Abstract
Cinnamaldehyde has an effective antimicrobial activity and therefore it received interest as a feed additive in swine nutrition. However, its high reactivity to amino acid residues might affect its efficacy and digestive processes. As an alternative, 20 chemical derivatives were evaluated. The in vitro antimicrobial activities were tested in an in vitro fermentation model. Thereof three compounds were selected and fed to newly weaned piglets. Five dietary treatments were replicated in six pens of four pigs per pen; i.e. control, cinnamaldehyde at 100 and 400 mg/kg, and 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde, both equimolar to 400 mg/kg cinnamaldehyde. In vitro results showed that 4-nitrocinnamaldehyde has the highest antimicrobial activity; however, this compound is carcinogenic and was not further issued. Cinnamaldehyde had the second-highest activity, particularly against coliform bacteria and Escherichia coli (E. coli), followed by 4-methoxycinnamaldehyde, 2-methoxycinnamaldehyde and hydrocinnamaldehyde. All other derivatives showed lower potency, but they were consistently more bactericidal against coliform bacteria and E. coli as compared to Gram-positive bacteria. At pH 7, aldehydes showed stronger bactericidal activity than their corresponding carboxylic acids, which was not the case at pH 5, suggesting a different mode of action. In the in vivo trial, no significant improvements in animal performance or antimicrobial effects were observed. To conclude, apart from 4-nitrocinnamaldehyde, none of the derivatives showed higher antimicrobial potency than cinnamaldehyde. Three selected compounds from in vitro trials failed to demonstrate major positive outcomes in the in vivo trial.
4-Nitrocinnamaldehyde has the highest antimicrobial activity in vitro.
Cinnamaldehyde had the second-highest activity, followed by 4-methoxycinnamaldehyde, 2-methoxycinnamaldehyde.
No significant improvements in animal performance or antimicrobial effects were observed.
Highlights
Introduction
Cinnamaldehyde is an α,β-unsaturated aldehyde and major constituent of cinnamon essential oil (Cinnamomum zeylanicum B.). This compound has been suggested as a potent antimicrobial to control pathogens in pig and poultry (Michiels et al. Citation2009; Verlinden et al. Citation2013). Given the increased limitations on the use of antibiotics as growth promoter and prophylactics worldwide and the phasing out of pharmacological ZnO in the EU by 2022 in pig production (Commission Implementing Decision of 26.6.2017, C(2017) 4,529 Final), the interest for this compound is legitimate (Bonetti et al. Citation2021). In animal production, cinnamaldehyde has been listed as authorised flavouring compound (category 2b) in EU Register of Feed Additives, and is also found as ingredients of some authorisations within the category of zootechnical feed additives (4d). The antimicrobial properties of cinnamaldehyde against intestinal pathogens have been well described (Helander et al. Citation1998; Doyle and Stephens Citation2019). Furthermore, in vitro simulations of the fermentation in the pig gut have demonstrated that cinnamaldehyde has a pronounced selective antimicrobial spectrum (Michiels et al. Citation2009). More specifically, in jejunal simulations, the growth of coliform bacteria was inhibited by 1.6 log10 CFU/mL at 100 mg/L of cinnamaldehyde in the medium, whereas for a similar effect on lactic acid bacteria a 10-fold concentration was required. Overall, this compound may restore the microbial balance in animals suffering from enteral infection and dysbiosis, such as the weaned piglet (Gresse et al. Citation2017).
Multiple target sites for antibacterial action are being described, which are likely species-dependent (Gill and Holley Citation2004; Kim et al. Citation2004; Gill and Holley Citation2006; Di Pasqua et al. Citation2007; Domadia et al. Citation2007; Visvalingam et al. Citation2013). Importantly, it has been shown that cinnamaldehyde does not induce acquired resistance in bacteria (Ali et al. Citation2005), supposedly as a consequence of its diverse modes of action. Furthermore, bacterial exposure (2 h) to cinnamaldehyde resulted in the induction of oxidative stress and reduced DNA replications, reduced synthesis of proteins and fimbriae by downregulation of the respective functional genes in Escherichia coli (E. coli). Longer exposure (4 h) resulted in the bacterial conversion of cinnamaldehyde to inactive cinnamyl alcohol. This suggests that the aldehyde moiety of the cinnamaldehyde molecule is essential to exert antimicrobial properties (Visvalingam et al. Citation2013). Evidence regarding the beneficial effects of single applied cinnamaldehyde in diets of pigs is scarce and inconsistent (Bikker et al. Citation2003; Andrés Elias et al. Citation2007; Yan and Kim Citation2012), although more reports are available testing mixtures of active ingredients including cinnamaldehyde (Manzanilla et al. Citation2004; Frankič et al. Citation2010; Blavi et al. Citation2016). Indeed, the in vivo application of cinnamaldehyde might come with some issues, such as its strong odour and taste. Also, its high reactivity with amino acid residues (Elahi et al. Citation2004), low-oxidative stability (Friedman et al. Citation2000), low-heat stability >70 °C (Gholivand and Ahmadi Citation2008), reduction of active transport in the gut by inhibition of Na+, K+-ATPase (Kreydiyyeh et al. Citation2000; Michiels, Missotten, Dierick, et al. Citation2010; Michiels, Missotten, Van Hoorick, et al. Citation2010) and fast absorption in the proximal gut (Michiels et al. Citation2008) are some major drawbacks.
Due to its electrophilic character, cinnamaldehyde can react with amino acids via two mechanisms: Schiff base formation and Michael addition (Elahi et al. Citation2004). It has been shown that the carbonyl carbon is more significant in terms of reactivity than the β-carbon. Potential participants in these concurrent and consecutive reactions of cinnamaldehyde in vitro and in vivo include α-NH2 groups or lysine ε-NH2 or SH groups of amino acids, peptides, proteins, as well as nucleic acid (DNA) side chains. Many of the biological actions of cinnamaldehyde can be ascribed to its protein reactive nature (Friedman Citation2017), such as the interaction with Transient Receptor Potential Channel Ankyrin 1 (TRPA1), a cation channel that plays an important role in the sensation of pain, inflammation, coughs and the intestinal feedback mechanism to alter the gastric function (McNamara et al. Citation2007; Nozawa et al. Citation2009; Grace and Belvisi Citation2011; Bautista et al. Citation2013). However, it can also be assumed that protein complexation might affect digestive processes negatively or reduce its potential antimicrobial activity in the gastro-intestinal tract.
