?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To investigate the effect of Flaxseeds (Flax) or vitex Agnus-castuson (Vitex), a total of 180 aged hens (58 weeks of age) were divided into 5 treatment groups with 36 each as followe: groups 2 and 3 were fed the basal diet supplemented with 1 or 2 g Flax/kg, respectively. And group 4 and 5 were fed the basal diet supplemented with a 2.5 or 5 g Vitex/kg, respectively. While group 1 was served as a control group. The experiment period was lasted for two months. Flax or Vitex in hens’ rations resulted in a significant (p ≤ .001) increase in EP and EM percentages compared to the control group, as well, EW was increased significantly with the high level of Vitex only. Although, Flax or vitex did not affect daily FI, a FCR values showed a significant improvement. The high level of both Flax and Vitex boosted (p ≤ .001) the egg-shell weight than the control group. Flax or Vitex treatment resulted in a slightly decline egg albumin weight (%),while, the egg yolk weight was increased significantly(p ≤ .001). There were a significant reduction (p ≤ .001) in blood total cholesterol and LDL cholesterol concentrations in Flax or Vitex groups, Contrariwise, HDL cholesterol was a significantly increased (p ≤ .001). Blood total antioxidant capacity concentration was a significant increase (p ≤ .001) of Flax and Vitex groups than the control. Adding Flax or Vitex to hens ration boosted blood oestradiol-17β hormone secretion (p ≤ .001) than the control group.
Egyptian chicken breeds are characterised by lower egg production rates compared to commercial breeds, and they reach the end of the egg production curve early. So, our aim was to use some plant materials as a phytoestrogens source to improve the reproductive and productive performance of these strains and prolong the period of egg production for them.
Highlights
Introduction
Several plant seeds contain bioactive components known as Phytochemicals and they are known to increase during germination in most plant species such as legumes, oilseeds, cereals (Alvarez-Jubete et al. Citation2010; Benincasa et al. Citation2015). These bioactive phytochemicals are called phytoestrogen such as phytosterols, isoflavones, lignans, etc. are considered to have a vital role in human health (Finley Citation2005; Webb and McCullough Citation2005). Plant oestrogens are plant-derived compounds that can mimic oestrogen action and cause oestrogenic effects via binding to the oestrogen receptor‐α and oestrogen receptor‐β and may mimic its hormonal actions potentially affecting the function of oestrogen throughout the animal body (Dusza et al. Citation2006; Yassein et al. Citation2015).
Flaxseed (Linum usitatissimum) is a common food and fibre crop it typically consists of 41% fat, 20% protein, and 28% fibre (Singh et al. Citation2011; Katare et al. Citation2012). Flaxseed is specifically high in omega-3 polyunsaturated fatty acids (PUFA) (Parker et al. Citation2012). There are several phenolic compounds in Flaxseeds, including flavonoids (35–70 mg/100 g) and lignans (1–26 mg/100 g). Secoisolariciresinol and secoisolariciresinol diglycoside are two major Flaxs lignans that have significant antioxidant and anti-inflammatory effects (Martinchik et al. Citation2012; Silva et al. Citation2013). Likewise, Flaxseeds are rich sources of antioxidants and phytoestrogens (Morton et al. Citation1994; Mazur et al. Citation1996) especially lignans, which act as steroid hormones and compete with oestrogen, thus decreasing endogenous levels and they can bind to cellular oestrogen receptors because they have structural similarity with 17-β-oestradiol (Rubilar et al. Citation2010). Phytoestrogens may have mimic biological responses similar to endogenous oestrogen in hens, hence, Flaxseeds are a good source of phytoestrogens and they might be added to laying hens’ diets. Flaxseeds contain up to 800 times more lignans than other plant foods (Mazur et al. Citation1996; Bergman Jungestrom et al. Citation2007). Yassein et al. (Citation2015) revealed that the inclusion of Flaxseeds in the diets of laying hens enhanced the laying rate and egg mass.
Vitex agnus-castus It is a world-famous plant known by various names, e.g. Fruit de gattilier (French), Sauzgatillo (Spanish), Mönchspfefferfrüchte (German), Panj-angosht (Persian), Frutto di Agnocasto (Italian), and Chaste tree (English) (King Citation2002). It is particularly known for its ability to improve conditions affecting a woman’s reproductive system. The Vitex is a small shrub that grows in the Mediterranean and its fruits have been used for some 2000 years as a natural treatment for female fertility and hormonal health (Blumenthal et al. Citation2000). As a result of the many studies that have proven its effectiveness, Vitex has become one of the most popular herbal remedies for hormonal imbalance. There are many types of research on the different useful health-promoting potentials of this plant, including antioxidant, immunomodulatory, cytotoxic, antimutagenic, antimicrobial, antifungal, antinociceptive, opioidergic, antiepileptic, and anti-inflammatory properties, as well as benefits for osteopenic syndromes (Chan et al. Citation2018; Heskes et al. Citation2018). Researchers believe that vitex works by decreasing levels of the hormone prolactin and this helps rebalance other hormones, including oestrogen and progesterone (Diana van Die et al. Citation2013). Petre Alina (Citation2019) wrote that Vitex Its most popular use is to relieve menopause symptoms and infertility issues. The major components of vitex essential oil were identified as 1,8-cineole (47.9%), terpinyl α-acetate (11.6%), sabinene (11.2%) and caryophyllene oxide (9.7%) (Lara et al. Citation2020)
In domestic fowls, ageing is associated with reproductive failure. At the end of the laying period, egg production rate and shell quality were declined which resulted in substantial economic losses. This decline is connected with declining of all levels of the hypothalamic-pituitary-gonadal axis (Johnson Citation2000). Moreover, a lot of physiological changes coincide with the end of the laying period including reduction of oestrogens secretion (Williams et al. Citation2005; Miao et al. Citation2019). This will inevitably influence the synthesis and transport of oestrogen-regulated yolk precursor of the liver, thereby affecting the reproduction performance of aged breeder hens (Liu et al. Citation2018). Moreover, decreased productivity in aged breeder hens is to be ascribed to ovarian senescence (Devine et al. Citation2012; Luderer Citation2014). However, found that Feeding Flaxseed to laying hens improved egg production (Scheideler et al. Citation1998) and increased egg weight (Novak and Scheideler Citation2001).
