Abstract
Parental executive functioning (EF) is considered one key contributing source, via direct and indirect routes, of inter-individual variation in offspring EF. The current study investigated the unexplored associations between maternal EF and infant EF as well as its precursor, sustained attention. Ninety-seven mother-infant-dyads from the FinnBrain Birth Cohort Study participated. Maternal EF was assessed using selected measures from the Cogstate test battery. At 8 months, infants completed Lab-TAB Blocks and modified A-not-B tasks. A modest but robust link between maternal EF and infant attention was revealed in girls. There was no association between mother and infant EF in either sex at 8 months. Notable directions for future research, and potential underlying mechanisms of sex differences are discussed.
Introduction
Executive functioning (EF) is a set of higher-order cognitive skills that are employed in goal-directed behavior and regulation of emotion (Nigg, Citation2017). Efficient early EF has high relevance for cognitive, behavioral and social well-being later in life, whereas EF difficulties are cardinal features of many psychiatric disorders emerging during childhood (Diamond, Citation2013). For instance, ADHD, a neuropsychiatric disorder typically characterized by EF dysfunction, has an early onset and global prevalence average of 5% (Sayal et al., Citation2018).
EFs can also be considered to consist of attentional skills, or ways of using attention (Zelazo, Citation2015). Therefore, maturation of attentional control is a key process in EF development, and attentional capacity has been suggested to be the source of common variance in the manifestation of different aspects of EF (Garon et al., Citation2008). Control of attention undergoes a major change during the first year of life. As reviewed by Hendry et al. (Citation2016), during the first months of life, when difficulties in disengaging attention from stimuli are typical, infant’s attention is strongly driven by environmental factors, i.e., novelty of events and objects. Across the first 6 months, shorter looking times for visual stimuli indicate easier disengagement and more mature attentional control, whereas after 6 months, longer looking times, particularly for novel and complex stimuli, mark more mature attentional control. Remarkable development in control of attention, which is manifested by increased focused attention and decreased distractibility, takes place at around 9 months.
Notably, infant attention is associated with EF in later infancy (Blankenship et al., Citation2019) and at ages 1–3 years (e.g., Kraybill et al., Citation2019). In the study by Blankenship et al. (Citation2019), infant 5-month attention, measured as the peak looking time and the shift rate, was found to have a direct effect on infant 10-month EF, assessed using the A-not-B task. In turn, Kraybill et al. (Citation2019) found a significant connection between 5-month attention and 3-year EF. These findings support the idea that infant attention and EF stem from the same rapidly developing neural networks and that infant attention might reflect later EF and later EF deficits. Furthermore, the development of attention and EF coincides with the emergence of the executive network, a neural network responsible for resolving conflicts, detecting errors and regulating inhibitory responses, towards the end of the first year (Garon et al., Citation2008).
Core executive functions, from which “higher-order” EF develops, include inhibition, working memory and cognitive flexibility (Miyake & Friedman, Citation2012). Although more readily observable as starting in toddlerhood, nascent EF abilities such as early inhibition and working memory (Garon et al., Citation2008) can be captured using laboratory tests (e.g., A-not-B task) during the second half of the first year of life; however, EFs are still relatively undifferentiated at this age (Hendry et al., Citation2016).
Individual differences in EF are moderately stable during its protracted development (Miyake & Friedman, Citation2012), but their rank-order stability is low to moderate in early childhood, suggesting a considerably dynamic development of these abilities during this period (Bridgett et al., Citation2015). Furthermore, the low stability may be partially explained by the heightened neural sensitivity during the first years of life (Weiss & Wagner, Citation1998); thus, environmental influences may have a more pronounced influence on the trajectories of attention/EF development during this period. Nevertheless, the sources of individual differences in early childhood EF remain only vaguely understood. Better understanding of these sources would be of significance in an understanding of the early milestones relevant to EFs, and any untypical developmental pathways of attention and EF.
One source of inter-individual variation in children’s EF is parental EF. Individual differences in EF are considered highly heritable (Miyake & Friedman, Citation2012), and moderate heritability of EF is observed in school age children (Polderman et al., Citation2006) and in adolescents (Jester et al., Citation2009). EF may also affect parenting, which in turn creates independent contributions to child EF (Bridgett et al., Citation2018). However, the literature linking maternal and offspring EF in early childhood remains inconclusive. One previous study found mother and child EFs to be positively associated between the ages 2–4 years (Cuevas et al., Citation2014), whereas another did not find at 27 months any mother-child EF association (Leve et al., Citation2013). Surprisingly, to our knowledge, no previous study has reported associations between maternal EF and child EF during infancy, i.e., at this important phase of development when the behavioral manifestations of EF first begin to emerge. Given the crucial role of EF for later development, a better understanding of the interrelations between maternal and infant EF is needed to better elucidate the mechanisms through which EF in infancy develops.
In summary, previous studies show that infant attention is associated with EF in early years. Moreover, evidence has been obtained of a positive link between mother and child EF at ages 2–4 years. However, associations between maternal EF and infant EF or attention have not been reported in the previous literature. The current study addresses these gaps by investigating the associations between maternal EF measured in pregnancy and infant EF, and one of its precursors, sustained attention, measured as duration of looking and facial displays of interest, at 8 months of age.
