ABSTRACT
Low complexity (LC) prion-like domains are over-represented among RNA-binding proteins (RBPs) and contribute to the dynamic nature of RNA granules. Importantly, several neurodegenerative diseases are characterized by cytoplasmic “solid” aggregates formed by mainly nuclear RBPs harboring LC prion-like domains. Although RBP aggregation in disease has been extensively characterized, it remains unknown how the process of aging disturbs RBP dynamics. Our recent study revealed that RNA granule components including 2 key stress granule RBPs with LC prion-like domains, PAB-1 and TIAR-2, aggregate in aged Caenorhabditis elegans in the absence of disease. Here we present new evidence showing that sustained stress granule formation triggers RBP aggregation. In addition, we demonstrate that mild chronic stress during aging promotes mislocalization of nuclear RBPs. We discuss the consequences of aberrant interactions between age-related RBP aggregation and disease-associated RBP aggregation. In particular, we show that FUST-1 and PAB-1 co-localize in aberrant cytoplasmic accumulations. Significantly, long-lived animals with reduced insulin/IGF-1 signaling abrogate stress granule RBP aggregation through activation of the transcription factors HSF-1 and DAF-16. We evaluate the different mechanisms that could maintain dynamic stress granules. Together these findings highlight how changes with age could contribute to pathogenesis in neurodegenerative diseases and disruption of RNA homeostasis.
INTRODUCTION
A number of diseases including certain neurodegenerative disorders are characterized by the presence of pathological highly intractable or “solid” protein aggregates formed by one or several distinct proteins. In the last decade, the list of proteins identified in aggregates associated with disease has been considerably extended with the addition of several RNA-binding proteins (RBPs). These include RBPs such as TDP-43 and FUS observed in aggregates of patients with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD).Citation1-3 RBPs associated with disease contain a low complexity (LC) “prion-like” domain similar to sequences identified in yeast prion proteins.Citation4,5 Highlighting the importance of this domain, familial cases of these diseases are frequently related to aggregation-promoting mutations in the LC prion-like domain.Citation6,7 In a non-disease context, RBPs with LC prion-like domains are key components of RNA granules. Depending on their composition, RNA granules such as stress granules, P-bodies, P-granules and neuronal granules perform different functions in the cell. RNA granules are highly dynamic membrane-less organelles and their assembly is mediated by association of RBPs through their LC prion-like domains and subsequent recruitment of RNA and associated proteins by RNA-binding domains.Citation8-10 In particular, weak interactions built between LC prion-like domains in RBPs promote a liquid-liquid phase separation in vitro consistent with the observed liquid-like proprieties of RNA granules in vivo.Citation11-14 Considering the special nature of the interactions between RBPs with LC prion-like domains and the growing number of RBPs forming hallmark aggregates in different neurodegenerative disorders, we hypothesized that aberrant aggregation of RBPs and RNA granules could be an important problem that the organism needs to actively avoid especially during aging.
RBPs with LC Prion-Like Domains and RNA Granule-Associated Proteins are Highly Prone to Aggregate with Age in C. elegans
Recently it has become clear that protein aggregation is not restricted to specific proteins in a disease context. Notably, we and others have shown that several hundred proteins are highly prone to aggregate during normal aging in different model organisms.Citation15-19 In our recent study,Citation20 the proteomic analysis of the aggregating proteome from long-lived C. elegans with reduced daf-2/insulin/IGF-1 receptor signaling highlighted a specific group of proteins namely RNA granule components. In particular we identified several stress granule components as well as 4 RBPs with LC prion-like domains that became highly insoluble with age in control animals but not in long-lived animals. These results imply that RNA granules or at least their core structures lose their dynamic characteristics with age. Significantly, preserving dynamic RBPs is a mechanism associated with longevity. We focused our main study on stress granule proteins as they are frequently found to co-localize with pathological TDP-43 and FUS inclusions and have been implied to play a role in pathogenesis.Citation21,22 We overexpressed 2 key stress-granule-related RBPs (sgRBPs) with LC prion-like domains, PAB-1, homolog of human polyadenylate-binding protein 1 (PABP-1) and TIAR-2, homolog of human T-cell-restricted intracellular antigen-1 (TIA-1) in C. elegans pharyngeal muscles.Citation20 Upon heat stress, both fluorescently-labeled proteins assembled into stress granules as expected. Importantly without exposing animals to additional stress, PAB-1 and TIAR-2 accumulated in stress-granule-like structures as well as large puncta in aged C. elegans. The absence of fluorescence recovery after photobleaching confirmed that the large PAB-1 and TIAR-2 puncta contained highly immobile protein demonstrating that these are solid aggregates. Importantly, we found that animals with higher levels of PAB-1 aggregation were smaller, less motile and shorter lived than animals without aggregation.Citation20 These results demonstrate that aggregation of sgRBPs is potentially toxic and could accelerate the aging process.
