Abstract
This review addresses our current understanding of the regulatory mechanism by which N-cadherin, a classical cadherin, affects neural progenitor cells (NPCs) during development. N-cadherin is responsible for the integrity of adherens junctions (AJs), which develop in the sub-apical region of NPCs in the neural tube and brain cortex. The apical domain, which contains the sub-apical region, is involved in the switching from symmetric proliferative division to asymmetric neurogenic division of NPCs. In addition, N-cadherin-based AJ is deeply involved in the apico-basal polarity of NPCs and the regulation of Wnt-β-catenin, hedgehog (Hh), and Notch signaling. In this review, we discuss the roles of N-cadherin in the maintenance, proliferation, and differentiation of NPCs through components of AJ, β-catenin and αE-catenin.
Abbreviations
| AJ | = | adherens junction |
| aPKC | = | atypical protein kinase C |
| EC | = | extracellular |
| Fox | = | forkhead box |
| Frz | = | frizzled |
| GFAP | = | glial fibrillary acidic protein |
| GSK3β | = | glycogen synthase kinase 3β |
| Hes | = | hairly/enhancer of split |
| Hh | = | hedgehog |
| IP | = | intermediate progenitor |
| iPSC | = | induced pluripotent stem cell |
| KO | = | knockout |
| LEF | = | lymphocyte enhancer factor |
| ngn2 | = | neurogenin 2 |
| NPC | = | neural progenitor cell |
| Par | = | partition defective complex protein |
| Ptc | = | Pached |
| shRNA | = | short hairpin RNA |
| Smo | = | smoothened |
| Sox2 | = | sry (sex determining region Y)-box containing gene 2 |
| TCF | = | T-cell factor |
| TA cell | = | transient amplifying cell; ZO-1, Zonula Occludens-1 |
Introduction
N-cadherin is a classical cadherin which is prominently expressed in the neural system.Citation1,2 Based on sequence comparison, the classical cadherins are divided into 2 subfamilies: type I and type II. N-cadherin is included in the type I classical cadherins.Citation3-5 The type I classical cadherins are defined by 5 extracellular (EC) domains (EC1-5), followed by a single-pass transmembrane domain and 2 well-conserved catenin-binding domains in the cytoplasmic portion.Citation1,2 In general, type I classical cadherins participate in Ca2+-dependent homophilic interactions with the EC1 domains.Citation1,6 During the development of the nervous system, N-cadherin mainly contributes to cell-cell adhesion in neural progenitor cells (NPCs) and neurons, and develops junctional complexes together with α-catenin, β-catenin, and actin fibers, called adherens junctions (AJs).Citation1,7 N-cadherin-based AJs are involved in various processes of neural development. There are several excellent reviews and papers detailing the roles of N-cadherin in neurulation, migration of neurons, axon elongation and guidance, and synaptogenesis.Citation1,2,8-12
N-cadherin-based AJs are also known to be related to the transition from symmetric proliferative division to asymmetric neurogenic division of NPCs. The proliferative division is responsible for the self-renewal of NPCs, and the neurogenic division generates nascent neurons and intermediate progenitors (IPs), which commit to become neurons in the ventricular zone of neural tube and brain cortex.Citation13-15 The balance between self-renewal and differentiation is tightly controlled to produce the organ of predetermined size.Citation16-18 In this review, we will concentrate on roles of N-cadherin in the mechanism underlying the maintenance of NPCs and the balance between the proliferation and differentiation of NPCs.
Tissue Architecture Mediated by N-cadherin-based AJ in Nervous System
One important role of N-cadherin on NPCs is to connect the NPCs tightly to each other for the tissue architecture of the nervous system.Citation19-23 Several investigations have observed that a loss of function of N-cadherin in the NPCs of the neural tube, brain cortex, and retina destroys the tissue architecture.Citation11,22-27 In this chapter, we describe the distribution of N-cadherin, the structure of N-cadherin-based AJ, and the AJ's maintainance of tissue architecture and apico-basal polarity of NPC.
Expression of N-cadherin in neuroepithilial cells and radial glia cells
Embryonic ectoderm first expresses E-cadherin, but this E-cadherin is replaced by N-cadherin during neurulation.Citation19,28 The neural tube wall constitutes a monolayer of neuroepithelial cells. At the onset of neurogenesis, neuroepithelial cells transform to radial glia cells.Citation29,30 With this transformation, the cells lose expression of occluding and functional tight junction.Citation31 However, the expression of Zonula Occludens-1 (ZO-1), a peripheral membrane protein of tight junction, remains in the sub-apical region of radial glia cells (). Radial glia cells directly or indirectly generate all neurons and, later in development, glia cells as NPCs.Citation32 Radial glia cells are polarized when the apical membrane is exposed to the ventricle and the basal side contacts the pial basal membrane ().
Figure 1. Description of radial glia cell as neural progenitor cell (NPC). Radial glia cells as NPCs exhibit a characteristic bipolar radial morphology with apical and basal processes. The apical and basal processes are responsible for 2 points of adhesion at the sub-apical region of N-cadherin-based AJs, and integrin-laminin interaction at their basal laminae, respectively. These two points of adhesion are responsible for the structural integrity and apico-basal polarity of the neural tube and brain cortex. Par3, ZO-1, and components of AJ, N-cadherin and β-catenin are localized at the apical domain of radial glia cells during development.
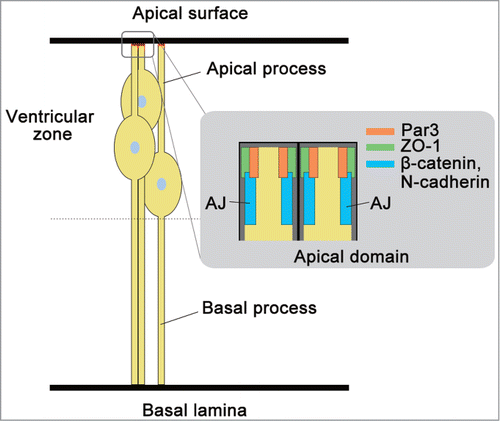
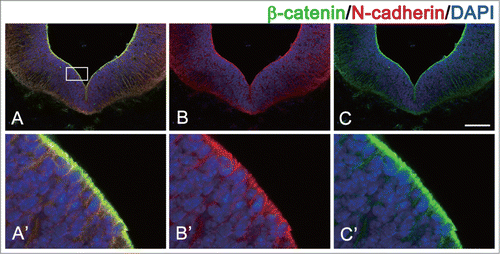
N-cadherin is broadly expressed in neuroepithelial cells and radial glia cells as NPCs, although this protein is most highly concentrated at the sub-apical region of these cells, where the AJs develop ().Citation20,33,34 We recently reported that N-cadherin is localized at the sub-apical region of the ventral midbrain at embryonic day 11.5 (E11.5) ().Citation34,35 These N-cadherin-based AJs contribute to the strong adhesion between the NPCs to maintain the tissue architecture.
Figure 2. Localization of N-cadherin and β-catenin in ventricular zone of ventral midbrain during development. N-cadherin and β-catenin is co-localized at the apical domain of NPCs in mouse embryo (embryonic day 11.5). (A) Merged image of N-cadherin and β-catenin staining. (B) image of β-catenin staining. (C) image of N-cadherin staining. Higher magnification views of the boxed areas in A, B, and C are shown in A’, B’, and C’, respectively. Scale bar indicates 100 μm.