Furthermore, although overall sex differences in EF within a healthy population are considered negligible, there might be sex-different developmental trajectories (Grissom & Reyes, Citation2019). Some previous literature indicates that in childhood, girls outperform boys in specific aspects of EF (e.g., inhibition, effortful attention, higher-order EF) (e.g., Carlson et al., Citation2004; Leve et al., Citation2013; Wierenga et al., Citation2019). There is also evidence that sex may interact with developmental processes linked with EF dysfunction in specific disorders. For instance, neuropsychiatric disorders with the EF dysfunction as a core symptom (e.g., ADHD, ASD), are much more often diagnosed in boys than in girls, and the presentation of these neuropsychiatric disorders may also differ between the sexes (Grissom & Reyes, Citation2019). For instance, the predominantly inattentive type of ADHD is significantly more prevalent in girls, whereas the predominantly hyperactive-impulsive type is more common in boys (Willcutt, Citation2012). Finally, it has been shown that genetic contributions to behavioral traits and disorders are often sex-dependent (Khramtsova et al., Citation2019). Thus, we additionally explored whether the links between maternal and infant attention/EF were dependent on infant sex at this particular period of development. We hypothesized that maternal EF is positively but modestly associated with infant EF (Cuevas et al., Citation2014) and attention, but due to the lack of previous research, we did not have a specific hypothesis regarding sex differences. To date, very few studies have been able to account for genetic contributions in the context of early EF development. Thus, in this study, to make preliminary conclusions about mechanisms, we additionally tested as an exploratory analysis whether the associations remained when a polygenic score (PGS) for educational attainment was included in the covariates; PGS is a genetic score related to cognitive and brain development (Judd et al., 2020).
Materials and methods
Participants
The participants were 97 healthy Finnish infants and their mothers drawn from the population of the Focus Cohort families participating in the FinnBrain Birth Cohort Study (Karlsson et al., Citation2018). For the present study, participation in both maternal neuropsychological assessment during pregnancy and the assessment of infant attention and EF at 8 months was required. The characteristics of the mother-infant dyads are displayed in .
Table 1 Sample characteristics (N = 97).
Procedures
Subjects were recruited at the gestational week (gw) 12 ultrasound visit. Maternal neuropsychological assessment was conducted between gws 26 and 30. Infant attention and EF were assessed at 8 months of age. The study was approved by The Ethics Committee of Hospital District of Southwest Finland (ETMK: 57/180/2011; ETMK: 59/1801/2013).
Maternal EF
Maternal EF was assessed using the Cogstate computerized test battery designed for use in research studies and clinical trials (www.cogstate.com; Maruff et al., Citation2009). The entire assessment comprised of nine tasks measuring various neuropsychological functions (Kataja et al., Citation2017) and, based on previous research, included three tasks tapping into a common factor representing EF/working memory performance (e.g., Nordenswan et al., Citation2020; Yoshida et al., Citation2011; Zhong et al., Citation2013): the Continuous Paired Associate Learning Test (CPAL; measures visuospatial working memory. The number of errors was used as the unit of measurement), the Groton Maze Learning Test (GML; EF/visuospatial working memory; number of errors), and the International Shopping List Test (ISL; verbal learning and memory; number of correct responses). A composite score of these three tests that were all modestly to moderately positive correlated (inter-correlations for CPAL and GML; CPAL and ISL; and ISL and GML were: r = .07, p > .05; r = .39, p < .001; r = .19, p = .058, respectively), with higher score indicating better performance was used in all subsequent analyses.
Infant attention
Infant sustained attention was assessed using the Blocks episode from The Laboratory Temperament Assessment Battery (Lab-TAB) Prelocomotor Edition (Goldsmith & Rothbart, Citation1999). In this task, the infant was provided with an opportunity to play freely with a set of blocks for 3 minutes. Infant’s sustained attention i.e. duration of looking at the blocks in seconds (0= not looking the blocks, 1 = 1–4 seconds, 2 = 5–8 seconds, 3 = 9–10 seconds) and intensity of facial interest in response to the blocks (0 = no interest, 1 = a low intensity interest, 2 = a high intensity interest) were coded from a video in 10 second epochs. Since the indicators were related to maternal EF in different direction, they were examined separately in further analyses. The correlations between the indicators in this study were low (r = .16, p >.05). Nevertheless, based on the theoretical background and a significant correlation between the duration of looking and facial interest in a larger population of the current study where maternal EF was not available (N = 419, r = .31, p < .001), we regarded both as reflecting sustained attention. Both measures showed adequate inter-rater reliability (Cohen’s Kappa for duration of Looking = 0.72 and for facial Interest = 0.79) and were used as continuous in the analyses.
