abstract
The objective of the present review is to synthesize the information on the cellular and molecular players responsible for maintaining a homeostatic balance between a naturally invasive human placenta and the maternal uterus in pregnancy; to review the roles of decorin (DCN) as a molecular player in this homeostasis; to list the common maladies associated with a break-down in this homeostasis, resulting from a hypo-invasive or hyper-invasive placenta, and their underlying mechanisms. We show that both the fetal components of the placenta, represented primarily by the extravillous trophoblast, and the maternal component represented primarily by the decidual tissue and the endometrial arterioles, participate actively in this balance. We discuss the process of uterine angiogenesis in the context of uterine arterial changes during normal pregnancy and preeclampsia. We compare and contrast trophoblast growth and invasion with the processes involved in tumorigenesis with special emphasis on the roles of DCN and raise important questions that remain to be addressed. Decorin (DCN) is a small leucine-rich proteoglycan produced by stromal cells, including dermal fibroblasts, chondrocytes, chorionic villus mesenchymal cells and decidual cells of the pregnant endometrium. It contains a 40 kDa protein core having 10 leucine-rich repeats covalently linked with a glycosaminoglycan chain. Biological functions of DCN include: collagen assembly, myogenesis, tissue repair and regulation of cell adhesion and migration by binding to ECM molecules or antagonising multiple tyrosine kinase receptors (TKR) including EGFR, IGF-IR, HGFR and VEGFR-2. DCN restrains angiogenesis by binding to thrombospondin-1, TGFβ, VEGFR-2 and possibly IGF-IR. DCN can halt tumor growth by antagonising oncogenic TKRs and restraining angiogenesis. DCN actions at the fetal-maternal interface include restraint of trophoblast migration, invasion and uterine angiogenesis. We demonstrate that DCN overexpression in the decidua is associated with preeclampsia (PE); this may have a causal role in PE by compromising endovascular differentiation of the trophoblast and uterine angiogenesis, resulting in poor arterial remodeling. Elevated DCN level in the maternal blood is suggested as a potential biomarker in PE.
Structure of the human placenta as an invasive organ
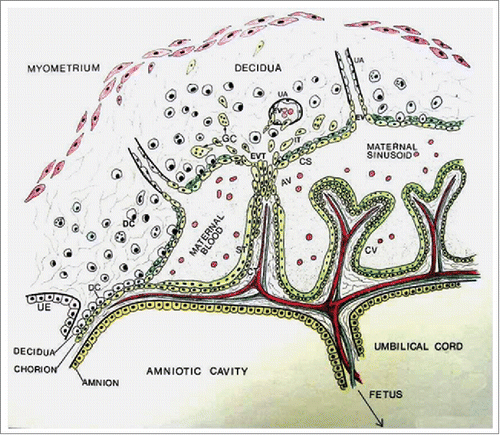
The human placenta is a highly invasive organ in which a subpopulation of trophoblast cells proliferate, migrate and invade the uterine endometrium and its arteries to nourish the fetus.Citation1,2 Adequate placental perfusion with maternal blood depends on highly regulated invasion and remodeling of utero-placental arteries by the trophoblast.Citation3,4 There are 2 distinct pathways of trophoblast differentiation from the bipotent stem cells in the cytotrophoblast layer of chorionic villi (): the villous pathway, in which cells proliferate and fuse, giving rise to the syncytiotrophoblast layer facing the maternal sinusoids, engaged primarily in exchange and endocrine functions; and the extravillous pathway in which cells break out of the villi as discrete cell columns which proliferate at their base,Citation5,6 migrate and invade the decidua and its arteries by adopting an “endovascular phenotype” (expressing certain endothelial cell markers) and replace the endothelial lining of the arteries.Citation7-9 Proliferation, migration and invasiveness of the extravillous trophoblast (EVT) cells are exquisitely regulated in situ,Citation1,2,10 and this may fail in certain pathological conditions. Poor EVT invasion and arterial remodeling underlies preeclampsia and certain forms of intrauterine growth restriction (IUGR),Citation4,11-13 whereas uncontrolled invasion features trophoblastic neoplasias, including invasive moles and choriocarcinomas.Citation13
Figure 1. Schematic diagram of human placenta at approximately 10 weeks of gestation showing the fetal compartment including the trophoblast and maternal sinusoids, and the maternal compartment including the decidua and uterine vessels (UV). Chorionic villi (CV) consist of 2 layers of trophoblast cells, the inner cytotrophoblast (CT) and the outer syncytiotrophoblast (ST). Cytotrophoblast cells in some villi known as anchoring villi (AV) migrate out as cell columns as extravillous trophoblasts (EVT) to invade the decidua. Some EVT cells remain dispersed in the deciduas as interstitial trophoblast (IT), some fuse to become trophoblast giant cells (GC) and others form cytotrophoblastic shell (CS) bordering the decidua and the maternal sinusoids. Some EVT cells from the CS enter uterine arteries as endovascular trophoblasts. It is also suggested that some IT cells invade the arteries from the exterior. (Reproduced with permission from Graham CH and Lala PK. Biochem Cell Biology 1992; 70: 867–874.).
Uterine angiogenesis and arterial remodeling during normal pregnancy and preeclampsia
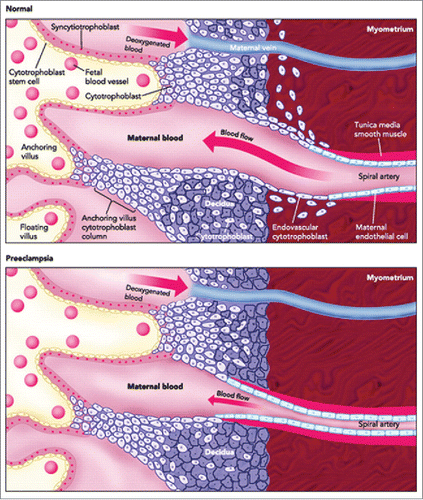
While blood vessels in the fetal compartment of the placenta arise by the process of “vasculogenesis” by recruitment of mesenchymally derived angioblasts, those in the maternal compartment represented by the decidualized endometrium arise by sprouting from pre-existing uterine vessels, or the process of “angiogenesis.” The arteries, but not the veins, in the endometrium and the inner third of the myometrium are extensively modified during normal pregnancy by the EVT cells. It has been suggested that EVT cell preference for migration toward the uterine arteries rather than veins is guided by the differential expression of chemokine receptors ephrin type-B receptor 2 (EPHB2) on arterial endothelium and ephrin type-B receptor 4 (EPHB4) on venous endothelium and their transmembrane ligand ephrin on EVT cells.Citation14 The modification or “remodeling” of the arteries is a process characterized by the disruption, and eventual loss of the smooth muscle cells, described as “fibrinoid necrosis” of the tunica media,Citation15,16 loss of elastic fibers and replacement of the endothelial lining with EVT cells which have adopted an “endovascular phenotype.”Citation3,4,15 Endovascular phenotype or “endovascular differentiation” of the trophoblast is characterized by gestational age-dependent acquisition of endothelial markers VE-cadherin, VCAM-1, PECAM-1 and N-cadherin.Citation15,17 Prior to the arrival of EVT cells, the uterine arteries, possibly primed by pregnancy-associated hormones, undergo certain morphological and molecular changes in the endothelium such as basophilia, swelling and expression of the endothelial activation marker VCAM. This is regarded as the trophoblast-independent phase of arterial remodeling,Citation18 while subsequent completion of the remodeling process is considered as trophoblast-dependent.Citation15 There are 2 views on EVT cell entry, presumably leading to the disruption of vascular smooth muscle cells (VSMC): (i) Certain EVT cells in the cytotrophoblastic shell () undergo endovascular differentiation and invade the distal ends of the arterioles within the endometrium to cause apoptosis of the endothelial cells; they crawl against the blood-flow to replace the endothelial lining as far as the distal third of the myometrium. (ii) Interstitial EVT cells located deeper in the decidua invade the arteries from outside.Citation19,20 While these 2 views are not mutually exclusive, presence of interstitial EVT cells has not been documented within the myometrium. In vitro studies suggest that EVT cells when confronting myometrial smooth muscle cells produce Mel-CAM (also known as MUC18 and CD146), an anti-migratory molecule.Citation21 Another feature of the arterial remodeling is the loss of elastic fibers in the tunica intima and tunica adventitia. It has been suggested that trophoblasts and VSMCs release MMP-12 in a cooperative manner to degrade elastin.Citation22 These events transform the arteries from narrow, pulsatile and high resistance tubes to wide, non-pulsatile and low resistance vessels that allow steady flow of maternal arterial blood to the placenta for fetal nourishment. A failure of or a compromised remodeling of the arteries is frequently associated with IUGR in the fetus and or preeclampsia (PE) in the mother ().
Figure 2. A schematic comparison of the structures of human placentas in normal and preeclamptic pregnancy, showing poor uterine spiral arterial remodeling in PE. Poor EVT invasion of the spiral arteries is believed to compromise the arterial remodeling. This causes reduced flow of maternal arterial blood to the placental sinusoids resulting in hypo-perfused placenta that may result in IUGR in the fetus and PE in the mother. (Adapted and modified from Wang A, Rana S, Karumanchi AS. Preeclampsia: The Role of Angiogenic Factors in Its Pathogenesis. Physiology. 2009 June; 24(3):147–158.).