Sustained Stress Granule Formation Triggers sgRBP Aggregation
In vitro studies have shown that liquid droplets formed by purified RBPs with LC prion-like domains will eventually transition into a more solid state over time, thus impairing their disassembly.Citation11-14 Solidification of liquid droplets and concomitantly the formation of fibrils was enhanced by disease-related mutations promoting aggregation and repeated cycles of phase separation.Citation11,13 These findings lead us to the hypothesis that sustained stress granule formation could enhance their aggregation in vivo. 24h after continuous mild stress at 25°C, confocal analysis revealed the formation of abundant stress granules in day-1-old adults (). Already on day 2, these animals started to form large PAB-1 aggregates visible at low-magnification. Overall, PAB-1 aggregation in a population of C. elegans grown at 25°C versus 20°C was greatly enhanced at all ages evaluated (, ). As control, we examined the rate of aggregate formation by KIN-19, an age-dependent aggregation-prone protein without a LC prion-like domain or RNA-binding domain. In this case, we observed only a minor enhancement of KIN-19 aggregation rate at 25°C that is probably due to the accelerated rate of C. elegans aging at this higher temperature (). These results demonstrate that continuous liquid-liquid phase separation over time plays an important role in initiating the aggregation of RBPs, potentially by allowing stress granules to stabilize and act as a nucleation site for aggregation.
FIGURE 1 (next page). Aging in combination with mild chronic stress enhances abnormal distribution of RNA granule components in C. elegans pharyngeal muscles. (A) Mild stress by elevation of growth temperature from 20°C to 25°C causes abundant tagRFP::PAB-1 stress granule formation in Pmyo-2::tagRFP::PAB-1 transgenics at day 1. Representative confocal images displayed as single plane. Scale bar 7 µm. (B) Mild chronic stress highly enhances tagRFP::PAB-1 aggregation with age in Pmyo-2::tagRFP::PAB-1 transgenics (Number of animals per group and time point is indicated in the columns; Fisher's exact test 2-tailed comparing low aggregation vs. all other categories at day 2, 4 and 7, 20°C vs. 25°C, ****p < 0.0001). (C) Representative images of tagRFP::PAB-1 aggregation levels in pharyngeal muscles (maximal z-stack projection). Low, up to 10 puncta in posterior bulb; intermediate, more than 10 puncta in posterior bulb; high, more than 10 puncta in anterior bulb; very high, more than 50 puncta in anterior bulb. Scale bar 10 µm. (D) Modest increase in KIN-19::mEOS aggregation in Pkin-19::KIN-19::mEOS animals aged at 25°C compared with 20°C. (Aggregation determined in anterior pharyngeal bulb: low, up to 10 puncta; intermediate, between 10 and 100 puncta; high, over 100 puncta. Fisher's exact test 2-tailed comparing high + intermediate against low aggregation levels at day 7, 20°C vs. 25°C p < 0.0001). (E) Mild chronic stress in combination with aging causes abnormal cytoplasmic tagRFP::HRP-1 localization in aged Pmyo-2::tagRFP::HRP-1 transgenics (Fisher's exact test 2-tailed at day 7, 20°C vs. 25°C, ****p < 0.0001). (F) High-magnification representative confocal images with nuclear Hoechst33342 staining showing cytoplasmic tagRFP::HRP-1 puncta at day 7 but not day 1 of adulthood, in Pmyo-2::tagRFP::HRP-1 transgenics aged at 25°C. Scale bar 5 µm. (G) Representative confocal images showing tagRFP::PAB-1 co-localization with small cytoplasmic FUST-1::Venus puncta in double transgenic animals expressing Pmyo-2::tagRFP::PAB-1; Pkin-19::FUST-1::Venus at day 7. Arrows mark co-localization. Scale bar: 4 µm.