The aim of this work is to study the phytoestrogen effect of Flaxseed and Agnus-castuson vitex on reproductive and productive performance, egg quality, plasma hormone profiles and antioxidant status of aged laying hens.
Materials and methods
Experimental design
To investigate the effect of some natural plant products (Flaxseeds (Flax) or vitex Agnus-castuson (Vtex)) on enhances hens’ reproductive and productive performance, a total of 180 hens at the end of egg production curve (58 weeks of age) from Mandarh strain (Egyptian local strain) with initial body weight (1570 g) were used during the spring period. The experiment was conducted at EL- Sabahia Poultry Research Station (Alexandria), Animal Production Research Institute, Agricultural Research Centre.
These prosedures suggest minimal stress to animal to ensure rights and welfare by eliminating harm or suffering to animals according to official decrees of the Ministry of Agriculture in Egyptregarding animal welfare (Decree No. 27 (1967) that enforces the humanity treatment of animals generally).
Birds have been fed a diet that contains 2745 kcal/kg ME and 16.2% crude protein. Feed and water were provided ad libtum throughout the experimental period. Hens were randomly assigned to 5 treatments of 3 replicates each (12 hens each) in floor pens under a 16‐h light: 8‐h dark lighting schedule. Group 1 was served as a control group that fed the basal diet, whereas, Groups 2 and 3 were fed the basal diet supplemented with 1 or 2 g Flax/kg basal diet, respectively. While, groups 4 and 5 were fed the basal diet supplemented with 2.5 or 5 g Vitex/kg basal diet, respectively. The experiment period lasted for two months. Flax and Vitex were prepared in powder shape before adding them to the basal diet.
Data collected
Daily egg production (EP) as (%), egg weight (EW) as (g), and egg mass (EM) as (g of egg/hen/day) were recorded for each replicate in each treatment group. As well, Daily feed intake (FI) (g/hen/day) and feed conversion ratio (FCR) (g feed/g egg) were recorded. 15 eggs from each treatment (5 eggs from each replicate) were randomly chosen and used to estimate egg-shell, albumin, and egg yolk weights as a percentage of egg weight. As well, egg-shell thickness (mm) was measured by a micrometer after the inner egg-shell membrane was removed, as well, Yolk colour intensity was measured based on the standard colour of the yolk using a Roche yolk colour fane with a range of score from 1 to 15 from light yellow to the dark yellow.
At the end of the experimental period, 5 hens from each treatment were randomly chosen and sacrificed then the ovary (without the ovary yolk follicle) and the oviduct was removed and weighted to the nearest 0.1 g and recorded as a percentage of final body weight. Ovary yolk follicles were separated and divided dependent on size to large yolk follicles (LYF > 10 mm diameter) and small yolk follicles (SYF > 5−10 mm diameter), as well, ovary large white follicles (LWF = 3−5 mm diameter) were counted (Renema et al. Citation1995).
At the end of experimental period, blood samples of each treatment were collected from birds during scarifying to obtain plasma or serum; blood samples were collected at 8 Am from hens who had already laid eggs to obtain blood samples at the same stage of the egg production cycle (Elkomy et al. Citation2012). Fresh blood samples were taken after collection to determine blood pictures including, the red blood cells count (RBCs). White blood cells count (WBCs), haemoglobin (Hb, g/dL), and haematocrit (PCV). Heparin was used as an anticoagulant but a part of the samples were withheld to obtain serum. Plasma or serum was obtained by centrifugation the blood at 3000 rpm for 20 min and stored at −20 °C for biochemical analysis. Plasma total protein (TP) concentration as (g/dL), plasma Total cholesterol (TCh) concentration as (mg/dL), high-density lipoproteins (HDL) cholesterol and low-density lipoproteins (LDL) cholesterol concentrations as (mg/dL), total antioxidant capacity (TAC mmol/L) were measured. Serum samples were used to determine the concentrations of oestradiol 17-β hormone (E2) (the Oestradiol ELISA Test Kit has a sensitivity of 6.5 pg/mL) by Immunoassay Technique Elisa Kits (Fortress Diagnostics Ltd, Antrim, UK, 2021).
Hatchability performance
During the last five days of each month, fertile eggs from each treatment were collected daily and stored in the controlled room till the time of incubation. During the incubation period, eggs of all treatments were received standard temperature (37.5 °C) and relative humidity (52%) for 18 days. During the last 3 days, eggs were incubated at 36.5°C and 65% relative humidity. All hatching chicks were counted and weighed within 45 min after hatch. Hatchability was calculated as a percentage from fertile eggs. The un-hatched eggs were broken out and the infertile eggs were counted to calculate the percentage of fertility.