The expression of N-cadherin in the developing neural tube and brain is regulated by sex-determining region Y (sry)-box containing gene 2 (Sox2), known as one of the early proneural transcription factors, forkhead box protein transcription factors Foxp2/4, and miR379-410 cluster microRNA (miRNA).Citation36-39 Sox2 is observed to be expressed prior to N-cadherin in the neural plate, and has been shown to activate N-cadherin expression in the regions.Citation28,38 Meanwhile, Foxp2 and Foxp4 repress the expression of N-cadherin during neurogenesis from NPCs in the development of the nervous system.Citation37 After neurogenic cell division of NPCs in a developing chick's spinal cord, the elevated expression of either of the Foxp transcription factors, promoted by a proneural gene neurogenin 2 (ngn2), represses the expression of N-cadherin, and thereby promotes the detachment of nascent neurons or IPs from the apical region of neuroepithelium.Citation37,40 This detachment is crucial for further neurogenesis and neuronal migration from the ventricular zone.Citation40,41
The expression of ngn2 in NPCs is regulated by Delta-Notch signaling during neurogenesis.Citation42,43 Delta activates the Notch receptor on directly adjacent NPCs to release the Notch intracellular domain that mediates the transcription of hairly/enhancer of split 1 (Hes1) gene.Citation43 Up-regulation of Hes1 expression in turn represses the expression of ngn2 in NPCs and keeps NPCs in a proliferative state.Citation42 In the developing mouse cerebral cortex, it has been reported that inhibition of Notch leads to down-regulation of Hes1 and sustained upregulation of ngn2.Citation42 After asymmetric neurogenic division in the developing mouse cortex, Delta-Notch signaling components are divided asymmetrically into daughter cells, and therefore Notch signaling in the nascent neuron or IP is lower than in the cell, which maintains the identity of NPCs.Citation44-46 These facts suggest the downregulation of Notch signaling may be important for the down-regulation of N-cadherin expression.
The expression of N-cadherin is also regulated by miRNAs of the miR379–410 cluster in NPCs during the development of the mouse cerebral cortex.Citation39 Overexpression of the miRNAs in NPCs represses the expression of N-cadherin and increases the neuronal differentiation, suggesting that the miRNA regulate neurogenesis in the developing mouse cerebral cortex.Citation39
Structure of AJ
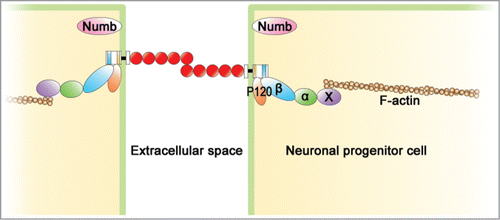
N-cadherin and related molecules are composed of an adhesive complex, AJ which is responsible for strong cell-cell adhesion between NPCs to maintain the tissue architecture ().Citation7,47 First, N-cadherin constitutes a cluster on the cell surface, and its extracellular domains on adjacent cells are bound to each other. The intracellular domain of N-cadherin is connected to β-catenin and p120-catenin. The β-catenin, in turn, is connected to α-catenin. The α-catenin was thought to bind to a cytoskeleton, actin fiber directly. Recently, it has been reported that a monomer of α-catenin bound to β-catenin does not interact with F-actin, and that only α-catenin dimers, isolated from the cadherin-β-catenin complex, bind actin bundles.Citation48-51 This finding suggests that some actin binding molecules mediate between α-catenin and actin fiber. The candidate of the molecule which mediates α-catenin and actin fiber is reported to be vinculin and EPLIN.Citation52,53 Moreover, p120 catenin also combines with the intracellular domain of N-cadherin.Citation7,54 These factors, α-catenin, β-catenin, and p120-catenin, are not only components of AJ, but also are responsible for the integrity of AJ. Various reports have observed that loss of α-catenin, β-catenin, or p120-catenin perturbs the integrity of AJ and destroys the adhesion.Citation55-58 Three subtypes of α-catenin, αE, αN, and αT, are known.Citation59-62 The expression of αE-catenin disappears with the differentiation of NPCs, and instead, αN-catenin is expressed in the differentiated neuron.Citation60,61
Figure 3. Structure of adherens junction (AJ) in NPCs. N-cadherin-based AJ is developed in the apical domain of NPCs. Its extracellular domains of N-cadherin on the adjacent cells bind to each other through homophilic interaction. The intracellular domain of N-cadherin, β-catenin (β), p120-catenin (p120), α-catenin (α), and a cytoskeleton, F-actin are connected to each other to construct AJ. Some actin binding molecules (X) mediate between α-catenin and actin fiber. Numb is involved in the mechanism by which AJ is formed in the apical region.
Integrity of apico-basal polarity in NPCs by N-cadherin
N-cadherin–based AJ is important for maintaining the apico-basal polarity in NPCs. Radial glia cells as NPCs exhibit a characteristic bipolar radial morphology with apical and basal processes.Citation32,63,64 The apical and basal processes are responsible for 2 points of adhesion at the sub-apical region of N-cadherin-based AJs, and integrin-laminin interaction at their basal laminae, respectively ().Citation32 The basal processes are attached to the sub-pial extracellular matrix through integrin-laminin interactions.Citation65,66
The destruction of apico-basal polarity is closely related to downregulation of N-cadherin in nascent neurons and IPs after asymmetric neurogenic division.Citation37,40,41 Nascent neurons often first adopt bipolar radial morphology, then quickly detach their process from the apical surface by down-regulation of N-cadherin.Citation37,40,41 Abnormal persistence of N-cadherin inhibits the detachment of the apical process.Citation40 Thus, N-cadherin regulates the detachment of the apical process, which results in the loss of AJ integrity and the apico-basal polarity of NPC.Citation37,40,41 Recently, it has been reported that Slit1b-Robo3 signaling and N-cadherin regulate apical process retraction in developing retinal ganglion cells.Citation41
Localization of AJ in the apical domain of NPCs
AJ is formed in the sub-apical region of NPCs in the ventricular zone of neural tube, cerebral cortex, and midbrain as shown in .Citation21,34,67,68 The locus determination of AJ is deeply involved in cell polarization. In various cells, cell polarization is known to be required for the cell polarity complex partition defective complex protein 3 (Par3)-atypical protein kinase C (aPKC)-Par6.Citation69,70 Par3 and aPKCζ are observed to be localized at the apical domain of radial glia cells in the mouse neocortex and of NPCs in the chick neural tube ().Citation71,72 In addition, mislocalization of aPKCζ results in the aberrant distribution of cell adhesion molecules ZO-1 and N-cadherin, and disrupts AJs and neural progenitor polarity in the developing chick neural tube. Besides the cell polarity complex, in NPCs, Numb plays a critical role in the mechanism in which AJ is formed in the apical domain.Citation67,73-76 Numb is an endocytic adaptor involved in the formation of apico-basal polarity.Citation77 The reported relationship between Numb and the cell polarity complex is that Numb interacts with a component of the cell polarity complex, Par3.Citation74 In NPCs during the development of the mouse cerebral cortex, Numb is localized in the apical domain of NPCs prior to N cadherin localization ().Citation67,73 According to the site of Numb, N-cadherin-based AJ is formed in the sub-apical region.Citation67,73 Numb is known to interact with p120-catenin,Citation58,77 so p120 catenin may be involved in the localization of N-cadherin-based AJ by Numb.
