Abstract
Growth cone guidance is driven by attractive and repulsive signaling cues. Until recently, repulsive signaling by semaphorins was thought to be mediated through Plexin receptors, whereas Slits-induced repulsion was solely mediated through Robo receptors. In a recent report published in Nature Neuroscience, Celine Delloye-Bourgeois and colleagues (2015) combined phenotypic analyses of transgenic mouse lines and in vitro biochemical experiments to identify PlexinA1 as a novel receptor for Slits. Strikingly, they uncovered for the very first time that the Slit2C-terminal fragment possesses some unique biological activity as binding partner for PlexinA1. Even more excitingly, the signaling cascade triggered by SlitC binding to PlexinA1 mediates growth cone collapse of commissural axons both in vivo and ex vivo and nicely complements Robo-Slit signaling in the developing spinal cord midline to prevent midline recrossing.
Abbreviations
| CNS | = | central nervous system |
| TRK | = | tropomyosine receptor kinases |
| MAPK | = | activated mitogen activated protein kinases |
| CGC | = | commissural growth cones |
| FP | = | ventral floor plate |
| Robo1/2 | = | Roundabout 1 and 2 |
| Sema | = | Semaphorin |
| P | = | phosphorylation site |
| GTP | = | guanosine triphosphate |
| ERK | = | extracellular-signal-regulated kinases |
| Nrp | = | neuropilin |
| FL | = | full length |
| C | = | C-terminal |
| N | = | N-terminal |
| DiI | = | 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate |
| WT | = | wild type. |
Navigation by Aversion: Dynamics of Commissural Axon Guidance
In the developing CNS (central nervous system), axons are guided to their future synaptic targets by a variety of secreted and cell-surface molecules and by the expression of guidance receptors on the growth cone plasma membrane.Citation1
Guidance receptors respond to signaling molecules by transducing the signal to intracellular cascades which, in turn, remodel the axonal cytoskeleton and allow for movement of the axon in the preferred direction.Citation2 Such a phenomenon may occur during development, when axons extend to reach their synaptic targets, or during adult stage following both injury or learning.
Commissural axons at the neural tube midline are a suitable model for studying axonal pathfinding.Citation3 As shown in , commissural axons extend past the ventral midline to establish functional connections with neurons which will innervate the contralateral side of the body.Citation4 Midline crossing by spinal commissural axons requires a tightly regulated switching of attractive and repulsive cues (including ephrins, semaphorins and netrins) and a proper level of specific receptors in the growth cone.Citation5 Upon receptor activation, growth cone motility is a result of tropomyosine receptor kinases (TRK) activated mitogen activated protein kinases (MAPK) signaling; MAPK in turn activate transcription factors that act upon the microtubule and cytoskeleton remodelling, adhesion to the extracellular environment and membrane turnover.Citation6
Figure 1. The figure illustrates a model of axonal midline crossing at the ventral floor plate of the neural tube. After axons have crossed the midline, recrossing is prevented by the combined action of Slit2C and PlexinA1. In PlexinA1−/− mutants, growth cones are either unable to cross the midline or re-cross it. Signaling of Slit2C via PlexinA1 after midline crossing is illustrated in the right-most panel. Abbreviations: P (phosphorylation site), GTP (guanosine triphosphate), ERK 1/2 (extracellular-signal-regulated kinase 1 and 2), Y1815 (Tyrosine residue in position 1815 in the amino acid sequence).