Statistical analysis
the data were tabulated and statistically analysed, where appropriate, by using the one-way ANOVA of SAS (SAS Institute Citation2009). The significance of the effects was tested at levels p ≤ .05 (*) and p ≤ .01 (**) and compared using the Turkey test (SAS Institute Citation2009). The statistical model used was as follows:
Where: Yij = the dependent variable, μ+ the overall mean, Ti= the effect of treatments, and eij= the random error.
Results
Data of Flax and Viite treatments on daily feed intake (FI) as (g/h/d), egg production (EP) as (%), egg weight (EW) as (g), egg mass (EM) as (g of egg/hen/day) and feed conversion ratio (FCR) as (g feed/g egg) were illustrated in the Table . It is worthy to note that all experimental groups have commenced with a nearly similar initial LBW which ranged between 1549.00 to 1592.67 g and all hens had egg production rate at approximately 55% (with insignificant differences).
Table 1. Productive performance of aged laying hens treated with different levels of Flax or Vitex.
The data showed no significant effects of Flax or Vitex at any of the studied levels on FI compared to the control group, as hens in the treated groups consumed similar amounts of feed. Despite, there was no effect on FI due to the inclusion of Flax or Vitex in hens’ rations, the FCR was improved, therefore, the FCR values showed a gradual and significantly decreasing and this effect was dependent on the level of Flax and Vitex. EP and EM results of inclusion Flax and Vitex in hens’ rations showed a highly significant (p ≤ .001) increase compared to the control group and this effect was Flax or Vitex level-dependent manner. As well, it could be noticed that the 2 g Flax level resulted in increasing EP and EM at levels similar to that of 2.5 g Vitex. Both Flax and Vitex at any studied levels led to an increase average EW compared to the control group and this increase was significant with the high level of Vitex level only.
The data of effect Flax and Viite on some egg quality measurements (Egg-shell weight, Egg-shell thickness, Albumin weight, and Egg yolk weight) are presented in Table . The high level of both tested groups boosted the egg-shell weight than the control group and this effect was significant (p ≤ .001), whilst, the low level did not differ from the control group. Although, the inclusion of the low level of Flax and Vitex in the hens’ rations did not affect the eggshell weight, the egg-shell thickness (mm) was increased, whereas, egg-shell thickness was increased significantly (p ≤ .05) in All tested groups than the control and this effect was level-dependent manner. Egg albumin weight (%) of Flax or Vitex groups showed to be slightly declined compared to the control group, however, the egg yolk weight was increased significantly (p ≤ .001). In addition, the yolk colour score of groups treated with Flax or Vitex was increased significantly (p ≤ .001) than the control.
Table 2. Egg quality characteristics of aged laying hens treated with different levels of Flax or Vitex.
Data of ovary, oviduct, and ovarian yolk follicles are summarised in (Table ). Ovary weight was not affected by tested material, while, There was a significant increase (p ≤ .001) in oviduct weight compared to the control group. As well, treated hens with Flax or Vitex at any of the studied levels resulted in a significant increase in the number of large yolk follicle (LYF) (p ≤ .05), small yolk follicle (SYF) (p ≤ .001), and LYFs weight (p ≤ .001) than the control group. On the other hand, there was no effect due to adding Flax or Vitex in hens ratio on ovary relative weight (%) and the number of large white follicles (LWF).
Table 3. Ovary, oviduct and follicles characteristics of aged laying hens treated with different levels of Flax or Vitex.
From Table , it could be summarised that adding Flax or Vitex to hens ration boosted blood oestradiol-17β hormone secretion, where blood oestradiol-17β hormone concentration was increased significantly (p ≤ .001) in the Flax and Vitex groups compared to the control group and this increase was in a level addition dependent manner. There were gradual and significant reductions (p ≤ .001) in blood cholesterol (Chol) and the harmful part of cholesterol (LDL) concentrations in treated groups compared to the control group and this reduction was level-dependent manner. Contrariwise, feeding hens on Flax or Vitex led to a gradual and significant increase in the benefit part of cholesterol (HDL) concentration (p ≤ .001) than the control group. Blood total protein concentration of 2 g Flax and 5 g Vitex groups appeared to be slightly decreased compared to the other Flax or Vitex treated groups and the control group, which was non-significant. Blood total antioxidant capacity (TAC) concentration was a highly significant increase (p ≤ .001) of Flax and Vitex groups compared to the control group with a preference for the group treated with 5 g Vitex/kg feed.
Table 4. Blood constituents and Oestradiol-17β hormone of aged laying hens treated with different levels of Flax or Vitex.
Blood Hb content, RBCs count, and PCV (Table ) in groups treated with Flax or Vitex were not affected compared to the control group, while, WBCs count was increased in Flax and Vitex treated groups and this increase was significant (p ≤ .001) with the high level of Falx and Vitex.
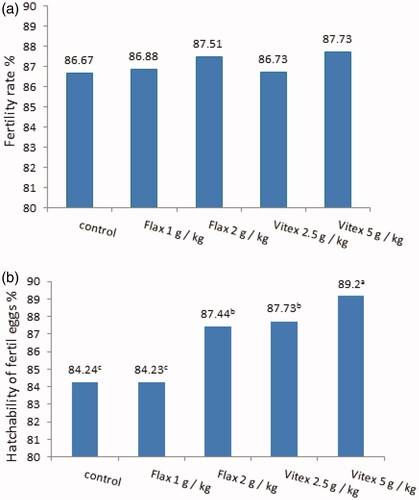
Table 5. Blood haematology, % fertility and % hatchability of aged laying hens treated with different levels of Flax or Vitex.