Role of N-cadherin on the Fate of NPCs
N-cadherin contributes not only to the integrity of AJ and the apico-basal polarity of NPCs, but also to the maintenance, proliferation, and differentiation of NPCs.
NPC niche during development
Adult neural stem cells have some properties of astrocytes, such as producing glial fibrillary acidic protein (GFAP), and reside in the subventricular zone along the wall of the lateral ventricle.Citation78-80 Adult neural stem cell niches are organized by specialized supporting cells, ependymal cells and endothelial cells of blood vessels.Citation81,82 N-cadherin is co-expressed with MT5-MMP in adult stem cells and ependymal cells. MT5-MMP-mediated cleavage of N-cadherin is dispensable for the regulation of adult neural stem cell generation and identity.Citation81 However, the signals from ependymal cells and endothelial cells to maintain the niche are mostly unknown. The neural stem cells can renew themselves and generate transient amplifying (TA) cells by asymmetric division, and subsequently the TA cells generate neuroblast cells.Citation78,83
Figure 4. Description of neural progenitor cell niche during development. The nuclei of radial glia cells move within the ventricular zone as the cell progresses through the cell cycle. During neurogenesis, radial glia cells perform 2 types of division: one is symmetric, proliferative division, and the other is asymmetric neurogenic division. The symmetric division provides both processes to the daughter cells; however, the asymmetric division does not provide the basal process to one daughter cell, and that cell loses the identity of NPCs and differentiates into nascent neuron or intermediate progenitor (IP).
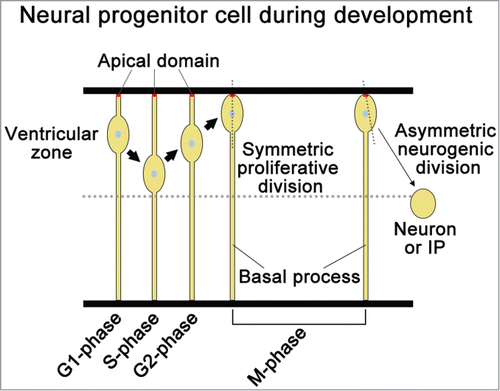
However, in the developing neural tube and brain cortex, NPCs reside in a neurogenic niche lacking distinct supporting cells. In the case of radial glia cells as NPCs, the apical and basal processes of radial glia cells are required for the formation of a self-supporting NPC niche ().Citation37,40,84,85 The nuclei of radial glia cells move within the ventricular zone as the cells progress through the cell cycle ().Citation86,87 During neurogenesis, radial glia cells perform 2 types of division: one is symmetric, proliferative division, and the other is asymmetric neurogenic division ().Citation88 Inheritance of the apical and basal process to the daughter cell is important for the maintenance of NPCs’ identity in mouse NPCs.Citation32,89,90 The symmetric division of NPCs provides both processes to the daughter cells; however, the asymmetric division does not provide the basal process, but only the apical process to one daughter cell, and then the cell loses the identity of NPCs and differentiates into a nascent neuron or IP.Citation32,88 Meanwhile, the other daughter cell inherits both processes and maintains the identity of NPCs.
Contribution of N-cadherin to niche formation
The maintenance of the NPC's niche is required for N-cadherin-based AJ.Citation39,84,85,91 In the apical domain of NPCs, the AJ exists with high density to form strong adhesions.Citation21,34,92 Moreover, in the case of adult NPCs in zebra fish, it has been shown clearly that adhesion through N-cadherin on NPCs of the subventricular zone where Rostral migration occurs constitutes the niche of NPCs in the brain of an adult zebra fish.Citation85
A high level of N-cadherin protein in the apical domain of NPCs seems to be important to undergo symmetric proliferative division and to maintain the identity of NPCs.Citation37,40 The nascent neurons and IPs derived from NPCs lose the identity of NPC while the expression of N-cadherin is down-regulated.Citation37,40,41 Furthermore, loss of function of N-cadherin by the conditional knockout (KO) and short hairpin RNA (shRNA) in the cerebral cortex, causes premature differentiation of NPCs.Citation84,93 These results demonstrate that N-cadherin plays a critical role in the self-supportive niche to keep the identity of NPCs.
Regulation of differentiation of NPCs by N-cadherin
It has been reported that downregulation of N-cadherin after asymmetric, neurogenic division is closely related to neuronal differentiation in various NPCs.Citation37,39-41,85,94 Down-regulation of N-cadherin is observed to be associated with the detachment of the apical process from the ventricular zone of the chick embryo spinal cord.Citation37,40 Abnormal persistence of N-cadherin expression inhibits the apical process withdrawal and cell cycle exit in prospective neurons.Citation40 These facts suggest the onset of neuronal differentiation is characterized by downregulation of N-cadherin.Citation37,40,41,94 Furthermore, experiments using N-cadherin gain- and loss-of-function approaches show that these perturb the neuronal differentiation in chick and zebra fish retina, mouse cerebral cortex, zebra fish cerebellum, and mouse ventral midbrain during development.Citation11,27,34,39,95-97 In adult zebra fish, down-regulation of N-cadherin is a trigger of the differentiation of NPCs during Rostral migration.Citation85 In addition, it has been reported that N-cadherin regulates neuronal differentiation from induced pluripotent stem cells (iPSCs).Citation98,99 These findings support the notion that the downregulation of the N-cadherin level in nascent neurons and IP is required for the onset of neuronal differentiation of NPCs.
Regulation of β-catenin Activity by N-cadherin
β-catenin is not only a component of AJ, but also a component of the canonical Wnt pathway.Citation100,101 Wnt allows β-catenin to translocate into the nucleus. In the nucleus, β-catenin combines with the transcription T-cell factor (TCF)/lymphocyte enhancer factor (LEF), and it causes the induction of proliferation-related genes such as cyclin D.Citation102-105 Thus, Wnt affects the level of β-catenin in the cytoplasm of NPCs. In addition, it is also known that N-cadherin affects the level of β-catenin signaling.Citation34,84,106 In this chapter, we would like to introduce the regulation of β-catenin signaling level by N-cadherin.
Structure of β-catenin
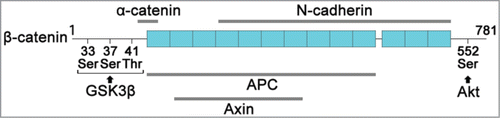
β-catenin contains a repetitive sequence (armadillo repeat) of 42 amino acids, located in the center of the molecule.Citation107,108 The portion of this repetitive sequence combines with the cytoplasmic region of N-cadherin, or APC, and Axin.Citation109 This N-cadherin binding site and the APC and Axin binding sites overlap, so β-catenin cannot combine with N-cadherin and the APC or Axin simultaneously (). It combines with the N-terminal domain of β-catenin with α-catenin ().Citation108 APC and Axin form a complex with glycogen synthase kinase 3β (GSK3β) to degrade β-catenin.Citation110-112
Figure 5. Structure of β-catenin. Human β-catenin has 781 amino acids and contains 13 repetitive sequences (armadillo repeat) of 42 amino acid is located in the center of the molecule. The N-terminal of β-catenin has 3 phosphorylation sites (33Ser, 37Ser, 41Thr) for GSK3β and the C-terminal of β-catenin has a phosphorylation site (Ser552) for Akt. β-catenin binds to α-catenin and cytoplasmic of N-cadherin to develop AJ. On the other hand, β-catenin binds to APC, and Axin to construct a phosphodestruction complex with GSK3β. The phosphodestruction complex phosphorylates β-catenin for the degradation.