Infant EF
Infant EF was assessed using a modified A-not-B (AB) procedure (see a more detailed description in Nolvi et al., Citation2018), which is considered to measure the core functions of EF: engaging attention, inhibition and working memory. In this modified task, the infant must recall the location of a toy under two identical hiding locations in a fixed pattern on three delay levels (0, 2 and 4 seconds), each level including 6 trials. For example, in the first delay level, the series of trials was R-L-L-R-R-L. If the infants scored correctly in half of the 6 trials, they proceeded to the next delay level. The total number of trials in the entire task ranged between 6 and 18. In each trial, infant′s reaching for a location was scored either as correct or incorrect. All reaching behaviors that were initiated within 8 seconds were considered reaching. Infant performance was scored by the experimenter and verified later by a trained coder. The coding showed adequate inter-rater reliability (81% agreement across trials). The sum of the infant’s performance in all the trials was used to develop one continuous total score (0–18) that was applied in the analyses.
Confounders
Maternal verbal intelligence
Maternal verbal intelligence may be associated with offspring EF (e.g., Leve et al., Citation2013) and was therefore controlled for in the analyses. The Verbal Comprehension Index from Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) (Wechsler, Citation2008, Citation2012), was used to assess verbal intelligence in a subgroup of mothers with the data available (N = 71).
Polygenic score (PGS) for educational attainment
DNA samples were extracted according to standard procedures at the National Institute for Health and Welfare. DNA samples were genotyped with Illumina Infinium PsychArray and Illumina Infinium Global Screening Array at Estonian Genome Centre. Quality control (QC) and a method for imputation have been described elsewhere (PMID: 32676648) (Acosta et al., 2020).
PGS is an overall measure of an individual’s genetic predisposition for a given trait, derived from genome-wide association study (GWAS) for certain trait. In this study, we calculated the PGS for educational attainment (in a subsample of N = 84) based on GWAS where 1131881 samples were studied (Lee et al., Citation2018). PGS for educational attainment refers to an individual’s genetic predisposition for educational attainment measured as the number of years of education, cognitive test performance, self-reported math ability and the highest math class taken (Lee et al., Citation2018). To generate PGS, we used all discovery cohorts from meta-analysis except 23andMe. The final sample size for GWAS was 766345. A PGS is constructed by calculating the weighted sum of risk alleles carried by an individual. The weight is determined by an effect size of an individual allele. We used the PRSice program (Euesden et al., Citation2015) to estimate the PGS for the studied individuals. PRSice provides several PGS with a range of different P-value thresholds based on GWAS. The p value threshold of .1 was used in the analyses, but the findings were similar using different thresholds. The analyses including the PGS were controlled for the principal components of gene-based ethnicity (PC1-PC3).
Maternal pre- and postnatal psychological distress
Maternal pre- and postnatal psychological distress were controlled for as potentially confounding factors as they may be associated with maternal and infant EF (Kataja et al., Citation2017; Nolvi et al., Citation2018). Mothers reported their symptoms by responding to the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., Citation1987), Symptom Checklist 90 anxiety subscale (SCL-90; Derogatis et al., Citation1973), and Pregnancy-related Anxiety Questionnaire Revised 2 (PRAQ-R2; Huizink et al., Citation2016) questionnaires. We used a principal component score comprising of all EPDS, SCL-90, and PRAQ-R2 measurements during pregnancy to reflect prenatal distress and one comprising of the EPDS and SCL-90 at 3 and 6 months postpartum to reflect postnatal distress.
Maternal education and age, infant age and sex
Maternal education (1 = low–3 = high) was obtained from questionnaires completed during pregnancy. Information on maternal age, infant sex and length of gestation were obtained from the national birth register (www.thl.fi).
Statistical analyses
Statistical analyses were conducted using SPSS 26.0. Associations between maternal EF, background variables and infant attention/EF in the whole sample, as well as separately for boys and girls, were examined using Pearson correlation coefficients. To test whether maternal EF or the interaction between maternal EF and infant sex predicted infant attention or EF we used a multivariable general linear model. Although none of the confounders were significantly associated with the infant outcomes in the bivariate analysis, sensitivity analyses were conducted with confounders (infant age, PGS for educational attainment, maternal age, maternal verbal intelligence, maternal education, maternal pre- and postnatal psychological distress). The significant interactions were probed conducting a simple slope analyses in Process Macro 3.5 (Hayes, Citation2017).
Results
Zero-order correlations are presented in . None of the selected confounders were significantly associated with child EF or attention. There were no significant differences in the performance of boys and girls in the attention/EF tasks or maternal EF performance by infant sex ().
Table 2 Associations between maternal EF and infant attention/EF and the confounding factors in the whole sample and separately by infant’s gender.
Table 3 Descriptive statistics for outcome variables.
In the entire sample, maternal EF was not significantly correlated with infant indicators of attention or EF. However, a positive correlation between maternal EF and infant facial interest, but not duration of looking or EF, was present for girls. Further, there was an interaction between maternal EF and infant sex in predicting infant facial interest (F1,92) =5.29, B= −0.13, p = 0.024, ƞ2p =.05). In girls, a higher maternal EF was associated with more frequent facial displays of attention in girls (B = 0.08, 95% CI [0.007, 0.149], p = 0.031) but not in boys (B= −0.05, 95% [−0.132, 0.036], p = 0.260) (). Similar interactions were not observed for duration of looking or infant EF (p>.53).
Figure 1 The association between maternal executive functioning and infant attention (facial interest) in boys and girls separately.