Altering the chemical structure of cinnamaldehyde (e.g. altering the functional group, substitution on the phenyl structure, saturation of the α,β-bond) will affect electron density and may convey hindrance for reaction, hence modifying affinity for nucleophiles, and ultimately, its biological activity may change. As such, cinnamic acid, acrylic acid and acrylamide, all of which contain both a carbonyl carbon and an unsaturated α,β-carbon–carbon double bond, are weakly electrophilic at the carbonyl carbon, and hence were observed to have little or no reactivity towards cellular proteins such as TRPA1 (Sadofsky et al. Citation2011). Furthermore, Lieder et al. (Citation2020) showed that a range of naturally occurring compounds that are structurally related to cinnamaldehyde did not induce serotonin release as potent as unsubstituted cinnamaldehyde, in both Caco-2 and QGP-1 cell lines, by interacting with TRPA1.
Therefore, it was hypothesised that altering the chemical structure of cinnamaldehyde would change its biological activity accordingly. In order to explore which structural features of cinnamaldehyde are important to exert its antimicrobial effect, chemical derivatives with reduced affinity for addition reactions, retaining an interesting antimicrobial spectrum, were screened. Hence, 1/the in vitro antimicrobial activity was tested in simulations of the fermentation of the pig foregut and 2/the in vivo efficacy of cinnamaldehyde and two selected derivatives (2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde) in weaned piglets on gut bacteria and indices of gut health as compared to cinnamaldehyde was studied.
Materials and methods
Cinnamaldehyde and chemical derivatives
Chemical derivatives were chosen, having a different functional moiety, i.e. alcoholic, ester or carboxylic acid moiety (Table , R1), having a different degree of α,β-saturation (Table , only hydrocinnamaldehyde), bearing substituents on the α-carbon atom (Table , R6); or bearing substituents on the phenyl ring (Table , R2–R5). None of the 21 compounds showed a good solubility in water, whereas most of them were soluble in ethanol. All compounds were soluble in DMSO and had a purity > 95%.
Table 1. Chemical structure, solubility and supplier reference for cinnamaldehyde and derivatives (21 compounds in total).
In vitro simulations of fermentation in the pig gut
Four series of in vitro simulations of the fermentation in the pig gut were performed. In the first and second series, all 21 compounds were tested at pH 5 and 7, respectively. Based on this screening, 11 compounds were selected for series 3 and 4. In each of these series, both pH 5 and 7 conditions were included. In all series, compounds were tested at equimolar doses equivalent to 100 and 400 mg cinnamaldehyde per litre incubation medium, next to a control treatment without added compound. Within each series, all treatments were run in duplicate. The in vitro simulations of the fermentation in the pig gut have been described in detail by Michiels et al. (Citation2009). In brief, the in vitro batch incubation medium was composed of 1 g of an artificial substrate, 10 mL of a buffer (either pH 5 or 7), 0.5 mL of a suspension of fresh pig gut bacteria (inoculum) and 50 μL of a solution of the compound in DMSO. The final concentration of DMSO in the incubation medium never exceeded 4.8 mL/L. The duration of the in vitro incubations was 4 h, allowing an exponential growth of the bacteria. They were carried out in 50 mL vessels in a shaking warm water bath (37 °C). For each series of incubations, two piglets fed a diet without any antimicrobial growth promoter and weaned for 3–4 weeks were euthanised and the gastrointestinal tract was removed. The contents of the small intestine were quantitatively collected and centrifuged (5′, 500 g, 5 °C). The supernatant that contained a suspension of the luminal bacteria of both pigs was mixed and used as inoculum. At the end of the incubation, samples were taken and processed for bacteriological enumerations based on plating techniques of major groups in the aerobic medium.
Animals, diets and treatments
The study was conducted in accordance with the ethical standards and recommendations for the accommodation and care of laboratory animals, covered by the European Directive 2010/63/EU on the protection of animals used for scientific purposes and the Belgian Royal Decree KB29.05.13 on the use of animals for experimental studies. No ethical approval was required for this trial as animals were kept under farm practices without interventions causing harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice (2010/63/EU). Hundred and twenty weaned piglets (Topigs × Piétrain, males and females, weaned on 26 d of age, 6.68 ± 1.73 kg) were assigned to five dietary treatments. Each treatment was replicated in six pens of four pigs per pen according to a randomised block design. Pigs were allocated according to sex and body weight. Pigs were housed with conventional ventilation, starting ambient temperature of 30 °C and decreased to 27.3 °C at d 13. The light schedule was 23 L:1D in the first 5 d and 16 L:8D afterwards (D period ending before 4 am). The piglets were fed a diet for weaners (Table ), including a digestibility marker (Celite 545 coarse, source of 4 mol/L HCl insoluble ash, 1%, Sigma-Aldrich, Bornem, Belgium) and a masking flavour additive to complement cinnamaldehyde’s pungent smell and taste and counter feed aversion (tailor-made; Scentarom nv, Merchtem, Belgium), but excluding organic acids, exogenous enzymes, and Zn beyond animal requirements. The dietary treatments were: C0: control, C1: control + 100 mg/kg cinnamaldehyde; C2: control + 400 mg/kg cinnamaldehyde; C3: control + 491 mg/kg 2-methoxycinnamaldehyde and C4: control + 491 mg/kg 4-methoxycinnamaldehyde.
Table 2. Ingredient and calculated and analysed nutrient composition of basal diet for weaned piglets from d 0 to 13 post-weaning, used in the in vivo trial.
The lowest dose, 100 mg/kg cinnamaldehyde is commercially relevant and currently implemented in pig feed. The higher dose, 400 mg/kg is experimentally relevant, in order to verify whether there is a dose-response effect. The latter is of great importance, as we should strive for an economically feasible compound. The two derivatives were added at an equimolar dose of cinnamaldehyde at the higher dose. Derivatives 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde were thus added at an equimolar dose of cinnamaldehyde at the higher dose, i.e. 400 mg/kg. Regarding cinnamaldehyde, this compound was added to the soybean oil fraction prior to mixing all ingredients. Utmost care was taken to properly mix the soybean oil with all ingredients ensuring homogenous distribution of cinnamaldehyde in the feed. The two solid derivatives were first finely ground. Then these derivatives were premixed manually in a small amount of the basal, before this premix was further mixed with the rest of the amount of basal diet to prepare the respective feeds. The pigs were weighed at d0, d5 and at the end of the trial, i.e. d12 (BW, kg). For each period (d0–5, d5–12 and total period, d0–12) average daily growth (ADG, g/d), average daily feed intake (ADFI, g/d), feed-to-gain ratio (F:G, g/g), average daily water intake (ADWI, mL/d) and water-to-feed ratio (W:F, mL/g) were recorded. All pigs were checked twice daily for general health during the experimental period. Faeces were visually scored for their consistency through the faecal scoring system: 1, normal soft formed stool; 2, (bloody) soft formed sticky faeces; 3, watery, liquid, unformed stool, (bloody) diarrhoea, wet backsides piglets. In case of score 3, the number of piglets showing wet backsides (indicative for diarrhoea) was counted (Van Noten et al. Citation2020).
