previous studies indicated that probiotics establish the balance of intestinal microbiome in hosts by inhabiting the growth and proliferation of undesirable opportunistic pathogens, like Salmonella, E. Coli, and Clostridium, and fortifying the beneficial microorganisms, such as Lactobacillus, Bacillus, and Bifidobacterium in the intestine (Ebeid et al. Citation2019; Jha et al. Citation2020). These positive effects might be related to the capability of probiotics to prohibit pathogens form attaching to the intestinal mucosa via competitive exclusion mechanism, competition for nutrients, promoting the physical barrier of the epithelium, producing a large number of antimicrobial peptides, and the activating immune modulation in the gut (Vieco-Saiz et al. Citation2019; Jha et al. Citation2020; Ebeid et al. Citation2021). As tabulated in Table , administration of organic acids mixture reduced intestinal E. coli and Salmonella counts in comparison with control. It was reported that dietary blend of organic acids minimised intestinal E. coli and Salmonella counts in broilers (Ghazalah et al. Citation2011; Emami et al. Citation2017; Nguyen et al. Citation2018). These effects might be connected with the ability of organic acids in inhibiting the growth and proliferation of pathogenic bacteria such as E. coli, Salmonella, and Clostridium perfringens because they are sensitive to low pH (Ghazalah et al. Citation2011; Ebeid and Al-Homidan Citation2021). Also, the non-dissociated organic acids, which are more lipophilic, could freely penetrate the bacterial cell membrane reaching to cytoplasm and release protons (H+), leading to reduction in the pH within the cell, disrupting the normal cell functioning and cell death (Mani-Lopez et al. Citation2012). Therefore, it might be suggested that the major benefit of probiotics and/or organic acids supplementation to poultry diets is maintaining a healthy microbial balance in GIT to establish the gut integrity, physiological function, intestinal immunity and health, which consequently translated into high performance and greater resistance to diseases.
Interestingly, in meat, adding probiotics and/or organic acids to broilers diets reduced the pathogenic bacterial count (Table ). Similarly, previous studies illustrated that dietary probiotic supplementation might be able to alleviate the challenge of pathogenic microbiota via reducing the undesirable opportunistic pathogens in gut and consequently improved meat safety (Shanmugasundaram et al. Citation2019; Ebeid et al. Citation2021). Dietary supplementation of probiotics reduced total bacterial count in breast meat compared with that in the control, and Salmonella was not detected in meat (Table ). Shanmugasundaram et al. (Citation2019) noted that dietary multiple-strains probiotic reduced the Salmonella load in carcase chickens. Also, Ebeid et al. (Citation2019) reported that dietary B. subtilis supplementation decreased the E. coli count in meat, and Salmonella was not detected in meat. Also, as shown in Table , supplementation of 0.06% organic acids reduced the total bacterial count and E. coli count in breast meat compared with that in the control. Similarly, Bourassa et al. (Citation2018) observed that dietary formic and propionic acids (1–5 kg/ton) reduced caecal Salmonella, and consequently carcase contamination in broiler chickens. It is noteworthy to indicate that the addition of probiotics in combination with organic acids blend was more effective in reducing Salmonella count in the gut and total bacterial count in meat (Figure ). Likewise, Nari et al. (Citation2020) documented that dietary butyric acid + Saccharomyces boulardii decreased intestinal E. coli and Clostridium. The synergic effects of probiotics and organic acids blend on reducing pathogenic bacteria in the gut and meat may be related to the reduction pH in the GIT, which suppresses the proliferation of pathogenic bacteria (Khan and Iqbal Citation2016). Therefore, it might be documented that the addition of probiotics in diet and/or organic acids in drinking water could be used as a successful strategy to reduced pathogenic bacteria in gut and carcase and consequently improve poultry meat safety.
Conclusions
It could be concluded that supplementation of probiotics and/or organic acids enhanced humoral- and cell-mediated immune response and decreased the counts of E. coli and Salmonella in the gut, and reduced total bacterial count in breast meat. Application of probiotics and organic acids might be recommended in the poultry industry as growth promoters and as alternatives to antibiotics. Getting such benefits is a necessity under practical conditions.
