Abstract
Type I collagen is a fibrillar protein, a member of a large family of collagen proteins. It is present in most body tissues, usually in combination with other collagens and other components of extracellular matrix. Its synthesis is increased in various pathological situations, in healing wounds, in fibrotic tissues and in many tumors. After extraction from collagen-rich tissues it is widely used in studies of cell behavior, especially those of fibroblasts and myofibroblasts. Cells cultured in a classical way, on planar plastic dishes, lack the third dimension that is characteristic of body tissues. Collagen I forms gel at neutral pH and may become a basis of a 3D matrix that better mimics conditions in tissue than plastic dishes.
Abbreviations
| AP-1 | = | activator protein 1 |
| ECM | = | extracellular matrix |
| ERK | = | extracellular signal-regulated kinase |
| FAK | = | focal adhesion kinase |
| GT | = | granulation tissue |
| HSC | = | hepatic stellate cells |
| JNK | = | c-Jun N-terminal kinase |
| MFB | = | myofibroblasts |
| MKL1 | = | megakaryoblastic leukemia 1 |
| MMP | = | metalloproteinases |
| NF-κB | = | nuclear factor kappa B |
| PI3K/Akt | = | phosphatidylinositide 3-kinase/Ak strain transforming |
| PEG | = | polyethylene glycol |
| α-SMA | = | α-smooth muscle actin |
| 3D | = | 3-dimensional |
| TGFβ-1 | = | transforming growth factor β 1 |
| TIMP | = | tissue inhibitor of metalloproteinases |
| TNF-α | = | tumor necrosis factor α |
Introduction
Cells in a tissue are surrounded with other cells and with ECM, which is a network containing proteins, glycoproteins, proteoglycans and glycosaminoglycans. ECM provides chemical and mechanical signals whose effects are interdependent. Chemical signals may originate in the chemical structure of ECM components or may be provided by cytokines and growth factors stored in the ECM and released under certain circumstances. ECM is more pliable than hard plastic surface and its mechanical properties contribute to diversity of physiological and pathological situations in the tissue. ECM is degradable and cells can migrate through it.Citation1,2 Cell culture on thin collagen film covering plastic substrate is useful in studies of some aspects of cell behavior, e.g. interaction with integrins,Citation3 but the contact with 3D environment makes the cells in tissue behave differently than the cells in conventional tissue culture on stiff plastic dishes do, as far as their morphology, differentiation, migration, and proliferation is concerned. The cells surrounded with an appropriate scaffold, usually collageneous, acquire tissue-like phenotype not observed in cells in monolayer. Mechanical signals from the ECM to the cells and contractile forces to the ECM are transmitted by protein complexes called focal adhesions. Three-dimensional matrix adhesions differ from adhesions on 2D substrates in protein composition and biological activity.Citation4 Force applied to integrins is transmitted through focal adhesions to the cytoskeleton.Citation5 The cells may integrate global signals coming from the entire surface and sense the spatial organization of activated adhesions.Citation6
Fibroblasts and myofibroblasts are cells involved in the healing of various tissues. Fibroblasts in the tissue surrounding the wound are activated and migrate into the provisional matrix containing fibrin and plasma fibronectin. Fibrin is a major component of the provisional matrix formed during wound healing and enables migration of inflammatory cells and fibroblasts. Collagen production becomes the main fibroblast function and the provisional matrix is gradually replaced with a collagenous ECM.Citation7-9 A part of fibroblasts may differentiate to protomyofibroblasts and further to MFB characterized by prominent stress fibers that contain α-SMA associated with non-muscle myosin. These proteins endow MFB with high contractional force that is combined with synthetic abilities of the MFB.Citation10,11 The wound contracts and the provisional matrix is replaced with GT that is gradually converted to a scar. Most cells then die by apoptosis. However, the reparative process may be dysregulated and result in fibrosis. The most abundant ECM component providing a scaffold that binds other proteins and proteoglycans is collagen I.Citation12,13
Solid tumors contain stroma that resembles GT in many aspects. The stroma is highly vascularized. Fibrin is formed by clotting extravasated fibrinogen and together with other plasma proteins it gives rise to a provisional matrix. Fibroblasts settle in the matrix and produce collagen I and other ECM components. Cancer-associated fibroblasts promote the growth of cancer cells and vice versa they respond to signals from epithelial cells by increased synthesis of collagen and other fibrogenic factors.Citation14,15
Three-dimensional matrices used as a model to study cell behavior in the tissue-like environment in normal and pathological situations are therefore often made of collagen.Citation2,16 Fibrin gels can also be used to provide 3D environment for cells but they have much smaller influence on cell behavior and their effects may be opposite to those of collagen.Citation17,18
Various models of collagen matrix aiming to mimic in vivo situations will be discussed in this review. Fibroblastic cells transferred from plastic to collagen gel change their morphology and functions and in response they modify their environment. Secretion of MMP by the cells has a particular role in these interactions. The contact of cells with collagen is mediated by specific receptors, integrins. Plasma fibronectin interacts both with collagen and cells in vivo and it is therefore often added to collagen matrix in vitro.
Collagen Matrix Models
Rodent tail tendons contain almost pure collagen I that can be extracted with diluted acids.Citation19-21 Collagen forms gel when its solution is neutralized. The concentration of 1 to 2 mg collagen/ml is frequently used to form matrix. Collagen contained in bovine skin is more crosslinked and its extraction requires the use of pepsin. This enzyme cleaves off telopeptides, the nonhelical amino acid sequences at the C- and N-ends of the collagen molecule. The lack of telopeptides may interfere with gel formation and change the properties of the gel. The pores in gel made of acid-extracted rat tail collagen are 1–2 μm in diameter. The pores in the gel from pepsin-digested collagen are larger and allow easier migration of the cells.Citation16 Cells can be seeded on top of the gel or be suspended in collagen solution. The cells on collagen may be covered with a second layer of collagen gel to add the cells the third dimension. Fibroblasts placed between 2 collagen layers migrate into them.Citation22,23
Fibroblasts cultured for a few weeks in the presence of L-ascorbic acid 2-phosphate form a multilayered structure surrounded by hydroxyproline-rich ECM.Citation24 The self-produced dermis-like structure formed in the presence of ascorbic acid in a long term culture contains collagens I and VI.Citation25
Fibroblasts embedded in collagen gel cause its contraction. When collagen gel formed in tissue culture dishes remains attached to the walls of the dish, fibroblasts can contract it only in the vertical direction. When the gel is detached from the dish immediately after gelation, it floats in the culture medium and contracts in all directions. The resulting matrix is called floating. The gel may be maintained under tension for 1 or more days before it is detached. This type of matrix is called stressed or stress-released. Contraction of floating collagen matrices gives rise to mechanically relaxed tissue resembling dermis, attached matrices resemble granulation tissue.Citation26-28 The shape of liver MFB growing on hard plastic and on collagen gels is shown in –. The cells respond to changing stiffness and tension of the substrate.
Figure 1. Rat liver myofibroblasts on plastic. Rat liver MFB were cultured on a plastic dish. (A) Cells 1 h, (B) 4 h and (C) 24 h after plating. The cells were initially rounded and then spread on the hard surface (Peterová and Kanta, unpublished results).