Sample collection
Piglets, one out of each pen with a weight closest to the average weight of the pen, were sampled either at d12 (15 piglets) or d13 (15 piglets), without previous fasting. Piglets were humanely sacrificed by inducing electronarcosis followed by exsanguination. Blood samples were taken to obtain serum. The abdomen was immediately opened to collect the digesta and intestinal sections. The gastro-intestinal tract was removed and partitioned into the following digesta sampling sites: stomach (M), 3 m of small intestine distal to the pylorus (SI1), small intestine from 1 to 4 m proximal to ileo-cecal valve (SI2), 1 m of the small intestine proximal to ileo-cecal valve (SI3) and last 20 cm of colon (RT). Following measurements were carried out on samples taken of digesta of M, SI1 and SI2: pH, weight of fresh digesta (g), bacterial groups by culturing techniques, short-chain fatty acids (SCFA) and lactic acid. Digesta of SI3 and RT were pooled per section and treatment (pooled from six piglets) and freeze-dried. The dry matter was used to determine 4 mol/L HCl insoluble ash and proximate analysis in order to calculate apparent ileal and faecal digestibility coefficients. Small intestinal segments at 3 m distal to pylorus were excised and used for histo-morphology measurements (segments were flushed with saline and immersed in formaline).
Chemical, bacteriological and histo-morphological analyses
Bacterial counts (viable counts; log10 CFU/g fresh sample) in samples of in vitro simulations and digesta of M, SI1 and SI2 of the in vivo trial were done using the ring-plate technique (Vanderheyde Citation1963). Serial 10-fold dilutions were made from 1 g aliquots of fresh material, using a sterilised peptone solution (1 g peptone + 0.4 g agar + 8.5 g NaCl in 1 L aq. dest.) and plated onto selective media in duplicate. Selective media were used for counting the following bacterial groups: total anaerobic bacteria (Reinforced Clostridial Agar, CM0151, Oxoid, Basingstoke, UK + 0.001% haemin; incubated for 48 h at 37 °C under 90% N2 and 10% CO2), coliform bacteria (Eosin Methylene Blue Agar, CM0069, Oxoid; incubated for 24 h at 37 °C aerobically), enterococci (Slanetz and Bartley Medium, CM0377, Oxoid; incubated for 48 h at 37 °C aerobically) and lactobacilli (Rogosa Agar, CM0627, Oxoid + 0.132% acetic acid; incubated for 48 h at 37 °C under 90% N2 and 10% CO2). The detection limit was 2 log10 CFU/mL. If no growth was observed, the detection limit was taken for further processing of data. Bacterial metabolites were determined in digesta of SI1 and SI2. SCFA and lactic acid were analysed by a chromatographic (GC) method (Jensen et al. Citation1995; Missotten et al. Citation2009). Diets and freeze-dried samples from SI3 and RT digesta were used to quantify 4 mol/L HCl insoluble ash as an indigestible marker to calculate apparent digestibility coefficients (Michiels, Missotten, Dierick, et al. Citation2010; Michiels, Missotten, Van Hoorick, et al. Citation2010). Analyses of dry matter, ether extract, nitrogen and 4 mol/L HCl insoluble ash were performed according to the standard methods as outlined by Van Nevel et al. (Citation2003). Measurement of the histo-morphological parameters villus length (V) and crypt depth (C) and enumeration of intra-epithelial lymphocytes (IELs) were carried out as described by Van Nevel et al. (2003). The acute-phase protein haptoglobine was determined in serum using a commercial kit (TP801, PhaseTM Range Haptoglobin Assay, Tridelta Development Ltd, Maynooth, Ireland).
Statistical analysis
In vitro incubation of series 1 and 2 served as the initial screening for antimicrobial effects of the 21 compounds. Data from series 1 (only the 11 selected compounds, pH 7), 2 (only the 11 selected compounds, pH 5), 3 (pH 5 and 7) and 4 (pH 5 and 7) were used for ANOVA for the two pH levels separately (SPSS Statistics version 25.0 program; SPSS Inc., Chicago, IL). Between each series, the inoculums differed, as they were obtained from other pigs. Therefore, series was taken into account as random factor. Statistical differences among treatments were separated by Tukey’s test. Alpha level used for significance determination was 0.05. All data are presented as estimated means. The linear model used was: yijkl = μ + ai + bj + aibj + cl + εijkl, whereby: μ = overall mean, ai = fixed effect of compound, bj = fixed effect of concentration, aibj = interaction term, cl = random effect of series and εijk = error. A hierarchical cluster analysis for both pH conditions separately of the 11 selected compounds with their antimicrobial profiles was done with the Ward’s linkage method based on Bray-Curtis dissimilarities in R with the hclust function. In the in vivo trial, pen was considered the experimental unit for all variables (for physiological variables one pig was taken from each pen, pen remains experimental unit). All data were subjected to ANOVA for testing the effect of treatment and block by using linear models (SPSS Statistics version 25.0 program; SPSS Inc., Chicago, IL). The linear model used was: yijk = μ + ai + bj + aibj + εijk, whereby: μ = overall mean, ai = fixed effect of dietary treatment, bj = random effect of block, aibj = interaction term and εijk = error. Initial body weight (d 0) was included as covariate for analysing performance data, if significant, and days post-weaning was introduced as within-factor for evaluating faeces consistency. Statistical differences among treatments were separated by Tukey’s test. Alpha level used for significance determination was 0.05. All data are presented as estimated means.
Results
Screening for in vitro antimicrobial activities of cinnamaldehyde and derivates
None of the 21 compounds showed a good solubility in water, whereas most of them were soluble in ethanol. All compounds were soluble in DMSO (Table ). At pH 5, all compounds at both 100 and 400 mg/L showed a moderate reduction of coliform bacteria (−0.5 to −2 log10 CFU/mL) as compared to control, except for 3,4-dihydroxycinnamic acid, 4-hydroxy-3-methoxycinnamic acid and 3,5-dimethoxy-4-hydroxycinnamic acid (data not shown).