Ethical approval
All experimental procedures were conducted according to the international procedures and approved by The Animal Ethics Committee of Qassim University in Saudi Arabia.
Acknowledgements
The authors would like to thank Al-Watania Poultry Company, Buraydah, Saudi Arabia for kindly providing broiler chickens and feeds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data and materials that support the results and analyses presented in this study are freely available upon request.
References
- Abdel-Moneim AME, Selim DA, Basuony HA, Sabic EM, Saleh AA, Ebeid TA. 2020. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod. 52(2):671–680.
- Abdelrazek HMA, Abuzead SMM, Ali SA, El-Genaidy HMA, Abdel-Hafez SA. 2016. Effect of citric and acetic acid water acidification on broiler’s performance with respect to thyroid hormones levels. Adv Anim Vet Sci. 4(5):271–278.
- Abudabos AM, Alyemni AH, Dafalla YM, Al-Owaimer AN. 2017. Effect of organic acid blend and Bacillus subtilis on growth, blood metabolites and antioxidant status in finishing broilers challenged with Clostridium perfringens. J Anim Plant Sci. 27(4):1101–1107.
- Agboola AF, Omidiwura BRO, Odu O, Popoola IO, Iyayi EA. 2016. Effects of organic acid and probiotic on performance and gut morphology in broiler chickens. SA J Sci. 45(5):494–501.
- Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T. 2017. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci. 96(1):74–82.
- Bourassa DV, Wilson KM, Ritz CR, Kiepper BK, Buhr RJ. 2018. Evaluation of the addition of organic acids in the feed and/or water for broilers and the subsequent recovery of Salmonella Typhimurium from litter and ceca. Poult Sci. 97(1):64–73.
- Cengiz Ö, Köksal BH, Tatlı O, Sevim Ö, Ahsan U, Üner AG, Ulutaş PA, Beyaz D, Büyükyörük S, Yakan A, et al. 2015. Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poult Sci. 94(10):2395–2403.
- Chaucheyras-Durand F, Durand H. 2010. Probiotics in animal nutrition and health. Benef Microbes. 1(1):3–9.
- Ebeid TA, Al-Homidan IH, Fathi MM. 2021. Physiological and immunological benefits of probiotics and their impacts in poultry productivity: a review. World's Poult Sci J. 77: 1–17.
- Ebeid TA, Al-Homidan IH. 2021. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry: a review. World's Poult Sci J. 77: 1–19.
- Ebeid TA, Fathi M, Al-Homidan I, Ibrahim Z, Al-Sagan A. 2019. Effect of dietary probiotics and the stocking density on carcass traits, meat quality, microbial populations and ileal histomorphology in broilers under hot climate conditions. Anim Prod Sci. 59(9):1711–1719.
- Emami NK, Daneshmand A, Naeini SZ, Graystone EN, Broom LJ. 2017. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: performance, microbiology, intestinal morphology, and immune response. Poult Sci. 96(9):3254–3263.
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria [Internet]. Report of a Joint FAO/WHO Expert Consultation.
- Fathi M, Al-Homidan I, Al-Dokhail A, Ebeid T, Abou-Emera O, Alsagan A. 2018. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital J Anim Sci. 17(3):804–814.
- Fathi MM, Al-Homidan I, Ebeid TA, Abou-Emera OK, Mostafa MM. 2020. Dietary supplementation of Eucalyptus leaves enhances eggshell quality and immune response in two varieties of Japanese quails under tropical condition. Poult Sci. 99(2):879–885.
- Fathi MM, Ebeid TA, Al-Homidan I, Soliman NK, Abou-Emera OK. 2017. Influence of probiotic supplementation on immune response in broilers raised under hot climate. Br Poult Sci. 58(5):512–516.
- Gadde UD, Oh S, Lillehoj HS, Lillehoj EP. 2018. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci Rep. 8(1):3592.