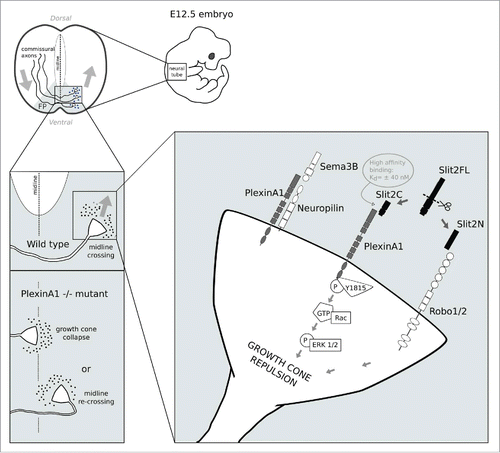
In the early stages of migration, axons are more sensitive to attractive cues and hence extend toward the ventral floor plate (FP). Soon after crossing the midline, they exhibit greater sensitivity to repulsive cues released by the FP, to which they were initially unresponsive, which leads them to grow dorsally and prevents them from re-crossing the midline.Citation7
This sensitivity switch is FP-mediated and triggers a change in the expression of a series of surface receptors found on the commissural growth cones (CGCs) of these neurons.Citation8
Among the known receptors involved in FP repulsion are the PlexinA1-Neuropilin2 receptor complex, which responds to signaling, and Roundabout 1 and 2 (Robo1/2) receptors, which respond to Slit proteins.Citation9 Sema3B belongs to the large semaphorin family of secreted and membrane proteins that function as repulsive cues in growth cone guidance—although some Semaphorins have also been reported to act as attractive cuesCitation10 and signal mainly through members of the Plexin family of transmembrane proteins.Citation11-12
Class 3 semaphorins bind to neuropilins which form receptor complexes with Plexins.Citation11 Other important proteins that function as repulsive cues in axonal guidance are Slit 1, 2 and 3, large extracellular matrix proteins expressed at the midline.
Their repulsive action was, up to recently, thought to be mediated exclusively by Robo receptors.Citation13 Both in vivo and in vitro, Slit molecules can be present in either full length (SlitFL), or as cleaved forms, which include a large N-terminal (Slit2N) and a small C-terminal (Slit2C) fragment.Citation14
Until recently, only the SlitFL and Slit2N mediated chemorepulsion had been reported, but the biological activity of SlitC remained elusive.Citation15-16 Recent research suggests that not all of the repulsive effects of Slits are mediated by Robos, since a knockout of all Slits results in more pronounced deficits than a knockout of Robo1 and Robo2, the 2 receptors of the Robo family that have a repulsive function in midline crossing.Citation17 The higher guidance errors in Slit−/− compared to Robo−/− mice suggest that, in addition to Robo1/2, certain Slits may have other receptors through which they exert their repulsive activity on CGCs.
Looking Further: Finding a Slit Receptor Other than Robo
Attention shifted to unconventional Slit receptors as potentially involved in commissural growth cone navigation after it was found that knocking out Robo1/2 induces fewer recrossing errors than Slit 1/2/3.Citation17 Hence, the research team led by Valerie Castellani decided to analyze in great detail the binding, signaling and functional properties of commissural neurons in transgenic mouse lines lacking PlexinA1, Slit1/2/3, Robo1/2 and Neuropilin2 (Nrp2) genes.Citation18 This comprehensive analysis of commissural axon trajectory at the midline revealed that PlexinA1-deficient mice exhibit a similar phenotype to robo-slit mutants, therefore placing PlexinA1 as a potential candidate for mediating Robo-independent Slit repulsion. Final validation of this novel signal-receptor interaction should implement in vitro binding assays, and complementary signal transduction experiments.