The inhibitory activity towards coliform bacteria at pH 7 was high for cinnamaldehyde, hydrocinnamaldehyde, 2-methoxycinnamaldehyde, 4-methoxycinnamaldehyde and 4-nitrocinnamaldehyde, mostly only at 400 mg/L; whereas the activity of the other compounds was equal or lower as compared to pH 5. With regard to Gram-positive enterococci and lactobacilli, only few compounds could consistently reduce bacterial growth, from which 4-nitrocinnamaldehyde and 2-nitrocinnamaldehyde (only lactobacilli) had clearly the highest activity. Other compounds, such as cinnamic acid -nhanced growth of Gram-positive bacteria at both concentrations. Based on these findings, 11 compounds showing the highest inhibitory activity towards coliforms were selected for additional incubations and statistical evaluation was done (Table ).
Table 3. Antimicrobial effect of cinnamaldehyde and selected derivatives in in vitro simulations of the fermentation in the pig gut (data are presented as log10 CFU/mL; n = 3),*significantly different from control, p < .05.
In vitro antimicrobial activities of 11 selected compounds and relation to chemical structure
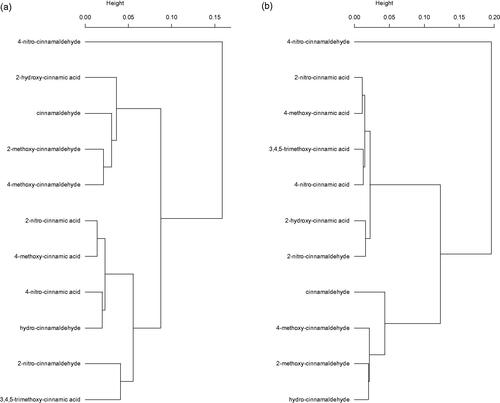
Significant reductions of coliform bacteria as compared to control at pH 5 were found for all selected compounds at 400 mg/L and for some compounds at the lower dose of 100 mg/L (Table ). The activity of substituted aldehydes was largely comparable to the equivalent acids (e.g. 2-nitrocinnamaldehyde vs. 2-nitrocinnamic acid, 4-methoxycinnamaldehyde vs. 4-methoxycinnamic acid) with the exception of the 4-nitro substituted compounds whereby the aldehyde was stronger than the acid. The results for the incubations at pH 7 demonstrate a different trend; i.e. only the aldehydes could reduce coliform growth as compared to control (p < .05), with the exception of 2-nitrocinnamaldehyde. The most active compounds were 4-nitrocinnamaldehyde and cinnamaldehyde itself. Derivatives substituted at the 4-position had a higher activity as compared to their analogues substituted at the 2-position (e.g. 4-methoxycinnamaldehyde vs. 2-methoxycinnamaldehyde); however, this is only true for aldehydes and not for acids. Inhibitory activity towards total anaerobic bacteria followed strikingly the findings for coliform bacteria, however only consistently for pH 7. Based on the antimicrobial spectrum of the 11 compounds, dendrograms were constructed based on Bray-Curtis dissimilarity (Figure ). These dendrograms confirm the above findings. At pH 5, 4-nitrocinnamaldehyde appears to have a different antimicrobial spectrum (i.e. less selective, but highest activity against coliforms), while cinnamaldehyde, its methoxy-derivatives and 2-hydroxycinnamic acid cluster because of the high activity against coliforms at pH 5 and the either or not significant reductions of lactobacilli at pH 5 and 400 mg/L (Figure ). The other compounds tend to have only an effect on coliforms or were rather weak antimicrobials and cluster separately. At pH 7, the picture is a clear cut, discriminating all compounds having no antimicrobial effect on the one hand and others showing reductions of coliforms or an even less selective spectrum (4-nitrocinnamaldehyde) (Figure ). Strangely, 2-hydroxycinnamic acid and hydrocinnamaldehyde switch clusters when both pH conditions are compared.
Effect of cinnamaldehyde, 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde on health, performance, gastro-intestinal bacteriology and histo-morphology in weaned piglets
No significant differences were found in BW, ADG, ADFI, F:G, ADWI and W:F between the dietary treatments in any of the monitored periods post-weaning (Table ). Serum haptoglobine tended to be different between treatments. C3 showed a concentration of 1.00 mg/mL, whereas concentrations for the other treatments ranged between 0.41 and 0.58 mg/mL (p = .10). Faecal consistency was not affected by treatment (mean values over experimental days were 2.2, 2.0, 2.2, 1.9 and 2.2 for C0, C1, C2, C3 and C4, respectively; p > .05). There were no treatment effects within a day. No differences were observed in the number of days during which piglets were showing diarrhoea, which was highly variable within treatment. Mean values for treatments were: 4.0, 3.7, 4.0, 3.7 and 4.8 for C0, C1, C2, C3 and C4, respectively (p >0.05). Mean faecal consistency and number of piglets showing diarrhoea were highly correlated across treatments (r = 0.84, p < .001). Table presents the bacterial counts and metabolites in stomach, SI1 and SI2. No effects were seen for gastric contents. Abundance of bacterial groups was neither affected in both segments of the small intestine. Overall, in all segments lactobacilli were the predominant group. Lactate levels in SI1 were affected by treatment (p < .05), without post-hoc differences. It suggests, however, that with feeding cinnamaldehyde, irrespective of supplementation levels, these lactate concentrations rose. Villus height, crypt depth, their ratio, nor number of IELs were different among treatments (Table ).
Table 4. Effect of cinnamaldehyde, 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde on performance of weaned piglets from d 0 to 12 post-weaning (n = 6) and ileal and faecal apparent digestibility (n = 1 on pooled samples per treatment).
Table 5. Effect of cinnamaldehyde, 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde on bacterial counts (log10, CFU/g) and metabolites (mmol/g) in digesta of piglets sampled on d 12 or 13 post-weaning (n = 6).
Table 6. Effect of cinnamaldehyde, 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde on histo-morphology of small intestine and IEL per 100 enterocytes at 3 m distal to pylorus of piglets sampled on d 12 or 13 post-weaning (n = 6)a.
