 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Directed cell migration is a crucial orchestrated process in embryonic development, wound healing, and immune response. The underlying substrate can provide physical and/or chemical cues that promote directed cell migration. Here, using electrospinning we developed substrates of aligned poly(lactic-co-glycolic acid) nanofibres to study the influence of glial cells on endothelial cells (ECs) in a 3-dimensional (3D) co-culture model. ECs build blood vessels and regulate their plasticity in coordination with neurons. Likewise, neurons construct nerves and regulate their circuits in coordination with ECs. In our model, the neuro-vascular cross-talk was assessed using a direct co-culture model of human umbilical vein endothelial cells (HUVECs) and rat Schwann cells (rSCs). The effect of rSCs on ECs behavior was demonstrated by earlier and higher velocity values and genetic expression profiles different of those of HUVECs when seeded alone. We observed 2 different gene expression trends in the co-culture models: (i) a later gene expression of angiogenic factors, such as interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF), and (ii) an higher gene expression of genes involved in actin filaments rearrangement, such as focal adhesion kinase (FAK), Mitogen-activated protein kinase-activated protein kinase 13 (MAPKAPK13), Vinculin (VCL), and Profilin (PROF). These results suggested that the higher ECs migration is mainly due to proteins involved in the actin filaments rearrangement and in the directed cell migration rather than the effect of angiogenic factors. This co-culture model provides an approach to enlighten the neurovascular interactions, with particular focus on endothelial cell migration.
Abbreviations
| HUVECs | = | human umbilical vein endothelial cells |
| rSCs | = | rat Schwann cells |
| ECs | = | endothelial cells |
| SCs | = | Schwann cells |
| IL-8 | = | interleukin-8 |
| VEGF | = | vascular endothelial growth factor |
| FAK | = | focal adhesion kinase |
| MAPKAPK13 | = | mitogen-activated protein kinase-activated protein kinase 13 |
| ECM | = | extracellular matrix |
| VCL | = | vinculin |
| PROF | = | profilin |
| FGFR3 | = | fibroblasts growth factor receptor-3 |
| CDC42 | = | cell division control 42 |
| PLGA | = | poly(lactic-co-glycolic acid). |
Introduction
During the development stage, blood vessels are formed in a closely coordinated manner with nerves, constructing closed circuits of neurovascular networks.Citation1-3 The vascular and neural systems mutually regulate their plasticity and affect pattern formation of their networks.Citation2,4 In response to their local need, blood vessels can modulate neuronal activities, and vice versa, nerves can guide changes in blood vessel patterns by branching, expanding, and pruning. The development of the neural and vascular systems occurs in similar and parallel patterns through branching, coupling, and guidance within target tissues.Citation2 The anatomical similarities and parallelism between the 2 systems are evident and likely due to the remarkable degree of cellular coordination, resulting in the development and maintenance of blood vessels and nerves as a functional neurovascular network.Citation2,3 ECs, neural, and glial cells respond to changing environment producing a wide panel of growth factors and their receptors, including angiogenic factors. These protein pairs transduce cellular information causing ECs and neural cells to proliferate, migrate and differentiate.Citation3 For example, neural cells are known to express angiogenic factors, such as VEGF that induces proliferation of ECs through its receptors. Conversely, ECs produce brain-derived neurotrophic factor (BDNF) that stimulate neurite outgrowth.Citation4 Other angiogenic factors generated from neurons and glia have been documented to affect ECs and neuronal cells behavior ().Citation5,6 With cell migration being a central step in angiogenesis, here we tailored aligned fibrous substrates that potentiate directed ECs migration.Citation7-9 Directional control of cell migration is critical to developmental morphogenesis and tissue homeostasis.Citation10 Several observations suggest that cells are capable of responding to mechanical and/or chemical cues from the substrates, by exerting contractile forces and then interpreting the substrate deformation to determine a preferred direction of their movement.Citation11-13
Table 1. Effect of angiogenic factors on the ECs.
Schwann cells (SCs) are the main glia of the peripheral nervous system, their processes are important for the elongation and guidance of regenerating axonsCitation14 and are known to produce neurotrophic factors and their receptors.Citation15 Gerber et al.Citation4 and Mukouyama et al.Citation16 showed that VEGF-related factors derived from SCs can induce arterial differentiation in ECs precursors, suggesting that peripheral nerves determine the pattern of blood vessel branching and arterial differentiation. On the other hand, VEGF prolongs the survival and stimulates the proliferation of SCs in explant cultures of superior cervical and dorsal root ganglia.Citation17 Gerber et al.Citation4 and Bates et al.Citation18 suggested that neuronal cells can induce ECs into arteriole differentiation and promote a specific vascular pattern. The crosstalk between SCs and ECs has been assessed, in most cases, by the influence of SCs in neuroblastoma angiogenesis. Huang et al.Citation19 showed that conditioned medium from SCs, derived from either adult nerve tissue or stroma-dominant neuroblastoma tumor tissue, inhibits ECs proliferation and migration. Other studies also showed the production of inhibitors of angiogenesis such as TIMP-2, by SCs.Citation20,21 More recent studies, using co-culture models of glioblastoma and ECs, suggested an increased activity of metalloproteinases (MMP-9) involved in vascular cell migration and invasion.Citation22 Gliobastomas are known to produce large amounts of basic fibroblast growth factor and VEGF, which may act to mediate the paracrine control of angiogenesis.Citation23,24
Several biomaterials have been investigated for vascular tissue engineering. Among these, poly(lactic-co-glycolic acid) (PLGA) scaffolds can provide a degradable structure and topographical cues to promote cell migration, attachment, proliferation, and tissue regeneration.Citation25 Here, ECs migration in response to SC co-culture on aligned PLGA electrospun fibers was used to determine the overall in vitro angiogenic potential of endothelial-Schwann cells co-culture. Migration studies and gene expression analysis were performed to identify which genes are activated in this process.