Patho-biology of preeclampsia
PE is a serious maternal syndrome of placental origin affecting 3–5% of pregnancies, associated with significant maternal as well as neonatal morbidity resulting from premature child birth. Clinical signs appear as unexplained hypertension and proteinuria as early as at 20 weeks of gestation in an otherwise normotensive mother. The disease is typically divided into 2 phases, the placental phase characterized by compromised trophoblast invasion and uterine arterial remodeling, and the maternal phase characterized by wide-spread maternal endothelial damage and dysfunction, possibly resulting from a variety of toxic products released from a hypo-perfused, hypoxic placenta.Citation23,24 These products may include lipid peroxidases, syncytiotrophoblast microvillous fragments, and inflammatory chemokines.Citation23-25 The placental origin of the disease is supported by the fact that a complete cure lies in the delivery of the placenta. Many studies indicate that a deficiency of trophoblast migration/invasion promoting factors or an increase in trophoblast migration/invasion restraining factors in situ may be associated with this disease (reviewed in refs. 13, 23, 24). Some of them may appear as blood biomarkers.
Factors regulating EVT cell proliferation, migration and invasiveness
A large number of molecules produced at the fetal-maternal interface were shown to exquisitely regulate trophoblast growth, migration and invasion in a positive or negative manner to maintain a healthy utero-placental homeostasis. These molecules include growth factors, inflammatory cytokines, growth factor binding proteins and proteoglycans and lipid derivatives produced by the trophoblast (autocrine) or the decidua including decidual leukocytes and immune cells (paracrine). Success in propagating pure human first trimester EVT cells in vitro, having the phenotype of EVT cells in situ,Citation26-28 has helped to identify some of the receptor-ligand interactions and signaling pathways regulating their proliferative, invasive and migratory functions and their alteration in PE.Citation13,29-31 While the mechanisms for EVT cell invasion are shared with cancer cells,Citation32-41 in contrast to cancer cells, EVT cell proliferation, migration and invasiveness are stringently regulated in situ.Citation1,2,7,13,41-45 To explore this regulation, a number of in vitro models have been utilised: (a) when villus cytotrophoblast cells are placed on matrigel, a subset (progenitor cells) differentiated along the invasive pathway,Citation46 a process stimulated by EGFCitation47 and IL-1β.Citation48 When grown on plastic, they differentiated into syncytiotrophoblast,Citation49 but become enriched for EVT cells when plated on laminin.Citation50 (b) Chorionic villus explants when plated on matrigel.Citation51 led to EVT cell sprouting that was stimulated with decidua derived activin.Citation52 and uterine NK cell derived IFN-γ.Citation53 (c) Our laboratory developed a method of propagating pure first trimester EVT cells from villus explants,Citation26-28 duplicated by others.Citation21 They express all the in situ markers of EVT: cytokeratin 7, HLA class 1 framework antigen, uPA-R, IGF-II mRNA and protein, and integrin chains α1, α5, αv,β1 and vitronectin receptor αvb3/β5 but not α6 or β4,Citation28 and HLA-G when grown on laminin or matrigel.Citation54 They senesce after 5–15 passages.Citation28 Subsequently, we produced an immortalised EVT cell line HTR-8/SVneo, by SV40-Tag transfection of a short-lived line HTR-8.Citation55 This cell line has fully retained the normal EVT cell phenotype including expression of cell surface HLA-G. Since in vitro derived cell lines can only serve as in vitro models for studies of molecular pathways, not otherwise identifiable, they need to be verified with primary isolates of trophoblast at least in limited experiments, whenever feasible. Utilizing HTR-8 and HTR-8/SVneo cells, and villus explant cultures on matrigel, our laboratory and others have established that locally-produced molecules which regulate EVT cell functions can be broadly placed into 3 functional groups.
Factors stimulating EVT proliferation
(a) Members of the EGF family EGF,Citation56 TGF-α,Citation56,57 and amphiregulin,Citation58 produced in situ stimulated EVT cell growth. EGF also promoted differentiation of cytotrophoblast stem cells along the invasive pathway.Citation48 (b) CSF-1 produced by pregnant endometrial glands,Citation59-62 stimulated EVT cell proliferation but not invasiveness.Citation63 (c) Decidua-derived VEGF-A,Citation64 and PLGF,Citation65 also stimulated EVT cell proliferation, only in the absence of heparin binding domains. EVT cells expressed receptors for both VEGF-A (Flt-1, KDR) and PLGF (Flt-1).Citation64,65
Factors stimulating EVT migration and invasiveness
Certain trophoblast-derived molecules including IGF-II and uPA, decidua-derived molecules including IGFBP-1, endothelin-1, PGE2, and chemokines CX3CL1, CCL4, CCL14 and villus mesenchymal cell derived molecules such as HGF were shown to stimulate EVT cell migration and invasiveness.
Roles of IGF-II and IGFBP1
In situ hybridization revealed that EVT cells invading the decidua expressed IGF-II mRNA, whereas decidual cells in the proximity of invading EVT cells expressed IGFBP-1 mRNA, suggesting a molecular cross talk at the fetal-maternal interface.Citation66 To our surprise both molecules independently stimulated EVT cell migration and invasiveness. IGF-II actions were mediated by binding IGF-IIR, involving inhibitory G proteins and activation of ERK1/2.Citation67 Decidua-derived IGFBP-1 also stimulated EVT cell migration independent of IGF-I or IGF-II binding. This was mediated by binding of the Arg-Gly-Asp (RGD) domain of IGFBP-1 to α5β1 integrin on the EVT cell surface, followed by activation of FAK and MAPK.Citation68 Patho-physiological significance of these findings was demonstrated by low IGFBP-1 levels in early gestation sera of women destined to develop PE.Citation69-72 and low IGFBP-1 mRNA expression by the decidua in PE.Citation73 Increased IGFBP-1 levels in maternal blood reported during overt PE.Citation74 appear to be non-decidual in origin.Citation73
Migration stimulation by uPA
EVT cells produced uPA,Citation75 expressed high affinity uPA receptors (uPAR).Citation76 and showed a polarized distribution of uPAR-bound uPA on cell surface at the invasion front in situ.Citation77 While EVT cells used the catalytic domain of uPA for matrix degradation,Citation1,7 the binding of its amino-terminal to the EVT cell surface uPAR stimulated EVT cell migration resulting from an elevation of [Ca2+]i, and activation of PLC, PI3K and MAPK.Citation78 These findings may explain the reasons for the observed uPA/uPAR imbalance in sera of PE mothers.Citation79
Migration stimulation by endothelin (ET)-1 and PGE2
ET1is produced by vascular endothelial cells within the placenta and the decidua. ET1 action on EVT cells was shown to follow PLC-dependent elevation of [Ca2+]i and activation of MAPK.Citation80 PGE2, a major decidual product, was shown to stimulate EVT cell migration.Citation81-83 by binding to PGE receptors, in particular EP1 and EP4. EP1-mediated migratory responses resulted from an increase in [Ca2+]i because of release from intracellular storage sites, and an activation of the calpain family of [Ca2+]i dependent proteases, required for cell detachment from the substratum.Citation81 Migratory responses resulting from EP1 or EP4 activity were dependent on RhoA-ROCK pathway, in which ROCK played an obligatory role, whereas RhoA action could be substituted by MAPK. However, RAC1 and CDC42 played an essential role in PGE2 or EP1/EP4 mediated migratory responses.Citation82,83 These findings are relevant to intervention of PE, in which trophoblast hypo-invasiveness and increased peripheral vascular resistance in the pregnant mother has been ascribed to decreased levels or responses to PGE2.