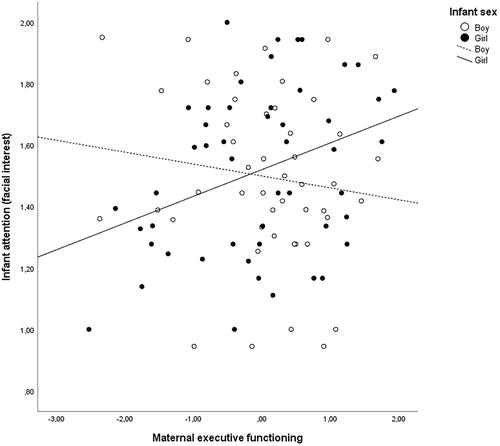
The results remained in the sensitivity analysis controlling for confounders. Further, the interaction remained when controlling for principal components for genetic background and the PGS for educational attainment (B=-.13, p=.022), which became positively associated with infant facial interest in the full model (B=.07, p=.018), suggesting that the associations may not be transmitted solely via genetic pathways.
Discussion
To our knowledge, this is the first exploration of connections between maternal EF and infant attention and emerging EF. In contrast to our hypothesis, an association between mother and infant EF was not observed among boys or girls at this early stage of EF development. However, interestingly, the results showed sex-specific evidence of a modest, but robust association between maternal EF and infant sustained attention (i.e., the antecedent of EF) measured as facial interest at the age of 8 months. A positive link between maternal EF and infant facial interest emerged among mother-daughter dyads, but no such link was observed for duration of looking.
There are several possible reasons for not observing the mother-infant resemblance among EF in this study. First, given the previous literature on attention development underlying the development of EFs, and attentional capacity being one probable source of inter-individual differences in EFs (Garon et al., Citation2008), the link between maternal EF and child attention might emerge earlier in development than the link between mother and child EF. At the age of 8 months, infants are settling into a significant transitional period in attention development, manifested by better concentration and less distraction from environmental stimuli. The latter half of the first year of life is also a remarkable period in EF development (Diamond, Citation1985). Thus, not finding an association between mother and infant EF at 8 months may be due to behavioral manifestations of EF just beginning to emerge at the age of the infants included in the current study. Given that specific cognitive abilities may exhibit stronger genetic stability from stages later than early childhood and continuing into adulthood (Alarcón et al., Citation1998), the heritability as well as the environmental influences affecting EF might not yet be evident during this early period of development.
Second, there are well-known methodological problems concerning the measurement of EF, particularly in the context of different developmental phases. First, in early childhood, EF is a comparatively undifferentiated construct that becomes more structured with age as a result of its protracted development (Friedman & Miyake, Citation2017). Furthermore, EF is a complex and multifaceted construct, with different measures used to capture different aspects of EF, which typically induces low intercorrelations among individual EF tasks (Friedman & Miyake, Citation2017). Second, even though measures assessing the same single aspect of EF would show good reliability, the existing correlation between the measures may be low (e.g., Rey-Mermet et al., Citation2017). Additionally, in young children, there are, for example, limitations in verbal abilities and attentional control that affect comprehension and the following of task rules and staying on a task (e.g., Carlson, Citation2005). Therefore, developmentally appropriate measures of EF are different from those of adults. Thus, it is possible that measures used in this study captured different aspects of EF resulting in no association being observed between maternal and infant EF.
We did not set any hypothesis regarding the sex differences. Nonetheless, there are several possibilities for the exploratory sex-specific findings that were observed. First, previous research has consistently demonstrated that genetic contributions to behavioral traits and diseases are sex-dependent and that these contributions are transmitted through sex-specific mechanisms (Khramtsova et al., Citation2019). In complex traits and diseases there may be sex differences in, for example, clinical presentation, age of onset, and susceptibility, such as differences in the symptoms of males and females with autism spectrum disorders, obsessive-compulsive disorder and schizophrenia (Khramtsova et al., Citation2019). Although our study did not focus on genetic transmission, these observations may provide insights into the mechanisms underlying the associations between maternal and infant EF. One potential underlying mechanism specific to attention and EF development might be found in sex differences in neural networks underlying EF. As reviewed by Manoli and Tollkuhn (Citation2018), there may be innate differences in neural functioning between sexes in infancy. In turn, innate divergencies underlie early behavioral differences between boys and girls both in normal development and in the context of neuropsychiatric disorders. Because of these differences, some findings may be detected at different ages for boys and girls. For instance, on the behavioral level, girls in the normal population are known to express higher effortful control (i.e., attention regulation and inhibitory control, which are also components of EF) than boys (Else-Quest et al., Citation2006). Overall, girls tend to outperform boys in attention focus, attention shifting and inhibitory control (Else-Quest et al., Citation2006; Silverman, Citation2021; Tao et al., Citation2014).