Results
Scaffold characterization
It is well established that, in the living system, the extracellular matrix (ECM) microenvironmental properties can directly influence the intracellular regulatory mechanisms that govern the migratory phenotype and determine how cell migration proceeds.Citation26 It was reported that the cells attach and organize well around fibers with diameters smaller than the diameter of the cells.Citation27 Therefore, to create an ideal scaffold which serves as an artificial ECM for tissue regeneration, it is crucial to replicate the dimensions of natural ECM. When cells are seeded on fibrous substrates, the fiber diameter plays an important role in cell morphology, particularly in cell polarization. The fabricated scaffolds were characterized in terms of their morphology and fiber diameter, which were mainly dependent on the polymer solution, flow rate, concentration, solvent used, and applied electric field. The relationship between the applied voltage and the formation of polymer fibers with a consistent morphology has already been extensively reported by previous studies.Citation28 As shown in , the produced substrates exhibited a good fiber alignment. The average fiber diameter was 473 ± 111 nm (addressed as electrospun (ESP) fibers onwards, ).
Figure 1. Imaging platform. After staining with a green fluorescent cell tracker and imaging, the original images are cropped into a region of interest (A) and converted into greyscale images (B). The images are then filtered using a Gaussian filter (C) and a 3-class threshold applied to distinguish the foreground from the background (D). The tissue assumed as foreground is measured in terms of its area and compared against the total area of the cropped image (E). The resulting images are then outlined (white line) and overlaid with the cropped image (F). Scale bar: 1 mm; x and y axxis in pixels
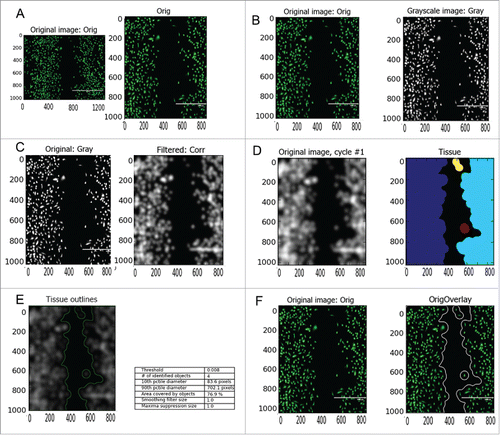
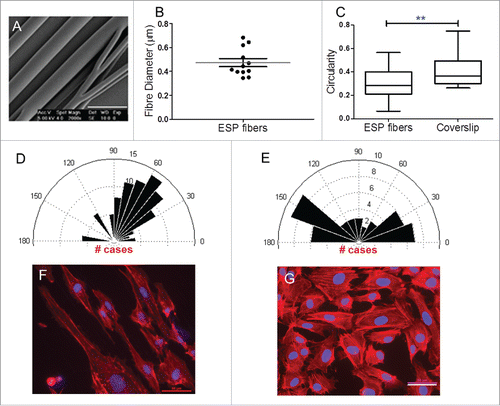
Figure 2. Scaffold characterization and cell morphology. (A) SEM micrographs of PLGA 50:50 ESP scaffolds showed a good fiber alignment (scale bar 1 µm). (B) Box diagram of fiber diameter (Whiskers Tukey, mean ± SD, n=12). (C) Cell circularity box diagram, cells seeded on fibrous substrates showed a more elongated morphology (1 is a perfect circle, while approaching 0 the cell become more elongated; unpaired t test ** P<0.05, mean ± SEM, n≥89 ). (D and E) Rose plot diagramsof HUVECs directionality response after one day in culture in fibers and coverslips, respectively (Rose plots are in degrees). (F and G) Immunofluorescence images show HUVECs morphology after one day in culture in ESP fibers and coverslips, respectively. The cells seeded in the fibrous substrates exhibited a more orientated alignment. (In red actin filaments are labeled using AlexaFluor 594 conjugated phalloidin, while in blue cell nuclei are labeled using DAPI, scale bar 50 µm). ESP: electrospun.
Cell morphology
The circularity value gives us an idea about how polarized the cells are. Values close to 0 suggest an elongated cell; values close to 1 suggest a circular cell. Here, HUVECs seeded on fibrous substrates exhibited lower values (0.30 ± 0.12) for circularity when compared with cells seeded on coverslips (0.43 ± 0.15), suggesting a more polarized and elongated morphology in those fibrous substrates (). Using aligned fibers we expected the cells to exhibit a higher alignment in accordance with the substrate underneath. As shown by the Rose plot diagram the majority of cells exhibited a confined alignment between 30 and 90 degrees (). In contrast when seeded on coverslips, cells exhibited a scattered alignment in several directions ().
Cell growth on scaffolds – cell viability
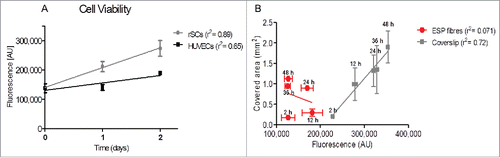
Cell viability was determined using a PrestoBlue® assay. As shown in , rSCs exhibited higher fluorescence values over time, with fluorescence increasing at higher rates (higher values of slope, m = 67690) when compared with HUVECs (m = 25280) seeded in single culture models. Besides their viability, an interesting correlation that can be made is the influence of cell viability on cell migration. As illustrated in , the percentage of covered area by HUVECs in single culture models seemed not to vary significantly with cell viability: low value of slope (m=-4.E-6), not significantly different from zero (p=0.45), and negligible value of r2 (0.071) suggested a poor dependence between cell viability and covered area. In contrast, HUVECs seeded on coverslips exhibited higher values of slope (m=1.2E-5), significantly different from zero (p < 0.0001), and higher values of r2 (0.72), suggesting a higher dependence between cell viability and covered area (cell migration).
Figure 3. Analysis of cell viability by PrestoBlue ® (A) Each single culture model was individually tested for their viability potential. rSCs have a higher viability rate when compared to HUVECs. (B) For each time-point used in the migration studies the viability potential of the HUVECs single culture model was measured. Viability is plotted against the covered area. The percentage of covered area in fibrous substrates is mainly due to cell migration rather than increased cell viability. (mean ± SD, n = 3, linear regression). ESP: electrospun.