There was no effect due to adding Flax or Vitex to hens’ rations on the percentage of egg fertile. Moreover, the percentage of hatching of fertilised eggs was significantly increased (p ≤ .001) as a result of feeding hens with high Flax level or low and high levels of Vitex compared to the control with a preference for the group treated with 5 g Vitex/kg feed.
Discussion
Productive performance
In the confrontation, a general belief within the feed industry is that egg production could be significantly reduced in layer flocks when the Flaxseed inclusion rate is greater than 8% (Gonzalez-Esquerra and Leeson Citation2001). Reduced production has been attributed to low nutrient utilisation or due to the presence of anti-nutritional factors that affect feed utilisation or it may be attributed to the decreased energy utilisation from Flax-containing diets (Slominski et al. Citation2006). So, several previous studies revealing the dietary inclusion of Flaxseed in the diet of 10% or more had no significant effect on the productive performance of laying hens (Novak and Scheideler Citation2001; Amini and Ruiz-Feria Citation2007; Abdouli et al. Citation2014; Omri et al. Citation2017). Other studies have shown that Flaxseeds are rich sources of antioxidants and phytoestrogens (Morton et al. Citation1994; Mazur et al. 1998) especially lignans, which act as steroid hormones and compete with endogenous oestrogen and they can bind to cellular oestrogen receptors because they have structural similarity with 17-β-oestradiol (Rubilar et al. Citation2010). Accordingly, Scheideler et al. (Citation1998), and Yassein et al. (Citation2015) revealed that the inclusion of Flaxseeds in the laying hens’ diets enhanced the EP and EM. Our finding was in agreement with the results of Saleh et al. (Citation2019) who reported that inclusion of Flax in aged hens’ diet at level 1% did not harm FI; thus, the FCR ratio was improved, whereas, the Flax treatment showed a significant improve in EP and EM of aged laying hens. Furthermore, Al-Nasser et al. (Citation2011) found that adding Flaxseed to the hen’ diet did not adversely affect feed efficiency. As well, feeding Flaxseed to laying hens improved EP rate (Scheideler et al. Citation1998) and EW (Novak and Scheideler Citation2001). In addition, Novak and Scheideler (Citation2001) found that feeding Flaxseed not only did not adversely affect the percentage of EP but resulted in heavier eggs than the control hens. Furthermore, Scheideler et al. (Citation1998) reported that improve EP rate indicated that Flaxseed is reaching omega-3 which is a healthy product that could also improve EP and EW. In contrast, Karakullukck et al. (Citation2016) demonstrated that supplementation of vitex volatile oil to the laying hen’ diet did not affect product performance and internal and external quality of the egg, except for egg-specific gravity and yolk colour during the peak egg production period. Previous studies supported the hypothesis that the improvement in the aged hens’ productive performance found herein may be attributed to the Flax and Vitex phytoestrogen compounds that enhanced the ovary follicle growth and stimulated the synthesis of yolk proteins and lipids in the liver and transfer them to the follicle, whereas, according to Kuiper et al. (Citation1998) and Rubilar et al. (Citation2010) Flax contains complex phenols, such as phytoestrogens, especially lignans, which act as oestrogenic compounds because they have structural similarities with 17-β-oestradiol and stimulate oestrogen receptors. Elkomy et al. (Citation2008) reported treating aged laying hens with Gibberellic acid (GA3) as a phytoestrogen significantly boosted blood oestrogen level, as well, increased EP rate and improved FC ratio than the control group. Dai et al. (Citation2021) summarised, flavonoids could improve reproduction performance by regulating liver lipid metabolism and improving ovarian function in aged breeder hens. In the same way, Researchers believe that vitex works by decreasing levels of the hormone prolactin and this helps rebalance other hormones, including oestrogen and progesterone (Diana van Die et al. Citation2013).
Egg quality characteristics
One of the clear results in the present study was that dietary Flaxseed or Vitex to aged hens resulted in an improving eggshell weight and eggshell thickness. This assumption agrees with the results of Saleh et al. (Citation2019) who mentioned that dietary Flaxseed supplementation significantly improved eggshell weight and eggshell thickness at the end of the egg‐laying cycle. Similarly, dietary phytoestrogens improved eggshell quality in Hyline brown laying hens (Ni et al. Citation2007; Gu et al. Citation2013) and quails (Sahin et al. Citation2007) during the late laying period. According to Sahin et al. (Citation2007); Gu et al. (Citation2013), and Saleh et al. (Citation2019) the improvements in eggshell quality characteristics were probably related to increasing serum calcium concentration that associated with phytoestrogens treatment. From another point of view, Wistedt et al. (Citation2012) declared that phytoestrogens are involved in regulating the carbonic anhydrase activity via oestrogen receptor-α and oestrogen receptor-β located in the shell gland. Thus, it might be speculated that aged laying hens might be more sensitive to phytoestrogen supplementation and increased calcium absorption and utilisation, which finally leads to enhancing eggshell quality characteristics (Saleh et al. Citation2019). Our results showed that increasing egg-shell thickness in the Flax and Vitex low-level groups compared to the control group despite the egg-shell weight was not differ may be due to the way the active components in the tested materials affect calcium deposition and the formation of cones during the formation of the eggshell in the oviduct.