An interesting example of sex differences in neural functioning in line with the findings of the current study, where no performance differences between sexes were found, is the study by Cuevas et al. (Citation2016). First, this study demonstrated that there are underlying sex differences in EEG power changes in EF related networks in the absence of performance differences in EF tasks in 4-year-olds. Second, girls exhibited more localized EEG changes during EF tasks than boys. Increased localization seems to be a normal developmental change in brain electrical activity during higher order cognitive processes (e.g., Bell & Wolfe, Citation2007); this could indicate more mature functional brain development in girls (e.g., Grabner et al., Citation2004), especially in infancy (Chen et al., Citation2021) . On the other hand, a faster integration of frontoparietal networks relevant for executive control in boys in comparison to girls in infancy has been observed in previous studies (Gao et al., Citation2015). In the context of our findings, the association between maternal EF and precursors of infant EF were perhaps found only in girls because of the potential underlying sex different developmental pathways in neural functioning. That is, girls’ EF may develop at a different pace in comparison to boys. Thus, similar associations for boys and girls may be detected in the whole sample at a later age (Chen et al., Citation2021), but this remains to be tested by future studies. Our findings suggest that sex might be a relevant factor to acknowledge in future research concerning early child EF and parental EF links.
Second, as Friedman and Miyake (Citation2017) present, although EFs are considered highly heritable, they are not invariable, but also responsive to environmental effects such as parenting. Consequently, maternal EF may impact child EF also through parenting behavior already in infancy, which is a critical time period for the emergence of EF (Bridgett et al., Citation2017). It has been concluded that maternal EF is crucial for regulation of parenting behavior (Cuevas et al., Citation2014). For instance, already in infancy a poorer maternal EF is known to associate with more negative parenting behaviors, such as displays of negativity and intrusiveness (Bridgett et al., Citation2017). In turn, negative caregiving practices are linked to children with a poorer EF (Valcan et al., Citation2018). It is also known that positive parenting behaviors (e.g., support and involvement) and parenting that involves cognitive support, such as cognitive stimulation, scaffolding and attention maintaining, are associated with a higher EF in children (Valcan et al., Citation2018). Interestingly, child sex may also moderate the link between parenting and child EF. For instance, boys may be especially vulnerable to social network-related resources, including, e.g., family stressors and parental satisfaction, in terms of the development of cognitive flexibility (Clark et al., Citation2013). Nevertheless, autonomy-supportive and controlling parenting behaviors appear to be very similar with girls and boys (Endendijk et al., Citation2016). However, in this study, we could not assess the parenting pathway and future studies should continue to study the parenting as one mechanism transmitting EF, and its sex differences, along with heritability in a larger sample of parent-infant dyads.
The current study has several strengths, one being that we used observational data to assess mother and infant EF, to the best of our knowledge, for the first time in the context of mother–infant EF resemblance. In addition, we accounted for several confounding factors, including PGS for educational attainment, which has not been used in prior studies. The limitations of the study include the fact that no multiple comparison corrections were applied, so the evidence should be considered preliminary and as a starting point for future studies. We used only one measurement point and task for infant EF. Though associations between maternal and infant EF did not emerge by 8 months of age, such associations may be present later in infancy (e.g., 12 months of age), when children’s EF has further developed. As discussed, the correspondence between mother and infant EF measures, which could have diminished the possibility for the tasks to measure different aspects of EF, was limited in this study. The general challenge with EF measurement is that it is frequently not possible to perform the same EF tasks for very young children and parents due to the lack of tasks that are suitable for covering this remarkably wide age range. Consequently, this leaves a possibility that no association was found between maternal and infant EF because different measures were used. As a final point, the sample of the present study was small. However, based on previous studies of Cuevas et al. (Citation2014) and Kim et al. (Citation2017) which examined associations between maternal EF and child EF at the ages of 2–4 and 3–6 years, the effect sizes ranged from r = .22 to .37. Our post hoc power analysis indicated that with a power of 0.7–0.8, such effects could be detected, and thus the detected effect in the present study were within in this effect size range; this indicates that the power was sufficient for detecting the anticipated effects. However, given the paucity of previous studies in this age range, larger samples are essential to replicate and expand on current findings.
To conclude, the association between sustained attention, specifically facial interest, and maternal EF is, in the absence of sex differences in task performance, evident in girls, but not in boys, by 8 months of age. Furthermore, evidence suggests, that these associations may not be transmitted solely via genetic pathways. Finally, our findings suggest that more robust associations between maternal and child EF may not emerge until later in life (e.g., toddlerhood, or early school age).
Acknowledgments
We thank the FinnBrain Study research personnel and the students involved in the data collection for their invaluable help with the processing and disseminating this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data protection legislation in Finland does not permit open distribution of data, but data could be delivered by special requests (which includes formal collaboration and material transfer agreements). If needed, the principal investigator, Professor Hasse Karlsson is able to provide you with further information about the data availability, if necessary ([email protected]).