Regulation of β-catenin distribution and degradation by N-cadherin
N-cadherin sequesters β-catenin by the binding of the cytoplasmic domain to β-catenin on the cytoplasmic membrane, and controls the level of β-catenin protein in the cytoplasm.Citation34,113-115 N-cadherin is localized in the apical domain of NPCs in the brain ventricular zone with a high level of β-catenin protein ().Citation34,35 It seems that N-cadherin can serve as a “pool” of β-catenin protein to control the level of β-catenin protein in the cytoplasm of NPCs. We recently observed that loss of N cadherin allows β-catenin to diffuse throughout the cytoplasm of NPCs and increase the β-catenin signaling in NPCs of dorsal midbrain during development.Citation34 The same phenomenon is observed also in E cadherin.Citation113-115 These findings suggest that N-cadherin may be a factor that regulates the distribution of β-catenin.
As described above, GSK3β plays a critical role in the degradation of β-catenin.Citation100,111,116 GSK3β activity is known to be affected by N-cadherin-based AJ.Citation117 Phosphodestruction complex, GSK3β, Axin, and APC complex are localized at the joint area of the apical domain of NPC in the mouse cerebral ventricular zone.Citation117 These complexes are activated by maintaining the adhesion by N-cadherin, and therefore have lowered the level of β-catenin in the cytoplasm.Citation117 Furthermore, detachment of the adhesion between the NPCs results in the decrease of the phosphodestruction complex for β-catenin.Citation117 On the other hand, N-cadherin is concerned with the stabilization of β-catenin through Akt.Citation84,93 The phosphorylation of Ser552 in β-catenin by Akt stabilizes β-catenin and allows β-catenin to translocate into the nucleus.Citation118,119 Regulation of Akt activity by N-cadherin is confirmed by the finding that a knockdown by the N-cadherin shRNA in the cerebral cortex causes the reduction of Akt activity.Citation84,93
Control of the Fate of NPCs by β-catenin Signaling
Regulation of niche formation by β-catenin signaling
A high level of β-catenin is involved in the niche formation of NPC in the apical region of the neural tube, brain cortex, and retina, just as N-cadherin.Citation68,84,120-122 Co-localization of β-catenin with N-cadherin is observed in the apical domain of NPCs in the cerebral cortex and midbrain ().Citation34,84 It has been observed that hyperstimulation of β-catenin/Wnt signaling inhibits normal retinal differentiation and expands the population of proliferative retinal progenitors in zebra fish.Citation120 Down-regulation of β-catenin signaling by loss of function of N-cadherin by the shRNA is associated with losing the identity of NPCs and premature differentiation in the developing mouse cerebral cortex.Citation84 Furthermore, loss of β-catenin in NPCs of mouse ventral midbrain during development perturbs the integrity of niche formation and leads to a severe reduction of dopamine neurogenesis.Citation35
In canonical Wnt signaling, β-catenin as a component of Wnt signaling up-regulates cyclin D1, which is involved in the progress of the cell cycle.Citation104,123 As described above, in the developing mouse midbrain. It has been reported that the expression of active β-catenin increases the proliferation of NPC and decreases the neurogenesis.Citation18,35,121
Contribution of β-catenin to differentiation of NPCs
The signaling of β-catenin is also related to progress not only in proliferation but also differentiation.Citation84,121,122,124 As it is for N-cadherin, β-catenin is deeply involved in the balance between the self-renewal and differentiation of NPC.Citation35,84,120,122 The level of β-catenin is regulated by various factors such as N-cadherin, Wnt, Akt and phosphodestruction complex.Citation34,84,102,117 During the differentiation of NPCs, the downregulation of β-catenin level is observed in nascent neuron and IP from NPCs.Citation84 In the developing cerebral cortex, loss of function of N-cadherin by the shRNA decreases Akt activity and thereby decreases the level of β-catenin by failure of phosphorylation level of Ser552 in β-catenin.Citation84 The decrease of the β-catenin level results in the premature differentiation of NPC.Citation122
Relationship of Hh and Notch Signaling to N-cadherin-based AJ
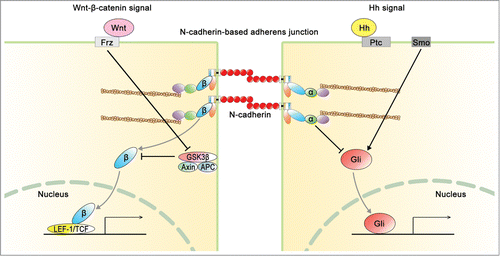
The fate of NPCs is regulated by Notch, Hh, and Wnt signaling during development. As mentioned above, Wnt signaling regulates the fate of NPCs through β-catenin. In this chapter, we discuss the relationship of Notch and Hh signaling to N-cadherin-based AJ in NPCs during development. Furthermore, the Hh signaling has been reported to interact with Wnt signaling, as shown in .Citation35,125
Figure 6. Interaction of N-cadherin-based AJ with Wnt signaling and hedgehog (Hh) signaling. N-cadherin regulates not only the integrity of AJ, but also the balance between Wnt signal and Hh signal. About Wnt signal, β-catenin (β) is mediated with N-cadherin-based AJ and canonical Wnt signal. On the other hand, α-catenin (α) is mediated with the junction and Hh signal. Ptc, patched; Smo, smoothened; Frz, frizzled.
Relationship of Notch signaling to N-cadherin-based AJ
Notch signaling plays a critical role in maintenance of NPCs niche and switching from proliferative cell division to neurogenic cell division.Citation46,126 After asymmetric neurogenic division, numerous nascent neurons and/or IPs expressing the ligand of Notch retain apical process transiently at the ventricular zone in the developing mouse cerebral cortex.Citation127 Both Notch1 and its ligand Delta-like 1 (Dll1) are distributed around AJs in the apical process.Citation127 The nascent neurons and IPs inherited lower level of Delta-Notch signaling components than the cell, which maintains the identity of NPC.Citation44,46 The down-regulation of Notch signaling is important for the downregulation of N-cadherin and subsequent apical process detachment.Citation127 As described above, some studies show a functional link between Notch signaling and cadherin-mediated AJ.Citation128,129
In addition, a crosstalk between Notch and Hh signaling pathway has been reported.Citation43 Hh activity in NPCs up-regulates the expression of Hes1 and brain lipid-binding protein (Blbp), downstream targets of Notch signaling and promotes symmetric proliferative division of NPCs.Citation43 As described in the above chapter, Hes1 suppresses the expression of ngn2 and maintains the expression of N-cadherin.Citation42
Regulation of Hh signal in NPCs by αE-catenin
αE-catenin has been reported to be able to detect the cell density of NPCs and is responsible for the negative feedback loop of proliferation in the cerebral cortex.Citation16 In a mouse cerebral cortex suffering from the loss of αE-catenin, the up-regulation of Hh signaling is observed and excess proliferation of NPC takes place in the cortex.Citation16 This finding is supported by the notion that Hh signaling is known to be concerned with proliferation of NPCs.Citation130,131-133 Furthermore, this finding proposes a negative feedback loop controlling the size of the developing cerebral cortex.Citation16 A proposed mechanism by which αE-catenin may detect the cell density in the cerebral cortex may serve αE-catenin as a tension-transducer, which senses the tension between NPCs and detects cell density in the cerebral cortex.Citation16,134 Recently, it has been reported that tension causes a conformational change of α-catenin, which allows a varied vinculin-binding site in α-catenin protein to expose and interact with α-catenin.Citation134,135 The force-dependent interaction between vinculin and αE-catenin might be involved in chemical response such as Hh signaling in NPC.