Discussion
Cinnamaldehyde shows a selective antimicrobial effect, affected by pH and in relation to the functional group
Apart from 4-nitrocinnamaldehyde, none of the derivatives showed higher antimicrobial potency than cinnamaldehyde. As previously shown, cinnamaldehyde showed a strong effect towards coliforms and hardly affected other groups of bacteria (Michiels et al. Citation2007). The greater hydrophobicity of the Gram-negative E. coli surface may explain its relative sensitivity to cinnamaldehyde compared to enterococci and lactobacilli, which are both Gram-positive bacteria. Hydrophobicity facilitates the interaction of the aromatic compound with the membrane (Gill and Holley Citation2006). Contradictory, Wei et al. (Citation2011) found that cinnamaldehyde was more active against Gram-positive bacteria (Bacillus subtilis) compared to Gram-negative bacteria (E. coli). They ascribed this effect to the significant differences in the outer layers of Gram-negative and Gram-positive bacteria. Gram-negative bacteria possess an outer membrane and a unique periplasmic space not found in Gram-positive bacteria (Nikaido Citation1996; Duffy and Power Citation2001). At neutral pH, cinnamaldehyde caused an effect on total anaerobic bacteria, which is mainly an effect of the reduction in coliforms, since binary fission is generally more rapid in coliforms. Coliform bacteria have shorter generation times and grow well in rich media, like the incubation medium used. Considering pH, both aldehydes and acids showed in general a higher activity in more acidic environment, for which the effect of 4-nitro-substituted aldehydes was higher compared to the corresponding acid. Regarding the aldehydes, the acidic environment promotes Schiff-base formation, compounds which are shown to be antimicrobial (Matar et al. Citation2015). Regarding the acids, studies showed that the antimicrobial effect of organic acids towards E. coli was pH-dependent, with lower pH values increasing the activity of the acids since a larger proportion of the acid is undissociated (Herald and Davidson Citation1983). Undissociated organic acids are lipophilic and can diffuse easier across the cell membrane. Once in the bacterial cell they dissociate at the pH of the cytoplasm causing metabolic uncoupling (Skřivanová and Marounek Citation2007). At neutral pH, a different trend was seen; i.e. only the aldehydes could reduce coliform growth as compared to control, with the exception of 2-nitrocinnamaldehyde.
The position of the nitro group affects the antibacterial properties
Compared to cinnamaldehyde, 4-nitrocinnamaldehyde showed a stronger effect towards all bacterial groups. Adding electron-withdrawing groups (halogens or nitro groups) on the aromatic ring of cinnamaldehyde may increase the antimicrobial properties. Results are in line with Song et al. (Citation2014), who demonstrated that higher antimicrobial activity was found by introducing a nitro group on the aromatic ring of cinnamaldehyde. Also, 4-nitrocinnamaldehyde showed a lower biofilm inhibitory concentration and higher quorum sensing inhibition towards Gram-positive bacteria, such as Streptococcus pyogenes, compared to cinnamaldehyde (Brackman et al. Citation2011; Beema Shafreen et al. Citation2014). Furthermore, Eder et al. (Citation1991) showed high mutagenicity of this compound, in the presence of bacterial nitroreductase. This effect was highly dependent on the position of the substituent. As such, genotoxicity of 4-nitrocinnamaldehyde (para-position) is dramatically higher compared to the ortho- and meta-isomers. This might explain the higher antimicrobial activity of 4-nitrocinnamaldehyde compared to 2-nitrocinnamaldehyde. As outlined above, cinnamaldehyde is particularly effective against coliform bacteria which are mainly present in the distal small intestine. This specific activity can result in a shift in the microbial ecology in favour of lactic acid-producing bacteria and a reduction of the number of (pathogenic) coliform bacteria. On the contrary, 4-nitrocinnamaldehyde does not exhibit this specific activity towards coliforms, and is therefore less compelling as an antimicrobial agent in pigs. Indeed, the commensal bacteria in the intestine, including Lactobacillus spp., play important roles in preventing the colonisation of pathogens through competitive exclusion and excretion of bacteriocins capable of bacterial lysis (Vieco-Saiz et al. Citation2019). Also, nitro-aromatic compounds are known for their mutagenic and carcinogenic properties, which has been shown in different studies (Chiu et al. Citation1978; Kovacic and Somanathan Citation2014). Therefore, this compound was not selected for the in vivo study.
2-Methoxycinnamaldehyde, 4-methoxycinnamaldehyde and hydrocinnamaldehyde show a specific antibacterial effect towards coliforms
Overall, aldehydes showed a higher antibacterial activity as compared to the acids. These effects are very likely due to an alteration in the function of membrane-associated proteins, which seems to be exerted mainly at the cell surface. The capability to penetrate the outer layer of cells and elicit a gross perturbation of the lipidic fraction of plasma membranes can help to explain their higher antibacterial activity compared to acids (Trombetta et al. Citation2002). Introducing an electron-donating group (e.g. methoxy group) on the aromatic ring of cinnamaldehyde may result in a compound with less antimicrobial activity (Song et al. Citation2014). However, this also decreases the Michael addition potential and concomitant reactivity and will result in less binding with endogenous proteins in piglets, such as TRPA1. This was shown by Lieder et al. (Citation2020), as they hypothesised that the change in the steric profile of the compound would be responsible for the lower affinity for TRPA1. Furthermore, they suggested that the ability to activate TRPA1 is associated with the ability to permeate the cell membrane (of the host organism). Since introducing additional groups might result in steric hindrance, this could also affect the potential to interact with the bacterial cell membrane and explain the lower antimicrobial activity. The position of the substituted group seems to affect the antibacterial properties of the compound. Adding a methoxy group on the 4-position induces a higher antibacterial effect towards coliforms at pH 5 compared to the substituent on the 2-position. In general, substituents on the para-position seem to be more effective compared to the ortho-position. Surprisingly, hydrocinnamaldehyde, which has a saturated α,β-bond, showed similar inhibiting effects towards coliforms to cinnamaldehyde, at the highest concentration and both pH’s. However, other findings show that hexanal and nonanal, as saturated aldehydes, do not exhibit significant antibacterial activity, while most unsaturated aldehydes have a broad antimicrobial spectrum (Bisignano et al. Citation2001). Moreover, these authors showed that the di-unsaturated aldehyde (E,E)-2,4-decadienal appears to be more toxic to bacterial cells than the corresponding mono-unsaturated aldehyde. Contrary, our results show that removing the unsaturated bond results in a less potent molecule compared to cinnamaldehyde, however without completely masking the antibacterial properties.