Additional information
Funding
REFERENCES
- Acosta, H., Kantojärvi, K., Hashempour, N., Pelto, J., Scheinin, N. M., Lehtola, S. J., Lewis, J. D., Fonov, V. S., Collins, D. L., Evans, A., Parkkola, R., Lähdesmäki, T., Saunavaara, J., Karlsson, L., Merisaari, H., Paunio, T., Karlsson, H., & Tuulari, J. J. (2020). Partial support for an interaction between a polygenic risk score for major depressive disorder and prenatal maternal depressive symptoms on infant right amygdalar volumes. Cerebral Cortex, 30(12), 6121–6134. https://doi.org/10.1093/cercor/bhaa158
- Alarcón, M., Plomin, R., Fulker, D. W., Corley, R., & DeFries, J. C. (1998). Multivariate path analysis of specific cognitive abilities data at 12 years of age in the Colorado adoption project. Behavior Genetics, 28(4), 255–264. https://doi.org/10.1023/A:1021667213066
- Bell, M. A., & Wolfe, C. D. (2007). Developmental neuropsychology changes in brain functioning from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental Neuropsychology, 31(1), 21–38. https://doi.org/10.1080/87565640709336885
- Blankenship, T. L., Slough, M. A., Calkins, S. D., Deater‐Deckard, K., Kim‐Spoon, J., & Bell, M. A. (2019). Attention and executive functioning in infancy: Links to childhood executive function and reading achievement. Developmental Science, 22(6), e12824. https://doi.org/10.1111/desc.12824
- Bridgett, D. J., Burt, N. M., Edwards, E. S., & Deater-Deckard, K. (2015). Intergenerational transmission of self-regulation: A multidisciplinary review and integrative conceptual framework. Psychological Bulletin, 141(3), 602–654. https://doi.org/10.1037/a0038662
- Bridgett, D. J., Ganiban, J. M., Neiderhiser, J. M., Natsuaki, M. N., Shaw, D. S., Reiss, D., & Leve, L. D. (2018). Contributions of mothers’ and fathers’ parenting to children’s self-regulation: Evidence from an adoption study. Developmental Science, 21(6), e12692. https://doi.org/10.1111/desc.12692
- Bridgett, D. J., Kanya, M. J., Rutherford, H. J. V., & Mayes, L. C. (2017). Maternal executive functioning as a mechanism in the intergenerational transmission of parenting: Preliminary evidence. Journal of Family Psychology, 31(1), 19–29. https://doi.org/10.1037/fam0000264
- Carlson, S. M. (2005). Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology, 28(2), 595–616. https://doi.org/10.1207/S15326942DN2802_3
- Carlson, S. M., Mandell, D. J., & Williams, L. (2004). Executive function and theory of mind: Stability and prediction from ages 2 to 3. Developmental Psychology, 40(6), 1105–1122. https://doi.org/10.1037/0012-1649.40.6.1105
- Chen, H., Liu, J., Chen, Y., Salzwedel, A., Cornea, E., Gilmore, J. H., & Gao, W. (2021). Developmental heatmaps of brain functional connectivity from newborns to 6-year-olds. Developmental Cognitive Neuroscience, 50, 100976. https://doi.org/10.1016/J.DCN.2021.100976
- Clark, C. A. C., Sheffield, T. D., Chevalier, N., Nelson, J. M., Wiebe, S. A., & Espy, K. A. (2013). Charting early trajectories of executive control with the shape school. Developmental Psychology, 49(8), 1481–1493. https://doi.org/10.1037/a0030578
- Cox, J. L., Holden, J. M., & Sagovsky, R. (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. https://doi.org/10.1192/bjp.150.6.782
- Cuevas, K., Calkins, S. D., & Bell, M. A. (2016). To Stroop or not to Stroop: Sex-related differences in brain-behavior associations during early childhood. Psychophysiology, 53(1), 30–40. https://doi.org/10.1111/psyp.12464
- Cuevas, K., Deater-Deckard, K., Kim-Spoon, J., Wang, Z., Morasch, K. C., & Bell, M. A. (2014). A longitudinal intergenerational analysis of executive functions during early childhood. British Journal of Developmental Psychology, 32(1), 50–64. https://doi.org/10.1111/bjdp.12021
- Cuevas, K., Deater-Deckard, K., Kim-Spoon, J., Watson, A. J., Morasch, K. C., & Bell, M. A. (2014). What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science, 17(2), 224–238. https://doi.org/10.1111/desc.12073
- Derogatis, L. R., Lipman, R. S., & Covi, L. (1973). SCL-90: An outpatient psychiatric rating scale-preliminary report. Psychopharmacology Bulletin, 9(1), 13–28.
- Diamond, A. (1985). Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development, 56(4), 868. https://doi.org/10.2307/1130099
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
- Else-Quest, N. M., Hyde, J. S., Goldsmith, H. H., & Van Hulle, C. A. (2006). Gender differences in temperament: A meta-analysis. Psychological Bulletin, 132(1), 33–72. https://doi.org/10.1037/0033-2909.132.1.33
- Endendijk, J. J., Groeneveld, M. G., Bakermans-Kranenburg, M. J., & Mesman, J. (2016). Gender-differentiated parenting revisited: Meta-analysis reveals very few differences in parental control of boys and girls. PLoS One, 11(7), e0159193. https://doi.org/10.1371/journal.pone.0159193
- Euesden, J., Lewis, C. M., & O’Reilly, P. F. (2015). PRSice: Polygenic risk score software. Bioinformatics, 31(9), 1466–1468. https://doi.org/10.1093/bioinformatics/btu848
- Friedman, N. P., & Miyake, A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. https://doi.org/10.1016/j.cortex.2016.04.023
- Gao, W., Alcauter, S., Smith, J., Keith, Gilmore, J. H., & Lin, W. (2015). Development of human brain cortical network architecture during infancy. Brain Structure and Function, 220(2), 1173–1186. https://doi.org/10.1007/s00429-014-0710-3
- Garon, N., Bryson, S. E., & Smith, I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134(1), 31–60. https://doi.org/10.1037/0033-2909.134.1.31
- Goldsmith, H. H., & Rothbart, M. K. (1999). The laboratory temperament assessment battery prelocomotor (Version 3). University of Oregon.