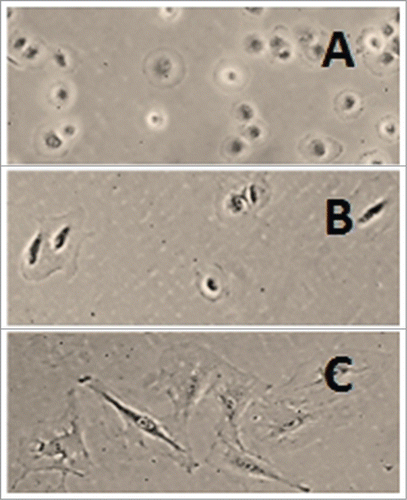
Figure 2. Rat liver myofibroblasts on attached collagen gel. Rat liver MFB were cultured on type I collagen gel. Cells (A) 1 h, (B) 2 h and (C) 24 h after seeding on the gel. The cells were rounded at first but they soon became stellate and aggregated. The gel was detached from the walls and the bottom of the dish 24 h after seeding the cells and the cells were allowed to grow for another 24 h (D) (Peterová and Kanta, unpublished results).
Figure 3. Rat liver myofibroblasts on stress-released collagen gel. Rat liver MFB were seeded on type I collagen gel. The gel was detached from the walls and the bottom of the dish 1 h later. The cells were still rounded 1 h after release (A), began to elongate after 2 h (B) and acquired a slender shape 24 h after release (C) (Peterová and Kanta, unpublished results).
Collagen properties can be modified by crosslinking collagen molecules and fibrils. Collagen I is a substrate of transglutaminase that introduces ϵ-(γ-glutamyl) lysine cross-links into its molecule at 37°C.Citation29,30 Fibroblast attachment, spreading and proliferation is enhanced on collagen polymerized as a result of transglutaminase treatment.Citation31 Collagen can also be crosslinked by 0.2% glutaraldehyde and used as a matrix for cell culture. No toxicity to fibroblasts is observed when collagen is treated with this glutaraldehyde concentration.Citation32
Mechanical properties of the matrix play a significant role in determining cell behavior. The stiffness of isotropic material is characterized by Young's modulus (elastic modulus). Its unit is Pascal (Pa). The stiffness of soft tissues is low; the stiffness of liver is 0.1 to 1 kPa, dermis 1–5 kPa and fibrotic tissues 20 to 100 kPa. Young's modulus of the provisional matrix in healing wounds (0.01 to 0.1 kPa) is comparable to that of collagen gel.Citation33 Collagen fibrils in 0.2–2.0% gel are similar to those in tumor ECM.Citation34
Collagen stiffness can be increased when a portion of liquid is removed by ultracentrifugation,Citation34 gel compressionCitation35 or by evaporating the solvent.Citation36 Fibroblasts seeded in matrix containing 20 or 40 mg collagen/ml are viable, migrate and proliferate. They reach a density similar to that found in human dermis.Citation36,37
The differences between cell growth on hard plastic surface and on soft collagen gel suggest that the rigidity of the substrate plays a crucial role. Cells can be placed between 2 sheets of collagen-coated polyacrylamide gel. The stiffness of polyacrylamide gel can be controlled by changing the percentage of the crosslinking compound, bis-acrylamide, in the reaction mixture and can be adjusted to correspond to the physiological stiffness of tissues. This treatment makes fibroblasts that are well spread in 2D culture change their morphology into bipolar or stellate characteristic of fibroblasts in vivo.Citation38 Increased rigidity affects not only stress fibers formation but also integrin expression on the cell surface.Citation39,40
Polyethylene glycol (PEG) can be covalently bonded to collagenous matrix extracted from porcine heart. PEG gels retain fibrillar structure, are more resistant to enzymatic degradation and do not inhibit metabolic activity of incorporated fibroblasts.Citation41
Interaction of Fibroblasts with Collagen Gel
The cells can be plated on the surface of collagen gel or incorporated into it to form a tissue-like structure.Citation19,20 Fibroblasts are rounded when they are embedded into collagen gel; they adopt stellate morphology within a few hours and they are spindle-shaped later.Citation42 Fibroblast and MFB morphology in 3D matrix is comparable to that in their original tissue but much different from polygonal appearance they adopt on a planar substratum.Citation18,43 Fibroblasts on collagen gel aggregate; this tendency decreases with increasing collagen concentration.Citation18,44
Fibroblasts embedded in collagen remodel surrounding matrix. They have few cell adhesions on their surface but they produce dendritic extensions that interact with collagen fibrils.Citation45,46 Fibroblasts align the flexible collagen meshwork around themselves and hold collagen fibrils in place. The fibrils are then stabilized by noncovalent interactions that do not require cell presence.Citation47,48 Fibroblasts in attached matrices develop isometric tension. The forces they generate do not depend on the stiffness of the substrate.Citation49 The forces produced by fibroblasts not expressing α-SMA increase rapidly within the first 6 hours after embedding the cells into collagen.Citation50
When collagen matrix is attached to the walls of the culture dish, distinct actin stress fibers that develop in fibroblasts can be visualized by phalloidin staining.Citation51,52 When fibroblasts are embedded in collagen layer cast on polyacrylamide gels, actin fibers staining with rhodamine phalloidin appear if Young's modulus is adjusted to 1.6 to 3.6 kPa. Stress fibers formation is facilitated by cell-cell contact. Direct linkage of the cytoskeleton stress fibers mediated by cell surface cadherins maintains tension between neighboring cells.Citation39,53 When tension generated by the cells reaches a critical level, α-SMA that is at first diffusely distributed in the cytosol is incorporated to preexisting β-actin-containing stress fibers.Citation54 Transcription factor MKL1 attached to globular actin (G-actin) is released after G-actin polymerization and translocated to the nucleus. It binds to the α-SMA gene promoter and initiates α-SMA expression. Matrix stiffening also activates the small GTPase RhoA and Rho kinase (ROCK) that control the balance between polymerized and depolymerized actin.Citation55 These changes correspond to the increasing tension in the forming GT in healing wounds. Cytoplasmic actin microfilament system containing α-SMA characterizes MFB.Citation56
Profibrogenic cytokine TGF-β1 and smad signaling are involved in gel contraction by fibroblasts derived from normal skin or hypertrophic scars.Citation57 TGF-β-pretreated fibroblasts cause significantly more rapid gel contraction.Citation58 The degree of substrate stiffness determined by underlying polyacrylamide gel modulates TGF-β-induced transdifferentiation of fibroblasts.Citation59 Both substrate stiffness and the presence of TGF-β are required for the differentiation of liver portal fibroblasts to MFB.Citation60
Generation of the threshold tension necessary for α-SMA incorporation requires formation of large, „supermature“ adhesion sites.Citation54 Contractility of both muscles and non-muscle cells is dependent on the interaction of actin and myosin. Non-muscle myosin II is closely associated with actin stress fibers in cochlear fibrocytes and the contraction of collagen matrix can be prevented by an inhibitor of myosin II function.Citation61 Aging dermal fibroblasts lose the ability of force generation in collagen gel which may be caused by decreased expression of myosin light chain kinase and Rho kinase.Citation62
Increasing collagen concentration supports cell proliferation and suppresses apoptosis.Citation63 Fibroblasts migrate along collagen concentration gradient to the stiffer regions of a collagen construct. This effect is called durotaxis.Citation64 α-SMA-positive MFB appear in wound GT when Young's modulus is about 20 kPa.Citation54 The stiffness of collagen matrices containing 1–2 mg collagen is about 50 Pa and the stiffness of plastic used in tissue culture is about 1 GPa.Citation2