Increasing egg yolk weight due to Flax and Vtex treatments may be attributed to their phytoestrogen compounds that have a mimic oestrogen-like action, which stimulates the liver to synthesise the yolk protein and yolk fat thus increasing the transmission activity of these components from the liver to the ovarian follicles. Similar results were demonstrated by Saleh et al. (Citation2019) they mentioned that treating aged hens with 1% Flax resulted in increasing eggshell quality characteristics and egg yolk weight.
In addition, the yolk colour score of groups treated with Flax or Vitex was increased significantly than the control and this effect may be due to increase yolk lipids content that contains feed pigments. In accordance to Saleh et al. (Citation2019) dietary supplementation of Flaxseeds to aged hens had a significant positive effect on yolk colour, whereas, it was significantly increased of treated groups than the control.
Due to the phytoestrogen effect of Flax and Vitex that resulted in boosting the blood oestradiol level in the Flax or Vitex treated hens groups (Table ), there was a significant increase (p ≤ .001) in oviduct weight compared to the control group, where, oviduct weight and length were subjected to oestradiol stimulation, whereas, there is a positive correlation between the oviduct activity and blood oestrogen levels. As well, treated hens with Flax or Vitex at any of the studied levels resulted in a significant increase in the number of large yolk follicle (LYF) (p ≤ .05), small yolk follicle (SYF) (p ≤ .001), and LYFs weight (p ≤ .001) than the control group and this increasing may be due to the phytoestrogen and endogenous oestradiol participated in stimulation the synthesis and transmission of protein and lipids from the liver to ovary yolk follicles. On the other hand, there was no effect due to adding Flax or Vitex in hens ratio on ovary relative weight (%) and the number of large white follicles (LWF). Our results were in agreement with the results of Dai et al. (Citation2021), who revealed that dietary hawthorn-leaves flavonoids supplementation could effectively maintain the number of primordial follicles in aged breeder hen’s ovary comparison with the control group. Therefore, they suggest that hawthorn-leaves flavonoids could improve the reproduction performance of the aged breeder hens by relieving ovarian oxidative stress and maintaining the number of primordial follicles.
Some blood constituents and 17-β estradiol level
Decrease blood protein concentration of high levels of Flax and Vitex groups may be correlated to increase the egg production rate of these treated groups, where stimulation occurred for the synthesis and transmission of yolk protein from the liver to ovary yolk follicles and the rate of egg albumin secretion was increased from oviduct glands. Yassein et al. (Citation2015) fed layer hens at rations contained at 0, 5, 10, and 15% Flax conducted to increase EP ratio, and the values of serum total protein and globulin were significantly higher, while, serum albumin and A/G ratio were significantly lower than the control group. Hussein (Citation2007) studied the effect of alcoholic extract of Vitex on mice total serum protein with two different doses (6 mg and 12 mg/mouse), his results showed a significant decrease in total serum protein compared to the control group.
From our results, it could be summarised that both Flax and Vitex at any studied doses resulted in a significant reduction (p ≤ .001) in blood total Chol and LDL concentrations, contrariwise, there was a gradual and significant increase in the benefit part of HDL concentration (p ≤ .001) than the control group. The reduction in total Chol and LDL concentration may be due to the flavonoides compounds in tested materials. Whereas, According to James et al. (Citation2007), the antioxidant and anti-inflammatory benefits of flavonoids led to a significant and repeatable reduction of total cholesterol and triglycerides. Saleh et al. (Citation2019) mentioned that the inclusion of Flax in the diets enhanced the serum antioxidant enzymes activities and reduced total cholesterol concentrations in old laying hens. Dai et al. (Citation2021) found that hawthorn-leaves flavonoids treatment showed the effect of anti-hyperlipidemic in aged breeder hens by decreasing the serum level of triglycerides, Total CHol, and LDL. Additionally, Hussein (Citation2007) found that alcoholic extract of Vitex showed a significant decrease in mice’s total serum cholesterol compared to the control group.
Respective to the influence of feeding Flax or Vited to aged hens, The present data revealed that blood total antioxidant capacity (TAC) concentration was a highly significant increase (p ≤ .001) of Flax and Vitex groups compared to the control group with a preference for the group treated with level of 5 g Vitex. Our finding was in agreement with that found by Saleh et al. (Citation2019) they observed that inclusion Flax in the diets enhanced the serum antioxidant enzymes (GSH‐Px and SOD) activities and reduced lipid peroxidation (MDA) in old laying hens. Moreover, Flaxseed has several other phenolic compounds, including flavonoids and lignans that have significant antioxidant, anti-inflammatory, and therapeutic effects (Oomah Citation2001; Davis et al. Citation2016). Likewise, Flax is a rich source of antioxidants and phytoestrogens (Morton et al. Citation1994; Mazur et al. 1998). Regarding Flax flavonoids, Secoisolariciresinol (SECO) and secoisolariciresinol diglycoside (SDG) are two major Flaxseed lignans that have significant antioxidants and anti-inflammatory effects (Martinchik et al. Citation2012; Silva et al. Citation2013). In addition, James et al. (Citation2011) suggested that the use of the aqueous extract of vitex possesses antidiabetic and antioxidant activity in rats and this effect could exert a beneficial action against the disease associated with free-radicals complications.