Migration stimulation by chemokines and HGF
Human decidual cells and endometrial gland cells produce a plethora of chemokines including CX3CL1, CCL4 and CCL14. They were shown to promote human trophoblast migration by binding to chemokine receptors on EVT cells.Citation84 Chorionic villus mesenchymal cells include a multipotent subset of stromal cells which produce many soluble cytokines and growth factors including HGF.Citation85 HGF exibited migration and invasion stimulatory effects on EVT cells.Citation85-87 HGF stimulated EVT cell motility depended on both MAPK and PI3K signaling pathways and iNOS expression.Citation86 HGF ligation of the c-Met receptor on EVT cells stimulated MMP-9 expression and elevation of cAMP leading to protein kinase A (PKA)-dependent Rap1 and integrin β1 activation.Citation85 These findings are consistent with the reports of reduced HGF production by the PE placentas Citation87 and reduced soluble Met (s-Met) in the blood of PE mothers.Citation88
Factors blocking EVT cell proliferation, migration and invasiveness
All these EVT cell functions are controlled mostly in a paracrine manner by at least 3 decidua-derived factors TGFβ, TNFα and DCN. TGFβ is produced by the syncytiotrophoblast and most abundantly by the decidua.Citation27,29 This molecule exerts anti-proliferative,Citation27 anti-migratory.Citation68,89 and anti-invasive.Citation7,35,90 actions on EVT cells. The anti-migratory function resulted from an up-regulation of integrins, making the cells more adhesive to the ECM.Citation89 The anti-invasive action was a combined result of reduced migration, a reduction of matrix-degrading ability because of down-regulation of uPA.Citation35,91 and upregulation of protease inhibitors TIMP-1.Citation35 and PAI-1.Citation91 EVT cells expressed all the TGFβ receptors (RI, II, III), downstream signaling molecules SMAD proteins (receptor associated smads 2, 3, co-smad 4, and inhibitory smads 6, 7) and responded to TGFβ by phosphorylation and nuclear translocation of smad 3Citation92 and smad-dependent up-regulation of PAI-1Citation93 and TIMP-1.Citation94 Trophoblast cells also express a TGFβ co-receptor endoglin which binds TGFβ-1 and β3 but not β2.Citation95,96 A stimulation of EVT cell sprouting in villus explant cultures noted after blocking endoglin.Citation96 is consistent with proliferation, migration and invasion-blocking roles of endogenous TGFβ.Citation31 A failure of downregulation of TGFβ-3 in villi was postulated as a precursor of preeclampsia.Citation97 Nodal, another member of the TGFβ superfamily inhibits EVT migration by activation of ALK7, and nodal/ALK7 overexpression is implicated in PE.Citation98 We discovered that choriocarcinoma cells are resistant to anti-proliferative and anti-invasive actions of TGFβ,Citation99 primarily due to loss of smad3 expression.Citation92-94 TNFα, a product of decidual macrophages was shown to inhibit EVT cell migration by up-regulating PAI-1.Citation100 We observed that a TGFβ-binding and inactivating proteoglycan DCN is co-localized with TGFβ in the decidual ECM, suggesting that DCN may serve as a reservoir for TGFβ in its inactive form,Citation29 until trophoblast-derived protease cascade at the invasion front breaks DCN-TGFβ complex and activates TGFβ to prevent over-invasion. However, to our surprise, DCN on its own was found to block EVT cell proliferation, migration and invasiveness, independent of TGFβ. An upregulation of p21 (a CDK inhibitor) explained, in part, the anti-proliferative effects. Again, choriocarcinoma cells proved to be resistant to these DCN actions.Citation30
Structural basis of DCN functions
DCN is a small leucine-rich proteoglycan (SLRP) produced in a variety of connective tissues, such as the dermis, cartilages, the decidua,Citation29,30 and mesenchymal cells of the chorionic villi.Citation29,101 A diagram of mature DCN structure derived from numerous studiesCitation102-104 is presented in . It consists of a 40 kDa core protein, harbouring 10 leucine-rich repeats (LRR), covalently linked with a tissue-specific glycosaminoglycan (GAG) chain (chondroitin or dermatan sulfate) at its N-terminus of domain II. The protein is encoded by chromosome 12q22 in the human. The mature protein is highly conserved across various species and responsible for many of the biological functions. It regulates matrix assembly, including collagen fibrillogenesis because of its ability to bind collagen type1. DCN null mice survive to adulthood, but show poor fertility and skin fragility due to disruption of collagen fibrils in the dermis.Citation105 Mutation of DCN gene has been associated with a rare autosomal dominant form of corneal dystrophy called “Congenital stromal corneal dystrophy (CSCD)," also named as “Witschel dystrophy.” Citation106 Mice genetically deficient in both DCN and biglycan genes undergo preterm labor and expulsion of the fetus owing to premature rupture of the fetal membranes.Citation107 Because of its TGFβ binding ability at LRR4 and 5, DCN can store TGFβ in the ECM.Citation103 It can inactivate mature TGFβ in some circumstancesCitation108 but not others,Citation109 apparently by interfering with TGFβ-binding to types I and III TGFβR. Whether this occurs universally in vivo is unclear, since the inactivation improves in vitro after removal of the GAG chains.Citation110 DCN can also bind myostatin, another member of the TGFβ superfamily and a myogenic inhibitory protein.Citation111 This binding has been shown to support myogenesis by inhibition of myostatin. For example, DCN immobilized on ECM in vitro sequesters myostatin into the ECM, preventing its inhibitory action on myoblast proliferation. Furthermore, ectopically introduced DCN into myoblasts promotes their proliferation and differentiation by inactivating endogenous myostatin.Citation112 DCN can modulate cell adhesion by binding with ECM molecules fibronectinCitation113,114 or thrombospondin.Citation115 However, as elaborated below, many DCN functions are also receptor-mediated.
DCN acts as an antagonistic ligand to numerous tyrosine kinase receptors
EGFR was the first DCN receptor identified by the Iozzo groupCitation116-120 having a lower affinity than EGF. DCN-binding led to a transient phosphorylation of EGFR kinase, followed by a reduction of the receptor number, attributed to accelerated receptor endocytosis by the caveolar pathway. DCN binding was mapped within a narrow region of the ligand binding domain 2 of the EGFR, partially overlapping with the EGF-binding domain.Citation119 A second DCN receptor IGF-IR was soon identified on endothelial cells that could bind DCN with an affinity comparable to IGF-1, showing DCN induced receptor phosphorylation, followed by receptor down-regulation.Citation121 However DCN could also bind IGF-I, compounding the biological effects of DCN on endothelial cells in the presence of IGF-I in the micro-environment.Citation121 Subsequently DCN was shown to be an antagonistic ligand for Met, the receptor for HGF.Citation122 Since DCN core protein and its 26-amino acid residue LRR5 were shown to inhibit VEGF-induced angiogenesis in endothelial cells in vitro,Citation123 we suspected that the anti-angiogenic action of DCN may have resulted from DCN binding to VEGFR-2.
TKR mediated DCN actions of EVT cells and endothelial cells
We investigated whether EGFR, IGF-IR and VEGFR-2 played a role in negative regulatory actions of DCN in EVT cells.Citation31 We noted that these TKRs are promptly phosphorylated by DCN similar to their natural ligands, and these events can be blocked with respective tyrosine kinase inhibitors. We found that DCN inhibits EVT cell outgrowth in situ from intact first trimester chorionic villous explants on matrigel. Using our EVT cell line HTR-8/SVneo, we further showed that anti-proliferative action of DCN is mediated primarily by EGFR and VEGFR-2, and anti-migratory action primarily by IGF-IR. Furthermore, VEGF-A mediated migration of EVT cells was blocked with DCN in a VEGFR-2 dependent manner.Citation124
Molecular localization of VEGFR-2 binding on DCN protein
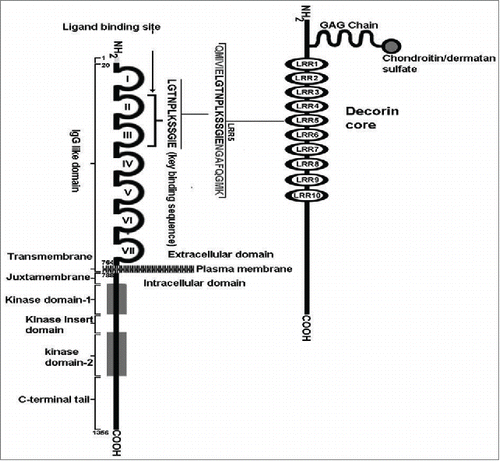
Far-Western blotting and co-immunoprecipitation studies revealed that DCN binds both native (EVT or endothelial cell expressed) and recombinant VEGFR-2 and that this binding is abrogated with a VEGFR-2 blocking antibody, indicating an overlap between the ligand-binding and the DCN-binding domains of VEGFR-2125 I-labeled VEGF-E (a virus-derived VEGFR-2 specific ligand) bound to EVT with a Kd of 566 pM, and DCN displaced this binding with a Ki of 3.93–5.78 nM, indicating a 7 to 10-fold lower affinity of DCN for VEGFR-2. DCN peptide fragments derived from its LRR- 5 domain that blocked DCN-VEGFR-2 interactions or VEGF-E binding in EVT cells also blocked VEGF-A and VEGF-E-induced EVT cell proliferation and migration, indicative of functional VEGFR-2-binding sites of DCN in a 12 AA span of LRR5 (). This binding inhibited VEGF-induced EVT migration by interfering with ERK1/2 activation. Subsequently we explored the mechanisms for VEGFR-2 dependent DCN antagonism of migration and endovascular differentiation of EVT cells.Citation125 We found that DCN inhibited VEGF-induced EVT cell migration and endothelial-like tube formation (an in vitro surrogate of endovascular phenotype). VEGF activated p38 MAPK, MEK3/6, and ERK1/2 in EVT cells, and the activation of these pathways was blocked by DCN. Employing selective MAPK inhibitors, we showed that both p38 MAPK and ERK pathways contributed independently to VEGF-induced EVT migration and tube formation. VEGF-mediated up-regulation of endothelial markers VE-cadherin and β-catenin in EVT as well as endothelial cells was blocked by DCN and MAPK inhibitors. These results revealed that DCN inhibits VEGF induced EVT migration and endovascular differentiation by interfering with p38 MAPK and ERK1/2 pathways in parallel.Citation125
Figure 3. A schematic diagram of the structure of human decorin. It contains backbone of a 40kDa core protein containing 4 domains. Domain-I contains a propeptide and a signal peptide (in green) which are cleaved in the matured protein. Domain-II is covalently linked with a GAG chain at its N-terminus. Domain-III contains 10 Leucine Rich Repeats (LRR) and N-linked Glycan side-chains (in orange). Domain -IV contains cysteine residues and forms a large loop.The TGFβ binding sites are located in LRR4 and LRR5. The VEGFR-2 binding site is located within LRR5. The binding site for myostatin is currently undetermined.