Fibroblasts, MFB and other cells involved in wound healing are affected by intrinsic forces produced by the ECM and extracellular fluid.Citation65 The final outcome of ECM remodeling is determined both by tissue stiffness and by mechanical loading of the tissue, i.e. the force applied to tissue. Mechanical forces influence both cell proliferation and gene expression. Prolonged mechanical loading may result in higher tissue stiffness.Citation66,67
Most cells grow only if their surfaces are attached to the ECM. The attachment of cells to ECM molecules is mediated by integrins. These receptors consisting of subunits α and β link ECM with the actin cytoskeleton and transmit signals from the outside to the cell and vice versa.Citation68 Integrins α1β1, α2β1, α10β1 and α11β1 are collagen receptors.Citation69 The engagement of integrins leads to the activation of signaling cascades, focal adhesion kinase (FAK), extracellular signal-regulated protein kinase (ERK) and Rho GTPases.Citation70,71 Discoidin domain receptors (DDR) 1 and 2 represent another family of cell-surface receptors. They are activated by collagens and regulate cell proliferation and ECM synthesis.They are expressed on fibroblasts in healing wounds and in tumors.Citation72,73
“Synthetic” Phenotype of Fibroblasts in Mechanically Stressed Collagen Matrices
Attached matrix
Physiological levels of tissue stiffness function as a brake on fibroblast proliferation and collagen I synthesis. Fibrotic diseases are accompanied by tissue stiffening which is no longer regarded as a mere consequence of the disease; it has become clear that it may drive the whole process.Citation74
Fibroblasts switch between proliferative and quiescence phenotypes. Fibroblasts in attached gels assume a “synthetic” phenotype.Citation52 They proliferate and synthesize collagen. The number of cells in attached gels increases rapidly while the culture in floating gels regresses. However, the cells remaining in the floating gels are viable and divide at the same rate when they return to standard culture conditions. Fibroblasts in attached gel are bipolar, fibroblasts in floating gel are stellate.Citation75 DNA synthesis measured by Citation3H-thymidine incorporation into DNA is almost one order of magnitude higher in the cells on plastic than in the cells in attached matrix and about 2 orders higher than in the cells in floating matrix.Citation76 Fibroblast proliferation is proportional to collagen concentration in the matrix when the gel is compressed and its Young's modulus increased.Citation77 DNA synthesis in attached gel is dependent on the ERK pathway. This signaling pathway is disrupted when the gels are released.Citation78 The cytoskeleton is then disorganized and DNA synthesis is inhibited. However, these 2 events are independent because only DNA synthesis in adhering gel is affected by an ERK inhibitor.Citation79
The release of attached matrix from the walls of the culture dish 24 hours after casting, (stress-released matrix), induces secretion of cytokines IL-6 and IL-8 by the embedded fibroblasts. The response of cells to TGF-β1 and TNF-α changes, growth inhibition is less severe in stress-released matrix than in the attached one. The signaling networks that include these cytokines are modified.Citation80
Collagen synthesis is downregulated on both RNA and protein levels when fibroblasts are transferred from plastic to 3D collagen matrix.Citation81 Both total protein synthesis and collagen synthesis measured by Citation3H-proline incorporation is several times higher in attached gels than in floating gels.Citation75,82 The expression of fibrillar collagens I and III in liver portal fibroblasts increases on a stiff substrate in paralel with α-SMA, while the expression of net-forming collagen IV decreases.Citation60 Tensile strength also controls the expression of collagen type XII that is associated with collagen fibrils.Citation83 Collagen α1(I) mRNA synthesis and steady-state level is decreased in fibroblasts transferred from plastic into collagen gel. No change is observed in the expression of fibronectin mRNA.Citation84 Total protein synthesis and collagen synthesis are high in the cells on plastic and in attached fibrin gel but low in floating collagen and fibrin gels. Mechanical forces seem to play a dominant role in this case.Citation85
Floating matrix
Contraction of freely-floating matrix by cells is dependent on α-SMA expression.Citation86,87 Fibroblasts in relaxed collagen gel lose stress fibers and focal adhesions and do not proliferate. They form dendritic extensions that have microtubule cores and actin rich-tips.Citation88,89 The extent of contraction is dependent on initial collagen concentration; lower density gels contract more rapidly. The release of mechanical tension triggers fibroblast apoptosis.Citation90,91 This effect is specific of collagen, apoptosis is not observed in contractile fibrin gels.Citation92 Signal transduction from the ECM is disturbed.Citation93 rRNA content is lower in collagen matrices than in the cells in monolayer.Citation94 mRNA expression of TGF-β1 increases in the order of plastic, attached matrix, stress-relaxed matrix and floating matrix. The expression of collagenase mRNA is higher in collagen matrix than in the cells on plastic.Citation95 The release of stressed matrix is followed by a burst of c-fos expression and ERK 1/2 kinase activation.Citation96
Cell survival, collagen synthesis and degradation are regulated by integrins. Antibodies to α2β1 integrins prevent the contraction and reduce apoptosis.Citation97-99 Integrin α11 mRNA and protein are up-regulated in attached collagen gel and down-regulated in fibroblasts grown in floating gel.Citation100 Rat liver MFB utilize α1β1 integrin for collagen matrix contraction as α2 subunit is not expressed in HSC, their precursors in vivo.Citation101 The expression of α2 subunit in fibroblasts cultured in collagen gel is dependent on NF-κB activity that is induced by contact of the cells with collagen.Citation102 The expression of the 2 receptors is regulated differentially and their functions are not identical. α1β1 mediates downregulation of collagen gene expression and α2β1 mediates induction of collagenase.Citation103 Both of them are able to mediate gel contraction but their expression in vitro depends on the environment and in vivo on the physiological state of the tissue.Citation101 Matrix contraction may be enhanced by collagen V that binds integrin αvβ3.Citation104
Increased synthesis of collagen is observed in the skin of α1-null mice. Col1(I) mRNA levels in both granulation tissue and fetal fibroblasts are higher in the cells isolated from α1-null animals and embedded in collagen gel than in wild-type cells. Integrins α1β1 provide a feedback inhibition of collagen synthesis.Citation105 Blocking of β1 subunit by a monoclonal antibody, which abrogates phoshorylation of Akt/protein kinase B, protects cells from contraction-induced apoptosis. Phosphatidyl-inositol 3-kinase (PI3K)/Akt signaling is a regulator of cell survival. Downregulation of PI3/Akt survival signal results in apoptosis. Constitutive expression of phophatidylinositol 3-kinase (PI3K) protects fibroblasts from both apoptosis and anoikis.Citation106
Secretion of metalloproteinases
MMP are a family of zinc-dependent proteinases that are secreted to the extracellular space or localized to the cell surface. They are collectivelly able to cleave all components of ECM and they can modify other biologically active molecules. A subgroup of collagenases comprises MMP-1, -8, -13 and -14 that degrade fibrillar collagens. Gelatinases MMP-2 and -9 cleave denatured collagen and collagen type IV.Citation107