- Grabner, R. H., Fink, A., Stipacek, A., Neuper, C., & Neubauer, A. C. (2004). Intelligence and working memory systems: Evidence of neural efficiency in alpha band ERD. Cognitive Brain Research, 20(2), 212–225. https://doi.org/10.1016/j.cogbrainres.2004.02.010
- Grissom, N. M., & Reyes, T. M. (2019). Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology, 44(1), 86–96. https://doi.org/10.1038/s41386-018-0179-5
- Hayes, A. F. (2017). Introduction to mediation, moderation and conditional process analysis: A regression-based approach in series methodology in the social sciences (2nd ed.). Guilford Press.
- Hendry, A., Jones, E. J. H., & Charman, T. (2016). Executive function in the first three years of life: Precursors, predictors and patterns. Developmental Review, 42, 1–33. https://doi.org/10.1016/j.dr.2016.06.005
- Huizink, A. C., Delforterie, M. J., Scheinin, N. M., Tolvanen, M., Karlsson, L., & Karlsson, H. (2016). Adaption of pregnancy anxiety questionnaire–revised for all pregnant women regardless of parity: PRAQ-R2. Archives of Women’s Mental Health, 19(1), 125–132. https://doi.org/10.1007/s00737-015-0531-2
- Jester, J. M., Nigg, J. T., Puttler, L. I., Long, J. C., Fitzgerald, H. E., & Zucker, R. A. (2009). Intergenerational transmission of neuropsychological executive functioning. Brain and Cognition, 70(1), 145–153. https://doi.org/10.1016/j.bandc.2009.01.005
- Judd, N., Sauce, B., Wiedenhoeft, J., Tromp, J., Chaarani, B., Schliep, A., van Noort, B., Penttilä, J., Grimmer, Y., Insensee, C., Becker, A., Banaschewski, T., Bokde, A. L. W., Quinlan, E. B., Desrivières, S., Flor, H., Grigis, A., Gowland, P., Heinz, A., … Klingberg, T. (2020). Cognitive and brain development is independently influenced by socioeconomic status and polygenic scores for educational attainment. Proceedings of the National Academy of Sciences of the United States of America, 117(22), 12411–12418. https://doi.org/10.1073/pnas.2001228117
- Karlsson, L., Tolvanen, M., Scheinin, N. M., Uusitupa, H. M., Korja, R., Ekholm, E., Tuulari, J. J., Pajulo, M., Huotilainen, M., Paunio, T., & Karlsson, H. (2018). Cohort profile: The FinnBrain Birth Cohort Study (FinnBrain). International Journal of Epidemiology, 47(1), 15–16j. https://doi.org/10.1093/ije/dyx173
- Kataja, E. L., Karlsson, L., Tolvanen, M., Parsons, C., Schembri, A., Kiiski-Mäki, H., & Karlsson, H. (2017). Correlation between the cogstate computerized measure and WAIS-IV among birth cohort mothers. Archives of Clinical Neuropsychology, 32(2), 252–258. https://doi.org/10.1093/arclin/acw099
- Khramtsova, E. A., Davis, L. K., & Stranger, B. E. (2019). The role of sex in the genomics of human complex traits. Nature Reviews Genetics, 20, 173–190. https://doi.org/10.1038/s41576-018-0083-1
- Kim, M. H., Shimomaeda, L., Giuliano, R. J., & Skowron, E. A. (2017). Intergenerational associations in executive function between mothers and children in the context of risk. Journal of Experimental Child Psychology, 164, 1–15. https://doi.org/10.1016/J.JECP.2017.07.002
- Kraybill, J. H., Kim-Spoon, J., & Bell, M. A. (2019). Infant attention and age 3 executive function. Yale Journal of Biology and Medicine, 92(1), 3–11.