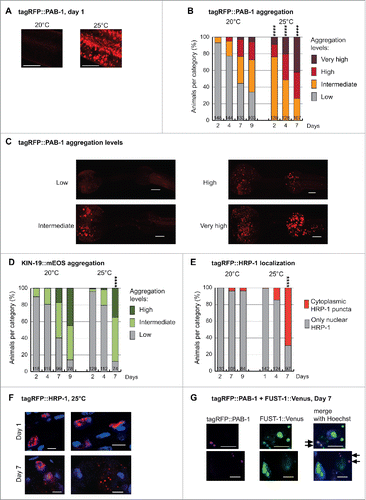
Mild Stress Combined with Aging Promotes Mislocalization of RBPs with LC Prion-Like Domains
Several neurodegenerative diseases such as ALS and FTLD are characterized by cytoplasmic inclusions of mainly nuclear-localized RBPs. Therefore RBP mislocalization from the nucleus to the cytoplasm is a key step toward pathogenesis. We investigated whether the aging process triggers mislocalization of nuclear RBPs. For this, we selected HRP-1, a nuclear RBP with a LC prion-like domain, which we identified in our proteomic analysis to become highly insoluble with age. Significantly, the human homologs of HRP-1, hnRNPA1 and hnRNPA3 form aberrant cytoplasmic inclusions in multisystem proteinopathy and they are also found to co-aggregate in inclusions in C9orf72 mutation-associated ALS/FTLD.Citation6,23 Using a fluorescent-tagged HRP-1, we confirmed its primary nuclear localization ( and ) and we observed that HRP-1 is not a normal constituent of cytoplasmic stress granules as it remained in the nucleus upon acute heat stress (2 hour heat shock at 32°C, data not shown). Next we tested if aging modulates HRP-1 localization. In C. elegans maintained in standard conditions at 20°C, HRP-1 remained in the nucleus in the majority of aged animals (). However, this was strikingly different when animals were exposed to an additional mild stress by aging them at 25°C. Whereas HRP-1 was localized in the nucleus in young animals, we observed the formation of distinct cytoplasmic puncta in aged animals (). Our quantification reveals that 69% of day-7-old animals grown at 25°C developed cytoplasmic HRP-1 puncta (). Therefore, the combination of changes related to aging and chronic exposure to mild environmental stress drives the aberrant cytoplasmic localization of a nuclear RBP. It remains to be investigated whether the aberrant cytoplasmic localization of RBPs that we observed in C. elegans is due to a disruption in nuclear integrity with age and/or a specific impairment in the nuclear import machinery.Citation24,25
Aberrant Interactions Between RBPs and Other Aggregation-Prone Proteins
An important question is whether RBP aggregation with age influences the aggregation of other RBPs. In vitro phase separated RBPs with LC prion-like domains recruit others into the same assembly.Citation10,12 Our data revealed that PAB-1 and TIAR-2 have very different aggregation patterns with age.Citation20 Significantly, TIAR-2 distribution in aged animals was remarkably reminiscent of stress granule assembly caused by heat shock in young animals whereas PAB-1 tended to form mostly large aggregates with age. Notably in double transgenics, TIAR-2 recruited PAB-1 preferentially into stress granule-like structures with age.Citation20 These results reveal that interactions between 2 RBPs with different aggregation propensities can change their aggregation patterns.