Effect of rSCs on HUVECs migration
It is well established that there is a cross-talk between the neural and vascular systems. Besides anatomical similarities, there are several angiogenic factors/receptors that affect both systems. Few studies have particularly focused on the role of IL-8 and VEGF.Citation29,30 However, to the best of our knowledge, none of previous studies have addressed how SCs affect the migration and the gene expression profile of ECs. Here, in order to assess the rSCs influence on HUVECs' migration, 4 co-culture ratios were chosen, namely 0%, 5%, 10%, 20% of rSCs relatively to HUVECs (rSCs:HUVECs), using a PLGA 50:50 nanofibrillar substrate, with fibers having an approximate diameter of 500 nm.
The covered area represents the displacement of the cell sheet to close the wound. The covered area after 48 hours changed from 1.12 ± 0.035 mm2 to 1.64 ± 0.19 mm2, when increasing the co-culture ratio from 0% to 5%, to decrease again to 1.13 ± 0.35 mm2 when the co-culture ratio was further increased to 20%. The covered area over time for 5% co-culture ratio was always higher than the other culture conditions, though not statistically different (p > 0.05, exception made at 24 hours where p < 0.05 for 5% versus 10%) ().
Figure 4. Effect of rSCs on HUVECs migration. Co-culture models with low ratios of rSCs have a positive effect on HUVECs migration, with increased percentage of covered area over time and higher values of cell migration speed. (A) Wound closure over time. The 5% ratio is the one that showed a higher covered area over time, with higher displacement of the cell sheet (One-way ANOVA, mean ± SD, n ≥ 2 # p < 0.05 (5% versus 10 %)). (B) Velocity profile of HUVECs using several rSCs:HUVECs ratios (One-way ANOVA, mean ± SD, n≥2 , second order polynomial (qudratic), #. p < 0.001 (5% vs. 20 %), # p < 0.05 (0% versus 20 % and 0% vs. 20 %), # p < 0.05 (10% versus 20 %)). (C) Results of the modified scratch wound healing assay; each color represent a different tissue that software will assume and measure as a cell sheet or single cell. The dashed gray vertical line represents the width of the initial gap. Scale bar 1 mm.
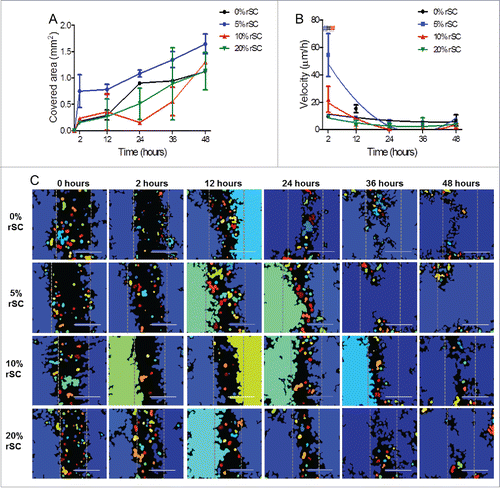
We observed that the average velocity of the cell front varied with the ratio rSCs:HUVECs, decreasing over time, with the highest value measured after 2 hours in all culture models: 9.54 ± 0.1 µm/h, 54.5 ± 15.74 µm/h, 22.17 ± 9.44 µm/h, 9.89 ± 0.48 µm/h for 0%, 5%, 10%, 20% co-culture ratios respectively. The 5% rSCs:HUVECs ratio clearly increased the initial velocity of HUVECs. Cell migration speed in this condition was statistically different when compared to almost all migration speed values in the other culture models, with exception made for the 10% rSCs:HUVECs ratio. When seeded alone, the speed of HUVECs migration increased until 12 hours, when it reached a peak of 15.29 ± 2.71 µm/h (). The displacement of the cell sheet is in accordance with results above stated, with the 5% ratio exhibiting higher values of displacement over time ().
Gene expression – qPCR
From a wide panel of angiogenic factors/receptors, we selected 3 well-known cell migration inducers (IL-8, FGFR3, and VEGF)Citation29-31; 2 proteins known to be involved in actin filaments dynamic turnover (PROF) and in linkage of integrin adhesion molecules to the actin cytoskeleton (VCL)Citation32; 2 protein kinases (FAK and MAPK) and a small GTPase of the Rho-subfamily that control diverse cellular functions including cell morphology and migration, as well as FAK phosphorylation (CDC42).Citation33-35 The gene expression of different cell migration effectors was analyzed using 5% rSCs:HUVECs co-culture condition compared to HUVECs alone, in the already stated scaffolds.
Angiogenic factors
The genetic analysis results for IL-8 showed a delay of 10 hours in the peak of gene expression in the co-culture model. In both models, the peak (7 and 8-fold increase in co-culture at 2 hours and single culture models at 12 hours, respectively) was followed by a decrease in fold induction up to 36 hours, when a slightly up-regulation was observed until 48 hours that corresponded to 3 and 2 fold increase in co-culture and single culture models, respectively ()
Figure 5. Influence of rSCs on angiogenic factors' gene expression expressed by HUVECs. The later up-regulation of IL-8 and VEGF in co-culture models seems to be insufficient to decrease HUVECs migration, being masked by significant higher expression of other genes involved in the actin filament rearrangement. PLGA 50:50 ESP fibers used as substrate. (Fold increase was calculated using ΔΔCt method, mean ± SD, n = 3, One-way ANOVA Tukey's multiple comparison Test, *p < 0.05, **. p < 0.01, *** p < 0.001, comparisons between single culture model vs. co-culture model).
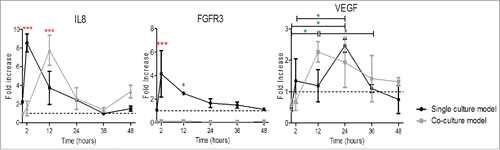
Regarding FGFR3, a similar trend was found for HUVECs cultured alone when compared to IL-8 gene expression, with an increase in gene expression until 2 hours (4 fold increase) followed by a decrease in gene expression until 48 hours. However, in the co-culture condition no significant changes in the FGFR3 could be appreciated ().