Relationship between apico-basal polarity and Hh signaling
How does apico-basal polarity in neural tube and brain cortex regulate the fate of NPC? The apico-basal polarity is known to be related to Hh signaling.Citation16,40,136,137 After asymmetric neurogenic division of NPCs, nascent neurons and IPs lose the apico-basal polarity and progress the differentiation in the developing neural tube and cerebral cortex.Citation40 The relation between the polarity and Hh signal is analyzed with mouse and chick neural tubes.Citation40 In prospective neurons in the neural tube, the reduction of N-cadherin expression and loss of polarity is observed.Citation40 As a result of losing polarity, the primary cilium are temporarily lost in the cells.Citation40 The primary cilium has Smo and Gli, which are components of Hh signaling pathway.Citation138,139 Therefore, loss of the primary cilium results in the suppression of Hh signaling.Citation40 In addition, the primary cilium activity is required for maintaining proper apico-basal polarity as neuroepithelium cells transform radial glia cells.Citation140 However, in the retina and neural tube of zebra fish, the destruction of the apico-basal polarity of NPC by N-cadherin knockout and mutants, or knockout of αE-catenin, increases the proliferative division and decreases the neurogenesis from the NPC.Citation16,136,137 This decrease of neurogenesis is related to Hh signaling.Citation16,136,137 These findings reveal that the apico-basal polarity of NPC is deeply involved in Hh signaling.
Relationship between Wnt signaling and Hh signaling
The interaction between Wnt-β-catenin signaling and Hh signaling is observed in the NPCs of the mouse neural tube and midbrain ventral region during development.Citation35,125,132 Upregulation of the Wnt-β-catenin signal in the developing ventral midbrain is observed to suppress Hh signaling.Citation35,125,141 Conversely, the inhibition of Wnt signaling is observed to increase Hh signaling.Citation35,125 In addition, in the neural tube of the embryo, Wnt signaling induces expression of the cyclin D1, and affects the G1 phase; on the other hand, Hh signaling affects the G2 phase.Citation104,123,132 These findings suggest that the balance between Wnt signaling and Hh signaling regulates the proliferation and differentiation of NPC.Citation35,125,141
Conclusion and Perspective
In this review, we discuss the role of N-cadherin-based AJ in not only the integrity of tissue architecture and apico-basal polarity, but also the integrity of various signaling such as β-catenin and αE-catenin in NPC. N-cadherin regulates the balance between the self-renewal and differentiation of NPC, and thereby controls the size and architecture of the neural system. However, the molecular mechanism underlying the control of NPC fate by N-cadherin remains unclear. Further study is needed to elucidate this mechanism. It has also been shown that N-cadherin is involved in the neuronal differentiation from an iPS cell.Citation99 If the mechanism underlying the determination of the NPCs by N-cadherin is found, it is expected to lead to a technique of development of efficient neurogenesis. It is further expected that development of this technique will become an effective method in the medical treatment of neurodegenerative diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev 2012; 92:597-634; PMID:22535893; http://dx.doi.org/10.1152/physrev.00014.2011
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 2007; 8:11-20; PMID:17133224; http://dx.doi.org/10.1038/nrn2043
- Suzuki S, Sano K, Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul 1991; 2:261-70; PMID:2059658
- Tanabe K, Takeichi M, Nakagawa S. Identification of a nonchordate-type classic cadherin in vertebrates: chicken Hz-cadherin is expressed in horizontal cells of the neural retina and contains a nonchordate-specific domain complex. Dev Dyn 2004; 229:899-906; PMID:15042713; http://dx.doi.org/10.1002/dvdy.10493
- Tanihara H, Sano K, Heimark RL, St John T, Suzuki S. Cloning of five human cadherins clarifies characteristic features of cadherin extracellular domain and provides further evidence for two structurally different types of cadherin. Cell Adhes Commun 1994; 2:15-26; PMID:7982033; http://dx.doi.org/10.3109/15419069409014199
- Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol 2001; 154:231-43; PMID:11449003; http://dx.doi.org/10.1083/jcb.200103143
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 2009; 1:a002899; PMID:20457565; http://dx.doi.org/10.1101/cshperspect.a002899
- Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development 2006; 133:3895-905; PMID:16943271; http://dx.doi.org/10.1242/dev.02560
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development 2006; 133:4839-47; PMID:17108003; http://dx.doi.org/10.1242/dev.02662
- Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci U S A 2010; 107:9873-8; PMID:20457910; http://dx.doi.org/10.1073/pnas.1003480107
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, et al. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 2003; 130:2479-94; PMID:12702661; http://dx.doi.org/10.1242/dev.00465
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron 2002; 35:77-89; PMID:12123610; http://dx.doi.org/10.1016/S0896-6273(02)00748-1
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 2004; 101:3196-201; PMID:14963232; http://dx.doi.org/10.1073/pnas.0308600100
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004; 131:3133-45; PMID:15175243; http://dx.doi.org/10.1242/dev.01173
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004; 7:136-44; PMID:14703572; http://dx.doi.org/10.1038/nn1172
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science 2006; 311:1609-12; PMID:16543460; http://dx.doi.org/10.1126/science.1121449
- Kang W, Wong LC, Shi SH, Hebert JM. The transition from radial glial to intermediate progenitor cell is inhibited by FGF signaling during corticogenesis. J Neurosci 2009; 29:14571-80; PMID:19923290; http://dx.doi.org/10.1523/JNEUROSCI.3844-09.2009
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 2002; 297:365-9; PMID:12130776; http://dx.doi.org/10.1126/science.1074192
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 1986; 320:447-9; PMID:3515198; http://dx.doi.org/10.1038/320447a0
- Inuzuka H, Redies C, Takeichi M. Differential expression of R- and N-cadherin in neural and mesodermal tissues during early chicken development. Development 1991; 113:959-67; PMID:1821862
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol 2007; 304:22-33; PMID:17222817; http://dx.doi.org/10.1016/j.ydbio.2006.12.014
- Dufour S, Saint-Jeannet JP, Broders F, Wedlich D, Thiery JP. Differential perturbations in the morphogenesis of anterior structures induced by overexpression of truncated XB- and N-cadherins in Xenopus embryos. J Cell Biol 1994; 127:521-35; PMID:7929592; http://dx.doi.org/10.1083/jcb.127.2.521