We also investigated the C. elegans ortholog of FUS, FUST-1. Like the human FUS, nematode FUST-1 has a LC prion-like domain, an RNA recognition motif, a zinc finger domain and a PY-NLS motif. In healthy cells, human FUS is predominately nuclear while in the disease state, FUS forms cytoplasmic inclusions. Similarly in C. elegans, FUST-1 was mostly located in the nucleus. However, its overexpression alone was sufficient to cause the formation of small cytoplasmic puncta visible at higher magnification in young and aged animals (data not shown). As heat shock did not result in a clear increase in cytoplasmic FUST-1 puncta (data not shown), these aberrant puncta are unlikely to be related to functional stress granules. This is similar to observations made with wild-type human FUS in mammalian cells and in C. elegans showing that FUS does not normally localize to stress granules upon exposure to heat stress.Citation26-28 Importantly when co-expressed, FUST-1 recruited PAB-1 into these aberrant cytoplasmic puncta in both aged as well as young animals ().
Several studies have highlighted an aberrant interaction between stress granules and misfolded proteins in yeast and in cell culture.Citation29-32 Significantly, recent work showed that stress granules containing misfolded proteins tend to be more stable.Citation31,32 In C. elegans we found that sgRBP PAB-1 forms large solid aggregates together with a non-RBP and age-dependent aggregation-prone protein KIN-19, when both proteins were overexpressed.Citation20 As KIN-19 greatly accelerated PAB-1 aggregation, cross-seeding mechanisms due to protein misfolding with age is likely to be a main cause of sgRBP aggregation. Important questions remain and in particular related to how this aberrant interaction is initiated: Are non-RBP aggregation-prone proteins first recruited into age-induced stress granules and then act as seeds for their solidification into large aggregates? Or do small age-dependent aggregates of non-RBPs trigger stress granule formation and co-aggregation? One argument in favor of the first possibility is the fact that stress granules induced by heat stress in HeLa cells subsequently recruit ALS-linked mutant SOD-1.Citation31
Overall, these findings show that RBPs with LC prion-like domains are prone to recruit other RBPs into aberrant cytoplasmic accumulations and to interact with other aggregation-prone proteins. This could explain why stress granule components are frequently observed not only in disease-associated RBP aggregates but also in other pathological aggregates identified in Alzheimer disease and Huntington's disease.Citation21 Moreover our results strongly suggest that sgRBPs are not passive players in neurodegenerative disease.
Strategies to Prevent Stress Granule Aggregation
We found that sgRBP aggregation is efficiently prevented by at least 3 independent longevity pathways in C. elegans, namely reduced daf-2/insulin/IGF-1-like receptor signaling, dietary restriction and reduced mitochondrial function.Citation20 Accordingly, maintaining dynamic stress granules is likely to be a common strategy to delay the course of aging. This concept is further supported by the reduced fitness of aged animals with sgRBP aggregation.Citation20 Two transcription factors, heat shock factor HSF-1 and DAF-16/FOXO, are strongly activated in animals with reduced daf-2 signaling