The co-culture model appeared to induce VEGF expression at earlier time points when compared to single culture models. In both culture models, a peak at 12 hours in the co-culture condition (2 fold increase) and at 24 hours in the single culture condition (2 fold increase) was measured, followed by a decrease in gene expression until 48 hours ().
Actin filaments rearrangement
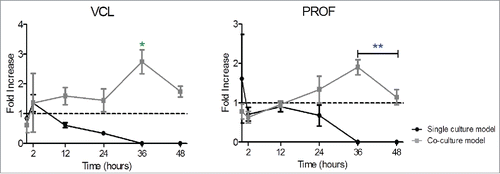
The VCL and PROF genes exhibited a similar trend for gene expression. A slight upregulation was verified at 2 hours in VCL gene expression, followed by a continuous decrease in gene expression for single culture conditions until 48 hours. In co-culture models, a small upregulation of VCL (2 fold increase) was followed by a relative constant gene expression until 24 hours. Whereas, a continuous up-regulation was exhibited up to 36 hours in PROF gene expression. In both gene expression profiles a considerable peak (3 fold increase and 2 fold increase in VCL and PROF respectively, p < 0.05) at 36 hours was verified in the co-culture conditions ().
Figure 6. Influence of rSCs on actin turnover proteins' gene expression expressed by HUVECs. In both graphics is exhibited a constant increase in gene expression in the co-culture model, peaking at 36 hours, suggesting a higher actin filaments rearrangement in those culture models. The setup used was the same as previously stated (Fold increase calculated using ΔΔCt method, mean ± SD, n = 3, One-way ANOVA Tukey's multiple comparison Test, *p < 0.05, **. p < 0.01, *** p < 0.001, comparisons between single culture model versus co-culture model).
Protein kinases
MAPK displayed a down regulation in single culture models after 0 hours followed by a plateau with minor oscillations in gene expression. Co-culture conditions exhibited a progressive statistically significant upregulation over time, peaking at 36 hours (7 fold increase) and followed by a down-regulation in gene expression until 48 hours ().
Figure 7. Influence of rSCs on protein kinases' gene expression expressed by HUVECs. The constant upregulation of these protein kinases suggested an increased substrate phosphorylation and an enhanced directed cell migration in co-culture models. The setup used was the same as previously stated. (Fold increase calculated using ΔΔCt method, mean ± SD, n = 3, One-way ANOVA Tukey's multiple comparison Test, *p < 0.05, **. p < 0.01, *** p < 0.001, comparisons between single culture model vs. co-culture model). Note: non-significant differences were observed for FAK between 48 hours versus 2 hours.
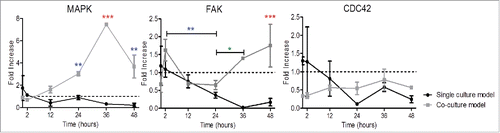
FAK expression was continuously down-regulated in single culture condition up to 36 hours, followed by a slight increase in gene expression. In co-culture conditions, the initial downregulation until 24 hours was rapidly counterbalanced. FAK expression was then constantly increased up to 48 hours (2 fold increase) ().
Regarding CDC42 there was a slight down regulation followed by a plateau over time, with minor oscillations in both culture models ().
Discussion
HUVEC morphology
Cell migration is usually initiated with cell morphology rearrangements in response to extracellular cues, including physical (mechanotaxis) and/or chemical (chemotaxis) cues from the extracellular matrix, and/or diffusible factors and signals from neighboring cells.Citation36-39 Among all the extracellular cues, the fiber diameter of the underneath substrate seems to play a crucial role on cell morphology. The effect of cell morphology on cell migration has been extensively studied.Citation40 The first step in cell migration is the achievement of direction through the establishment of cell polarity.Citation41
When seeded in constraint-free surfaces, the cells exhibited multiple lateral lamellipodia, leading to an area augmentation, and subsequently an increase in cell circularity. In contrast, cell association with aligned fibers seems to provide an important physical cue to initiate cell polarization, by regulating cell shape and orientation of cellular organelles, which results in a decreased cell circularity. Consequently, the fiber alignment per se is a geometrical cue that influences the cell alignment into a restricted direction, the fiber direction.
Cell growth on scaffolds – cell viability
Cell viability in polymeric substrates is a crucial parameter that was taken into account in the process of scaffold manufacturing. As shown in , the correlation between cell viability (an indirect count of cell number) and cell migration seems to be poorer when cells were seeded in ESP fibers (r2=0.071). Consequently, the covered area observed on our substrates seemed to be caused mainly by cell migration and not due to an increase in cell number, suggesting that the migration speed was enhanced by the directionality in aligned substrates, as already shown by Doyle et al.Citation8,26 and Provenzano et al.Citation42 Because the cells were kept in culture for 48 hours before removing the metal strip, and due to the higher viability of cells in coverslips, the fluorescence values in those conditions were higher than in fibrous substrates prior to the migration experiment per se.
Effect of rSCs on HUVECs migration
Recent studies demonstrated a positive effect of neural cells on ECs in co-culture. Ford et al.Citation43,44, seeding ECs and neural progenitor cells (NPCs) together, showed a 3 to 5-fold increase in the number of functional blood vessels. Using the same culture model, Li et al.Citation45 showed that NPCs play an important role in forming stable bold vessels. Some investigations attempted to correlate the influence of neural cells on angiogenesis or the effect of ECs on neurogenesis.Citation44-46
In our co-culture 3D models, the covered area in 5% ratios samples after 48 hours was superior to single HUVECs culture, suggesting a positive effect of glial cells on ECs migration. Interestingly, in the 5% ratio condition an increase in the initial HUVECs migration speed could also be measured. The rSCs exhibited a higher proliferative rate () when compared with HUVECs, which could lead to an overgrowth of glial cells over endothelial cells, reducing the space for endothelial cells to migrate. This could lead to the observed decreased value in migration speed of HUVECs when seeded together with high ratios of rSCs.