The addition of a single or multiple groups on the aryl ring of cinnamic acid causes variation in antimicrobial properties
Cinnamic acid is known for its weak antibacterial effect against most Gram-negative and Gram-positive bacteria (Chang et al. Citation2001; Olasupo et al. Citation2003; Wen et al. Citation2003). On the contrary, in the first screening, this compound enhanced bacterial growth of Gram-positive bacteria at both concentrations. Adding substituents on the aryl ring of the acid, such as 4-methoxycinnamic acid, resulted in potent inhibition of coliforms at low pH in the highest dose. Other authors found an equal inhibition towards Gram-negative and Gram-positive bacteria (Narasimhan et al. Citation2004; Nakazono et al. Citation2005), however, only a significant inhibition of coliforms was found in our study. In our study, the Gram-positive bacteria were not significantly inhibited by this compound. Also, the natural phenolic 4-hydroxycinnamic acid, also known as p-coumaric acid, has been found to be more potent compared to cinnamic acid (Guzman Citation2014). Our study confirmed this for its ortho-isomer, since it showed a potent inhibition towards coliforms at the lowest pH. However, 2-hydroxycinnamic acid also showed a significant reduction of lactobacilli at the highest dose, which is not beneficial. Caffeic acid (3,4-dihydroxycinnamic acid), ferulic acid (4-hydroxy-3-methoxycinnamic acid) and sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) showed the least reduction towards coliform bacteria. These naturally abundant cinnamic acids have been studied for their antimicrobial activities and they all showed a weak growth inhibition against Gram-negative bacteria and not towards Gram-positive bacteria and fungi (Barber et al. Citation2000; Olasupo et al. Citation2003; Zabka and Pavela Citation2013). Lee et al. (2001)[AQ4] showed that the growth-inhibiting activity against E. coli was much more pronounced for 4-hydroxy-3-methoxycinnamic acid than for 3,4-dihydroxycinnamic acid, which indicates that the methoxy group seems to be essential for growth-inhibiting activity against E. coli. When multiple substituents are present, steric hindrance or hydrophobicity might cause alterations in antimicrobial properties. In general, derivatives where only one substituent was added, showed better activities compared to multiple substituents.
No significant improvements in animal performance were observed
Out of all compounds, cinnamaldehyde, 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde were chosen to test the effect on health and performance in weaned piglets. These latter two compounds, both constituents of Agastache rugosa, showed a specific inhibition towards coliforms at both pH’s, which is most beneficial for the weaned piglet. Ileal digestibility of crude protein and ether extract seemed lower in feed supplemented with 100 mg kg−1 cinnamaldehyde and feed supplemented with the para-methoxy derivative. Surprisingly, higher supplementation of cinnamaldehyde seemed not to result in improved digestibility. However, it has to be taken into account that digestibility is analysed on pooled samples, without statistical substantiation. In literature, several studies showing the improved digestibility of energy and nutrients with the supplementation of essential oils are registered. For example, weaned pigs fed a diet supplemented with 0.01% of an essential oil blend containing thymol and cinnamaldehyde improved apparent digestibility of dry matter and crude protein (Li et al. Citation2012). This effect could be attributable to different factors, such as the enhanced secretion of bile rich in bile acids, stimulation of digestive enzyme activities and greater acidification of the gastric contents. Furthermore, the pivotal ability of the stomach to empty gastric contents into the duodenum seems to play a key role in this. A plant extract mixture-containing carvacrol, cinnamaldehyde and capsicum oleoresin, fed to weaned pigs, increased stomach contents concomitant to percentage of dry matter, suggesting an increased gastric retention time (Manzanilla et al. Citation2004). Fledderus et al. (Citation2007) showed that a 10% slower gastric emptying rate caused by the inclusion of 1% carboxymethylcellulose, resulted in an increased protein hydrolysis in the gastric fraction of the pig. Above mentioned evidence urged us to hypothesise that cinnamaldehyde would improve apparent ileal digestibility, which was not the case here. Especially since cinnamaldehyde is a known activator for TRPA1 and since it has been shown that TRPA1 activation in rodents might delay gastric emptying through serotonin release, which in turn can lead to an increased protein digestion (Doihara et al. Citation2009; Nozawa et al. Citation2009). Recently, these channels have been described in the pig, and their potential to modulate gastric function has been confirmed (Van Liefferinge et al. Citation2020). Lieder et al. (Citation2020) showed potent activation of TRPA1 by cinnamaldehyde, resulting in serotonin release in a human intestinal cell model. However, the introduction of an additional methoxy group largely reduced the serotonin-releasing potential and concomitantly decreased the activation of this cation channel via covalent modification of conserved cysteine or lysine residues within the cytoplasmic N-terminus. Accordingly, supplementing 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde to the diet, showing less affinity towards TRPA1, did not result in an effect on gastric emptying and protein digestion. Furthermore, the application of cinnamaldehyde and both methoxy derivatives did not result in an effect on bacterial counts and metabolites in the digesta of piglets in the current experiment. We suggest that the lack of in vivo antibacterial and pro-digestive effect is mainly caused by the fast absorption in the stomach and the proximal small intestine, in which mixing with pancreatic juice and bile enhances the absorption even more (Michiels et al. Citation2008), which both affects its antibacterial efficacy and the ability to regulate gastric emptying. To exert its full potential, cinnamaldehyde would need to reach the distal SI. Therefore, a protection method should be developed, enabling a controlled and targeted release of cinnamaldehyde in this intestinal region of interest. Regarding the activation of TRPA1 and the regulation of gastric emptying, the impact of the ‘ileal brake’ mechanism regulating gastric emptying is stronger than the duodenal brake (Maljaars et al. Citation2007). Also, cinnamaldehyde is particularly effective against coliform bacteria which are mainly present in the distal SI.
Conclusion
To conclude, apart from 4-nitrocinnamaldehyde, none of the derivatives showed higher antimicrobial potency than cinnamaldehyde. The selected compounds 2-methoxycinnamaldehyde and 4-methoxycinnamaldehyde showed specific inhibiting effects towards coliforms in vitro, but failed to demonstrate major positive outcomes in the in vivo trial. Thus, none of the derivatives outperformed cinnamaldehyde. However, and important to mention, administration of this compound in pig industry should be closely monitored, since the activation of TRPA1 might increase satiety and decrease feed intake. To counter a decrease in feed intake, the implementation of methoxy-derivatives, displaying lower affinity towards endogenous protein such as TRPA1 while retaining a specific antimicrobial profile, might be interesting. This research provides fundamental knowledge on which structural features of cinnamaldehyde are important to exert its antimicrobial effect.