A New Ligand for an Old Receptor: The SlitC-PlexinA1 Interaction
First, anterograde tracing of commissural fibers was performed by insertion of DiI crystals into open-book preparations of E12.5 spinal cords isolated from PlexinA1-null and Sema3B-null mutant mice. This approach showed that PlexinA1−/− commissural neurons exhibited abnormal axonal guidance, including FP recrossing (Fig. 1), which was not observed in Sema3B−/− mice, pointing to a Sema3B-independent function of PlexinA1. As this phenotype was reminiscent of Robo-Slit knockouts,Citation19 the authors compared a double mutant missing Robo1 and Robo2 and a triple mutant missing Robo1/2 and PlexinA1 and observed a significant increase in the FP recrossing phenotype in the triple, compared to the double mutant.Citation18 Next, coimmunoprecipitation experiments on E12.5 mouse spinal cord lysates showed that both the Slit2-FL (full length) and Slit2C (C-terminal) fragments, but not SlitN (N-terminal), co-precipitated with PlexinA1, thus indicating a specific in vivo interaction between PlexinA1 and Slit2C. In line with these data, in vitro binding assays performed on COS7 cells transfected with PlexinA1 and incubated with conditioned medium from HEK293T cells overexpressing AP-tagged Slits confirmed a nanomolar affinity of Sit2C (but not of Slit2N) for PlexinA1. Further experiments in transfected COS7 cells determined that Slit2C co-precipitated with the PlexinA1 extracellular domain. Moreover, treating cultured neurons isolated from E12.5 WT and Robo-null mice with Slit2C induced a collapse of CGCs, which was abolished in PlexinA1−/− cultures. Similarly, co-cultured dorsal spinal cord explants and cell aggregates secreting the different Slit fragments showed that CGCs were repelled by Slit2C in WT, but not in PlexinA1−/− explants, further suggesting that PlexinA1 interacts specifically with the Slit-C fragment. Furthermore, the authors employed a bioinformatics prediction-guided generation of single, double and triple tyrosine mutants and assessed the receptor phosphorylation after treatment with Slit2C. This approach revealed that Slit2C induced PlexinA1 phosphorylation on the Y1815 tyrosine residue of its intracellular domain (Fig. 1). The importance of Y1815 phosphorylation for growth cone collapse was further confirmed after Y1815 substitutions resulted in significant collapse response decrease. Finally, Slit2C induced growth cone collapse in isolated commissural neurons of both WT and Nrp2−/− mice and co-precipitated with PlexinA1-only expressing cells, but not PlexinA1-Nrp2 expressing cells, suggesting that PlexinA1-Slit signaling does not depend on the presence of Nrp2.
A Novel Signaling Pathway: Implications and Future Perspectives
In this publication, the authors employed various biochemical, functional and phenotypic analysis to demonstrate that Slit2C is biologically active through PlexinA1 and is responsible for chemorepulsion during midline recrossing.Citation18 Slit2C was formerly thought not to play a significant role during midline crossing, but its novel role through PlexinA1 signaling enhances our understanding of growth cone guidance mechanisms.
Importantly, the authors showed that Slit2N and Slit2C bind to distinct receptors and the repulsive activity of Slit2C is independent of Robo and Nrp2 receptors. Furthermore, the mechanisms of Plexin activation by Slit2C differ from those by Sema3B. The growth cone collapsing activity of both Plexin-Slit2C and Plexin-Sema3B hence depends on different phosphorylation sites, triggers specific intracellular signaling cascades and selectively contributes to growth cone guidance.
One of the pitfalls in axonal guidance research is the transfer of findings from in vitro models to in vivo models, since the efficiency of signaling molecules in axonal pathfinding is spatio-temporally determined. Here, the reported role of SlitA2C-PlexinA1 dependent growth cone dynamics was demonstrated in vivo, highlighting the significance of the findings.
However, the newly discovered role of Slit2C signaling through PlexinA1 raises several questions. Firstly, the mechanisms underlying the cleavage of SlitFL into SlitC and SlitN needs further investigation with a special focus on SlitN, SlitC and SlitFL spatiotemporal distribution with a particular emphasis on their relative expression and availability. So far, a recent study revealed the restricted position of the SlitC and SlitFL in E11 embryo sections in the floor plate and in a gradient along the basal surface of the spinal cord.Citation20
Secondly, the potential additive action of Slit2C-PlexinA1 and Slit2N-Robo1/2 deserves to be further investigated to fully elucidate the potential benefit of such a synergistic mechanism. Furthermore, Robo3.2 (a splice variant of the Robo3 receptor also acting as a classical Slit receptor) might contribute to growth cone repulsion after midline crossing.Citation17
Despite the ability of all PlexinA receptor subtypes to bind SlitC in vitro, the authors suggest that PlexinA1 is the Slit receptor in spinal commissures. Further analysis of its structural features and expression profile differences among PlexinAs could enhance our understanding of the specific contribution of PlexinA1 during commissural axon guidance and provide new insights on the possible roles of other PlexinAs in various biological contexts.