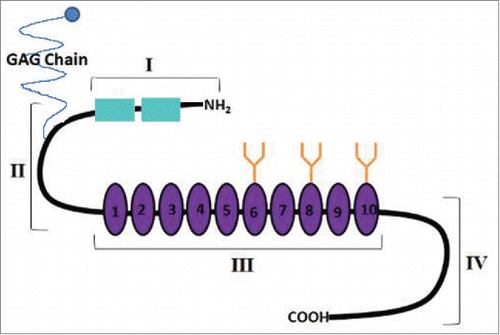
Figure 4. A schematic diagram of the VEGFR-2 binding site in a 12 aa span of LRR5 in decorin protein core. The VEGF-A and decorin binding sites of VEGFR-2 are overlapping. (Reproduced with permission from Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011 Aug; 25(8):1431–43.).
Mechanisms of DCN actions on the trophoblast
Decidua-derived DCN appears to be a powerful negative regulator of trophoblast migration and invasion independent of TGFβ binding; this was primarily due its binding and antagonizing several TKRs including EGFR, IGF-IR, Met, and VEGFR-2 which promote these functions when stimulated by their natural ligands. The underlying mechanisms are most likely similar to those reported with other cell types. In the case of EGFR, DCN binding is followed by a rapid receptor endocytosis and degradation by the caveolar pathway and a retardation of the receptor recycling.Citation120 A rapid receptor internalization and receptor shedding were noted after DCN binding to Met, followed by downregulation of β-catenin and Myc.Citation122 DCN antagonized IGF-IR functions in tumor cells by interfering with IGF-IR activity and attenuating downstream signaling, but no change in receptor endocytosis.Citation126 In the case of VEGFR-2 binding to DCN, we found no evidence of accelerated receptor endocytosis in comparison to VEGF binding on EVT cells (Lala PK and Girish GV, unpublished data). VEGFR-2 binding compromised trophoblast migration and endovascular differentiation by interfering with p38 MAPK and ERK1/2 pathways in parallel.Citation125 It is highly likely that other TKRs with DCN binding ability will be identified in the future on cells including trophoblast.
Role of DCN in angiogenesis
While most studies have established that DCN blocks angiogenesis, some studies suggested a pro-angiogenic role. This difference is ascribed to differences in the cellular and molecular environment in DCN action, elegantly reviewed by Jarvelainen et al.Citation127 Anti-angiogenic actions: Many studies including ours have identified DCN as a potent anti-angiogenic molecule due to its actions on vascular endothelial cells, mediated by multiple mechanisms: (i) In vitro studies with vascular endothelial cells revealed that DCN binding to the ECM molecule thrombospondin-1 enhances anti-angiogenic role of this molecule.Citation128 (ii) DCN binding to IGF-IR blocked angiogenesis in tumor cells,Citation126 in contrast to increased motility reported for endothelial cells grown on type 1 collagen matrix.Citation129 (iii) VEGFR-2, the most potent pro-angiogenic receptor on endothelial cells, antagonizes VEGF-mediated migration and tube formation both by the trophoblast and endothelial cells.Citation124,125 Inhibition of VEGF-induced angiogenesis by DCN or certain DCN peptides reported earlierCitation123 can be explained on the basis of VEGFR-2 binding of these molecules demonstrated by us.Citation124 (iv) DCN mediated down regulation of VEGF production by tumor cells was shown as another mechanism for suppression of tumor-induced angiogenesis in vitro and in vivo. This was demonstrated by addition of exogenous DCN to tumor cells or ectopic DCN expression in tumor cells.Citation130 (v) DCN can also act on VSMC by binding to PDGF, a growth promoter of VSMC. DCN binding to PDGF inhibited PDGF-stimulated PDGFR phosphorylation and proliferation of VSMC, required for angiogenesis.Citation131 Pro-angiogenic actions: DCN null mice exhibited reduced corneal angiogenesis in response to injury, suggesting a pro-angiogenic role of DCN.Citation127 However, this may possibly be explained by upregulation of pro-angiogenic biglycans.Citation132 or activation of endogenous TGFβ which can have indirect angiogenic functions.Citation133 DCN null mice were shown to up-regulate biglycansCitation107 which were reported to have angiogenic ability.Citation132 Finally, targeted DCN gene therapy blocked angiogenesis in mouse cornea,Citation134 reinforcing its anti-angiogenic role. Other pro-angiogenic actions of DCN can possibly be explained in the context of DCN-ECM interactions, favoring the assembly of a pro-angiogenic matrix.Citation127,135 For example, endothelial tube formation was stimulated in DCN over-expressing cells placed in a 3D matrix which allowed DCN binding to both IGF-IR and IGF-I.Citation121 It is also possible that the nature of microvasculature may dictate DCN action. For example, ectopic DCN over-expression in mouse cerebral endothelial cells upregulated VEGF-A to promote angiogenesis.Citation136
Role of DCN in tumor progression
Interestingly, many of the DCN actions in restraining placental invasion of the pregnant uterus and its vasculature are also applicable to tumor progression. Currently there is a substantial amount of literature suggesting that DCN has tumoristatic functions in certain cancers because of its dual roles: limiting angiogenesis and antagonising TKRs having oncogenic roles because of their mutational activation. Additional mechanisms have also been found. It has been suggested DCN is a natural tumor-suppressor molecule that can be harnessed for therapy. Indeed, SLRPs including DCN have been shown to play an important role in health and disease including cancer.Citation137 These molecules produced by stromal cells in ECM within the tumor micro-environment are believed to be important contributors in controlling tumor growth.Citation137,138 In support, the relative level of DCN expression in soft tissue tumors has been correlated with prognosis.Citation139 Some studies examined the effects of either ectopic introduction of DCN gene, or a deletion of DCN gene. For example, Grant et al.Citation130 demonstrated reduced angiogenic abilities of DCN transfected fibrosarcoma, osteosarcoma and carcinoma cell lines tested in vitro and in vivo. DCN transfection of breast carcinoma cells was shown to antagonise an elaborate angiogenic network. There was concurrent inhibition of Met, HIF-1α and induction of angiostatic molecules thrombospondin-1 and TIMP-3.Citation140 Deletion of DCN gene promoted colorectal carcinogenesis in a mouse model.Citation141 Using a DCN knockout mouse model, these authors demonstrated that colorectal carcinogenesis resulting from DCN knockdown was associated with epithelial mesenchymal transition (EMT), as marked by down-regulation of E-cadherin and up-regulation of β-catenin.Citation142 Goldoni et al.Citation143 demonstrated anti-metastatic role of exogenous DCN treatment in experimental breast cancer. They compared the effects of DCN alone or in combination with an ErbB2 kinase inhibitor AG879 on the growth of an ErbB2-over-expressing mammary carcinoma cell line. DCN was shown to inhibit ErbB2 kinase in tumor cells. When DCN and AG879 were used in combination, the inhibitory effects were synergistic or additive on tumor growth in vitro. While the combination therapy did not improve anti-tumor effects of DCN on primary tumor growth in vivo, DCN significantly reduced lung metastasis. Similarly DCN and carboplatin were shown to have synergistic growth-inhibitory effects on ovarian cancer cells.Citation144 However, caution has been raised against DCN therapy in human pancreatic cancer.Citation145 In pancreatic cancer tissues DCN overexpression was noted in stromal satellite cells possibly as a protective defense mechanism. While DCN alone had cytostatic effects on several pancreatic cancer cell lines in vitro, it attenuated cytostatic effects of carboplatin or gemcitabine in some cell lines. It is likely that DCN will be useful in combination therapies in tumors with low DCN expression in situ. Furthermore, genetic mutations may lead to DCN resistance in certain cancers. For example, in contrast to the migration and invasion restraining roles of decidua-produced DCN as well as TGFβ on the normal human trophoblast cells, as detailed earlier, we found that human choriocarcinoma cells are resistant to both molecules,Citation30,92-94,99 explaining their emancipation from decidua-mediated controls.
Possible role of DCN over-expression in the decidua in pathogenesis of PE
DCN mediated dual impediment of endovascular differentiation of the EVT and angiogenesis,Citation124,125hall marks of PE, may imply that DCN overexpression at the fetal-maternal interface may contribute to the pathogenesis of PE. We tested this contention by quantitating DCN expression in placentas from 16 healthy and 16 PE pregnancies at comparable gestational ages using 2 approaches.Citation146 DCN mRNA expression at the tissue level was quantified with real time qPCR in chorionic villus tissues. DCN mRNA at the cellular level was measured both in chorionic villi and decidua basalis employing in situ hybridization with35S labeled antisense cRNA probe. Hybridization with sense cRNA probe served as controls. Using both approaches we found that DCN expression by chorionic villus mesenchymal cells was unaltered in PE. In contrast, DCN mRNA expression by decidual cells in the basal plate (where EVT cells invade) was profoundly up-regulated in PE placentas, as compared to healthy placentas of equivalent gestational ages. Immunocytochemical localization of DCN in the same tissues confirmed these findings at the protein level. We suggest that over-expression of DCN in the basal decidua contributes to the pathogenesis of PE because of a dual action of DCN on the trophoblast and endothelial cells. In the former DCN causes a hypo-invasive phenotype and poor endovascular differentiation. Anti-angiogenic action of DCN on endothelial cells in combination with poor endovascular differentiation of the trophoblast contribute to poor uterine arterial remodeling. In addition, we discovered that exposure to exogenous DCN or ectopic overexpression of DCN in EVT cells significantly upregulated the expression of sFlt-1. This anti-angiogenic molecule produced by the trophoblast in PE placentas is recognized as a blood biomarker in PE mothers.Citation147 All these events are patho-biological hall marks of placentas associated with PE. We suggest that DCN in the maternal blood is another potential biomarker in PE. This suggestion has recently been validated by longitudinal studies of maternal plasma samples in pregnant subjects during the second trimester (Siddiqui M et al. submitted for publication).