- Ganzler-Odenthal SI, Redies C. Blocking N-cadherin function disrupts the epithelial structure of differentiating neural tissue in the embryonic chicken brain. J Neurosci 1998; 18:5415-25; PMID:9651223
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev Biol 2001; 234:454-69; PMID:11397013; http://dx.doi.org/10.1006/dbio.2001.0251
- Luo Y, Ferreira-Cornwell M, Baldwin H, Kostetskii I, Lenox J, Lieberman M, Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development 2001; 128:459-69; PMID:11171330
- Erdmann B, Kirsch FP, Rathjen FG, More MI. N-cadherin is essential for retinal lamination in the zebrafish. Dev Dyn 2003; 226:570-7; PMID:12619142; http://dx.doi.org/10.1002/dvdy.10266
- Matsunaga M, Hatta K, Takeichi M. Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron 1988; 1:289-95; PMID:3078519; http://dx.doi.org/10.1016/0896-6273(88)90077-3
- Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn 2012; 241:1333-49; PMID:22684994; http://dx.doi.org/10.1002/dvdy.23813
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 2000; 26:395-404; PMID:10839358; http://dx.doi.org/10.1016/S0896-6273(00)81172-1
- Sahara S, O'Leary DD. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron 2009; 63:48-62; PMID:19607792; http://dx.doi.org/10.1016/j.neuron.2009.06.006
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure–remodeling of the neuroepithelium prior to neurogenesis. Dev Biol 1996; 180:664-79; PMID:8954735; http://dx.doi.org/10.1006/dbio.1996.0336
- Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci 2011; 31:3683-95; PMID:21389223; http://dx.doi.org/10.1523/JNEUROSCI.4773-10.2011
- Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol 1987; 120:215-27; PMID:3817290; http://dx.doi.org/10.1016/0012-1606(87)90119-9
- Sakane F, Miyamoto Y. N-cadherin regulates the proliferation and differentiation of ventral midbrain dopaminergic progenitors. Dev Neurobiol 2013; 73:518-29; PMID:23420609; http://dx.doi.org/10.1002/dneu.22077
- Tang M, Villaescusa JC, Luo SX, Guitarte C, Lei S, Miyamoto Y, Taketo MM, Arenas E, Huang EJ. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci 2010; 30:9280-91; PMID:20610763; http://dx.doi.org/10.1523/JNEUROSCI.0860-10.2010
- Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A 2013; 110:E3017-26; PMID:23884650; http://dx.doi.org/10.1073/pnas.1220176110
- Rousso DL, Pearson CA, Gaber ZB, Miquelajauregui A, Li S, Portera-Cailliau C, Morrisey EE, Novitch BG. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron 2012; 74:314-30; PMID:22542185; http://dx.doi.org/10.1016/j.neuron.2012.02.024
- Matsumata M, Uchikawa M, Kamachi Y, Kondoh H. Multiple N-cadherin enhancers identified by systematic functional screening indicate its Group B1 SOX-dependent regulation in neural and placodal development. Dev Biol 2005; 286:601-17; PMID:16150435; http://dx.doi.org/10.1016/j.ydbio.2005.08.005
- Rago L, Beattie R, Taylor V, Winter J. miR379-410 cluster miRNAs regulate neurogenesis and neuronal migration by fine-tuning N-cadherin. EMBO J 2014; 33:906-20; PMID:24614228; http://dx.doi.org/10.1002/embj.201386591
- Das RM, Storey KG. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014; 343:200-4; PMID:24408437; http://dx.doi.org/10.1126/science.1247521
- Wong GK, Baudet ML, Norden C, Leung L, Harris WA. Slit1b-Robo3 signaling and N-cadherin regulate apical process retraction in developing retinal ganglion cells. J Neurosci 2012; 32:223-8; PMID:22219284; http://dx.doi.org/10.1523/JNEUROSCI.2596-11.2012
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 2008; 58:52-64; PMID:18400163; http://dx.doi.org/10.1016/j.neuron.2008.02.014
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One 2011; 6:e14680
- Nelson BR, Hodge RD, Bedogni F, Hevner RF. Dynamic interactions between intermediate neurogenic progenitors and radial glia in embryonic mouse neocortex: potential role in Dll1-Notch signaling. J Neurosci 2013; 33:9122-39; PMID:23699523; http://dx.doi.org/10.1523/JNEUROSCI.0791-13.2013
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 2007; 449:351-5; PMID:17721509; http://dx.doi.org/10.1038/nature06090
- Dong Z, Yang N, Yeo SY, Chitnis A, Guo S. Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 2012; 74:65-78; PMID:22500631; http://dx.doi.org/10.1016/j.neuron.2012.01.031
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 2008; 36:149-55.
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903-15; PMID:16325583; http://dx.doi.org/10.1016/j.cell.2005.09.021
- Maiden SL, Hardin J. The secret life of alpha-catenin: moonlighting in morphogenesis. J Cell Biol 2011; 195:543-52; PMID:22084304; http://dx.doi.org/10.1083/jcb.201103106
- Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem Soc Trans 2008; 36:141-7; PMID:18363554; http://dx.doi.org/10.1042/BST0360141
- Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol 2010; 189:339-52; PMID:20404114; http://dx.doi.org/10.1083/jcb.200910041
- Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol 2003; 160:399-407; PMID:12566430; http://dx.doi.org/10.1083/jcb.200212057
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A 2008; 105:13-9; PMID:18093941; http://dx.doi.org/10.1073/pnas.0710504105
- Chauvet N, Prieto M, Fabre C, Noren NK, Privat A. Distribution of p120 catenin during rat brain development: potential role in regulation of cadherin-mediated adhesion and actin cytoskeleton organization. Mol Cell Neurosci 2003; 22:467-86; PMID:12727444; http://dx.doi.org/10.1016/S1044-7431(03)00030-7
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem 2001; 276:12301-9; PMID:11121423; http://dx.doi.org/10.1074/jbc.M010377200
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci 2004; 7:357-63; PMID:15034585; http://dx.doi.org/10.1038/nn1212
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol 1998; 142:847-57; PMID:9700171; http://dx.doi.org/10.1083/jcb.142.3.847
- Ozaki C, Yoshioka M, Tominaga S, Osaka Y, Obata S, Suzuki ST. p120-Catenin is essential for N-cadherin-mediated formation of proper junctional structure, thereby establishing cell polarity in epithelial cells. Cell Struct Funct 2010; 35:81-94; PMID:20859058; http://dx.doi.org/10.1247/csf.10009
- Piven OO, Kostetskii IE, Macewicz LL, Kolomiets YM, Radice GL, Lukash LL. Requirement for N-cadherin-catenin complex in heart development. Exp Biol Med (Maywood) 2011; 236:816-22; PMID:21680756; http://dx.doi.org/10.1258/ebm.2011.010362
- Ajioka I, Nakajima K. Switching of alpha-catenin from alphaE-catenin in the cortical ventricular zone to alphaN-catenin II in the intermediate zone. Brain Res Dev Brain Res 2005; 160:106-11; PMID:16185771; http://dx.doi.org/10.1016/j.devbrainres.2005.08.004