Both mechanical forces and the chemical nature of collagen play an important role in regulating MMP expression. Collagenase mRNA expression and activity are higher in fibroblasts cultured on type I collagen gel when compared to cells on plastic.Citation108 Releasing stress by treating fibroblasts on plastic with cytochalasin D that disrupts cell cytoskeleton enhances expresion and secretion of MMP-1, -2, -3, -13 and membrane-bound MMP-14. The active form of MMP is more strongly expressed in cells cultured in floating matrices than in cells in monolayer.Citation109 Increased expression of MMP-2 and its inhibitor TIMP-2 mRNAs is observed in fibroblasts when collagen gel with cultured cells is prestrained.Citation110 Contact of human MFB with collagen I gel results in the activation of proMMP-2 that is not observed in the cells grown on plastic or plastic coated with a thin layer of collagen I or IV, laminin or Matrigel. The induction of MMP-2 by accumulating collagen I may contribute to the remodeling of ECM in fibrotic liver.Citation111,112 Increased expression of the active form of MMP-2 on collagen gel is accompanied by up-regulation of MT1-MMP protein. Metalloproteinase MT1-MMP (MMP-14) is known to activate MMP-2.Citation113
Collagen degradation is more rapid in floating matrices than in attached gels.Citation114 Active forms of MMP-1 and MMP-2 can be detected around human HSC cultured on collagen gel by in situ zymography and in the culture medium, respectively.Citation115 The expression of MMP-13 increases in rat liver MFB when the cells are embedded in attached collagen gel. However, the observed collagen degradation is a result of a joint action of a few proteinases.Citation18
Collagen contraction is enhanced by MMP activity and impaired by MMP inhibition. MMP activity is stimulated in floating gel.Citation116-118 Gel contraction by fibroblasts is greatly accelerated when the matrix is treated with plasmin that may activate MMP-1 secreted by the cells. Fibroblasts in healing wounds are in close proximity to keratinocytes that produce plasminogen activator in response to cytokines. Plasmin may play a role in provisional matrix remodeling.Citation119 Contraction of floating collagen matrix gives rise to a mechanically relaxed tissue resembling dermis. The cells in floating matrix show low capacity to synthesize DNA and proliferate, decreased responsiveness to growth factors and decreased ability to synthesize collagen.Citation26 Three-dimensional matrix, especially at higher stiffness, impedes cell proliferation and migration. The cells may secrete proteinases and degrade adjacent matrix to create space for these activities. Interconnected multicellular networks are formed in gels with low Young's modulus.Citation120
Integrins α1β1 and α2β1 have different functions in the regulation of MMP expression. MMP-1 mRNA level in fibroblasts cultured in retracting collagen gel is higher than that in cells on plastic. The diference can be eliminated when α2β1 integrin is blocked by a specific antibody. In contrast, the difference is much larger when α1 subunit is blocked. Changes in MMP expression are paralleled by changed expression of Ets-1 transcription factor.Citation103,121 The induction of MMP-13 in periodontal ligament fibroblasts is dependent on integrin α11β1.Citation122 The induction of MMP in collagen gel may be affected by signaling pathways downstream of integrin ligand binding. Contact of human skin fibroblasts with 3D collagen results in simultaneous activation of 3 groups of mitogen-activated protein kinases, ERK 1/2, JNK and p38. Tyrosine kinase inhibition suppresses MMP-13 expression, ERK1/2 inhibition enhances the expression.Citation123 Collagen activates a member of protein kinase C family PKCξ and NFκB DNA binding.Citation124 The expression of MMP-3, -9, -13, and -14 mRNA as well as the activation of MMP-9 is enhanced in activated rat HSC embedded in collagen I gel. The stimulation of MMP-9 expression requires NF-κB and AP-1 activities.Citation125,126
The Influence of Fibronectin on Collagen Properties
Type I collagen is a major component of connective tissues but its action can be modified by other components of the ECM as well as by cytokines and agents used for treating the cells. Fibronectin contained in blood plasma and to a smaller extent in fetal bovine serum used in cell culture influences the events in collagen matrix substantially.
Medium containing fetal bovine serum is procontractile. Collagen gel contraction by human dermal fibroblasts is inhibited when serum used in culture medium is depleted of fibronectin by affinity chromatography.Citation127 The inhibition can be abolished by adding plasma fibronectin or vitronectin to culture medium containing fibronectin-depleted fetal bovine serum.Citation128 Fibronectin is much more efficient. The stimulated gel contractility is inhibited by peptides containing arg-gly-asp (RGD) sequences.Citation129 Fibronectin fibrils are associated with stress fibers formed in the cells in attached collagen gel. Fibroblasts in floating gel do not form stress fibers or form fibronectin fibrils.Citation130 Fibronectin added to collagen sponges accommodating chick fibroblasts enhances DNA synthesis in the cells. Fibronectin-coated sponges enhance wound healing in vivo.Citation131
F-actin and α5 integrin are induced by fibronectin in trabecular meshwork cells cultured in collagen gel.Citation132 Fibronectin promotes gel contraction by human corneal fibroblasts. It stimulates the formation of stress fibers in the cells and increases the amounts of integrin subunits α5 and β1 and of paxillin, a component of focal adhesions.Citation133 Fibroblasts cultured on collagen matrix form clusters that are stabilized by fibronectin fibrils. Cell clustering requires α5β1 integrins and can be prevented by blocking Rho kinase or myosin II activity.Citation44,134 A succession of events taking place in the first hours of matrix contraction has been proposed. The earliest stage involving fibronectin and the integrin subunit α5 is followed by vitronectin-mediated cell attachment and the sequence is completed by the appearance of α2 integrin subunit and its interaction with collagen.Citation135 Fibronectin produced by the fibroblasts themselves may enhance matrix contraction.Citation136,137 Fibronectin is present on cell surfaces and associated with collagen in attached matrices. It disappears from the cell surface after the matrix is released. Protein and DNA synthesis decrease.Citation138 Fibronectin-null mouse embryonic fibroblast adhere to collagen gel in the absence of serum but do not spread or proliferate. Addition of plasma fibronectin results in an increase in the cell number and formation of multicellular structures. Inhibition of fibronectin polymerization prevents cell proliferation.Citation139
Conclusion and Perspectives
The number of studies of cell behavior utilizing various 3D tissue models is increasing. Three-dimensional structure produces cells that differ in many aspects from their counterparts cultured on flat plastic dishes. Both morphology and metabolism of the cells are changed. Type I collagen as the most abundant protein found in tissues is the basis of many 3D models. Its chemical properties, the ability to interact with cells and to bind other ECM components, contribute a distinct specificity to the contact with cells. Collagen can be further modified by crosslinking or by casting on a stiffer substrate to better mimic matrix development in healing wounds or in other tissues under physiological or pathological situations. Future collagen matrix models may come even closer to situations in vivo by better controling physico-chemical properties of the matrix, by incorporating into it other ECM proteins, glycoproteins and proteoglycans and by allowing fibroblastic cell interaction with inflammatory cells, epithelial and endothelial cells that also participate in the events going on in tissues.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by PRVOUK P37/1.