Gaps in knowledge and future directions
DCN is a multifunctional molecule, the functions mediated by a variety of binding events that regulate cell adhesion, migration and invasion. DCN can inactivate TGFβ under certain conditions, but whether this happens in situ in the decidua where they are co-localized, remains to be explored. DCN binds numerous TKRs which have been identified; it may possibly bind to other TKRs that remain to be identified. It is highly likely that some of the DCN actions on the trophoblast are receptor-independent, mediated by binding to ECM molecules that remain to be identified. We have preliminary evidence that DCN binding to EVT cells compromises many other functional events such as intercellular communications that remain to be fully characterized at the molecular level. The role of DCN on VSMC and trophoblast-VSMC interactions remain an area of investigation in the context of uterine arterial remodeling. Very little information is available as to whether DCN acts as a protective molecule in trophoblastic tumorigenesis or metastasis to the placenta from pregnancy-associated cancers in maternal organs including the breast. Recently Borbely et al.Citation148 reported immunohistochemical localization of DCN protein in trophoblast cells of invasive pregnancies including placenta acreta accreta, invasive moles and choriocarcinomas, the significance of which remains unknown. One possibility is that these hyper-invasive placental pathologies involve epithelial-mesenchymal transition of the trophoblast, allowing cells to make this mesenchymal molecule. In situ hybridization is needed to confirm the cellular source of DCN in these pathologies.
Abbreviations
| BP | = | binding protein |
| DCN | = | decorin |
| ECM | = | extracellular matrix |
| EGF | = | epidermal growth factor |
| EVT | = | extravillous trophoblast |
| EPH | = | ephrin |
| ET | = | endothelin |
| EP | = | PGE receptor |
| GAG | = | glycosaminoglycan |
| HGF | = | hepatocyte growth factor |
| HIF | = | hypoxia inducible factor |
| HLA | = | human leukocyte antigen |
| IGF | = | insulin like growth factor |
| IUGR | = | intra-uterine growth restriction |
| IL | = | interleukin |
| IFN | = | interferon |
| Met | = | HGF receptor |
| MMP | = | matrix metalloprotease |
| PDGF | = | platelet derived growth factor |
| PE | = | preeclampsia |
| PECAM1 | = | platelet/endothelial cell adhesion molecule 1 |
| PGE | = | prostaglandin E |
| PAI | = | plasminogen activator inhibitor |
| R | = | receptor |
| SLRP | = | small leucine rich proteoglycan |
| TGF | = | transforming growth factor |
| TKR | = | tyrosine kinase receptor |
| TNF | = | tumor necrosis factor |
| TIMP | = | tissue inhibitor of metalloproteinase |
| uPA | = | urokinase type plasminogen activator |
| VEGF | = | vascular endothelial growth factor |
| VCAM | = | vascular cell adhesion protein |
| VSMC | = | vascular smooth muscle cell |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research collaboration from Dr. Victor Han (Department of Pediatrics at the University of Western Ontario) is gratefully acknowledged.
Funding
Studies from the authors' lab reported here were funded by grants from the Canadian Institute of Health Research (CIHR) and the Children's Health Research Institute (London, ON).
References
- Lala PK, Hamilton GS. Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta 1996; 17:545-55; PMID:8916202; http://dx.doi.org/10.1016/S0143-4004(96)80071-3
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the developmental puzzle. Science 1994; 266:1508-18; PMID:7985020; http://dx.doi.org/10.1126/science.7985020
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, VanAsshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994; 101:669-74; PMID:7947500; http://dx.doi.org/10.1111/j.1471-0528.1994.tb13182.x
- Burton GJ, Woods AW, Jauniaux E, Kingdom JJP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009; 30:473-82; PMID:19375795; http://dx.doi.org/10.1016/j.placenta.2009.02.009
- Bulmer JN, Morrison L, Johnson PM. Expression of the proliferation markers Ki67 and transferrin receptor by human trophoblast populations. J Reprod Immunol 1988; 14:291-302; PMID:3225818; http://dx.doi.org/10.1016/0165-0378(88)90028-9
- Genbacev O, McMaster MT, Fisher SJ. A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol 2000; 157:1337-51; PMID:11021837; http://dx.doi.org/10.1016/S0002-9440(10)64648-2
- Graham CH, Lala PK. Mechanisms of placental invasion of the uterus and their control. Biochem Cell Biol 1992; 70:867-74; PMID:1297352; http://dx.doi.org/10.1139/o92-135
- Kurman RJ. The morphology, biology, and pathology of intermediate trophoblast A look back to the present. Hum Pathol 1992; 22:847-55; http://dx.doi.org/10.1016/0046-8177(91)90173-M
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003 Jul; 69(1):1-7; http://dx.doi.org/10.1095/biolreprod.102.014977
- Guzeloglu-Kaayisli O, Guzeloglu-Kaayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 2009; 27:62-79; PMID:19197806; http://dx.doi.org/10.1055/s-0028-1108011
- Pijnenborg R. The placental bed. Hypertension Pregnancy 1996; 15:7-23; http://dx.doi.org/10.3109/10641959609015685
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592-4; PMID:15947178; http://dx.doi.org/10.1126/science.1111726
- Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to preeclampsia and fetal injury. Placenta 2003; 24:575-87; PMID:12828917; http://dx.doi.org/10.1016/S0143-4004(03)00063-8
- Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development 2005; 132:4097-106; PMID:16107476; http://dx.doi.org/10.1242/dev.01971
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Path Bact 1967; 93:569-79; PMID:6054057; http://dx.doi.org/10.1002/path.1700930218
- Kam EPY, Gardner L, Loke YW, King A. The role of trophoblast in the physiological changes in decidual spiral arteries. Hum Reprod 1999; 14:213-2138; http://dx.doi.org/10.1093/humrep/14.8.2131
- Damsky CH, Fisher SJ. Trophoblast psedovasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998; 10:660-6; http://dx.doi.org/10.1016/S0955-0674(98)80043-4
- Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998; 19:241-52; PMID:9639319; http://dx.doi.org/10.1016/S0143-4004(98)90055-8
- Whitley GSTJ, Cartwright JE. Cellular and Molecular Regulation of Spiral Artery Remodelling: Lessons from the Cardiovascular Field. Placenta 2010; 31:465-74; PMID:20359743; http://dx.doi.org/10.1016/j.placenta.2010.03.002
- Pijnenborg R, Vercruysse L, Hanssens M. The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies. Placenta 2006; 27:939-58; PMID:16490251; http://dx.doi.org/10.1016/j.placenta.2005.12.006
- Shih IM, Wang TL, Wu TC, Kurman RJ, Gearhart JD. Expression of Mel-CAM in implantation site intermediate trophoblast cell line, IST-1, limits its migration on uterine smooth muscle cells. J Cell Science 1998; 111:2655-44; PMID:9701564
- Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knöfler M, Cartwright JE, Whitley GSTJ, Aplin JD. Trophoblast- and Vascular Smooth Muscle Cell-Derived MMP-12 Mediates Elastolysis during Uterine Spiral Artery Remodeling. Am J Pathol 2010; 177:2103-15; PMID:20802175; http://dx.doi.org/10.2353/ajpath.2010.100182
- Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta 2002; 23:359-72; PMID:12061851; http://dx.doi.org/10.1053/plac.2002.0819
- Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Semin Reprod Endocrinol 1998; 16:65-73; PMID:9654609; http://dx.doi.org/10.1055/s-2007-1016254
- Siwetz M, Dieber-Rotheneder M, Cervar-Zivkovic M, Kummer D, Kremshofer J, Weiss G, Herse F, Huppertz B, Gauster M. Placental fractalkine is up-regulated in severe early-onset preeclampsia. Am J Pathol 2015; 185:1334-43; PMID:25769431; http://dx.doi.org/10.1016/j.ajpath.2015.01.019
- Yagel S, Casper RF, Powel W, Parhar RS, Lala PK. Characterization of pure human first trimester cytotrophoblast cells in long term culture; growth pattern, markers and hormone production. Am J Obstet Gynecol 1989; 160:938-45; PMID:2469330; http://dx.doi.org/10.1016/0002-9378(89)90314-1
- Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-β at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod 1992; 46:561-72; PMID:1374270; http://dx.doi.org/10.1095/biolreprod46.4.561
- Irving JA, Lysiak JJ, Graham CH, Han VKM, Hearn S, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta 1995; 16:413-33; PMID:7479613; http://dx.doi.org/10.1016/0143-4004(95)90100-0
- Lysiak JJ, Hunt J, Pringle GA, Lala PK. Localization of transforming growth factor β and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta 1995; 16:221-31; PMID:7638106; http://dx.doi.org/10.1016/0143-4004(95)90110-8