The present data summarised that adding Flax or Vitex to hens’ ration boosted blood oestradiol-17β hormone and this effect was significant (p ≤ .001) in the Flax and Vitex groups compared to the control group. it could be assumed that increasing oestradiol-17β in Flax or Vitex groups Maybe due to the tested materials containing phytoestrogen that have a mimic oestrogen-like action, which able to conjugate with oestrogen-receptors, as result there was an abundance of endogenous oestrogen secreted from the ovary that led to an increase in its level in the blood. Previous studies demonstrated that Flaxseeds are rich sources of antioxidants and phytoestrogens (Morton et al. Citation1994; Mazur et al. 1998) especially lignans, which act as steroid hormones and compete with oestrogen, thus decreasing endogenous levels and they can bind to cellular oestrogen receptors because they have structural similarity with 17-β-oestradiol (Rubilar et al. Citation2010). Dusza et al. (Citation2006) reported that phytoestrogens affected ovarian steroidogenesis in females. In birds, phytoestrogens had developmental reproductive changes in chickens (Berry et al. Citation1999). Elkomy et al. (Citation2007) stated that GA3 as a phytoestrogen not only has a mimic’s oestradiol biological effect but also have effects similar to those of oestrogen with a suggestion that GA3 can also stimulate oestrogen secretion (Elkomy et al. Citation2008). Offer rations containing 1% Flax to elderly hens resulted in elevated plasma levels of LH, FSH, and oestradiol‐17β (Saleh et al. Citation2019). Also, the inclusion of plant phytoestrogen (daidzein) increased the ovulation rate in ducks (Zhao et al. Citation2004). Serum oestrogen levels in hawthorn-leaves flavonoids treated groups were elevated significantly compared with the control group; also, improved the activity of antioxidant enzymes (Dai et al. Citation2021). Furthermore, Dusza et al. (Citation2006) stated that phytoestrogens are involved in up‐regulating the oestrogen receptor‐β mRNA hepatocytes and induce vitellogenin mRNA expression in chickens. On the other side, Christie and Walker (Citation1998) demonstrated that vitex has been used for hundreds of years to regulate the function of female reproductive organs. It is thought to exhibit a normalising or balancing effect on reproductive hormone production (Schellenberg Citation2001).
As shown in Table , treated aged hens with Flax and Vitex did not exert an effect on hematological measurements (Hb content, RBCs count, and PCV) except WBCs count that was a significant increase in Flax and Vitex high-level groups only. Despite that, some previous studies suggested that plant flavonoids can decrease the number of red blood cells, packed cell volume, and haemoglobin content (Wang et al. Citation1985; Hsu et al. Citation1987). Likewise, essential oils and flavonoids were also shown in another study to affect blood parameters resulting in inhibition of synthesis of red blood cells (Wang et al. Citation1985) or decreased production of its elements (Blumenthal et al. Citation2000). Our data revealed that the hematological measurements evaluated here were unaffected by the addition of Flax or Vtex to aged hens.
The results are graphically illustrated in Figure the percentage of fertilised eggs and hatchability of fertilised eggs for the Flax and Vitex groups compared to the control group. There was no effect due to feeding the hens with rations containing different levels of Flax or Vitex on the percentage of fertilised eggs. While improving the percentage of hatching of fertilised eggs was occurred in Flax and Vitex groups. According to Dai et al. (Citation2021), the hatchability was elevated in hawthorn-leaves flavonoids groups without differences were observed in fertilisation rate and embryo survival rate among the three groups.
Conclusion
Flax or Vitex as a source of phytoestrogens can be used to improve the performance of older laying hens, prolong their productive life and thus increase the economic yield of the flock. Moreover, an improvement occurred in the antioxidative status, hormonal profile, steroidogenesis, and egg quality in aged laying hens.
Geolocation information
The experiment was conducted at EL- Sabahia Poultry Research Station, Animal Production Research Institute, Agricultural Research Centre, Alexandria governorate, Egypt.
Disclosure statement
The authors declare that there is no known conflict of interest associated with this publication.
Data availability statement
Not applicable. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abdouli H, Omri B, Tayachi L. 2014. Effect of whole fenugreek seed before and after its maceration in water on Hen’s laying performance and egg cholesterol profile. J New Sci. 8:28–34.
- Al-Nasser AY, E. Al-Saff A, K. Abdulla F, E. Al-Baho M, Ragheb G, M. Mashaly M., 2011. Effect of adding flaxseed in the diet of laying hens on both production of omega-3 enriched eggs and on production performance. Int J Poult Sci. 10(10):825–831.
- Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E. 2010. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat, and wheat as affected by sprouting and baking. Food Chem. 119(2):770–778.
- Amini K, Ruiz-Feria CA. 2007. Evaluation of pearl millet and flaxseed effects on egg production and n-3 fatty acid content. Br Poult Sci. 48(6):661–668.
- Benincasa P, Galieni A, Manetta AC, Pace R, Guiducci M, Pisante M, Stagnari F. 2015. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat. J Sci Food Agric. 95(9):1795–1803.
- Bergman Jungestrom M, Lilian TU, Charlotta D. 2007. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 13(3):1061–1067.
- Berry WD, Zhang X, Liu P, McDanie L. 1999. Chick oviduct growth in response to genistein. Poultr Sci. 78(Suppl 1):21.
- Blumenthal M, Goldberg A, Brinckmann J, editors. Herbal medicine: expanded commission E monographs. Newton (MA): Integrative Medicine Communications; 2000.
- Chan E, Wong SK, Chan HT. 2018. Casticin from Vitex species: a short review on its anticancer and anti-inflammatory properties. J Integr Med. 16(3):147–152.