References
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006; 7:211-24; PMID:16496023; http://dx.doi.org/10.1038/nrm1858
- Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol 2010; 26:335-61; PMID:19575667; http://dx.doi.org/10.1146/annurev.cellbio.042308.113318
- Plant AL, Bhadriraju K, Spurlin TA, Elliott JT. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta 2009; 1793:893-902; PMID:19027042; http://dx.doi.org/10.1016/j.bbamcr.2008.10.012
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001; 294:1708-12; PMID:11721053; http://dx.doi.org/10.1126/science.1064829
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem 2004; 279:12001-4; PMID:14960578; http://dx.doi.org/10.1074/jbc.R300038200
- Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng 2005; 33:1469-90; PMID:16341917; http://dx.doi.org/10.1007/s10439-005-8159-4
- Laurens N, Koolwijk P, De Maat MPM. Fibrin structure and wound healing. J Thromb Haemostas 2006; 4:932-9; http://dx.doi.org/10.1111/j.1538-7836.2006.01861.x
- Clark RAF. Fibrin and wound healing. Ann NY Acad Sci 2001; 936:355-67; PMID:11460492; http://dx.doi.org/10.1111/j.1749-6632.2001.tb03522.x
- McClain SA, Simon M, Jones E, Nandi A, Gailit JO, Tonnesen MG, Newman D, Clark RAF. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol 1996; 149:1257-70; PMID:8863674
- Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 2011; 92:158-67; PMID:21355940; http://dx.doi.org/10.1111/j.1365-2613.2011.00764.x
- Kraman R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol 2013; 231:273-89; PMID:24006178
- Bienkowski RS, Gotkin MG. Control of collagen deposition in mammalian lung. Proc Soc Exp Biol Med 1995; 209:118-40; PMID:7770462; http://dx.doi.org/10.3181/00379727-209-43886a
- Lorena D, Uchio K, Costa AMA, Desmouliere A. Normal scarring: importance of myofibroblasts. Wound Rep Regen 2002; 10:86-92; http://dx.doi.org/10.1046/j.1524-475X.2002.00201.x
- Dvorak HF. Tumors: wounds that do not heal. New England J Med 1986; 315:1650-9; http://dx.doi.org/10.1056/NEJM198612253152606
- Tripathi M, Billet S, Bhowmick NA. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhes Migr 2012; 6:231-5; http://dx.doi.org/10.4161/cam.20419
- Wolf K, Alexander S, Schacht V, Coussens LM, von Adrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 2009; 20:931-41; PMID:19682592; http://dx.doi.org/10.1016/j.semcdb.2009.08.005
- Han Y-P, Zhou L, Wang J, Xiong S, Garner WL, French SW, Tsukamoto H. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J Biol Chem 2004; 279:4820-8; PMID:14617627; http://dx.doi.org/10.1074/jbc.M310999200
- Jiroutová A, Peterová E, Bittnerová L, Slavkovský R, Čevelová P, Řezáčová M, Cerman J, Mičuda S, Kanta J. Collagenolytic potential of rat liver myofibroblasts. Physiol Res 2013; 62:15-25
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 1972; 54:626-37; PMID:4339818; http://dx.doi.org/10.1083/jcb.54.3.626
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979; 76:1274-8; PMID:286310; http://dx.doi.org/10.1073/pnas.76.3.1274
- Hu XW, Knight DP, Chapman JA. The effect of non-polar liquids and non-ionic detergents on the ultrastructure and assembly of rat tail tendon collagen fibrils in vitro. Biochim Biophys Acta 1997; 1334:327-37; PMID:9101729; http://dx.doi.org/10.1016/S0304-4165(96)00112-2
- Artym VV, Matsumoto K. Imaging cells in three-dimensional collagen matrix. Curr Protoc Cell Biol 2010; Suppl 48:10.18.1-10.18.20; PMID:20853341
- Haas VR, Santos AR Jr, Wada MLF. Behaviour of fibroblastic cells cultured in collagen I using the sandwich technique. Cytobios 2001; 106 Suppl 2:255-67; PMID:11545452
- Ishikawa O, Kondo A, Okada K, Miyachi Y, Furumura M. Morphological and biochemical analyses on fibroblasts and self-produced collagens in a novel three-dimensional culture. Br J Dermatol 1997; 136:6-11; PMID:9039287; http://dx.doi.org/10.1111/j.1365-2133.1997.tb08738.x
- Hazeki N, Yamato M, Imamura Y, Sasaki T, Nakazato K, Yamamoto K, Konomi H, Hayashi T. Analysis of matrix protein components of the dermis-like structure formed in a long-term culture of human fibroblasts: type VI collagen is a major component. J Biochem 1998; 123:587-95; PMID:9538247; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021977
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994; 124:401-4; PMID:8106541; http://dx.doi.org/10.1083/jcb.124.4.401
- Rhee S, Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev 2007; 59:1299-305; PMID:17825456; http://dx.doi.org/10.1016/j.addr.2007.08.006
- Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen 2004; 12:134-47; PMID:15086764; http://dx.doi.org/10.1111/j.1067-1927.2004.012208.x
- Erwanto Y, Kawahara S, Katayama K, Takenoyama S, Fujino H, Yamauchi K, Morishita T, Kai Y, Watanabe S, Muguruma M. Microbial transglutaminase modifies gel properties of porcine collagen. Asian-Aust J Anim Sci 2003; 16:269-76; http://dx.doi.org/10.5713/ajas.2003.269
- Stachel I, Schwarzenbolz U, Henle T, Meyer M. Cross-linking of type I collagen with microbial transglutaminase: identification of cross-linking sites. Biomacromolecules 2010; 11:698-705; PMID:20131754; http://dx.doi.org/10.1021/bm901284x
- Chau DYS, Collighan RJ, Verderio EAM, Addy VL, Griffin M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005; 26:6518-29; PMID:15927250; http://dx.doi.org/10.1016/j.biomaterials.2005.04.017
- Sheu M-T, Huang J-C, Yeh G-C, Ho H-O. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 2001; 22:1713-9; PMID:11396874; http://dx.doi.org/10.1016/S0142-9612(00)00315-X
- Hinz B. Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 2009; 11:120-6; PMID:19296884; http://dx.doi.org/10.1007/s11926-009-0017-1
- Erikson A, Nortvedt HN, Naess SN, Sikorski P, Davies C de L. Physical and chemical modifications of collagen gels: impact on diffusion. Biopolymers 2008; 89:135-43; PMID:17957715; http://dx.doi.org/10.1002/bip.20874
- Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater 2005; 15:1762-70; http://dx.doi.org/10.1002/adfm.200500042
- Besseau L, Coulomb B, Lebreton-Decoster C, Giraud-Guille M-M. Production of ordered collagen matrices for three-dimensional cell culture. Biomaterials 2002; 23:27-36; PMID:11762846; http://dx.doi.org/10.1016/S0142-9612(01)00075-8
- Helary C, Foucault-Bertaud A, Godeau G, Coulomb B, Giraud Guille MM. Fibroblast populated dense collagen matrices: cell migration, cell density and metalloproteinases expression. Biomaterials 2005; 26:1533-43; PMID:15522755; http://dx.doi.org/10.1016/j.biomaterials.2004.05.016
- Benningo KA, Dembo M, Wang Y-L. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. PNAS 2004; 101:18024-9; PMID:15601776; http://dx.doi.org/10.1073/pnas.0405747102