- Xu G, Guimond MJ, Chakraborty C, Lala PK. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual product. Biol Reprod 2002; 67:681-9; http://dx.doi.org/10.1095/biolreprod67.2.681
- Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty C, Bhattacharjee RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology 2008; 149:6187-97; PMID:18703624; http://dx.doi.org/10.1210/en.2008-0780
- Yagel S, Parhar RS, Lysiak JJ, Lala PK. Normal nonmetastatic human trophoblast cells share in vitro invasive properties of malignant cells. J Cell Physiol 1988; 136:455-62; PMID:3170642; http://dx.doi.org/10.1002/jcp.1041360309
- Emonard H., Christiane Y, Smet M, Grimaud JA, Foidart JM. Type IV and interstitial collagenolytic activities in normal and malignant trophoblast cells are specifically regulated by extracellular matrix. Invasion Metastasis 1990; 10:170-77; PMID:2159447
- Moll UM, Lane BL. Proteolytic activity of first trimester human placenta: localization of interstitial callagenase in villous and extravillous trophoblast. Histochemistry 1990; 94:555-60; PMID:2178159; http://dx.doi.org/10.1007/BF00272621
- Graham CH, Lala PK. Mechanism of control of trophoblast invasion in situ. J Cell Physiol 1991; 148:228-34; PMID:1652588; http://dx.doi.org/10.1002/jcp.1041480207
- Bischof P, Friedli E, Martelli M, Campana A. Expression of extracellular-matrix degrading metalloproteinases by cultured human cytotrophoblast cells: Effects of cell adhesion and immunopurification. Am J of Obstet Gynecol 1991; 165:1791-801; http://dx.doi.org/10.1016/0002-9378(91)90034-O
- Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kD type IV collagenases in human trophoblasts: A possible mechanism for control of trophoblast invasion. Am J of Obstet and Gynecol 1994; 171:832-8; http://dx.doi.org/10.1016/0002-9378(94)90107-4
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92 kD type IV collagenase mediates invasion of human cytotrophoblasts. J of Cell Biol 1990; 113:437-49; http://dx.doi.org/10.1083/jcb.113.2.437
- Nawrocki B, Polette M, Marchand V, Maquoi E, Beorchia A, Tournier, JM, Foidart JM, Birembaut P. Membrane-type matrix metalloproteinase expression at the site of human placenta implantation. Placenta 1996; 17:565-72; PMID:8916204; http://dx.doi.org/10.1016/S0143-4004(96)80073-7
- Yagel S, Kerbel RS, Lala PK, Elder-Gara T, Dennis JW. Basement membrane invasion by first trimester human trophoblast: requirement for branched complex-type Asn-linked oligosaccharides. Clin Exp Metastasis 1990; 8:305-17; PMID:2112436; http://dx.doi.org/10.1007/BF01810677
- Lala PK, Graham CH. Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer Metastasis Rev 1990; 9:369-79; PMID:2097085; http://dx.doi.org/10.1007/BF00049525
- Lala PK, Lysiak JJ. Role of locally produced growth factors in human placental growth and invasion with special reference to transforming growth factors. In: Immunology of Reproduction, (ed.) Hunt JS. 1994; pp:57-81, New York, Springer-Verlag Inc
- Lala PK, Lysiak, JJ. Autocrine-paracrine regulation of human placental growth and invasion by locally active growth factors. In: Immunology of Human Reproduction. (eds.) Kurpisz M, Fernandez N. 1995; pp:235-59. Oxford, Bios. Scientific Publishers
- Lala PK, Graham CH, Lysiak JJ, Khoo NKS, Hamilton GS. TGF-β regulation of placental function. Troph Res 1998; 11:149-57
- Lala PK, Hamilton GS. Athanassiades A. Role of growth factors and other placental signals in extravillous trophoblast cell function. Troph Res 1998; 12:327-39
- Fisher SJ, Cui T, Zhang L, Hartman L, Grahl K, Guo-Yang Z, Tarpey J, Damsky CH. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol 1989; 109:891-902; PMID:2474556; http://dx.doi.org/10.1083/jcb.109.2.891
- Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol 1994; 164:550-61; PMID:8045351; http://dx.doi.org/10.1006/dbio.1994.1223
- Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, Quigley JP, French DL, Fisher SJ. Interleukin-1 β regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem 1994; 269:17125-31; PMID:8006017
- Yui J, Garcia-Lloret M, Brown AJ, Berdan RC, Morrish DW, Wegmann TG, Guilbert LJ. Functional long-term cultures of human term trophoblasts purified by column-elimination of CD-9-expressing cells. Placenta 1994; 15:231-46; PMID:8066048; http://dx.doi.org/10.1016/0143-4004(94)90015-9
- Loke YW, Gardner L, Grabowska A. Isolation of human extravillous trophoblast cells by attachment to laminin-coated magnetic beads. Placenta 1989; 7:221-31; http://dx.doi.org/10.1016/S0143-4004(86)80160-6
- Genbacev O, Schubach SA, Miller RK. Villous culture of first trimester human placenta: Model to study extravillous trophoblast (EVT) differentiation. Placenta 1992; 13:439-61; PMID:1470605; http://dx.doi.org/10.1016/0143-4004(92)90051-T
- Caniggia I, Lye SJ, Cross, JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrin 1997; 138:3976-86
- Hu Y, Dutz JP, MacCalman CD, Young P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role of IFN-gamma. J Immunol 2006; 177:8522-30; PMID:17142750; http://dx.doi.org/10.4049/jimmunol.177.12.8522
- Zdravkovic M, Aboagye-Mathiesen G, Guimond MJ, Hager H, Ebbesen P, Lala PK. Susceptibility of MHC class 1 expressing human extravillous trophoblast cells to killing by natural killer cells., Placenta 1999; 20:431-40; PMID:10419808; http://dx.doi.org/10.1053/plac.1999.0393
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206:204-11; PMID:7684692; http://dx.doi.org/10.1006/excr.1993.1139
- Lysiak JJ, Connelly IH, Khoo NKS, Stetler-Stevenson W, Lala PK. Role of transforming growth factor-α and epidermal growth factor (EGF) on proliferation and invasion by first trimester human trophoblast. Troph Res 1994; 8:455-67
- Lysiak JJ, Han VKM, Lala PK. Localization of transforming growth factor-α in the human placenta and decidua: role in trophoblast growth. Biol Reprod 1993; 49:885-94; PMID:8286584; http://dx.doi.org/10.1095/biolreprod49.5.885
- Lysiak JJ, Johnson GR, Lala PK. Localization of amphiregulin in the human placenta and decidua throughout gestation: role in trophoblast growth. Placenta 1995; 16::359-66; PMID:7567798; http://dx.doi.org/10.1016/0143-4004(95)90093-4
- Saji F, Azuma C, Kimura T, Koyama M, Ohashi K, Tanizawa O. Gene expression of macrophage colony stimulating factor and its receptor in human placenta and decidua. Am J Reprod Immunol 1990; 24:99-104; PMID:1707631; http://dx.doi.org/10.1111/j.1600-0897.1990.tb01046.x
- Kauma SW, Aukerman SL, Eierman D, Turner T. Colony stimulating factor-1 and c-fms expression in human endometrial tissues and placenta during the menstrual cycle and early pregnancy. J Clin Endocrinol Metabol 1991; 73:746-51; http://dx.doi.org/10.1210/jcem-73-4-746
- Daiter E, Pampfer S, Yeung YG, Barad D, Stanley ER, Pollard JW. Expression of colony-stimulating factor-1 (CSF-1) in the human uterus and placenta. J Clin Endocrinol Metabol 1992; 74:850-8; http://dx.doi.org/10.1210/jc.74.4.850
- Pampfer S, Daiter E, Barad D, Pollard JW. Expression of colony stimulating factor-1 receptor (c-fms) proto-oncogene product in the human uterus andplacenta. Biol Reprod 1992; 46:48-57; PMID:1532134; http://dx.doi.org/10.1095/biolreprod46.1.48
- Hamilton GS, Lysiak JJ., Watson AJ, Lala PK. Effects of colony stimulating factor (CSF)-1 on human extravillous trophoblast growth and invasion. J Endocrinol 1998; 159:69-77; PMID:9795343; http://dx.doi.org/10.1677/joe.0.1590069
- Athanassiades A, Hamilton GS, Lala PK. Vascular endothelial growth factor stimulates proliferation but not migration or invasiveness in human extravillous trophoblast. Biol Reprod 1998; 59:643-54; PMID:9716565; http://dx.doi.org/10.1095/biolreprod59.3.643
- Athanassiades A, Lala PK. Role of placental growth factor (PlGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 1998; 19:465-73; PMID:9778119; http://dx.doi.org/10.1016/S0143-4004(98)91039-6
- Han VKM, Bassett N, Walton J, Challis JRG. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membrane: evidence for IGF-IGFBP interaction at the feto-maternal interface. J Clin Endocrinol Metabol 1996; 81:2680-93
- McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK. Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein (s) and phosphorylation of MAPK. J Clin Endocrinol Metabol 2001; 86:3665-74; http://dx.doi.org/10.1210/jcem.86.8.7711
- Gleeson LM, Chakraborty C, McKinnon T, Lala PK. Insulin-like growth factor binding protein-1 stimulates human trophoblast migration by signalling through α5β1 integrin via MAPK pathway. J Clin Endocrinol Metab 2001; 86:2484-93