- Lee, J. J., Wedow, R., Okbay, A., Kong, E., Maghzian, O., Zacher, M., Nguyen-Viet, T. A., Bowers, P., Sidorenko, J., Karlsson Linnér, R., Fontana, M. A., Kundu, T., Lee, C., Li, H., Li, R., Royer, R., Timshel, P. N., Walters, R. K., Willoughby, E. A., … Turley, P. (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50(8), 1112–1121. https://doi.org/10.1038/s41588-018-0147-3
- Leve, L. D., DeGarmo, D. S., Bridgett, D. J., Neiderhiser, J. M., Shaw, D. S., Harold, G. T., Natsuaki, M. N., & Reiss, D. (2013). Using an adoption design to separate genetic, prenatal, and temperament influences on toddler executive function. Developmental Psychology, 49(6), 1045–1057. https://doi.org/10.1037/a0029390
- Manoli, D. S., & Tollkuhn, J. (2018). Gene regulatory mechanisms underlying sex differences in brain development and psychiatric disease. Annals of the New York Academy of Sciences, 1420(1), 26–45. https://doi.org/10.1111/nyas.13564
- Maruff, P., Thomas, E., Cysique, L., Brew, B., Collie, A., Snyder, P., & Pietrzak, R. H. (2009). Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology, 24(2), 165–178. https://doi.org/10.1093/arclin/acp010
- Miyake, A., & Friedman, N. P. (2012). The nature and organization of individual differences in executive functions. Current Directions in Psychological Science, 21(1), 8–14. https://doi.org/10.1177/0963721411429458
- Nigg, J. T. (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(4), 361–383. https://doi.org/10.1111/jcpp.12675
- Nolvi, S., Pesonen, H., Bridgett, D. J., Korja, R., Kataja, E. L., Karlsson, H., & Karlsson, L. (2018). Infant sex moderates the effects of maternal pre- and postnatal stress on executive functioning at 8 months of age. Infancy, 23(2), 194–210. https://doi.org/10.1111/infa.12206
- Nordenswan, E., Kataja, E.-L., Deater-Deckard, K., Korja, R., Karrasch, M., Laine, M., Karlsson, L., & Karlsson, H. (2020). Latent structure of executive functioning/learning tasks in the cogstate computerized battery. SAGE Open, 10(3), 215824402094884. https://doi.org/10.1177/2158244020948846
- Polderman, T. J. C., Gosso, M. F., Posthuma, D., van Beusterveldt, T. C. E. M., Heutink, P., Verhulst, F. C., & Boomsma, D. I. (2006). A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurologica Belgica, 106, 191–207.
- Rey-Mermet, A., Gade, M., & Oberauer, K. (2017). Should we stop thinking about inhibition? Searching for individual and age differences in inhibition ability. Journal of Experimental Psychology: Learning, Memory, and Cognition, 44(4), 501. https://doi.org/10.1037/XLM0000450
- Sayal, K., Prasad, V., Daley, D., Ford, T., & Coghill, D. (2018). ADHD in children and young people: Prevalence, care pathways, and service provision. The Lancet. Psychiatry, 5(2), 175–186. https://doi.org/10.1016/S2215-0366(17)30167-0
- Silverman, I. W. (2021). Gender differences in inhibitory control as assessed on simple delay tasks in early childhood: A meta-analysis. International Journal of Behavioral Development, 45(6), 533–544. https://doi.org/10.1177/01650254211020385
- Tao, T., Wang, L., Fan, C., & Gao, W. (2014). Development of self-control in children aged 3 to 9 years: Perspective from a dual-systems model. Scientific Reports, 4, 7272. https://doi.org/10.1038/srep07272
- Valcan, D. S., Davis, H., & Pino-Pasternak, D. (2018). Parental Behaviours Predicting Early Childhood Executive Functions: a Meta-Analysis. Educational Psychology Review, 30(3), 607–649. https://doi.org/10.1007/s10648-017-9411-9
- Wechsler, D. (2008). Wechsler Adult Intelligence Scale-IV. Wechsler, D. Psychological Corporation.
- Wechsler, D. (2012). Wechsler Adult Intelligence Scale-IV. Wechsler, D. Psykologien kustannus Oy. Psychological Corporation.
- Weiss, M. J. S., & Wagner, S. H. (1998). What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. American Journal of Preventive Medicine, 14(4), 356–360.
- Wierenga, L. M., Bos, M. G. N., van Rossenberg, F., & Crone, E. A. (2019). Sex effects on development of brain structure and executive functions: Greater variance than mean effects. Journal of Cognitive Neuroscience, 31(5), 730–753. https://doi.org/10.1162/jocn_a_01375
- Willcutt, E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics, 9(3), 490–499. https://doi.org/10.1007/s13311-012-0135-8
- Yoshida, T., Suga, M., Arima, K., Muranaka, Y., Tanaka, T., Eguchi, S., Lin, C., Yoshida, S., Ishikawa, M., Higuchi, Y., Seo, T., Ueoka, Y., Tomotake, M., Kaneda, Y., Darby, D., Maruff, P., Iyo, M., Kasai, K., Higuchi, T., … Hashimoto, K. (2011). Criterion and construct validity of the cogstate schizophrenia battery in japanese patients with schizophrenia. PLoS One, 6(5), e20469. https://doi.org/10.1371/journal.pone.0020469
- Zelazo, P. D. (2015). Executive function: Reflection, iterative reprocessing, complexity, and the developing brain. Developmental Review, 38, 55–68. https://doi.org/10.1016/j.dr.2015.07.001
- Zhong, N., Jiang, H., Wu, J., Chen, H., Lin, S., Zhao, Y., Du, J., Ma, X., Chen, C., Gao, C., Hashimoto, K., & Zhao, M. (2013). Reliability and validity of the CogState battery Chinese language version in schizophrenia. PLoS One, 8(9), e74258. https://doi.org/10.1371/journal.pone.0074258