Among the genes analyzed, IL-8,Citation47,48 FGFR3,Citation31 and VEGFCitation49 are known to be a potent angiogenic factors, implicated in EC proliferation and migration both in vivo and in vitro. Thus, their up-regulation at early time points might act as a trigger signal to cells to start migrating. Furthermore, it has been shown that VEGF in mitogenic concentrations induced stress fiber formation and recruitment of vinculin to focal adhesions in a pattern that is typical of migrating ECs, where well-formed focal adhesions and stress fibers aligned in the direction of migration were described.Citation49
The complex formed by vinculin and profilin will serve as an anchorage point to actin filaments. The peak observed at 36 hours suggested the formation of strong focal adhesion complexes and, therefore, the end of cell migration. In fact, the recruitment of vinculin to focal adhesion sites has been shown to have a role in strengthening the adhesion between cells and ECM, while reduction of adhesion and increased migration occur with down-regulation of vinculin.Citation32 We did not expect downregulation of PROF at earlier time points, since during cell migration the turn-over and the restriction of the actin cytoskeleton are constant.
Rousseau et al.Citation50 showed that MAPK is not an obligatory regulator of cell migration, but seems to be associated with the directionality of cell migration.Citation35 Other studies showed that MAPKs regulate the expression of metalloproteinases. Those proteins are crucial for the cleavage of basement membrane and subsequent EC migration.Citation22 The increased upregulation in co-culture models suggests a more directed cell migration toward the denuded gap, resulting in cells completely closing the gap and stop migrating earlier in these co-culture models. Here, at early time points and mainly in co-culture models, we observed a slight up regulation of FAK suggesting an increased focal adhesion turnover. Similarly, after 36 hours, when the gap closure was nearly completed, an increase in FAK gene expression was measured, suggesting an increase in focal adhesion turnover, yet in this case in order to form strong and mature adhesion complexes.
The inorganic phosphate, resulted from the hydrolysis of the GTP molecule into GDP and Pi inorganic phosphate by CDC42,Citation51-53 can be used in phosphorylation mechanisms, particularly in FAK phosphorylation. In fact, the lower gene expression of this gene in our co-culture models at earlier time points suggests that the focal adhesion complexes, dependent on the phosphorylation of several proteins, appeared to be smaller and more unstable.
Summarizing, the enhanced cell migration observed in co-culture models can be explained by the upregulation of FAK at 2 hours, and IL-8, FGFR3 and VEGF at 12 hours. The later upregulation of VEGF and IL-8 expression in our co-culture model seems to be insufficient to decrease cell migration speed, being "masked" by the significant upregulation of other genes at earlier time points (2 hours). Another interesting result is the constant increased gene expression of Profilin, and MAPK in co-culture models, in contrast with a continuous decrease over time in single culture models. The increased gene expression of Profilin, FAK, VCL and MAPK suggest a pronounced turn-over in actin filaments rearrangements, intrinsic in cell migration. In particular, FAK and MAPK activity suggested an increase in substrate phosphorylation, with an enhanced phosphate group transfer, which will enhance not only the cell migration but the directed cell migration as well.
Materials and Methods
Scaffold fabrication
Scaffolds were manufactured from poly(lactic-co-glycolic acid) with 50:50 ratio between lactic and glycolic acid segments (PLGA 50:50, cat. Number PURASORB PDLG 5002, Corbion Purac Biomaterials). The polymer was dissolved in 1,1,1,3,3,3-Hexafluoro-2-propanol (HFiP, cat. Number 8335101, BioSolve BV) overnight at a final concentration of 25% (wt/v) and then electrospun using a standard electrospinning apparatus in a chamber with environmental control (25˚C, 30% humidity) using a parallel plate collector to obtain aligned fibers .
The electrospun fibers started to occur around 15 kV, but a more stable jet appeared to be at 17.5 kV. Increasing the voltage resulted on higher electrostatic forces on the apparatus and instability of the jet, which would slip and splay.Citation54 The parameter to adjust the voltage was based on the instability of the Taylor cone. The advantage of using this technique lies in the simplicity of the setup and the ease of collecting single fibers. The air gap between the electrodes created residual electrostatic repulsion between the spun fibers, which helped the alignment of the fibers.Citation54
For all the experiments, the voltage was kept constant at 17.5 kV, the flow rate 1.5 mL/h, and the polymer was pushed out the syringe for 12 minutes. A 14 mm diameter coverslip (thickness 0.13–0.16mm, cat. Number: GG-14-pll, neuVitro) was used as a collector.
Scaffold characterization
Scaffolds fiber morphology and diameter were characterized by scanning electron microscopy (SEM) (Philips XL 30 ESEM-FEM). Samples were attached to stubs using double sided carbon tape and gold sputter coated before acquiring images. The fiber diameter was calculated from 12 images using a semi-automated script form ImageJ (National Institutes of Health, Bethesda, MD). Briefly, a Li threshold method was applied to the images followed by the identification of distinctly segmented fibers by the user. The diameter was approximated by creating a selection of the identified fiber length using the Level Set segmentation plugin, following by manual check and adjustment to ensure good approximation of the fiber diameter.
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) (cat. Number: C2517A, Lonza, passage 4–8) were cultured in endothelial growth medium (EGM, cat. Number: CC-3162), which consisted of endothelial basic medium supplemented with (%v/v): 2% foetal bovine serum (FBS), 0.04 % hydrocortisone, 0.4% human fibroblasts growth factor B (hFGFB), 0.1% VEGF, 0.1% R3-Insulin-like Growth Factor-I (R3-IGF-1), 0.1% ascorbic acid, 0.1% human endothelial growth factor (hEGF), 0.1% Gentamicin/Amphotericin-B (GA-1000), and 0.1% heparin (all from Lonza).
Rat Schwann cells (rSCs), cell line RT4-D6P2T (ATCC), were cultured in Dulbecco's Modified Eagle Medium (DMEM, cat. Number: 41966–029) high glucose supplemented with (%v/v) 10% FBS (heat inactivated, cat. Number 10500–064, Life Technologies) and 1% Pen/Strep (Life technologies™). Cells were grown at 37˚C in a humid atmosphere with 5% CO2. The medium was refreshed every other day.