- Anim-Niyami N, Hills FA, Sooranna SR, Steer PJ, Johnson MR. A longitudinal study of maternal plasma insulin-like growth factor binding protein-1 concentrations during normal pregnancey and pregnancies complicated by pre-eclampsia. Hum Reprod 2000; 15:2215-9; PMID:11006202; http://dx.doi.org/10.1093/humrep/15.10.2215
- De Groot CJ, O'Brien TJ, Taylor RN. Biochemical evidence of impaired trophoblastic invasion of decidual stroma in women destined to have preeclampsia. Am J Obstet Gynecol 1996; 175:24-9; PMID:8694068; http://dx.doi.org/10.1016/S0002-9378(96)70245-4
- Grobman WA, Kazer RR. Serum insulin, insulin-like growth factor-I, and insulin-like growth factor binding protein-1 in women who develop preeclampsia. Obstet Gynecol 2001; 97:521-6; PMID:11275021; http://dx.doi.org/10.1016/S0029-7844(00)01193-5
- Hietala R, Pohja-Nylander P, Rutanen EM, Laatikainen T. Serum insulin-like growth factor binding protein-1 at 16 weeks and subsequent preeclampsia. Obstet Gynecol 2002; 95:185-9; http://dx.doi.org/10.1016/S0029-7844(99)00489-5
- Gratton RJ, Asano H, Han VKM. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with preeclampsia. Placenta 2002; 23:303-10; PMID:11969341; http://dx.doi.org/10.1053/plac.2001.0780
- Giudice LC, Martina NA, Crystal RA, Tazuke S, Druzin ML. Insulin-like growth factor binding protein-1 (IGFBP-1) at the maternal-fetal interface and IGF-I, IGFII, and IGFBP-1 in the circulation of women with severe pre-eclampsia. Am J Obstet Gynecol 1997; 176:751-7; PMID:9125598; http://dx.doi.org/10.1016/S0002-9378(97)70598-2
- Queenan JT, Kao LC, Arboldeda CE, Ulloa-Aqurre A, Golos TG, Cines DB, Strauss JF. Regulation of urokinase-type plasminogen activator production by cultured human cytotrophoblasts. J of Biol Chem 1987; 262:10903-6
- Zini JM, Murray SC, Graham CH, Lala PK, Barnathan ES, Mazar A, Henkin J, Cines DB, McCrae KR. Characterization of urokinase receptor expression by human placental trophoblast. Blood 1992; 79:2917-29; PMID:1316787
- Multhaupt HAB, Mazar A, Cines DB, Warhol MJ, McCrae KR. Expression of urokinase receptors by human trophoblast; a histochemical and ultrastructural analysis. Lab Invest 1994; 71:392-401; PMID:7933989
- Liu J, Chakraborty C, Graham CH, Barbin YP, Dixon SJ, Lala PK. Noncatalytic domain of uPA stimulates extravillous trophoblast migration by using phospholipase C, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp Cell Res 2003; 286:138-51; PMID:12729802; http://dx.doi.org/10.1016/S0014-4827(03)00089-2
- Lindoff C, Astedt B. Plasminogen activator of urokinase type and its inhibitor of placental type in hypertensive pregnancies and in intrauterine growth retardation: possible markers of placental function. Am J Obstet Gynecol 1994; 171:60-4; PMID:8030735; http://dx.doi.org/10.1016/S0002-9378(94)70078-8
- Chakraborty C, Barbin YP, Chakraborty S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAPKinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol 2003; 201:63-73; PMID:12706295; http://dx.doi.org/10.1016/S0303-7207(02)00431-8
- Nicola C, Timoshenko AV, Dixon J, Lala PK, Chakraborty C. EP1 receptor-mediated migration of the first trimester human extravillous trophoblast: the role of intracellular calcium and calpain. J Clin Endocrinol Metab 2005; 90:4736-46; PMID:15886234; http://dx.doi.org/10.1210/jc.2005-0413
- Nicola C, Chirpac A, Lala PK, Chakraborty C. Roles of Rho guanosine triphosphatase A, Rho kinases and extracellular signal regulated kinases (1/2) in Prostaglandin E2-mediated migration of first trimester human extravillous trophoblast. Endocrinology 2008; 149:1243-51; PMID:18079197; http://dx.doi.org/10.1210/en.2007-1136
- Nicola C, Lala PK, Chakraborty C. Prostaglandin(PG)E2-mediated migration of human trophoblast requires Rac1 and Cdc42 GTPases. Biol Reprod 2008; 78:976-82; PMID:18235104; http://dx.doi.org/10.1095/biolreprod.107.065433
- Hannan NJ, Jones RL, White CA, Salamonsen LA. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface. Biol Reprod 2006; 74:896-904; PMID:16452465; http://dx.doi.org/10.1095/biolreprod.105.045518
- Chen CP. Placental villous mesenchymal cells trigger trophoblast invasion. Cell Adhesion Migration 2014; 8:94-7; P; PMID:24622731; http://dx.doi.org/10.4161/cam.28347
- Cartwright JE, Tse IWK, Whitle GStJ. Hepatocyte Growth Factor Induced Human Trophoblast Motility Involves Phosphatidylinositol-3-Kinase, Mitogen-Activated Protein Kinase, and Inducible Nitric Oxide Synthase. Exp Cell Res 2002; 279:219-26; PMID:12243747; http://dx.doi.org/10.1006/excr.2002.5616
- Kauma SW, Bae-Jump V, Walsh SW. Hepatocyte growth factor stimulates trophoblast invasion: A potential mechanism for abnormal placentation in preeclampsia. J Clin Endocrinol Metab 1999; 84:4092-6; PMID:10566655
- Naghshvar F, Torabizadeh Z, Zadeh NM, Mirbaha H, Gheshlaghi P. Investigating the Relationship between Serum Level of s-Met (Soluble Hepatic Growth Factor Receptor) and Preeclampsia in the First and Second Trimesters of Pregnancy. Obstet Gynecol 2013; 2013:925062
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGFβ, IGF-II and IGFBP-1. Exp Cell Res 1995; 217:419-27; PMID:7535237; http://dx.doi.org/10.1006/excr.1995.1105
- Graham CH, McCrae KR, Lala PK. Molecular mechanisms controlling trophoblast invasion of the uterus. Troph Res 1993; 7:237-50
- Graham CH. Effect of transforming growth factor β in the plasminogen activator system in cultured first trimester human cytotrophoblast. Placenta 1997; 18:137-43; PMID:9089774; http://dx.doi.org/10.1016/S0143-4004(97)90085-0
- Xu G, Chakraborty C, Lala PK. Expression of TGFβ Signaling Genes in the Normal, Premalignant, and Malignant Human Trophoblast: Loss of Smad3 in Choriocarcinoma Cells. Biochem Biophys Res Commun 2001; 287:47-55; http://dx.doi.org/10.1006/bbrc.2001.5533
- Xu G, Chakraborty C, Lala PK. Restoration of TGF-β regulation of plasminogen activator inhibitor-1 in Smad3-restituted human choriocarcinoma cells. Biochem Biophys Res Commun 2002; 294:1079-86; PMID:12074587; http://dx.doi.org/10.1016/S0006-291X(02)00605-8
- Xu G, Chakraborty C, Lala PK. Reconstitution of Smad3 restores TGF-β response of tissue inhibitor of metalloprotease-1 upregulation in human choriocarcinoma cells. Biochem Biophys Res Commun 2003; 300:383-90; PMID:12504095; http://dx.doi.org/10.1016/S0006-291X(02)02845-0
- St.-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin, a transforming growth factor-β binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biol Reprod 1994; 51:405-13; PMID:7528549; http://dx.doi.org/10.1095/biolreprod51.3.405
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinol 1997; 138:4977-88
- Caniggia I, Grisaru-Gravnosky S, Kkuliszewsky M, Post M, Lye SJ. Inhibition of TGFβ3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest 1999; 103:1641-50; PMID:10377170; http://dx.doi.org/10.1172/JCI6380
- Nadeem L, Munir S, Fu G, Dunk C, Baczyk D, Caniggia I, Lye S and Peng C. Nodal Signals through Activin Receptor-Like Kinase 7 to Inhibit Trophoblast Migration and Invasion: Implication in the Pathogenesis of Preeclampsia. Am J Pathol 2011; 178:1177-89; PMID:21356369; http://dx.doi.org/10.1016/j.ajpath.2010.11.066
- Graham CH, Connelly I, MacDougall JR, Kerbel RS, Sterler-Stevenson WG, Lala PK. Resistance of malignant trophoblast cells to both the antiproliferative and antiinvasive effects of transforming growth factor-β. Exp Cell Res 1994; 214:93-9; PMID:8082752; http://dx.doi.org/10.1006/excr.1994.1237
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knöfler M. Tumor Necrosis Factor-α Inhibits Trophoblast Migration through Elevation of Plasminogen Activator Inhibitor-1 in First-Trimester Villous Explant Cultures. J Clin Endocrinol Metab 2004; 89:812-22; PMID:14764800; http://dx.doi.org/10.1210/jc.2003-031351
- Delorme MA, Xu L, Berry L, Mitchell L, Andrew M. Anticoagulant Dermatan Sulfate proteoglycan in the term human placenta. Thromb Res 1998; 90:147-53; PMID:9692613; http://dx.doi.org/10.1016/S0049-3848(98)00035-8
- Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A 1986; 83:7683-7; PMID:3484330; http://dx.doi.org/10.1073/pnas.83.20.7683
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 1998; 67:609-52; PMID:9759499; http://dx.doi.org/10.1146/annurev.biochem.67.1.609
- Stander M, Naumann U, Wick W, Weller M. Transforming growth factor-β and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res 1999; 296:221-7; PMID:10382266; http://dx.doi.org/10.1007/s004410051283