- Stocker AM, Chenn A. Differential expression of alpha-E-catenin and alpha-N-catenin in the developing cerebral cortex. Brain Res 2006; 1073-1074:151-8; PMID:16457793
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell 1992; 70:293-301; PMID:1638632; http://dx.doi.org/10.1016/0092-8674(92)90103-J
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001; 409:714-20; PMID:11217860; http://dx.doi.org/10.1038/35055553
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res 2001; 41:51-60; PMID:11535293; http://dx.doi.org/10.1016/S0168-0102(01)00259-0
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 2001; 31:367-79; PMID:11516395; http://dx.doi.org/10.1016/S0896-6273(01)00374-9
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci 2007; 27:13854-65; PMID:18077697; http://dx.doi.org/10.1523/JNEUROSCI.4494-07.2007
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 2007; 10:819-27; PMID:17589506; http://dx.doi.org/10.1038/nn1924
- Tang M, Miyamoto Y, Huang EJ. Multiple roles of beta-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development 2009; 136:2027-38; PMID:19439492; http://dx.doi.org/10.1242/dev.034330
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta 2008; 1778:614-30; PMID:18005931; http://dx.doi.org/10.1016/j.bbamem.2007.08.029
- Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 2013; 319:1357-64; PMID:23535009; http://dx.doi.org/10.1016/j.yexcr.2013.03.021
- Ghosh S, Marquardt T, Thaler JP, Carter N, Andrews SE, Pfaff SL, Hunter T. Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctions in neural progenitors. Proc Natl Acad Sci U S A 2008; 105:335-40; PMID:18162555; http://dx.doi.org/10.1073/pnas.0705713105
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 2009; 63:189-202; PMID:19640478; http://dx.doi.org/10.1016/j.neuron.2009.07.004
- Kim S, Walsh CA. Numb, neurogenesis and epithelial polarity. Nat Neurosci 2007; 10:812-3; PMID:17593942; http://dx.doi.org/10.1038/nn0707-812
- Wang Z, Sandiford S, Wu C, Li SS. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 2009; 28:2360-73; PMID:19609305; http://dx.doi.org/10.1038/emboj.2009.190
- Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development 2010; 137:1315-25; PMID:20332149; http://dx.doi.org/10.1242/dev.043190
- Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci 2004; 7:803-11; PMID:15273690; http://dx.doi.org/10.1038/nn1289
- Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, et al. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell 2011; 22:3103-19; PMID:21775625; http://dx.doi.org/10.1091/mbc.E11-03-0274
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923; http://dx.doi.org/10.1016/S0092-8674(00)80783-7
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science 1994; 264:1145-8; PMID:8178174; http://dx.doi.org/10.1126/science.8178174
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev 2003; 13:543-50; PMID:14550422; http://dx.doi.org/10.1016/j.gde.2003.08.012
- Porlan E, Marti-Prado B, Morante-Redolat JM, Consiglio A, Delgado AC, Kypta R, Lopez-Otin C, Kirstein M, Farinas I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat Cell Biol 2014; 16:629-38; PMID:24952463; http://dx.doi.org/10.1038/ncb2993
- Culver JC, Vadakkan TJ, Dickinson ME. A specialized microvascular domain in the mouse neural stem cell niche. PLoS One 2013; 8:e53546; PMID:23308251; http://dx.doi.org/10.1371/journal.pone.0053546
- Zuccotti A, Le Magueresse C, Chen M, Neitz A, Monyer H. The transcription factor Fezf2 directs the differentiation of neural stem cells in the subventricular zone toward a cortical phenotype. Proc Natl Acad Sci U S A 2014; 111:10726-31; PMID:25002477; http://dx.doi.org/10.1073/pnas.1320290111
- Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell 2010; 18:472-9; PMID:20230753; http://dx.doi.org/10.1016/j.devcel.2009.12.025
- Yagita Y, Sakurai T, Tanaka H, Kitagawa K, Colman DR, Shan W. N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation. J Neurosci Res 2009; 87:3331-42; PMID:19301425; http://dx.doi.org/10.1002/jnr.22044
- Takahashi T, Nowakowski RS, Caviness VS, Jr. Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci 1993; 13:820-33; PMID:8426239
- Frade JM. Interkinetic nuclear movement in the vertebrate neuroepithelium: encounters with an old acquaintance. Prog Brain Res 2002; 136:67-71; PMID:12143404; http://dx.doi.org/10.1016/S0079-6123(02)36007-2
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 2014; 15:351-64; PMID:24639559; http://dx.doi.org/10.1002/embr.201438447
- Kosodo Y, Huttner WB. Basal process and cell divisions of neural progenitors in the developing brain. Dev Growth Differ 2009; 51:251-61; PMID:19379277; http://dx.doi.org/10.1111/j.1440-169X.2009.01101.x
- Shitamukai A, Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. Dev Growth Differ 2012; 54:277-86; PMID:22524601; http://dx.doi.org/10.1111/j.1440-169X.2012.01345.x
- Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013; 140:4510-21; PMID:24154521; http://dx.doi.org/10.1242/dev.090738
- Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn 2005; 233:528-39; PMID:15844200; http://dx.doi.org/10.1002/dvdy.20365
- Zhang J, Shemezis JR, McQuinn ER, Wang J, Sverdlov M, Chenn A. AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev 2013; 8:7; PMID:23618343; http://dx.doi.org/10.1186/1749-8104-8-7
- Kurusu M, Katsuki T, Zinn K, Suzuki E. Developmental changes in expression, subcellular distribution, and function of Drosophila N-cadherin, guided by a cell-intrinsic program during neuronal differentiation. Dev Biol 2012; 366:204-17; PMID:22542600; http://dx.doi.org/10.1016/j.ydbio.2012.04.006
- Klingener M, Chavali M, Singh J, McMillan N, Coomes A, Dempsey PJ, Chen EI, Aguirre A. N-Cadherin Promotes Recruitment and Migration of Neural Progenitor Cells from the SVZ Neural Stem Cell Niche into Demyelinated Lesions. J Neurosci 2014; 34:9590-606; PMID:25031401; http://dx.doi.org/10.1523/JNEUROSCI.3699-13.2014
- Noles SR, Chenn A. Cadherin inhibition of beta-catenin signaling regulates the proliferation and differentiation of neural precursor cells. Mol Cell Neurosci 2007; 35:549-58; PMID:17553695; http://dx.doi.org/10.1016/j.mcn.2007.04.012
- Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol 2009; 7:e1000240; PMID:19901980; http://dx.doi.org/10.1371/journal.pbio.1000240
- Haque A, Yue XS, Motazedian A, Tagawa Y, Akaike T. Characterization and neural differentiation of mouse embryonic and induced pluripotent stem cells on cadherin-based substrata. Biomaterials 2012; 33:5094-106; PMID:22520296; http://dx.doi.org/10.1016/j.biomaterials.2012.04.003
- Su H, Wang L, Huang W, Qin D, Cai J, Yao X, Feng C, Li Z, Wang Y, So KF, et al. Immediate expression of Cdh2 is essential for efficient neural differentiation of mouse induced pluripotent stem cells. Stem Cell Res 2013; 10:338-48; PMID:23416351; http://dx.doi.org/10.1016/j.scr.2013.01.003