- Christie S, Walker AF. 1998. Vitex agnus-castus: a review of its traditional and modern therapeutic use. Eur J Herbal Med. 3:29–45.
- Dai H, Lv Z, Huang Z, Ye N, Li S, Jiang J, Cheng Y, Shi F. 2021. Dietary hawthorn-leaves flavonoids improve ovarian function and liver lipid metabolism in aged breeder hens. Poultr Sci. 100(12):101499–101411.
- Davis JE, Cain J, Small C, Hales DB. 2016. The therapeutic effect of Flax-based diets on fatty liver in aged laying hens. Poult Sci. 95(11):2624–2632.
- Devine PJ, Perreault SD, Luderer U. 2012. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 86(2):1–10.
- Diana V D M, Burger HG, Teede HJ, Bone KM. 2013. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med. 79(7):562–575.
- Dusza L, Ciereszko R, Skarzynski DJ. 2006. Mechanism of phytoestrogens action in reproductive processes of mammals and birds. Reprod Biol. 6(Suppl 1):151–174.
- Elkomy EA, Elghalid OA, Elnagar SA. 2012. Evaluation of the estrogenic effect of Gibberellic acid in aging hens. Egypt J Anim Prod. 49(3):303–308.
- Elkomy AE, Elnagar SA, El-Sebai A. 2007. Steroidogenic effects of Gibberellic Acid (GA3) on chicks. Egypt Poult Sci J. 27(IV):1239–1255.
- Elkomy AE, El-Shaarrawi G, El-Ansary E, Elnagar AA. 2008. Evaluation of estrogenic response to subcutaneous injection of Gibberellic acid (GA3) in aged female fowl. Egypt Poult Sci J. 28(4): 1265–1286.
- Finley JW. 2005. Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids, glucosinolates, polyphenols and selenocompounds. Ann Bot. 95(7):1075–1096.
- Fortress Diagnostics Ltd, Antrim, UK (Unit 9A, The Technology Park, Belfast Road, Co. Antrim, N Ireland, BT41 1QS - E: [email protected]), 2021.
- Gonzalez-Esquerra R, Leeson S. 2001. Alternatives for the enrichment of eggs and chicken meat with omega-3 fatty acids. Can J Anim Sci. 81(3):295–305.
- Gu H, Shi SR, Chang LL, Tong HB, Wang ZY, Zou JM. 2013. Safety evaluation of daidzein in laying hens: part II. Effects on calcium-related metabolism. Food Chem Toxicol. 55:689–692.
- Heskes AM, Sundram TCM, Boughton BA, Jensen NB, Hansen NL, Crocoll C, Cozzi F, Rasmussen S, Hamberger B, Hamberger B, et al. 2018. Biosynthesis of bioactive diterpenoids in the medicinal plant Vitex agnus-castus. Plant J. 93(5):943–958.
- Hsu MF, Young JH, Wang JP, Teng CM. 1987. Effect of hsien-ho-t’sao (Agrimonia pilosa) on experimental thrombosis in mice. Am J Chin Med. 15(1–2):43–51.
- Hussein MAA. 2007. Effect of vitex agnus- castus extract T on some physiological parameters of mice (Mus musculus L.). Med J Basrah Univ. 25(2):57–59.
- James DB, Owolabi OA, Oluloto AO, Mohammed H, Muhammed OA. 2011. Change in organs weight and antioxidant potential of combined effects of aqueous extracts of Phyllanthus amarus and Vitex doniana stem bark on streptozotocin-induced dibetic rats. Asian J Med Sci. 3(6):237–242.
- James MR, Xian-Liu Z, Guthrie N. 2007. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern Ther. 13(6):44–46.
- Johnson AL. 2000. Reproduction in the female. In: Whittow GC, editor. Sturkie’s avian physiology. 5th ed. New York (NY): Academic Press; p. 569–596.
- Karakullukck MZ, Guclu BK, Kara K, Tugruly S. 2016. Effect of some essential oil supplementation to laying hen diet on performance and egg quality. J Fac Vet Med Istanbul Univ. 42(1):31–37.
- Katare C, Saxena S, Agrawal S, Prasad G, Bisen P. 2012. Flaxseed: a potential medicinal food. J Nutr Food Sci. 2(1):1–8.
- King SR. 2002. Medicinal plants of the world volume 2: chemical constituents, traditional and modern medicinal uses by Ivan, A. Ross (U.S. Food and Drug Administration). Humana Press, Inc., Totowa, NJ. J Nat Prod. 65(7):1085–1085.
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 139(10):4252–4263.
- Lara PR, Bezerra GP, Romero NR, Silva HCD, Lemos TLG, Arriaga AMC, Alves PB, Dos Santos MB, Militão GCG, Silva TDS, et al. 2020. Chemical composition and biological activities of the essential oils from Vitex-agnus castus, Ocimum campechianum and Ocimum carnosum. An Acad Bras Cienc. 92(1):e20180569.
- Liu XT, Lin X, Mi Y-L, Zeng W-D, Zhang C-Q. 2018. Age-related changes of yolk precursor formation in the liver of laying hens. J Zhejiang Univ Sci B. 19(5):390–399.
- Luderer U. 2014. Ovarian toxicity from reactive oxygen species. Vitam Horm. 94:99–127.