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997; 136:729-43; PMID:9024701; http://dx.doi.org/10.1083/jcb.136.3.729
- Bredrup C, Knappskog PM, Majewski J, Rødahl E, Boman H. Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Invest Ophthalmol Vis Sci 2005; 46:420-6; PMID:15671264; http://dx.doi.org/10.1167/iovs.04-0804
- Calmus ML, Macksoud EE, Tucker R, Iozzo RV, Lechner BE. A mouse model of spontaneous preterm birth based on the genetic ablation of biglycan and decorin. Reproduction 2011; 142:183-94; PMID:21502335; http://dx.doi.org/10.1530/REP-10-0387
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 1990; 346:281-4; PMID:2374594; http://dx.doi.org/10.1038/346281a0
- Hausser H, Groning A, Hasilik A, Schonherr E, Kresse H. Selective inactivity of TGF-β/decorin complexes. FEBS Lett 1994; 353:243-5; PMID:7957866; http://dx.doi.org/10.1016/0014-5793(94)01044-7
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J 1994; 302:527-34; PMID:8093006; http://dx.doi.org/10.1042/bj3020527
- Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ, Sharma M, Kambadur R, Nishimura T. Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun 2006; 340(2):675-80; PMID:16380093; http://dx.doi.org/10.1016/j.bbrc.2005.12.060
- Kishioka Y, Thomas M, Wakamatsu J, Hattori A, Sharma M, Kambadur R, Nishimura T. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J Cell Physiol 2008; 215:856-67; PMID:18163379; http://dx.doi.org/10.1002/jcp.21371
- Schmidt G, Hausser H, Kresse H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem J 1991; 280:411-4; PMID:1747115; http://dx.doi.org/10.1042/bj2800411
- Winnemoller M, Schmidt G, Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol 1991; 54:10-17; PMID:1827765
- Winnemoller M, Schon P, Vischer P, Kresse H. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol 1992; 59:47-55; PMID:1468447
- Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 carcinoma cells. J Biol Chem 1998; 273:3121-4; PMID:9452417; http://dx.doi.org/10.1074/jbc.273.6.3121
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 1999; 274:4489-92; PMID:9988678; http://dx.doi.org/10.1074/jbc.274.8.4489
- Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. Sustained downregulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem 2000; 275:32879-87; PMID:10913155; http://dx.doi.org/10.1074/jbc.M005609200
- Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem 2002; 38:35671-81; http://dx.doi.org/10.1074/jbc.M205317200
- Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem 2005; 280:32468-79; PMID:15994311; http://dx.doi.org/10.1074/jbc.M503833200
- Schonherr E, Sunderkotter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem 2005; 280:15767-72; PMID:15701628; http://dx.doi.org/10.1074/jbc.M500451200
- Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 2009; 185:743-54; PMID:19433454; http://dx.doi.org/10.1083/jcb.200901129
- Sulochana KN, Fan H, Jois S, Subramanian V, Sun F, Kini RM, Ge R. Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis. J Biol Chem 2005; 280:27935-48; PMID:15923192; http://dx.doi.org/10.1074/jbc.M414320200
- Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol 2011 Aug; 25(8):1431-43; http://dx.doi.org/10.1210/me.2010-0426
- Lala N, Gannareddy GV, Cloutier-Bosworth A, Lala PK. Mechanisms in Decorin Regulation of Vascular Endothelial Growth Factor-Induced Human Trophoblast Migration and Acquisition of Endothelial Phenotype. Biol Reprod 2012; 87:1-14; http://dx.doi.org/10.1095/biolreprod.111.097881
- Iozzo RV, Buraschi S, Genua M, Xu Shi-Q, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A. Decorin Antagonizes IGF Receptor I (IGF-IR) Function by Interfering with IGF-IR Activity and Attenuating Downstream Signaling. J Biol Chem 2011; 286:3412-21; http://dx.doi.org/10.1074/jbc.M111.262766
- Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol 2015; 43:15-26; http://dx.doi.org/10.1016/j.matbio.2015.01.023
- Davies CL, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res 2001; 62:26-42; PMID:11421658; http://dx.doi.org/10.1006/mvre.2001.2311
- Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG Aeschlimann D, Eble JA. Decorin Regulates Endothelial Cell Motility on Collagen I through Activation of Insulin-like Growth Factor I Receptor and Modulation of a2b1 Integrin Activity. J Biol Chem 2008; 283:17406-15; PMID:18413316; http://dx.doi.org/10.1074/jbc.M710025200
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 2002; 21:4765-77; http://dx.doi.org/10.1038/sj.onc.1205595
- Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, Eskandarian MR, Smeets M, Butany J, Pasterkamp G, et al. Decorin Inhibition of PDGF-Stimulated Vascular Smooth Muscle Cell Function: Potential Mechanism for Inhibition of Intimal Hyperplasia after Balloon Angioplasty. Am J Pathol 2003; 163:869-78; PMID:12937128; http://dx.doi.org/10.1016/S0002-9440(10)63447-5
- Yamamoto K, Ohga N, Hida Y, Maishi N, Kawamoto T, Kitayama K, Akiyama K, Osawa T, Kondoh M, Matsuda K, et al. Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. Br J Cancer 2012; Mar 13; 106(6):1214-23; http://dx.doi.org/10.1038/bjc.2012.59
- Yang EY, Moses H. Transforming growth factor-β induced changes in cell migration, proliferation and angiogenesis in chicken chorio-allantoic membrane. J Cell Biol 1990; 111:731-41; PMID:1696268; http://dx.doi.org/10.1083/jcb.111.2.731
- Mohan RR, Tovey JC, Sharma A, Schultz GS, Cowden JW, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularisation in vivo. PLoS One 2011; 6:26432; http://dx.doi.org/10.1371/journal.pone.0026432
- Fiedler LR, Eble JA. Decorin regulates endothelial cell-matrix interactions during angiogenesis. Cell Adhesion Migration 2009; 3:3-6; PMID:19372733; http://dx.doi.org/10.4161/cam.3.1.7275
- Santra M, Santra S, Zhang J, Chopp M. Ectopic decorin expression upregulates VEGF expression in mouse cerebral endothelial cells via activation of the transcription factors Sp1, HIF1alpha and Stat3. J Neurochem 2008; 105:324-37; PMID:18021292; http://dx.doi.org/10.1111/j.1471-4159.2007.05134.x
- Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 2010 Oct; 277(19):3864-75; http://dx.doi.org/10.1111/j.1742-4658.2010.07797.x
- Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer 2008; 123:2473-9; PMID:18798267; http://dx.doi.org/10.1002/ijc.23930
- Matsumine A, Shintani K, Kusuzaki K, Matsubara T, Satonaka H, Wakabayashi T, Iino T, Uchida A. Expression of decorin, a small leucine-rich proteoglycan, as a prognostic factor in soft tissue tumors. J Surg Oncol 2007; 96:411-8; PMID:17579351; http://dx.doi.org/10.1002/jso.20745
- Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP Schaefer L, Iozzo RV. Decorin Antagonizes the Angiogenic Network : concurrent inhibition of MET, hypoxia inducible factor 1a, vascular endothelial growth factor A, and induction of thromospondin and TIMP3. J Biol Chem 2012; 287:5492-506; PMID:22194599; http://dx.doi.org/10.1074/jbc.M111.283499
- Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 2008; 29:1435-40; PMID:18550571; http://dx.doi.org/10.1093/carcin/bgn141
- Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 2012; 33:326-30; PMID:22159220; http://dx.doi.org/10.1093/carcin/bgr293
- Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens, RT, McQuillan DJ, Iozzo RV. An antimetastatic role of decorin in breast cancer. Am J Pathol 2008; 173:844-55; PMID:18688028; http://dx.doi.org/10.2353/ajpath.2008.080275
- Nash MA, Loercher AE, Freedman RS. In vitro growth inhibition of ovarian cancer cells by decorin: synergism of action between decorin and carboplatin. Cancer Res 1999; 59:6192-6; PMID:10626812
- Koninger J, Giese NA, di Mola FF, Berberat P, Giese T, Esposito I, Bachem MG, Büchler MW, Friess H. Overexpressed Decorin in Pancreatic Cancer: Potential Tumour Growth Inhibition and Attenuation of Chemotherapeutic Action. Clin Cancer Res 2004; 10:4776-83; PMID:15269152; http://dx.doi.org/10.1158/1078-0432.CCR-1190-03
- Nandi P, Siddiqui MF, Han V, Lala PK. Possible role of decorin over-expression by decidual cells in pre-ecclampsia. Proc 36th Annual Meeting of the American soc Reprod Immunol Kingsto,ON, June 3-5, 2015, G26; Am J Reprod Immunol 2015; 73(suppl 2):48, ( Abstract). Full manuscript submitted for publication
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N Eng J Med 2004; 350:672-683; http://dx.doi.org/10.1056/NEJMoa031884
- Borbely AU, Daher S, Ishigai MM, Mattar R, Sun SY, Knöfler M, Bevilacqua E, Oliveira SF. Decorin and biglycan immunolocalization in non-villous structures of healthy and pathological human placentas. Histopathology 2014 Apr; 64(5):616-25; http://dx.doi.org/10.1111/his.12304