and play an essential role in mediating longevity and improving proteostasis. We demonstrated that both factors are involved in promoting dynamic RBPs.Citation20 Notably HSF-1 activity during development was essential to set-up an active quality-control system to avoid sgRBP aggregation both in wild-type and in long-lived daf-2 mutants. As HSF-1 is the master regulator of the chaperone system, we sought to determine which specific chaperones may be involved in abrogating sgRBP aggregation. Interestingly, we did not find a connection between sgRBP aggregation and the activity of the C. elegans homologs of HSP40, HSP70 and HSP110, which have been previously implicated in stress granule dynamics in S. cerevisiae and/or cell culture.Citation9,29,30,33 In addition, HSP40, HSP70 and HSP110 chaperones act together to promote protein disaggregation.Citation34,35 However, we did not observe the involvement of the nematode disaggregase machinery in the modulation of sgRBP aggregation (in particular DNJ-13, HSP-1 and HSP-110, Citation20 and data not shown). In a small RNA interference screen targeting 15 C. elegans chaperones individually, only the knock-down of small heat shock protein HSP-16.11 accelerated PAB-1 aggregation (statistically significant in 3 out of 4 repeats, data not shown). In future work, it will be important to test the chaperone complex HSPB8-BAG3-HSP70 which is required to avert defective ribosomal products from accumulating in stress granules Citation32 and thus might also play a role in sgRPB aggregation regulation. Furthermore a recent study demonstrated that mini-chromosome maintenance MCM proteins stabilize stress granules.Citation36 As several mcm genes are upregulated in hsf-1 mutants in C. elegans,Citation37 these could be promising candidates as pro-aggregation factors contributing to sgRBP aggregation with age. In addition to strategies enhancing stress granule disassembly, our results implicate that preventing cross-seeding by the aggregating proteome will be an effective way to reduce RBP aggregation. Significantly, increasing DAF-16 activity could be used as a general strategy to reduce widespread protein aggregation with age.Citation20
CONCLUSIONS
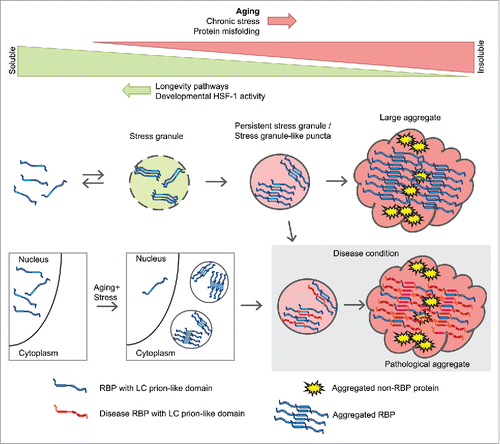
We propose the model depicted in to explain our results and their possible implications for neurodegenerative diseases. First, RBPs with LC prion-like domains form stress granules during aging and undergo a change in structure allowing them to stabilize the whole stress granule and avoid its disassembly. The presence of other age-dependent aggregation-prone proteins helps accelerate a further change in structure and leads to the formation of large aggregates. Second, the occurrence of a mild chronic stress during aging triggers the mislocalization of certain nuclear RBPs in cytoplasmic accumulations. Third, we hypothesize that aberrant interactions between age-dependent aggregating RBPs and disease-associated RBPs also affects disease protein aggregation. As stress granule proteins are found not only in disease-associated RBP aggregates but also in tau aggregates and Huntingtin aggregates,Citation21 their inherent propensity to aggregate with age could impact pathogenesis in a range of neurodegenerative diseases.
FIGURE 2. Model depicting aggregation of sgRBPs during aging and the relationship with disease RBPs.