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskel 2005; 60:24-34; http://dx.doi.org/10.1002/cm.20041
- Jones C, Ehrlich HP. Fibroblast expression of α-smooth muscle actin, α2β1 integrin and αvβ3 integrin: influence of surface rigidity. Exp Mol Pathol 2011; 91:394-9; PMID:21530503; http://dx.doi.org/10.1016/j.yexmp.2011.04.007
- Grover GN, Rao N, Christman KL. Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology 2014; 25:014011; PMID:24334615; http://dx.doi.org/10.1088/0957-4484/25/1/014011
- Dodd NJF, Schor SL, Rushton G. The effects of a collagenous extracellular matrix on fibroblast membrane organization. Exp Cell Res 1982; 141:421-31; PMID:6291961; http://dx.doi.org/10.1016/0014-4827(82)90230-0
- Tomasek JJ, Hay ED. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J Cell Biol 1984; 99:536-49; PMID:6146628; http://dx.doi.org/10.1083/jcb.99.2.536
- da Rocha-Azevedo B, Grinnell F. Fibroblast morphogenesis on 3D collagen matrices: the balance between cell clustering and cell migration. Exp Cell Res 2013; 319:2440-6; PMID:23664837; http://dx.doi.org/10.1016/j.yexcr.2013.05.003
- Mercier I, Lechaire J-P, Desmouliere A, Gaill F, Aumailley M. Interactions of human skin fibroblasts with monomeric or fibrillar collagens induce different organization of the cytoskeleton. Exp Cell Res 1996; 225:245-56; PMID:8660912; http://dx.doi.org/10.1006/excr.1996.0174
- Jiang H, Grinnell F. Cell-matrix entanglement and mechanical anchorage of fibroblasts in three-dimensional collagen matrices. Mol Biol Cell 2005; 16:5070-6; PMID:16107563; http://dx.doi.org/10.1091/mbc.E05-01-0007
- Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981; 290:249-51; PMID:7207616; http://dx.doi.org/10.1038/290249a0
- Guidry C, Grinnell F. Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Collagen Rel Res 1986; 6:515-29; http://dx.doi.org/10.1016/S0174-173X(87)80050-X
- Freyman TM, Yannas IV, Yokoo R, Gibson LJ. Fibroblast contractile force is independent of the stiffness which resists the contraction. Exp Cell Res 2002; 272:153-62; PMID:11777340; http://dx.doi.org/10.1006/excr.2001.5408
- Eastwood M, Porter R, Khan U, McGrouther G, Brown R. Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J Cell Physiol 1996; 166:33-42; PMID:8557773; http://dx.doi.org/10.1002/(SICI)1097-4652(199601)166:1%3c33::AID-JCP4%3e3.0.CO;2-H
- Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol 2000; 10:362-5; PMID:10932093; http://dx.doi.org/10.1016/S0962-8924(00)01802-X
- Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B. Fibroblasts in mechanically stressed collagen lattices assume a „synthetic“ phenotype. J Biol Chem 2001; 276:36575-85; PMID:11468280; http://dx.doi.org/10.1074/jbc.M101602200
- Ehrlich HP, Allison GM, Leggett M. The myofibroblast, cadherin, α smooth muscle actin and the collagen effect. Cell Biochem Funct 2006; 24:63-70; PMID:15584087; http://dx.doi.org/10.1002/cbf.1188
- Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J Cell Biol 2006; 172:259-68; PMID:16401722; http://dx.doi.org/10.1083/jcb.200506179
- Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 2012; 47:340-8; PMID:22461426; http://dx.doi.org/10.1165/rcmb.2012-0050OC
- Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost 2003; 90:993-1002; PMID:14652629
- Kopp J, Preis E, Said H, Hafemann B, Wickert L, Gressner AM, Pallua N, Dooley S. Abrogation of transforming growth factor-β signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem 2005; 280:21570-6; PMID:15788410; http://dx.doi.org/10.1074/jbc.M502071200
- Liu XD, Umino T, Ertl R, Veays T, Skold CM, Takigawa K, Romberger DJ, Spurzem JR, Zhu YK, Kohyama T, et al. Persistence of TGF-β1 induction of increased fibroblast contractility. In Vitro Cell Dev Biol-Anim 2001; 37:193-201; PMID:11370814; http://dx.doi.org/10.1290/1071-2690(2001)037%3c0193:POTIOI%3e2.0.CO;2
- Meyer-ter-Vehn T, Han H, Grehn F, Schlunck G. Extracellular matrix elasticity modulates TGF-β-induced p38 activation and myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 2011; 52:9149-55; PMID:22058331; http://dx.doi.org/10.1167/iovs.10-6679
- Li Z, Dranoff JA, Chan EP, Uemura M, Sévigny F, Wells RG. Transforming growth factor-β and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 2007; 46:1246-56; PMID:17625791; http://dx.doi.org/10.1002/hep.21792
- Kelly JJ, Forge A, Jagger DJ. Contractility in Type III cochlear fibrocytes is dependent on non-muscle myosin II and intercellular gap junctional coupling. JARO 2012; 13:473-84; PMID:22476723; http://dx.doi.org/10.1007/s10162-012-0322-7
- Fujimura T, Hotta M, Kitahara T, Takema Y. Loss of contraction force in dermal fibroblasts with aging due to decreases in myosin light chain phosphorylation enzymes. Arch Pharm Res 2011; 34:1015-22; PMID:21725823; http://dx.doi.org/10.1007/s12272-011-0619-9
- Helary C, Zarka M, Giraud-Guille MM. Fibroblasts within concentrated collagen hydrogels favour chronic skin wound healing. J Tissue Eng Regen Med 2012; 6:225-37; PMID:22362469; http://dx.doi.org/10.1002/term.420
- Hadjipanayi E, Mudera V, Brown RA. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton 2009; 66:121-8; PMID:19170223; http://dx.doi.org/10.1002/cm.20331
- Agha R, Ogawa R, Pietramaggiori G, Orgill DP. A review of the role of mechanical forces in cutaneous wound healing. J Surg Res 2011; 171:700-8; PMID:22005503; http://dx.doi.org/10.1016/j.jss.2011.07.007
- Prajapati RT, Eastwood M, Brown RA. Duration and orientation of mechanical loads determine fibroblast cyto-mechanical activation: monitored by protease release. Wound Repair Regen 2000; 8:238-46; PMID:10886814; http://dx.doi.org/10.1046/j.1524-475x.2000.00238.x
- Petersen A, Joly P, Bergman C, Korus G, Duda GN. The impact of substrate stiffness and mechanical loading on fibroblast-induced scaffold remodeling. Tissue Eng Part A 2012; 18:1804-17; PMID:22519582; http://dx.doi.org/10.1089/ten.tea.2011.0514
- Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011; 209:139-51; PMID:21307119; http://dx.doi.org/10.1530/JOE-10-0377
- White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol 2004; 36:1405-10; PMID:15147720; http://dx.doi.org/10.1016/j.biocel.2003.08.016
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 2003; 15:572-82; PMID:14519392; http://dx.doi.org/10.1016/S0955-0674(03)00109-1
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol 2008; 18:347-52; PMID:18514521; http://dx.doi.org/10.1016/j.tcb.2008.05.002
- Márquez J, Olaso E. Role of discoidin domain receptor 2 in wound healing. Histol Histopathol 2014; 29:1355-64.
- Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012; 31:295-321; PMID:22366781; http://dx.doi.org/10.1007/s10555-012-9346-z
- Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppresion. J Cell Biol 2010; 190:693-706; PMID:20733059; http://dx.doi.org/10.1083/jcb.201004082
- Nakagawa S, Pawelek P, Grinnell F. Long-term culture of fibroblasts in contracted collagen gels: effects on cell growth and biosynthetic activity. J Invest Dermatol 1989; 93:792-8; PMID:2584746; http://dx.doi.org/10.1111/1523-1747.ep12284425
- Lambert CA, Soudant EP, Nusgens BV, Lapiere CM. Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest 1992; 66:444-51; PMID:1316527
- Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med 2009; 3:77-84; PMID:19051218; http://dx.doi.org/10.1002/term.136
- Rosenfeldt H, Grinnell F. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem 2000; 275:3088-92; PMID:10652290; http://dx.doi.org/10.1074/jbc.275.5.3088
- Fringer J, Grinnell F. Fibroblast quiescence in floating or released collagen matrices. Contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem 2001; 276:31047-52; PMID:11410588; http://dx.doi.org/10.1074/jbc.M101898200
- Carlson MA, Smith LM, Cordes CM, Chao J, Eudy JD. Attachment-regulated signaling networks in the fibroblast-populated 3D collagen matrix. Sci Rep 2013; 3:1880; PMID:23697962; http://dx.doi.org/10.1038/srep01880
- Ivarsson M, McWhirter A, Borg TK, Rubin K. Type I collagen synthesis in cultured human fibroblasts: regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers. Matrix Biol 1998; 16:409-25; PMID:9524361; http://dx.doi.org/10.1016/S0945-053X(98)90014-2
- Le J, Rattner A, Chepda T, Frey J, Chamson A. Production of matrix metalloproteinase 2 in fibroblast reaction to mechanical stress in a collagen gel. Arch Dermatol Res 2002; 294:405-10; PMID:12522578
- Trächslin J, Koch M, Chiquet M. Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp Cell Res 1999; 247:320-8; http://dx.doi.org/10.1006/excr.1998.4363
- Eckes B, Mauch C, Hüppe G, Krieg T. Downregulation of collagen synthesis in fibroblasts within three-dimensional collagen lattices involves transcriptional and posttranscriptional mechanisms. FEBS Lett 1993; 318:129-33; PMID:8440370; http://dx.doi.org/10.1016/0014-5793(93)80006-G
- Gillery P, Leperre A, Coustry F, Maquart F-X, Borel J-P. Different regulation of collagen I transcription in three-dimensional lattice cultures. FEBS Lett 1992; 296:297-9; PMID:1537408; http://dx.doi.org/10.1016/0014-5793(92)80308-4
- Arora PD, McCulloch CAG. Dependence of collagen remodelling on α-smooth muscle actin expression by fibroblasts. J Cell Physiol 1994; 159:161-75; PMID:8138584; http://dx.doi.org/10.1002/jcp.1041590120
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001; 12:2730-41; PMID:11553712; http://dx.doi.org/10.1091/mbc.12.9.2730
- Grinnell F, Ho C-H, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell 2003; 14:384-95; PMID:12589041; http://dx.doi.org/10.1091/mbc.E02-08-0493
- Rhee S, Jiang H, Ho C-H, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. PNAS 2007; 104:5425-30; PMID:17369366; http://dx.doi.org/10.1073/pnas.0608030104
- Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, Rennard SI. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol-Anim 2001; 37:10-16; PMID:11249200; http://dx.doi.org/10.1290/1071-2690(2001)037%3c0010:COFCCG%3e2.0.CO;2
- Grinnell F, Zhu M, Carlson MA, Abrams JM. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res 1999; 248:608-19; PMID:10222153; http://dx.doi.org/10.1006/excr.1999.4440
- Fluck J, Querfeld C, Cremer A, Niland S, Krieg T, Sollberg S. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol 1998; 110:153-7; PMID:9457911; http://dx.doi.org/10.1046/j.1523-1747.1998.00095.x
- Fringer J, Grinnell F. Fibroblast quiescence in floating collagen matrices. Decrease in serum activation of MEK and Raf but not Ras. J Biol Chem 2003; 278:20612-7; PMID:12663662; http://dx.doi.org/10.1074/jbc.M212365200
- Gillery P, Georges N, Wegrowski J, Randoux A, Borel J-P. Protein synthesis in collagen lattice-cultured fibroblast is controlled at the ribosomal level. FEBS Lett 1995; 357:287-9; PMID:7835429; http://dx.doi.org/10.1016/0014-5793(94)01375-B
- Varedi M, Tredget EE, Ghahary A, Scott PG. Stress-relaxation and contraction of a collagen matrix induces expression of TGF-β and triggers apoptosis in dermal fibroblasts. Biochem Cell Biol 2000; 78:427-36; PMID:11012081; http://dx.doi.org/10.1139/o00-014
- Rosenfeldt H, Lee DJ, Grinnell F. Increased c-fos mRNA expression by human fibroblasts contracting stressed collagen matrices. Mol Cell Biol 1998; 18:2659-67.
- Schiro JA, Chan BMC, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin α2β1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell 1991; 67:403-10; PMID:1913826; http://dx.doi.org/10.1016/0092-8674(91)90191-Z
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by α2β1A and αvβ3 integrins. J Cell Sci 2000; 113:2375-83; PMID:10852817
- Niland S, Cremer A, Fluck J, Eble JA, Krieg T, Sollberg S. Contraction-dependent apoptosis of normal dermal fibroblasts. J Invest Dermatol 2001; 116:686-92; PMID:11348456; http://dx.doi.org/10.1046/j.1523-1747.2001.01342.x
- Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin α11β1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem 2010; 285:10434-45; PMID:20129924; http://dx.doi.org/10.1074/jbc.M109.078766
- Racine-Samson L, Rockey DC, Bissell DM. The role of α1β1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem 1997; 272:30911-7; PMID:9388237; http://dx.doi.org/10.1074/jbc.272.49.30911
- Xu J, Zutter MM, Santoro SA, Clark RAF. A three-dimensional collagen lattice activates NF-κB in human fibroblasts: role of integrin α2 gene expression and tissue remodeling. J Cell Biol 1998; 140:709-19; PMID:9456329; http://dx.doi.org/10.1083/jcb.140.3.709
- Langholz O, Röckel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol 1995; 131:1903-15; PMID:8557756; http://dx.doi.org/10.1083/jcb.131.6.1903
- Berendsen AD, Bronckers ALJJ, Smit TH, Wallboomers XF, Everts V. Collagen type V enhances matrix contraction by human periodontal ligament fibroblasts seeded in three-dimensional collagen gels. Matrix Biol 2006; 25:515-22; PMID:16973341; http://dx.doi.org/10.1016/j.matbio.2006.07.006
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 1999; 112:263-72; PMID:9885280
- Tian B, Lessan K, Kahm J, Kleidon J, Henke C. β1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem 2002; 277:24667-75; PMID:11986332; http://dx.doi.org/10.1074/jbc.M203565200
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 2000; 14:2123-33; PMID:10970876; http://dx.doi.org/10.1101/gad.815400