Previously sterilized scaffolds (soaked in 70% ethanol for 2 hours) were incubated in cell culture medium overnight. After media removal, a physical metal barrier was placed in the center of the scaffold, in order to create a gap for the cells to migrate into, and held using O-rings (Eriks, The Netherlands). Cells were then seeded at 2×104 cells/cm2 and returned to the incubator. Upon reaching confluence, the physical barrier was removed using sterile tweezers and the samples washed thrice with Phosphate buffered saline solution (PBS, cat. Number: 10010–023, Life Technologies). Fresh medium was added.
Co-culture
In order to evaluate the effect of rSCs on HUVECs' behavior and cell migration, a direct contact co-culture model was created using 3 cell ratios: 5%, 10% and 20% (%rSCs). The used medium ratio depended on cell ratio, where for example for a 5% cell ratio a correspondent 95%:5% HUVEC:rSC media (%v/v) ratio was used.
Imaging platform
The developed imaging platform is shown in . Briefly, after staining with cell tracker and imaging, the original images were cropped in order to all have the same width and height (). The cropped images were then converted into gray scale images (). The images were submitted to a blurred Gaussian filter twice (). The 4th step was the identification of primary objects (i.e. objects that were assumed as tissue) and measurements (). For this purpose, we used a 3-class thresholding method (Otsu global) in order to: (i) distinguish the background from the foreground and (ii) overcome the issue that the intensity pixel value varies throughout the image. The different colors mean different tissues that have been identified as foreground. The quantified area was the total area of all different tissues. The lasts 2 steps are the outlining of the identified primary objects (), and the overlay of that outlined image with the original cropped image. All the steps were done in an automated way.
Figure S1A shows the cell sheet displacement over time prior to the analysis. As the cells move onwards, the area of the denuded gap (green) is measured. From the difference between the gaps at different times resulted 2 new sections (blue). The area of these sections is summed and then divided by the height of the image and by the interval of time between 2 consecutive images, obtaining the cell velocity (Figure S1B).
Imaging platform – technical specifications
Three classes of thresholding allow defining 3 categories: foreground, background and a middle intensity between the 2. The middle intensity class will be added to the foreground in order to generate the final 2-class output. This thresholding method allows assigning a value to the foreground or background as desired. The threshold value ranges from 0 to 1 (foreground/background). This is a safety precaution when the threshold is calculated automatically, by overriding the automatic threshold. The low value suggests high differences between foreground and background.
A smoothing filter is used only when distinguishing between clumped objects is required. Low values should be used if many objects are merged together that ought to be separated. When too many objects, that have to be merged, are separated the values should be higher, as seen in our studies. Using the value 1, we "transform" the cell layers on each side of the gap as a cell sheet, whose migration would be only assessed toward the denuded gap and not the single cell migration within the cell layer.
Migration assay
Among several cell tracers, Cell Tracker™ Green CMFDA has extensively been used in studies of HUVECs' live cell tracking.Citation55,56 Moreover, the cytotoxicity of these probes have been studied by the supplier in many cell types, including HUVECs, which appears to exert a minimal impact on several cell processes including cell proliferation and motility.
Only HUVECs were treated with 10 µM Cell Tracker ™ Green CMFDA (Molecular Probes®, cat. Number: C7025) for 45 minutes at 37 ˚C. When stained, rSCs and ECs were cultured together, according to the previously mentioned ratios, at a total density 2×104 cells/cm2. When the physical barrier was removed, 3 images of each sample were taken (EVOS® XL microscope with 3MP CMOS color camera, cat. Number AME3300, with GFP LED light cube ex.470/22 EM: 525/50 cat. Number: AMEP4651, Life Technologies) at 0, 2, 12, 24, 36, and 48 hours using x10 lens (exposure time 500 ms, brightness 80%). The images were then analyzed using a basic Cell Profiler (Broad Institute) pipeline, developed by the authors.
The following equations were used to assess the percentage of covered area (Equationequation 1(1)
(1) ) and cell migration velocity (Equationequation 2
(2)
(2) ):
(1)
(1)
(2)
(2) Where
is the area of the initial gap,
is the area of the gap after
hours,
is the height of the image, and
is the time occurred between
and
.
Viability assay
Cell viability was assessed using PrestoBlue® assay according to the manufacturer's protocol (Life Technologies, cat. Number: A-13262). Briefly, 10% (v/v) of PrestoBlue® reagent was added in each well and the samples (n = 3) incubated at 37 ˚C for 2 hours. Three 100 µL media samples were taken from each well into a Nunc™ 96-well plate (Thermo Scientific, cat. Number: 267350). Fluorescence was measured at 540–570 nm excitation 580–610 nm emission in VICTOR3™ 1420 Multilabel Counter (PerkinElmer). The readout from the scaffolds was corrected with a blank (medium plus PrestoBlue® reagent).
PCR
To analyze the expression of migration markers by HUVECs, the total RNA was isolated using a combination of the TRIzol® method with the NucleoSpin® RNA II isolation kit (Bioké, cat. Number 740955.50). Briefly, scaffolds (n = 3) were washed once with PBS and 500 µl of TRIzol reagent (Invitrogen, cat. Number: 15596026) was added to the samples. After chloroform addition (200 µL) and phase separation by centrifugation (15 mins, 12000g, 4°C), the aqueous phase containing the RNA was collected, mixed with 250 µL of 70% ethanol and loaded onto the RNA binding column of the kit. Subsequent steps were done in accordance with the manufacturer's protocol. RNA was collected in RNase-free water. The quantity and quality of RNA was analyzed using an ND100 spectrophotometer (Nanodrop technologies). 139.5 ng of RNA were used for first strand cDNA synthesis using iScript (Bio-Rad) according to the manufacturer's protocol. One μL of undiluted cDNA was used for subsequent analysis. PCR was performed in an iQ5 real time PCR machine (Bio-Rad) using SYBR Green supermix (Bio-Rad) and fold increase calculated using ΔΔCt method.Citation57 Primer sequences are shown in .
Table 2. List of primers used.
Statistical analysis
A one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (unless otherwise specified) were used to determine statistically significant differences (GraphPad Prism, *p < 0.05, **p < 0.01, ***p < 0.001). Data are expressed as mean ± standard deviation (unless otherwise specified).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplemental data for this article can be accessed on the publisher's website.