Ethical approval
The study was conducted in accordance with the ethical standards and recommendations for the accommodation and care of laboratory animals, covered by the European Directive 2010/63/EU on the protection of animals used for scientific purposes and the Belgian Royal Decree KB29.05.13 on the use of animals for experimental studies. No ethical approval was required for this trial as animals were kept under farm practices without interventions causing harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice (2010/63/EU).
Acknowledgements
Scentarom nv (Merchtem, Belgium) is kindly acknowledged for providing the masking flavour. The authors are also grateful to A. Matthys, T. Van der Eecken, S. Coolsaet, G. Verhofstée, J. Provoost, K. Monkerhey and G. Vanheule for skilful technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, E.V.L., upon reasonable request.
Additional information
Funding
References
- Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. 2005. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob. 4(1):20– 27.
- Andrés Elias N ,Badiola I ,Pujols J ,Torrallardona D. 2007. Effects of cinnamaldehyde, benzoic acid and grapefruit extract on gut health of weanling pigs Book of Abstracts of the 58th Annual Meeting of EAAP; August 26–29; Dublin, Ireland.
- Barber MS, McConnell VS, DeCaux BS. 2000. Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways. Phytochemistry. 54(1):53–56.
- Bautista DM, Pellegrino M, Tsunozaki M. 2013. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol. 75:181–200.
- Beema Shafreen RM, Selvaraj C, Singh SK, Karutha Pandian S. 2014. In silico and in vitro studies of cinnamaldehyde and their derivatives against LuxS in Streptococcus pyogenes: effects on biofilm and virulence genes. J Mol Recognit. 27(2):106–116.
- Bikker P, Fontanillas R, Roura E. 2003. Dietary supplementation with botanical compounds depresses piglet feed intake while faecal E. coli counts remain unchanged. J Anim Sci. 81(1):203.
- Bisignano G, Laganà MG, Trombetta D, Arena S, Nostro A, Uccella N, Mazzanti G, Saija A. 2001. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol Lett. 198(1):9–13.
- Blavi L, Solà-Oriol D, Mallo J, Pérez J. 2016. Anethol, cinnamaldehyde, and eugenol inclusion in feed affects postweaning performance and feeding behavior of piglets. J Anim Sci. 94(12):5262–5271.
- Bonetti A, Tugnoli B, Piva A, Grilli E. 2021. Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals. 11(3):642.
- Brackman G, Celen S, Hillaert U, Van Calenbergh S, Cos P, Maes L, Nelis HJ, Coenye T. 2011. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS One. 6(1):e16084.
- Chang ST, Chen PF, Chang SC. 2001. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol. 77(1):123–127.
- Chiu CW, Lee LH, Wang CY, Bryan GT. 1978. Mutagenicity of some commercially available nitro compounds for Salmonella typhimurium. Mutation ResGenet Toxicol. 58(1):11–22.
- Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. 2007. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 55(12):4863–4870.
- Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. 2009. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn-Schmied Arch Pharmacol. 380(4):353–357. ].
- Domadia P, Swarup S, Bhunia A, Sivaraman J, Dasgupta D. 2007. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem Pharmacol. 74(6):831–840.
- Doyle AA, Stephens JC. 2019. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia. 139:104405.
- Duffy CF, Power RF. 2001. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int J Antimicrob Agents. 17(6):527–529.
- Eder E, Deininger C, Muth D. 1991. Genotoxicity of p-nitrocinnamaldehyde and related alpha, beta-unsaturated carbonyl compounds in two bacterial assays. Mutagenesis. 6(4):261–269.
- Elahi EN, Wright Z, Hinselwood D, Hotchkiss SA, Basketter DA, Smith Pease CK. 2004. Protein binding and metabolism influence the relative skin sensitization potential of cinnamic compounds. Chem Res Toxicol. 17(3):301–310.
- Fledderus J, Bikker P, Kluess JW. 2007. Increasing diet viscosity using carboxymethylcellulose in weaned piglets stimulates protein digestibility. Livest Sci. 109(1–3):89–92.
- Frankič T, Levart A, Salobir J. 2010. The effect of vitamin E and plant extract mixture composed of carvacrol, cinnamaldehyde and capsaicin on oxidative stress induced by high PUFA load in young pigs. Animal. 4(4):572–578.
- Friedman M, Kozukue N, Harden LA. 2000. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J Agric Food Chem. 48(11):5702–5709.
- Friedman M. 2017. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J Agric Food Chem. 65(48):10406–10423.
- Gholivand MB, Ahmadi F. 2008. Simultaneous determination of trans-cinnamaldehyde and benzaldehyde in different real samples by differential pulse polarography and study of heat stability of trans-cinnamaldehyde. Anal Lett. 41(18):3324–3341.
- Gill A, Holley R. 2006. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol. 108(1):1–9.
- Gill AO, Holley RA. 2004. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol. 70(10):5750–5755.
- Grace MS, Belvisi MG. 2011. TRPA1 receptors in cough. Pulm Pharmacol Ther. 24(3):286–288.
- Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. 2017. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 25(10):851–873.
- Guzman JD. 2014. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules. 19(12):19292–19349.
- Helander IM, Alakomi H-L, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LG, von Wright A. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem. 46(9):3590–3595.
- Herald P, Davidson P. 1983. Antibacterial activity of selected hydroxycinnamic acids. J Food Sci. 48(4):1378–1379.
- Jensen M, Cox R, Jensen B. 1995. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim Sci. 61(2):293–304.
- Kim HO, Park SW, Park HD. 2004. Inactivation of Escherichia coli O157: H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 21(1):105–110.
- Kovacic P, Somanathan R. 2014. Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J Appl Toxicol. 34(8):810–824.
- Kreydiyyeh S, Usta J, Copti R. 2000. Effect of cinnamon, clove and some of their constituents on the Na(+)-K(+)-ATPase activity and alanine absorption in the rat jejunum. Food Chem Toxicol. 38(9):755–762.
- Li P, Piao X, Ru Y, Han X, Xue L, Zhang H. 2012. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian Austral J Anim Sci. 25(11):1617–1626.
- Lieder B, Hoi J, Burian N, Hans J, Holik A-K, Beltran Marquez LR, Ley JP, Hatt H, Somoza V. 2020. Structure-dependent effects of cinnamaldehyde derivatives on TRPA1-induced serotonin release in human intestinal cell models. J Agric Food Chem. 68(13):3924–3932.