- Emonard H, Christiane Y, Munaut C, Foidart JM. Reconstituted basement membrane matrix stimulates interstitial procollagenase synthesis by human fibroblasts in culture. Matrix 1990; 10:373-7; PMID:1964716; http://dx.doi.org/10.1016/S0934-8832(11)80144-7
- Lambert CA, Colidge AC, Munaut C, Lapiere CM, Nusgens BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol 2001; 20:397-408; PMID:11691580; http://dx.doi.org/10.1016/S0945-053X(01)00156-1
- Karamichos D, Brown RA, Mudera V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res 2007; 83A:887-94; http://dx.doi.org/10.1002/jbm.a.31423
- Théret N, Lehti K, Musso O, Clément B. MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology 1999; 30:462-8; http://dx.doi.org/10.1002/hep.510300236
- Wang D-R, Sato M, Li L-N, Miura M, Kojima N, Sanoo H. Stimulation of pro-MMP-2 production and activation by native form of extracellular type I collagen in cultured hepatic stellate cells. Cell Struct Funct 2003; 28:505-13; PMID:15004420; http://dx.doi.org/10.1247/csf.28.505
- Préaux A-M, Mallat A, Van Nhieu JT, D’Ortho M-P, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology 1999; 30:944-50; http://dx.doi.org/10.1002/hep.510300432
- Von den Hoff JW. Effects of mechanical tension on matrix degradation by human periodontal ligament cells cultured in collagen gels. J Periodont Res 2003; 38:449-57; PMID:12941067; http://dx.doi.org/10.1034/j.1600-0765.2003.00404.x
- Li Y-L, Sato M, Kojima N, Miura M, Senoo H. Regulatory role of extracellular matrix components in expression of matrix metalloproteinases in cultured hepatic stellate cells. Cell Struct Funct 1999; 24:255-61; PMID:15216880; http://dx.doi.org/10.1247/csf.24.255
- Phillips JA, Bonassar LJ. Matrix metalloproteinase activity synergizes with α2β1 integrins to enhance collagen remodeling. Exp Cell Res 2005; 310:79-87; PMID:16098964; http://dx.doi.org/10.1016/j.yexcr.2005.03.039
- Scott KA, Wood EJ, Karran EH. A matrix metallopoteinase inhibitor which prevents fibroblast-mediated collagen lattice contraction. FEBS Lett 1998; 441:137-40; PMID:9877181; http://dx.doi.org/10.1016/S0014-5793(98)01542-7
- Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci 2003; 44:1104-10; PMID:12601036; http://dx.doi.org/10.1167/iovs.02-0412
- Pins GD, Collins-Pavao ME, Van De Water L, Yarmush ML, Morgan JR. Plasmin triggers rapid contraction and degradation of fibroblast-populated collagen lattices. J Invest Dermatol 2000; 114:647-53; PMID:10733668; http://dx.doi.org/10.1046/j.1523-1747.2000.00858.x
- Bott K, Upton Z, Schrobback K, Ehrbat M, Hubbell JA, Lutolf MP, Rizzi SC. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials 2010; 31:8454-64; PMID:20684983; http://dx.doi.org/10.1016/j.biomaterials.2010.07.046
- Znoyko I, Trojanowska M, Reuben A. Collagen binding α2β1 and α1β1 integrins play contrasting roles in regulation of Ets-1 expression in human liver myofibroblasts. Mol Cell Biochem 2006; 282:89-99; PMID:16317516; http://dx.doi.org/10.1007/s11010-006-1400-0
- Barczyk MM, Lu N, Popova SN, Bolstad AI, Gullberg D. α11β1 integrin-mediated MMP-13-dependent collagen lattice contraction by fibroblasts: evidence for integrin-coordinated collagen proteolysis. J Cell Physiol 2013; 228:1108-19; PMID:23065814; http://dx.doi.org/10.1002/jcp.24261
- Ravanti L, Heino J, López-Otín C, Kähäri V-M. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 1999; 274:2446-55; PMID:9891015; http://dx.doi.org/10.1074/jbc.274.4.2446
- Xu J, Clark RAF. A three-dimensional collagen lattice induces protein kinase C-ξ activity: role in α2 integrin and collagenase mRNA expression. J Cell Biol 1997; 136:473-83; PMID:9015316; http://dx.doi.org/10.1083/jcb.136.2.473
- Takahara T, Zhang LP, Yata Y, Xue F, Minemura M, Sato H, Watanabe A. Modulation of matrix metalloproteinase-9 in hepatic stellate cells by three-dimensional type I collagen: its activation and signaling pathway. Hepatol Res 2003; 26:318-26; PMID:12963432; http://dx.doi.org/10.1016/S1386-6346(03)00169-4
- Takahara T, Smart DE, Oakley F, Mann DA. Induction of myofibroblast MMP-9 transcription in three-dimensional collagen I cultures: regulation by NF-κB, AP-1 and Sp1. Int J Biochem Cell Biol 2004; 36:353-63; PMID:14643899; http://dx.doi.org/10.1016/S1357-2725(03)00260-7
- Gillery P, Maquart F-X, Borel J-P. Fibronectin dependence of the contraction of collagen lattices by human skin fibroblasts. Exp Cell Res 1986; 167:29-37; PMID:3758208; http://dx.doi.org/10.1016/0014-4827(86)90201-6
- Taliana L, Evans MDM, Dimitrijevich SD, Steele JG. Vitronectin or fibronectin is required for corneal fibroblast-seeded collagen gel contraction. Invest Ophthalmol Vis Sci 2000; 41:103-9; PMID:10634608
- Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem 2000; 275:10673-82; PMID:10744764; http://dx.doi.org/10.1074/jbc.275.14.10673
- Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly. Exp Cell Res 1995; 217:109-17; PMID:7867709; http://dx.doi.org/10.1006/excr.1995.1069
- Doillon CJ, Silver FH, Berg RA. Fibroblast growth on a porous collagen sponge containing hyaluronic acid and fibronectin. Biomaterials 1987; 8:195-200; PMID:3607152; http://dx.doi.org/10.1016/0142-9612(87)90063-9
- Nakamura Y, Sagara T, Seki K, Hirano S, Nishida T. Permissive effect of fibronectin on collagen gel contraction mediated by bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci 2003; 44:4331-6; PMID:14507877; http://dx.doi.org/10.1167/iovs.03-0068
- Liu Y, Yanai R, Lu Y, Kimura K, Nishida T. Promotion by fibronection of collagen gel contraction mediated by human corneal fibroblasts. Exp Eye Res 2006; 83:1196-204; PMID:16914141; http://dx.doi.org/10.1016/j.exer.2006.06.008
- da Rocha-Azevedo B, Ho C-H, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res 2013; 319:546-55; PMID:23117111; http://dx.doi.org/10.1016/j.yexcr.2012.10.005
- Sethi KK, Yannis IV, Mudera V, Eastwood M, McFarland C, Brown RA. Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Repair Regen 2002; 10:397-408; PMID:12453144; http://dx.doi.org/10.1046/j.1524-475X.2002.10609.x
- Asaga H, Kikuchi S, Yoshizato K. Collagen gel contraction by fibroblasts requires cellular fibronectin but not plasma fibronectin. Exp Cell Res 1991; 167-74; PMID:1995291; http://dx.doi.org/10.1016/0014-4827(91)90552-6
- Yoshizato K, Tsukahara M, Oki T, Hayashi M, Morpho MOY. The interaction of cellular fibronectin with collagen during fibroblast-mediated contraction of collagen gels. J Invest Dermatol Symp Proc 1999; 4:190-5; http://dx.doi.org/10.1038/sj.jidsp.5640207
- Mochitate K, Pawelek P, Grinnell F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res 1991; 193:198-207; PMID:1995294; http://dx.doi.org/10.1016/0014-4827(91)90556-A
- Sevilla CA, Dalecki D, Hocking DC. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng Part A 2010; 16:3805-19; PMID:20673131; http://dx.doi.org/10.1089/ten.tea.2010.0316