Supplementary_file.pptx
Download MS Power Point (3.3 MB)Funding
This study was funded by the Dutch Ministry of Economic affairs and Province of Gelderland & Overijssel (Pieken in de Delta program, number PID101020) and by the Natural Sciences and Engineering Research Council (NSERC) of Canada.
References
- Risau W. Mechanisms of angiogenesis. Nature 1997; 386:671-4; PMID:9109485; http://dx.doi.org/10.1038/386671a0
- Shima DT, Mailhos C. Vascular developmental biology: getting nervous. Curr Opin Genet Dev 2000; 10:536-42; PMID:10980432; http://dx.doi.org/10.1016/S0959-437X(00)00124-6
- Park J-A, Choi K-S, Kim S-Y, Kim K-W. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun 2003; 311:247-53; PMID:14592405; http://dx.doi.org/10.1016/j.bbrc.2003.09.129
- Gerber H-P, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway: REQUIREMENT FOR Flk-1/KDR ACTIVATION. J Biol Chem 1998; 273:30336-43; PMID:9804796; http://dx.doi.org/10.1074/jbc.273.46.30336
- Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 Regulates Migration and Angiogenesis of Human Endothelial Cells. Endocr J 1999; 46:S59-S62; PMID:12054122; http://dx.doi.org/10.1507/endocrj.46.Suppl_S59
- Valable S, Bellail A, Lesné S, Liot G, MacKenzie ET, Vivien D, Bernaudin M, Petit E. Angiopoietin-1-induced phosphatidyl-inositol 3-kinase activation prevents neuronal apoptosis. FASEB J 2003; 17(3):443-5; PMID:12514118
- Sheets K, Wunsch S, Ng C, Nain AS. Shape-dependent cell migration and focal adhesion organization on suspended and aligned nanofiber scaffolds. Acta Biomaterialia 2013; 9:7169-77; PMID:23567946; http://dx.doi.org/10.1016/j.actbio.2013.03.042
- Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol 2013; 25:642-9; PMID:23850350; http://dx.doi.org/10.1016/j.ceb.2013.06.004
- Curtis A, Wilkinson C. Topographical control of cells. Biomaterials 1997; 18:1573-83; PMID:9613804; http://dx.doi.org/10.1016/S0142-9612(97)00144-0
- Plotnikov SV PA, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 2012; 151:1513-27; PMID:23260139; http://dx.doi.org/10.1016/j.cell.2012.11.034
- Pelham RJ Jr, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 1997; 94:13661-5; PMID:9391082; http://dx.doi.org/10.1073/pnas.94.25.13661
- Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: Regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol 1998; 8:51-4; PMID:9695809; http://dx.doi.org/10.1016/S0962-8924(98)80005-6
- Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 2004; 25:877-86; PMID:14609676; http://dx.doi.org/10.1016/S0142-9612(03)00593-3
- Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem 2007; 102:686-98; PMID:17437540; http://dx.doi.org/10.1111/j.1471-4159.2007.04580.x
- Niapour A, Karamali F, Karbalaie K, Kiani A, Mardani M, Nasr-Esfahani MH, Baharvand H. Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnol Lett 2010; 32:781-6; PMID:20213527; http://dx.doi.org/10.1007/s10529-010-0230-z
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002; 109:693-705; PMID:12086669; http://dx.doi.org/10.1016/S0092-8674(02)00757-2
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999; 19:5731-40; PMID:10407014
- Bates D, Taylor GI, Newgreen DF. The Pattern of Neurovascular Development in the Forelimb of the Quail Embryo. Dev Biol 2002; 249:300-20; PMID:12221008; http://dx.doi.org/10.1006/dbio.2002.0771
- Huang D, Rutkowski JL, Brodeur GM, Chou PM, Kwiatkowski JL, Babbo A, Cohn SL. Schwann Cell-conditioned Medium Inhibits Angiogenesis. Cancer Res 2000; 60:5966-71; PMID:11085514
- Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev 2008; 27:57-66; PMID:18058195; http://dx.doi.org/10.1007/s10555-007-9105-8
- Thomas SL, De Vries GH. Angiogenic expression profile of normal and neurofibromin-deficient human Schwann cells. Neurochem Res 2007; 32:1129-41; PMID:17404841; http://dx.doi.org/10.1007/s11064-007-9279-z
- Chandrasekar N, Mohanam S, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Glial cell-induced endothelial morphogenesis is inhibited by interfering with extracellular signal-regulated kinase signaling. Clin Cancer Res 2003; 9:2342-9; PMID:12796404
- Stefnik DF, Rizkalla LR, Soi A, Goldblatt SA, Rizkalla WM. Acidic and Basic Fibrolast Growth Factors Are Present in Glioblastoma Multiforme. Cancer Res 1991; 51:5760-5; PMID:1717153
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992; 359:845-8; PMID:1279432; http://dx.doi.org/10.1038/359845a0
- Chang NJ, Lam CF, Lin CC, Chen WL, Li CF, Lin YT, Yeh ML. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthritis Cartilage 2013; 21:1613-22; PMID:23927932; http://dx.doi.org/10.1016/j.joca.2013.07.016
- Doyle AD WF, Matsumoto K, Yamada KM. One dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 2009; 184:481-90; PMID:19221195; http://dx.doi.org/10.1083/jcb.200810041
- Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng 1999:19-46; http://dx.doi.org/10.1146/annurev.bioeng.1.1.19
- Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001; 42:261-72; http://dx.doi.org/10.1016/S0032-3861(00)00250-0
- Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res 1993; 600:49-55; PMID:8422590; http://dx.doi.org/10.1016/0006-8993(93)90400-H
- Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 2000; 11:617-24; PMID:11102799; http://dx.doi.org/10.1016/S0958-1669(00)00153-1
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocrine Rev 1997; 18:26-45.