Moreover, one could further investigate the intracellular Rho GTPase signaling pathway, as it has been shown that Rac acts upstream and downstream of PlexinA1 by modulating growth cone collapse in Sema3a-PlexinA1 signalling18 and SlitC-PlexinA1 signaling.Citation21
Furthermore, it would be interesting to examine the role of PlexinA1-Slit2C interactions beyond the FP crossing model. For example, studying this complex in spinal cord longitudinal neurons might provide interesting insights, given they run close to the FP and that Slits play a role in their navigation,Citation22 or at the level of the corpus callosum for cortico-cortical projection guidance.Citation23 Such experiments could help to elucidate whether the chemorepulsion mediated by the PlexinA1 complex is determined by its high concentration at the FP or whether it may be required elsewhere along the midline. Another possible target for future research could be the interaction of PlexinA1 complexes with extracellular proteoglycans, which modulate growth cone chemosensitivity.Citation24
Another variable contributing to axonal guidance organization was discovered by Wright et al. (2012) who hypothesized that dystroglycans are important for the organization of endogenous Slit proteins at the floor plate. Indeed, the authors could demonstrate that the interaction between dystroglycans and the C-terminal LG domain present in SlitC and SlitFL (but not in SlitN) is an essential factor in determining the distribution of Slit proteins at the floor plate during midline crossing of commisural axons.Citation20 Thus, determining the interaction of SlitC and dystroglycans while searching for more patterning molecules for Slits could provide interesting insights on the fine tuning of the spatiotemporal regulation of midline crossing and extend our understanding of axonal guidance disorders.
The findings of this work might be of special importance for research outside the nervous system, since both Semaphorin-Plexin and the Slit-Robo signaling pathway have important regulatory functions in tumorigenesis, cancer progression and immune regulation.Citation25,26 Recent research indicates that there is frequent dysregulation of axonal guidance molecules, including Slits and Semaphorins, during tumor progression and tumorigenesis.Citation27 In fact, several reports implicate the Slit/Robo pathway as a regulator for multiple oncogenic signaling pathways. However, the effects of Slits vary in different systems, which suggests activation of distinct signaling cascades. Current data implies that Slit2 inhibits tumor progression in most tumors.Citation25
In the context of the newly discovered functional interaction of Slit2C and PlexinA1, it would be interesting to investigate if this signaling is responsible for the antitumor effects of Slit2. Notably, the present study substantially contributes to our current understanding of chemorepellent processes occurring in the neural tube floor plate during axon guidance and may also have important implications for cancer research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Dominique Bagnard for his guidance and for his thoughtful comments.
Funding
The Joint Master in Neuroscience is supported by NEUREX (www.neurex.org) and by a specific IDEX (Excellence Initiative) pedagogy grant from the University of Strasbourg.