Furthermore, aggregation of RNA granule components with age could have dramatic consequences for the normal function of RNA granules. For example, the inability of stress granules to disassemble after stress subsides and the permanent sequestration of stress granule components including mRNAs would stunt cellular adaptations and recovery from stress. As such, stress granule aggregation could explain reduced stress resistance with age. In the age-dependent aggregating proteome we also identified CAR-1, an RBP with LC prion-like domain with homology to the P-body component LSM14B.Citation20 This could indicate that P-bodies may solidify with age. This would have important consequences for the removal of aberrant mRNAs and could contribute to the decline of nonsense-mediated decay observed with age.Citation38 Significantly the function of other membrane-less structures such as the nucleolus are likely to be negatively affected by the aggregation of RBPs with LC prion-like domains. For instance, we identified that the nucleolus RBP Fibrillarin (FIB-1) was highly prone to aggregate with age.Citation20 Previously FIB-1 was shown to undergo liquid-liquid phase separation and became less dynamic with age.Citation39,40
The conservation of LC prion-like domains throughout evolution from yeast to mammals underlines the importance of this domain in the cellular biology. In the last 10 years, an increasing number of dementias have been associated with the aberrant aggregation of specific RBPs. Overall our study highlights the vulnerability of membrane-less organelles organized by RBPs with LC prion-like domains to transition into a solid state during normal aging in a multicellular organism. This has important implications for our understanding of the role of aging in pathogenesis and toxicity in neurodegenerative diseases.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This work was supported by funding from the DZNE and a Marie Curie International Reintegration Grant (322120 to DCD)
REFERENCES
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602-11. doi:10.1016/j.bbrc.2006.10.093. PMID:17084815
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-3. doi:10.1126/science.1134108. PMID:17023659
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132(Pt 11):2922-31. doi:10.1093/brain/awp214. PMID:19674978
- King OD, Gitler AD and Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61-80. doi:10.1016/j.brainres.2012.01.016. PMID:22445064
- Alberti S, Halfmann R, King O, Kapila A and Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137(1):146-58. doi:10.1016/j.cell.2009.02.044. PMID:19345193
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467-73. doi:10.1038/nature11922. PMID:23455423
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J and Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284(30):20329-39. doi:10.1074/jbc.M109.010264. PMID:19465477
- Couthouis J, Hart MP, Shorter J, DeJesus-Hernandez M, Erion R, Oristano R, Liu AX, Ramos D, Jethava N, Hosangadi D, et al. A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci U S A. 2011;108(52):20881-90. doi:10.1073/pnas.1109434108. PMID:22065782
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM and Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15(12):5383-98. doi:10.1091/mbc.E04-08-0715. PMID:15371533
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149(4):753-67. doi:10.1016/j.cell.2012.04.017. PMID:22579281
- Murakami T, Qamar S, Lin JQ, Schierle GS, Rees E, Miyashita A, Costa AR, Dodd RB, Chan FT, Michel CH, et al. ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron. 2015;88(4):678-90. doi:10.1016/j.neuron.2015.10.030. PMID:26526393
- Lin Y, Protter DS, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015;60(2):208-19. doi:10.1016/j.molcel.2015.08.018. PMID:26412307
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T and Taylor JP. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163(1):123-33. doi:10.1016/j.cell.2015.09.015. PMID:26406374
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015;162(5):1066-77. doi:10.1016/j.cell.2015.07.047. PMID:26317470
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL and Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8(8):e1000450. doi:10.1371/journal.pbio.1000450. PMID:20711477
- Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ et al. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11(1):120-7. doi:10.1111/j.1474-9726.2011.00765.x. PMID:22103665
- Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813-25. doi:10.1016/j.cell.2010.10.007. PMID:21111239