- Maljaars J, Peters H, Masclee A. 2007. The gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Alimentary pharmacology & therapeutics. 26:241–250.
- Manzanilla EG, Perez JF, Martin M, Kamel C, Baucells F, Gasa J. 2004. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 823210–3218.
- Matar SA, Talib WH, Mustafa MS, Mubarak MS, AlDamen MA. 2015. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 3′-diaminodipropylamine. Arabian J Chem. 8(6):850–857.
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. 2007. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 104(33):13525–13530.
- Michiels J, Missotten J, Dierick N, Fremaut D, De Smet S. 2010. Thymol and trans-cinnamaldehyde reduce active nutrient absorption and chloride secretion in the pig jejunal Ussing chamber model. Livestock Science. 134(1–3):27–29.
- Michiels J, Missotten J, Dierick N, Fremaut D, Maene P, De Smet S. 2008. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J Sci Food Agric. 88(13):2371–2381.
- Michiels J, Missotten J, Fremaut D, De Smet S, Dierick N. 2007. In vitro dose-response of carvacrol, thymol, eugenol and trans-cinnamaldehyde and interaction of combinations for the antimicrobial activity against the pig gut flora. Livest Sci. 109(1–3):157–160.
- Michiels J, Missotten J, Fremaut D, De Smet S, Dierick N. 2009. In vitro characterisation of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. Anim Feed Sci Technol. 151(1–2):111–127.
- Michiels J, Missotten J, Van Hoorick A, Ovyn A, Fremaut D, De Smet S, Dierick N. 2010. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch Anim Nutr. 64(2):136–154.
- Missotten J, Goris J, Michiels J, Van Coillie E, Herman L, De Smet S, Dierick N, Heyndrickx M. 2009. Screening of isolated lactic acid bacteria as potential beneficial strains for fermented liquid pig feed production. Anim Feed Sci Technol. 150(1–2):122–138.
- Nakazono Y, Watanabe Y, Hashinaga F, Tadera K. 2005. Studies on antimicrobial and antioxidative substance of Yuzu (Citrus junos hort. ex Tanaka) seed. J Biol Sci. 6(1):135–139.
- Narasimhan B, Belsare D, Pharande D, Mourya V, Dhake A. 2004. Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem. 39(10):827–834.
- Nikaido H. 1996. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 178(20):5853–5859.
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, et al. 2009. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 106(9):3408–3413.
- Olasupo N, Fitzgerald D, Gasson M, Narbad A. 2003. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett Appl Microbiol. 37(6):448–451.
- Sadofsky LR, Boa AN, Maher SA, Birrell MA, Belvisi MG, Morice AH. 2011. TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids. Pharmacol Res. 63(1):30–36.
- Skřivanová E, Marounek M. 2007. Influence of pH on antimicrobial activity of organic acids against rabbit enteropathogenic strain ofEscherichia coli. Folia Microbiol. 52(1):70–72.
- Song X, Yang Y, Zhao J, Chen Y. 2014. Synthesis and antibacterial activity of cinnamaldehyde acylhydrazone with a 1, 4-benzodioxan fragment as a novel class of potent β-ketoacyl-acyl carrier protein synthase III (FabH) inhibitor. Chem Pharm Bull. 62:1110–1118.
- Thoroski J, Blank G, Biliaderis C. 1989. Eugenol induced inhibition of extracellular enzyme production by Bacillus subtilis. J Food Prot. 52(6):399–403.
- Trombetta D, Saija A, Bisignano G, Arena S, Caruso S, Mazzanti G, Uccella N, Castelli F. 2002. Study on the mechanisms of the antibacterial action of some plant alpha,beta-unsaturated aldehydes. Lett Appl Microbiol. 35(4):285–290.
- Van Liefferinge E, Van Noten N, Degroote J, Vrolix G, Van Poucke M, Peelman L, Van Ginneken C, Roura E, Michiels J. 2020. Expression of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 in the gut of the peri-weaning pig is strongly dependent on age and intestinal site. Animals. 10(12):2417.
- Van Nevel C, Decuypere J, Dierick N, Molly K. 2003. The influence of Lentinus edodes (Shiitake mushroom) preparations on bacteriological and morphological aspects of the small intestine in piglets. Archives of Animal Nutrition. 57(6):399–412.
- Van Noten N, Degroote J, Van Liefferinge E, Taminiau B, De Smet S, Desmet T, Michiels J. 2020. Effects of thymol and thymol α-D-glucopyranoside on intestinal function and microbiota of weaned pigs. Animals. 10(2):329.
- Vanderheyde H. 1963. Zur Vereinfachung der quantitativen und qualitativen Bestimmung der Bakterien unter Verwendung von Ringplatten. Zentr Bakteriol Parasitenk Infekt Hygiene Abteilung. 189(2):224.
- Verlinden M, Pasmans F, Mahu M, Maele LV, De Pauw N, Yang Z, Haesebrouck F, Martel A. 2013. In vitro sensitivity of poultry Brachyspira intermedia isolates to essential oil components and in vivo reduction of Brachyspira intermedia in rearing pullets with cinnamaldehyde feed supplementation. Poultr Sci. 92(5):1202–1207.
- Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D. 2019. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol. 10:57.
- Visvalingam J, Hernandez-Doria JD, Holley RA. 2013. Examination of the genome-wide transcriptional response of Escherichia coli O157:H7 to cinnamaldehyde exposure. Appl Environ Microbiol. 79(3):942–950.
- Wei QY, Xiong JJ, Jiang H, Zhang C, Ye W. 2011. The antimicrobial activities of the cinnamaldehyde adducts with amino acids. Int J Food Microbiol. 150(2–3):164–170.
- Weibel H, Hansen J. 1989. Interaction of cinnamaldehyde (a sensitizer in fragrance) with protein. Contact Dermatitis. 20(3):161–166.
- Wen A, Delaquis P, Stanich K, Toivonen P. 2003. Antilisterial activity of selected phenolic acids. Food Microbiol. 20(3):305–311.
- Yan L, Kim I. 2012. Effect of eugenol and cinnamaldehyde on the growth performance, nutrient digestibility, blood characteristics, fecal microbial shedding and fecal noxious gas content in growing pigs. Asian Austral J Anim Sci. 25(8):1178–1183.
- Zabka M, Pavela R. 2013. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere. 93(6):1051–1056.