- Ghazalah AA, Atta AM, Elkloub K, Moustafa MEL, Shata RFH. 2011. Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. Int J Poult Sci. 10(3):176–184.
- Gong L, Wang B, Mei X, Xu H, Qin Y, Li W, Zhou Y. 2018. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim Sci J. 89(11):1561–1571.
- He S, Yin Q, Xiong Y, Liu D, Hu H. 2020. Effects of dietary fumaric acid on the growth performance, immune response, relative weight and antioxidant status of immune organs in broilers exposed to chronic heat stress. Czech J Anim Sci. 65(3):104–113.
- He T, Long S, Mahfuz S, Wu D, Wang X, Wei X, Piao X. 2019. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals. 9(11):985.
- Horn KG, Gedris CA, Rodney KM. 1996. Selective isolation of vancomycin-resistant enterococci. J Clin Microbiol. 34(4):924–927.
- Hossain MM, Begum M, Kim IH. 2016. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Veterinarni Medicina. 60(2):77–86.
- Isabel B, Santos Y. 2009. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. J Appl Poult Res. 18(3):472–476.
- Jha R, Das R, Oak S, Mishra P. 2020. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Animals. 10(10):1863.
- Kaczmarek SA, Barri A, Hejdysz M, Rutkowski A. 2016. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult Sci. 95(4):851–859.
- Khan SH, Iqbal J. 2016. Recent advances in the role of organic acids in poultry nutrition. J Appl Poult Res. 44(1):359–369.
- Mani-Lopez E, García HS, López-Malo A. 2012. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int. 45(2):713–721.
- McDonald LC, Hackney CR, Ray B. 1983. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl Environ Microbiol. 45(2):360–365.
- Nari N, Ghasemi HA, Hajkhodadadi I, Farahani AHK. 2020. Intestinal microbial ecology, immune response, stress indicators, and gut morphology of male broiler chickens fed low-phosphorus diets supplemented with phytase, butyric acid, or Saccharomyces boulardii. Livest Sci. 234 (4):103975.
- Natsir MH, Hartutik OS, Widodo E, Widyastuti ES. 2017. Use of acidifiers and herb-acidifier combinations with encapsulated and non-encapsulated intestinal microflora, intestinal histological and serum characteristics in broiler. AIP Conf Proc. 1844(5):020012.
- Nguyen DH, Lee KY, Mohammadigheisar M, Kim IH. 2018. Evaluation of the blend of organic acids and medium-chain fatty acids in matrix coating as antibiotic growth promoter alternative on growth performance, nutrient digestibility, blood profiles, excreta microflora, and carcass quality in broilers. Poult Sci. 97(12):4351–4358.
- SAS Institute. 2013. JMP version 11 user’s guide. Cary (NC): SAS Institute Inc.
- Shanmugasundaram R, Mortada M, Cosby DE, Singh M, Applegate TJ, Syed B, Pender CM, Curry S, Murugesan GR, Selvaraj RK. 2019. Synbiotic supplementation to decrease Salmonella colonization in the intestine and carcass contamination in broiler birds. PLoS One. 14(10):e0223577.
- Sikandar A, Zaneb H, Younus M, Masood S, Aslam A, Khattak F, Ashraf S, Yousaf MS, Rehman H. 2017. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas J Anim Sci. 30(5):690–699.
- Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D. 2019. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol. 10(11):57.
- Wu Y, Wang B, Zeng Z, Liu R, Tang L, Gong L, Li W. 2019. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult Sci. 98(10):5028–5039.
- Yang X, Xin H, Yang C, Yang X. 2018. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim Nutr. 4(4):388–393.
- Yazhini P, Visha P, Selvaraj P, Vasanthakumar P, Chandran V. 2018. Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet World. 11(9):1344–1348.
- Zhou ZY, Packialakshmi B, Makkar SK, Dridi S, Rath NC. 2014. Effect of butyrate on immune response of a chicken macrophage cell line. Vet Immunol Immunopathol. 162(1–2):24–32.