- Tanase M, Urbanska AM, Zolla V, Clement CC, Huang L, Morozova K, Follo C, Goldberg M, Roda B, Reschiglian P, Santambrogio L. Role of Carbonyl Modifications on Aging-Associated Protein Aggregation. Sci Rep. 2016;6:19311. doi:10.1038/srep19311. PMID:26776680
- Ayyadevara S, Mercanti F, Wang X, Mackintosh SG, Tackett AJ, Prayaga SV, Romeo F, Shmookler Reis RJ and Mehta JL. Age- and Hypertension-Associated Protein Aggregates in Mouse Heart Have Similar Proteomic Profiles. Hypertension. 2016; 67(5):1006-13. doi:10.1161/HYPERTENSIONAHA.115.06849. PMID:26975704
- Lechler MC, Crawford ED, Groh N, Widmaier K, Jung R, Kirstein J, Trinidad JC, Burlingame AL and David DC. Reduced Insulin/IGF-1 Signaling Restores the Dynamic Properties of Key Stress Granule Proteins during Aging. Cell Rep. 2017;18(2):454-467. doi:10.1016/j.celrep.2016.12.033. PMID:28076789
- Bentmann E, Haass C and Dormann D. Stress granules in neurodegeneration–lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma. FEBS J. 2013;280(18):4348-70. doi:10.1111/febs.12287. PMID:23587065
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013;201(3):361-72. doi:10.1083/jcb.201302044. PMID:23629963
- Mori K, Lammich S, Mackenzie IR, Forne I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013;125(3):413-23. doi:10.1007/s00401-013-1088-7. PMID:23381195
- D'Angelo MA, Raices M, Panowski SH and Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136(2):284-95. doi:10.1016/j.cell.2008.11.037. PMID:19167330
- Dormann D, Haass C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 2011;34(7), 339-48. doi:10.1016/j.tins.2011.05.002. PMID:21700347
- Sama RR, Ward CL, Kaushansky LJ, Lemay N, Ishigaki S, Urano F and Bosco DA. FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress. J Cell Physiol. 2013;228(11):2222-31. doi:10.1002/jcp.24395. PMID:23625794
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr., Sapp P, McKenna-Yasek D, Brown RH, Jr., Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19(21):4160-75. doi:10.1093/hmg/ddq335. PMID:20699327
- Murakami T, Yang SP, Xie L, Kawano T, Fu D, Mukai A, Bohm C, Chen F, Robertson J, Suzuki H, et al. ALS mutations in FUS cause neuronal dysfunction and death in Caenorhabditis elegans by a dominant gain-of-function mechanism. Hum Mol Genet. 2012;21(1):1-9. doi:10.1093/hmg/ddr417. PMID:21949354
- Cherkasov V, Hofmann S, Druffel-Augustin S, Mogk A, Tyedmers J, Stoecklin G, Bukau B. Coordination of translational control and protein homeostasis during severe heat stress. Curr Biol. 2013;23(24):2452-62. doi:10.1016/j.cub.2013.09.058. PMID:24291094
- Kroschwald S, Maharana S, Mateju D, Malinovska L, Nuske E, Poser I, Richter D and Alberti S. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife. 2015; 4:e06807. doi:10.7554/eLife.06807. PMID:26238190
- Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017;36(12):1669-1687. doi:10.15252/embj.201695957. PMID:28377462
- Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, Seguin SJ, Morelli FF, Vinet J, Leo G, et al. A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism. Mol Cell. 2016;63(5):796-810. doi:10.1016/j.molcel.2016.07.021. PMID:27570075
- Walters RW, Muhlrad D, Garcia J and Parker R. Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. RNA. 2015;21(9):1660-71. doi:10.1261/rna.053116.115. PMID:26199455
- Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One. 2011;6(10):e26319. doi:10.1371/journal.pone.0026319. PMID:22022600
- Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, Guilbride DL, Wade RC, Morimoto RI, Mayer MP and Bukau B. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524(7564):247-51. doi:10.1038/nature14884. PMID:26245380
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A and Parker R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell. 2016;164(3):487-98. doi:10.1016/j.cell.2015.12.038. PMID:26777405
- Brunquell J, Morris S, Lu Y, Cheng F and Westerheide SD. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genomics. 2016;17:559. doi:10.1186/s12864-016-2837-5. PMID:27496166
- Son HG, Seo M, Ham S, Hwang W, Lee D, An SW, Artan M, Seo K, Kaletsky R, Arey RN, et al. RNA surveillance via nonsense-mediated mRNA decay is crucial for longevity in daf-2/insulin/IGF-1 mutant C. elegans. Nat Commun. 2017;8:14749. doi:10.1038/ncomms14749. PMID:28276441
- Berry J, Weber SC, Vaidya N, Haataja M and Brangwynne CP. RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A. 2015;112(38):E5237-45. doi:10.1073/pnas.1509317112. PMID:26351690
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP.. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell.2016;165(7):1686-97. doi:10.1016/j.cell.2016.04.047. PMID:27212236