- Coll JLB-ZeA, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG, Adamson ED. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology,adhesion, and locomotion. Proc Natl Acad Sci U S A 1995; 92:9161-5; PMID:7568093; http://dx.doi.org/10.1073/pnas.92.20.9161
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995; 377 (6549):539-44; PMID:7566154; http://dx.doi.org/10.1038/377539a0
- Zhao X, Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011; 63:610-5; PMID:21118706; http://dx.doi.org/10.1016/j.addr.2010.11.001
- Hannigan MO, Zhan LJ, Ai YX, Kotlyarov A, Gaestel M, Huang CK. Abnormal migration phenotype of mitogen-activated protein kinase-activated protein kinase 2(–/–) neutrophils in Zigmond chambers containing formyl-methionyl-leucyl-phenylalanine gradients. J Immunol 2001; 167:3953-61; PMID:11564814; http://dx.doi.org/10.4049/jimmunol.167.7.3953
- Miller ED, Li K, Kanade T, Weiss LE, Walker LM, Campbell PG. Spatially directed guidance of stem cell population migration by immobilized patterns of growth factors. Biomaterials 2011; 32:2775-85; PMID:21272933; http://dx.doi.org/10.1016/j.biomaterials.2010.12.005
- Zhiqiang Zhao LQ, Brian Reid, Jin Pa, Takahiko Hara, Min Zhaoc. Directing migration of endothelial progenitor cells with applied DC electric fields. Stem Cell Res 2012; 8:38-48; PMID:22099019; http://dx.doi.org/10.1016/j.scr.2011.08.001
- Lulu Han ZM, Jindan Wu, Yang Guo, Tanchen Ren, Changyou Gao. Directional cell migration through cellecell interaction on polyelectrolyte multilayers with swelling gradient. Biomaterials 2013; 34:975-84; PMID:23127331; http://dx.doi.org/10.1016/j.biomaterials.2012.10.041
- Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 2013; 34:1942-53; PMID:23245926; http://dx.doi.org/10.1016/j.biomaterials.2012.11.012
- Petrie RJ, Doyle AD, Yamada KM. Random vs. directionally persistent cell migration. Nat Rev Mol Cell Biol 2009; 10:538-49; PMID:19603038; http://dx.doi.org/10.1038/nrm2729
- Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat Cell Biol 2005; 7:336-7; PMID:15803131; http://dx.doi.org/10.1038/ncb0405-336
- Provenzano PP ID, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J 2008; 95:5374-84; PMID:18775961; http://dx.doi.org/10.1529/biophysj.108.133116
- Ford MC BJ, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. PNAS 2006; 103:2512-7; PMID:16473951; http://dx.doi.org/10.1073/pnas.0506020102
- Rauch MF, Hynes SR, Bertram J, Redmond A, Robinson R, Williams C, Xu H, Madri JA, Lavik EB. Engineering angiogenesis following spinal cord injury: A coculture of neural progenitor and endothelial cells in a degradable polymer implant leads to an increase in vessel density and formation of the blood-spinal cord barrier. Eur J Neuroscience 2009; 29:132-45; PMID:19120441; http://dx.doi.org/10.1111/j.1460-9568.2008.06567.x
- Li Q FM, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF and BDNF mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J Neurosci Res 2006; 84:1656-68; PMID:17061253; http://dx.doi.org/10.1002/jnr.21087
- Ford MC BJ, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. PNAS 2006; 103:2512-7; PMID:16473951; http://dx.doi.org/10.1073/pnas.0506020102
- Lai Y SY, Liu XH, Zhang Y, Zeng Y, Liu YF. Interleukin-8 Induces the Endothelial Cell Migration through the Activation of Phosphoinositide 3-Kinase-Rac1/RhoA Pathway. Int J Biol Sci 2011; 7(6):782-91; PMID:21750647; http://dx.doi.org/10.7150/ijbs.7.782
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114(15):2713-22; PMID:11683406
- Romer LH, McLean N, Turner CE, Burridge K. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol Biol Cel 1994; 5:349-61; http://dx.doi.org/10.1091/mbc.5.3.349
- Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 1997; 15:2169-77; PMID:9393975; http://dx.doi.org/10.1038/sj.onc.1201380
- Ma J, Xue Y, Liu W, Yue C, Bi F, Xu J, Zhang J, Li Y, Zhong C, Chen Y. Role of Activated Rac1/Cdc42 in Mediating Endothelial Cell Proliferation and Tumor Angiogenesis in Breast Cancer. PLoS ONE 2013; 8:e66275; PMID:23750283; http://dx.doi.org/10.1371/journal.pone.0066275
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356-70; PMID:17373658; http://dx.doi.org/10.1002/bies.20558
- de Toledo M, Anguille C, Roger L, Roux P, Gadea G. Cooperative Anti-Invasive Effect of Cdc42/Rac1 Activation and ROCK Inhibition in SW620 Colorectal Cancer Cells with Elevated Blebbing Activity. PLoS ONE 2012; 7:e48344; PMID:23144867; http://dx.doi.org/10.1371/journal.pone.0048344
- Kim GH KW. Formation of oriented nanofibers using electrospinning. Appl Phys Lett 2006; 88:233101; http://dx.doi.org/10.1063/1.2210972
- Aubin H, Kranz A, Hulsmann J, Pinto A, Barth M, Fomin A, Lichtenberg A, Akhyari P. A novel native derived coronary artery tissue-flap model. Tissue Eng Part C Methods 2013; 19:970-80; PMID:23631507; http://dx.doi.org/10.1089/ten.tec.2012.0712
- Wang XY, Pei Y, Xie M, Jin ZH, Xiao YS, Wang Y, Zhang LN, Li Y, Huang WH. An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab Chip 2015; 15:1178-87; PMID:25565271; http://dx.doi.org/10.1039/C4LC00973H
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
- Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 2014; 3 (10):647-61; http://dx.doi.org/10.1089/wound.2013.0517
- Mouneimne G HS, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH, Mullins RD, Brugge JS. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell 2012; 22(5):615-30; PMID:23153535; http://dx.doi.org/10.1016/j.ccr.2012.09.027
- Kouklis P, Konstantoulaki M, Malik AB. VE-cadherin-induced Cdc42 signaling regulates formation of membrane protrusions in endothelial cells. J Biol Chem 2003; 278:16230-6; PMID:12595527; http://dx.doi.org/10.1074/jbc.M212591200