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 2002; 16:1066-76; PMID:12000790; http://dx.doi.org/10.1101/gad.230302
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 2004; 167:339-49; PMID:15492040; http://dx.doi.org/10.1083/jcb.200402153
- Vleminckx K, Kemler R, Hecht A. The C-terminal transactivation domain of beta-catenin is necessary and sufficient for signaling by the LEF-1/beta-catenin complex in Xenopus laevis. Mech Dev 1999; 81:65-74; PMID:10330485; http://dx.doi.org/10.1016/S0925-4773(98)00225-1
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382:638-42; PMID:8757136; http://dx.doi.org/10.1038/382638a0
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 1999; 96:5522-7; PMID:10318916; http://dx.doi.org/10.1073/pnas.96.10.5522
- Wisniewska MB. Physiological role of beta-catenin/TCF signaling in neurons of the adult brain. Neurochem Res 2013; 38:1144-55; PMID:23377854; http://dx.doi.org/10.1007/s11064-013-0980-9
- Liu Q, Wang W, Zhang L, Zhao L, Song W, Duan X, Zhang Y. Involvement of N-cadherin/beta-catenin interaction in the micro/nanotopography induced indirect mechanotransduction. Biomaterials 2014; 35:6206-18; PMID:24818888; http://dx.doi.org/10.1016/j.biomaterials.2014.04.068
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8:1711-7; PMID:2788574
- Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length beta-catenin. Structure 2008; 16:478-87; PMID:18334222; http://dx.doi.org/10.1016/j.str.2007.12.021
- Tucci V, Kleefstra T, Hardy A, Heise I, Maggi S, Willemsen MH, Hilton H, Esapa C, Simon M, Buenavista MT, et al. Dominant beta-catenin mutations cause intellectual disability with recognizable syndromic features. J Clin Invest 2014; 124:1468-82; PMID:24614104; http://dx.doi.org/10.1172/JCI70372
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16:3797-804; PMID:9233789; http://dx.doi.org/10.1093/emboj/16.13.3797
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 2002; 277:17901-5; PMID:11834740; http://dx.doi.org/10.1074/jbc.M111635200
- Roberts DM, Pronobis MI, Poulton JS, Waldmann JD, Stephenson EM, Hanna S, Peifer M. Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol Biol Cell 2011; 22:1845-63; PMID:21471006; http://dx.doi.org/10.1091/mbc.E10-11-0871
- Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/beta-catenin signalling during epithelial-mesenchymal transition. PLoS One 2011; 6:e23899; PMID:21909376; http://dx.doi.org/10.1371/journal.pone.0023899
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci 1999; 112(Pt 8):1237-45; PMID:10085258
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. J Cell Biol 1996; 132:1105-14; PMID:8601588; http://dx.doi.org/10.1083/jcb.132.6.1105
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002; 108:837-47; PMID:11955436; http://dx.doi.org/10.1016/S0092-8674(02)00685-2
- Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol 2009; 186:219-28; PMID:19620634; http://dx.doi.org/10.1083/jcb.200811108
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 2007; 39:189-98; PMID:17237784; http://dx.doi.org/10.1038/ng1928
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007; 282:11221-9; PMID:17287208; http://dx.doi.org/10.1074/jbc.M611871200
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev 2012; 7:30; PMID:22920725; http://dx.doi.org/10.1186/1749-8104-7-30
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 2003; 258:406-18; PMID:12798297; http://dx.doi.org/10.1016/S0012-1606(03)00123-4
- Draganova K, Zemke M, Zurkirchen L, Valenta T, Cantu C, Okoniewski M, Schmid MT, Hoffmans R, Gotz M, Basler K, et al. Wnt/beta-catenin signaling regulates sequential fate decisions of murine cortical precursor cells. Stem cells 2015; 33: 170-82; PMID: 25182747; http://dx.doi.org/10.1002/stem.1820
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398:422-6; PMID:10201372; http://dx.doi.org/10.1038/18884
- Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci 2004; 117:5731-7; PMID:15522889; http://dx.doi.org/10.1242/jcs.01505
- Tang M, Luo SX, Tang V, Huang EJ. Temporal and spatial requirements of Smoothened in ventral midbrain neuronal development. Neural Dev 2013; 8:8; PMID:23618354; http://dx.doi.org/10.1186/1749-8104-8-8
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci 2007; 25:1006-22; PMID:17331197; http://dx.doi.org/10.1111/j.1460-9568.2007.05370.x
- Hatakeyama J, Wakamatsu Y, Nagafuchi A, Kageyama R, Shigemoto R, Shimamura K. Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 2014; 141:1671-82; PMID:24715457; http://dx.doi.org/10.1242/dev.102988
- Mizuhara E, Nakatani T, Minaki Y, Sakamoto Y, Ono Y, Takai Y. MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J Biol Chem 2005; 280:26499-507; PMID:15908431; http://dx.doi.org/10.1074/jbc.M500375200
- Sasaki N, Sasamura T, Ishikawa HO, Kanai M, Ueda R, Saigo K, Matsuno K. Polarized exocytosis and transcytosis of Notch during its apical localization in Drosophila epithelial cells. Genes Cells 2007; 12:89-103; PMID:17212657; http://dx.doi.org/10.1111/j.1365-2443.2007.01037.x
- Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development 2005; 132:143-54; PMID:15576403; http://dx.doi.org/10.1242/dev.01566
- Cayuso J, Ulloa F, Cox B, Briscoe J, Marti E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development 2006; 133:517-28; PMID:16410413; http://dx.doi.org/10.1242/dev.02228
- Alvarez-Medina R, Le Dreau G, Ros M, Marti E. Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development 2009; 136:3301-9; PMID:19736325; http://dx.doi.org/10.1242/dev.041772
- Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev 2006; 20:3036-48; PMID:17079690; http://dx.doi.org/10.1101/gad.391106
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533-42; PMID:20453849; http://dx.doi.org/10.1038/ncb2055
- Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, et al. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun 2014; 5:4525; PMID:25077739
- Yamaguchi M, Imai F, Tonou-Fujimori N, Masai I. Mutations in N-cadherin and a Stardust homolog, Nagie oko, affect cell-cycle exit in zebrafish retina. Mech Dev 2010; 127:247-64; PMID:20362667; http://dx.doi.org/10.1016/j.mod.2010.03.004
- Chalasani K, Brewster RM. N-cadherin-mediated cell adhesion restricts cell proliferation in the dorsal neural tube. Mol Biol Cell 2011; 22:1505-15; PMID:21389116; http://dx.doi.org/10.1091/mbc.E10-08-0675
- Oro AE. The primary cilia, a 'Rab-id' transit system for hedgehog signaling. Curr Opin Cell Biol 2007; 19:691-6.
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature 2005; 437:1018-21; PMID:16136078; http://dx.doi.org/10.1038/nature04117
- Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 2013; 16:1000-7; PMID:23817546; http://dx.doi.org/10.1038/nn.3451
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci 2009; 12:125-31; PMID:19122665; http://dx.doi.org/10.1038/nn.2243