- Martinchik AN, Baturin AK, Zubtsov VV, Molofeev V. 2012. Nutritional value and functional properties of Flaxseed. Vopr Pitan. 81(3):4–10.
- Mazur W, Fotsis T, Wähälä K, Ojala S, Salakka A, Adlercreutz H. 1996. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem. 233(2):169–180.
- Miao LP, Li LL, Zhu MK, Dong XY, Elwan HAM, Zou XT. 2019. Excess dietary fluoride affects laying performance, egg quality, tissue retention, serum biochemical indices, and reproductive hormones of laying hens. Poult Sci. 98(12):6873–6879.
- Morton MS, Wilcox G, Wahlqvist ML, Griffiths K. 1994. Determination of lignans and isoflavonoids in human female plasma following dietary supplementation. J Endocrinol. 142(2):251–259.
- Ni Y, Zhu Q, Zhou Z, Grossmann R, Chen J, Zhao R. 2007. Effect of dietary daidzein on egg production, shell quality, and gene expression of ER-alpha, GH-R, and IGF-IR in shell glands of laying hens. J Agric Food Chem. 55(17):6997–7001.
- Novak C, Scheideler SE. 2001. Long–term effects of feeding Flaxseed–based diets. 1. Egg production parameters, components, and eggshell quality in two strains of laying hens. Poultr Sci. 80(10):1480–1489.
- Omri B, Chalghoumi R, Abdouli H. 2017. Study of the effects of dietary supplementation of linseeds, fenugreek seeds, and tomato pepper mix on laying hen’s performances, egg yolk lipids, and antioxidants profiles, and lipid oxidation status. J Anim Sci Livestock Prod. 1(2):1–9.
- Oomah BD. 2001. Flaxseed is a functional food source. J Sci Food Agric. 81(9):889–894.
- Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. 2012. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Journal of Hepatology. 56(4):944–951.
- Petre Alina MS. 2019. Avalible at RD (NL) on August 9. https://www.healthline.com/nutrition/vitex#what-it-is
- Renema RA, Robinson FE, Melnychuk VL, Hardin RT, Bagley LG, Emmerson DA, Blackman JR. 1995. The use of feed restriction for improving reproductive traits in male-line large white turkey Hens.: 2. Ovary morphology and laying traits. Poultr Sci. 74(1):102–120.
- Rubilar M, Gutiérrez C, Verdugo M, Shene C, Sineiro J. 2010. Flaxseed as a source of functional ingredients. J Soil Sci Plant Nutr. 10(3):373–377.
- Sahin N, Onderci M, Balci TA, Cikim G, Sahin K, Kucuk O. 2007. The effect of soy isoflavones on egg quality and bone mineralisation during the late laying period of quail. Br Poult Sci. 48(3):363–369.
- Saleh AA, Ahmed EAM, Ebeid TA. 2019. The impact of phytoestrogen source supplementation on reproductive performance, plasma profile, yolk fatty acids, and antioxidative status in aged laying hens. Reprod Dom Anim. 54(6):846–854.
- SAS Institute. 2009. SAS user’s guide: statistics version. 5th ed. Cary (NC): SAS Inst., Inc.
- Scheideler SE, Jaroni D, Froning GW. 1998. Strain and age effects on egg composition from hens fed diets rich in n-3 fatty acids. Poult Sci. 77(2):192–196.
- Schellenberg R. 2001. Treatment of premenstrual syndrome with Vitex agnus-castus extract prospective. A randomized, placebo-controlled study. BMJ. 322(7279):134–137.
- Silva FGD, O’Callagahan Y, O’Brien NM, Netto FM. 2013. Antioxidant capacity of Flaxseed products: the effect of in vitro digestion. Plant Foods Hum Nutr. 68(1):24–30.
- Singh KK, Mridula D, Rehal J, Barnwal P. 2011. Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr. 51(3):210–222.
- Slominski BA, Meng X, Campbell LD, Guenter W, Jones O. 2006. The use of enzyme technology for improved energy utilization from full-fat oilseeds. Part II: flaxseed. Poultr Sci. 85(6):1031–1037.
- Wang JP, Hsu MF, Teng CM. 1985. Antiplatelet effect of hsien-ho-t’sao (Agrimonia pilosa). Am J Chin Med. 13(1–4):109–118.
- Webb AL, McCullough ML. 2005. Dietary lignans: potential role in cancer prevention. Nutr Cancer. 51(2):117–131.
- Williams TD, Ames CE, Kiparissis Y, Wynne-Edwards KE. 2005. Laying-sequence-specific variation in yolk oestrogen levels, and relationship to plasma oestrogen in female zebra finches (Taeniopygia guttata). Proc Biol Sci. 272(1559):173–177.
- Wistedt A, Ridderstråle Y, Wall H, Holm L. 2012. Effects of phytoestrogen supplementation in the feed on the shell gland of laying hens at the end of the laying period. Anim Reprod Sci. 133(3–4):205–213.
- Yassein SA, El‐Mallah GM, Sawsan MA. 2015. Response of laying hens to dietary Flaxseed levels on performance, egg quality criteria, the fatty acid composition of egg, and some blood parameters. Int J Res Stud Biosci. 3(10):27–34.
- Zhao R, Wang Y, Zhou Y, Ni Y, Lu L, Grossmann R, Chen J. 2004. Dietary daidzein influences laying performance of ducks (Anas platyrhynchos) and early post-hatch growth of their hatchlings by modulating gene expression. Comp Biochem Physiol A Mol Integr Physiol. 138(4):459–466.