References
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 2007; 56:807-22; PMID:18054858; http://dx.doi.org/10.1016/j.neuron.2007.10.019
- Nawabi H, Briançon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev 2010; 24:396-410; PMID:20159958; http://dx.doi.org/10.1101/gad.542510
- Black DL, Zipursky SL. To cross or not to cross: alternatively spliced forms of the Robo3 receptor regulate discrete steps in axonal midline crossing. Neuron 2008; 58:297-8; PMID:18466738; http://dx.doi.org/10.1016/j.neuron.2008.04.019
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Neuron 1995; 14:941-8; PMID:7748561; http://dx.doi.org/10.1016/0896-6273(95)90332-1
- Nawabi H, Castellani V. Axonal commissures in the central nervous system: how to cross the midline? Cell Mol Life Sci 2011; 68:2539-53; PMID:21538161; http://dx.doi.org/10.1007/s00018-011-0691-9
- Vitriol EA, Zheng JQ. Growth cone travel in space and time. Neuron 2012; 73:1068-81; PMID:22445336; http://dx.doi.org/10.1016/j.neuron.2012.03.005
- Dickson BJ, Zou Y. Navigating intermediate targets: the nervous system midline. Cold Spring Harb Perspect Biol 2010; Aug;2(8):a002055; PMID:20534708; http://dx.doi.org/10.1101/cshperspect.a002055
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: A role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 2000; 102:363-75; PMID:10975526; http://dx.doi.org/10.1016/S0092-8674(00)00041-6
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 2011; Mar; 3(3):a001800; PMID:21106647; http://dx.doi.org/10.1101/cshperspect.a001800
- Fiore R, Püschel AW. The function of semaphorins during nervous system development. Front Biosci 2003; 8:484-99; http://dx.doi.org/10.2741/1080
- Yazdani U, Terman JR, The semaphorins. Genome Biol. 2006; 7:211; PMID:16584533; http://dx.doi.org/10.1186/gb-2006-7-3-211
- Negishi M, Katoh H. Rho family GTPases as key regulators for neuronal network formation. J Biochem 2002; 132:157-66; PMID:12153710; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a003205
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the Robo receptor in Drosophila. Cell 1999; 96:785-94; PMID:10102267; http://dx.doi.org/10.1016/S0092-8674(00)80589-9
- Ba-Charvet KTM, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and Specificity of Actions of Slit2 Proteolytic Fragments in Axon Guidance. J Neurosci 2001; 21:4281-9; PMID:11404413
- Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci 2001; 21:1548-56; PMID:11222645
- Chédotal A. Slits and their receptors. Adv Exp Med Biol 2007; 621:65-80; http://dx.doi.org/10.1007/978-0-387-76715-4_5
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci 2010; 30:9445-53; PMID:20631173; http://dx.doi.org/10.1523/JNEUROSCI.6290-09.2010
- Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci 2015; 18:36-45; PMID:25485759; http://dx.doi.org/10.1038/nn.3893
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 2004; 42:213-23; PMID:15091338; http://dx.doi.org/10.1016/S0896-6273(04)00179-5
- Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 2012; 76:931-44; PMID:23217742; http://dx.doi.org/10.1016/j.neuron.2012.10.009
- Turner LJ, Nicholls S, Hall A. The Activity of the Plexin-A1 Receptor Is Regulated by Rac. J Biol Chem 2004; 279:33199-205; PMID:15187088; http://dx.doi.org/10.1074/jbc.M402943200
- Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development 2008; 135:3643-53; PMID:18842816; http://dx.doi.org/10.1242/dev.023325
- Shu T, Sundaresan V, McCarthy MM, Richards LJ. Slit2 Guides Both Precrossing and Postcrossing Callosal Axons at the Midline In Vivo. J Neurosci 2003; 23:8176-84; PMID:12954881
- Conway CD, Howe KM, Nettleton NK, Price DJ, Mason JO, Pratt T. Heparan Sulfate Sugar Modifications Mediate the Functions of Slits and Other Factors Needed for Mouse Forebrain Commissure Development. J Neurosci 2011; 31:1955-70; PMID:21307234; http://dx.doi.org/10.1523/JNEUROSCI.2579-10.2011
- Gara RK, Kumari S, Ganju A, Yallapu MM, Jaggi M, Chauhan SC. Slit/Robo pathway: a promising therapeutic target for cancer. Drug Discov Today 2015; 20:156-64; PMID:25245168; http://dx.doi.org/10.1016/j.drudis.2014.09.008
- Műzes G, Sipos F. Relation of immune semaphorin/plexin signaling to carcinogenesis.Eur J Cancer Prev 2014; 23:469-76; http://dx.doi.org/10.1097/CEJ.0000000000000059
- Harburg GC, Hinck L. Navigating breast cancer: axon guidance molecules as breast cancer tumorsuppressors and oncogenes. J Mammary Gland Biol Neoplasia 2011; 16:257-70; PMID:21818544; http://dx.doi.org/10.1007/s10911-011-9225-1
