ABSTRACT
The use of synthetic surfaces and materials to influence and study cell behavior has vastly progressed our understanding of the underlying molecular mechanisms involved in cellular response to physicochemical and biophysical cues. Reconstituting cytoskeletal proteins and interfacing them with a defined microenvironment has also garnered deep insight into the engineering mechanisms existing within the cell. This review presents recent experimental findings on the influence of several parameters of the extracellular environment on cell behavior and fate, such as substrate topography, stiffness, chemistry and charge. In addition, the use of synthetic environments to measure physical properties of the reconstituted cytoskeleton and their interaction with intracellular proteins such as molecular motors is discussed, which is relevant for understanding cell migration, division and structural integrity, as well as intracellular transport. Insight is provided regarding the next steps to be taken in this interdisciplinary field, in order to achieve the global aim of artificially directing cellular response.
Introduction
Synthetic biology is an emerging interdisciplinary field which is of paramount importance in several research fields, including regenerative medicine, cell therapy and genetic and tissue engineering. The global aim of synthetic biologists is to exploit the complex behavior of natural biological systems and extend to artificial systems, in order to either understand better the native biological function or for use in a large variety of biomedical or therapeutic applications.Citation1 In the context of tissue engineering and regenerative medicine, a chief hurdle to overcome is the ability of the replacement tissue to function similarly to that which should occur in vivo. However, a major challenge which has impeded successful tissue regeneration clinically, is the lack of control and reproducibility over artificially directing stem cell fate.Citation2 Achieving this will pave the way for innumerable regenerative therapies. In order to overcome these challenges, researchers must go back to the fundamentals and study the influence of each individual parameter of the surrounding substratum on cellular behavior.
Historically, the complexity of in vivo conditions led to in vitro techniques whereby cells are removed from the organism and their properties are measured in a controlled environment. This allows for precise control over external parameters (temperature, pH etc.) and thus their effect on cellular behavior can be quantitatively studied. However, it has long been reckoned that cell behavior measured outside the living organism using the conventional “flat glass” in vitro experiment is markedly different to that which occurs in vivo. This is because many cellular processes cannot be precisely replicated outside of the native environment. These challenges have warranted the use of synthetic biology techniques in order to successfully replicate the desired biological function. As we will highlight throughout this review, this is only possible when the synthetic material mimics the native 3D extracellular environment.
Interaction of cells with their microenvironment
Understanding cellular response to the properties of its extracellular environment (or extracellular matrix (ECM)) is fundamentally important for tissue engineering/regenerative medicine applications, i.e., for biocompatibility of implants or tissue replacements. In addition, it is now well-known that stem cell fate is influenced by the physical characteristics of its surrounding microenvironment.Citation3-5 However, obtaining a complete understanding of cell-substratum response is extremely difficult due to the complexity of these processes. Therefore, investigating the effect of specific parameters of the ECM on cellular response in an in vitro environment is an attractive option, since defined boundary conditions can be set. The past decade has seen a surge in research using artificial microenvironments to investigate cell response.Citation6,7
Most cells are anchorage dependent, and are in need of a substrate/surface to adhere to in order to function normally. Surfaces play a vital role in biology since most biological reactions occur at a surface or interface.Citation8 The interaction between the ECM and adhered cells is a functional one and they work together to yield a dynamic system capable of self-organization. For example, osteoblasts and osteoclasts adhered to a hydroxyapatite and collagen composite yields the capability of bone to remodel itself.Citation9 In general, cells are subjected to a huge variety of stimuli in their local environment such as charges present on the surface of the substrate or in the surrounding medium and stresses induced from the motion of surrounding fluid or neighboring cells/tissues. At the same time, they adhere to a mechanical substrate of a certain stiffness, topology and chemical composition. Huge research efforts are dedicated to understanding these effects, and experimental results have shown that each situation (e.g. stiff vs. soft substrate,Citation10 flat vs. roughened topology,Citation11 positive vs. negatively charged surface,Citation12 static vs. stretched surfaceCitation13 etc.) plays a role in governing cellular dynamics and function.
Local topology effect on cells
A significant drawback of the traditional in vitro experiment is that cellular response is examined from cells adhered to 2-dimensional (2D) planar glass substrates or well-plates, whereas cells in living tissue are interacting with a 3-dimensional (3D) ECM. Cell migration, polarization, morphology and adhesion have all been shown to be influenced by the topographical properties of the substrates they are surrounded by.Citation14 And strikingly, it was shown that cells adhered to surfaces presenting nano- and microscale topographical details compared to 2D planar substrates more closely mimicked the cell migratory behavior seen in a 3D ECM.Citation15 Research efforts concentrated on engineering cell-compatible 3D biomaterials are largely focused on having micro- and nanoscale surface features, since now there is a wealth of evidence showing local topographic control of cell function. Studies of fibroblast interactions with topographic nanostructures revealed their preference for wedge/nanograte topographies as opposed to nanoposts.Citation16 Fibroblasts have also been shown to co-align and polarize along the axial direction of collagen fibers, whereas fibroblasts adhered to randomly oriented collagen fibers have an anisotropic orientation.Citation17 Perhaps even more significant, increased proliferation of fibroblasts adhered to aligned collagen scaffolds compared to the random scaffold was shown, with fibroblast morphology on aligned collagen comparing well with that seen in native tissues.Citation18 Elias et al. showed that osteoblasts proliferate significantly more on a network of ∼100 nm diameter carbon fibers rather than on flat glass.Citation19 An increase in the synthesis of alkaline phosphatase and deposited calcium was also reported on the nanofibers, both of which are essential for bone formation in vivo.
Considering the size of the topographical details that cells sense are typically the same order of magnitude as the cell itself, this results in cell contact guidance. However, there exists a fine limit as shown by a study on human mesenchymal stem cell (hMSC) adhesion, where cell attachment was observed to be a factor of 2 lower on surfaces having an isotropic roughness of the same size as the cell, as opposed to above or below that of the cell size.Citation20 Indeed, it is evident from the wide variety of literature on the topic that different cell types all react to topology changes, but not to the same degree.21 Progress made in microfabrication techniques such as lithography, machining and etching has allowed for precise control over nanoscale surface features (e.g., cells on pillars,Citation22,23 grooves,Citation11,24 ridges,Citation25 pits,Citation26 gratings,Citation27 fibersCitation28) and has massively advanced our knowledge on the local topology effect on cellular response. In addition, these surfaces have many other applications in synthetic biology. For example, cells seeded on surfaces presenting micropillars/needles allow for direct measurement of cell traction forces.Citation22 This ground-breaking study revealed a close link between biochemical and mechanical cues which regulate cell adhesion and mechanics. More recently, the experimental combination of live cells on micropillars with super-resolution microscopy has been demonstrated and allows for the relation between cell traction forces and the number of molecules (fluorescently tagged paxillin in this case) in a focal adhesion complex to be determined.Citation29
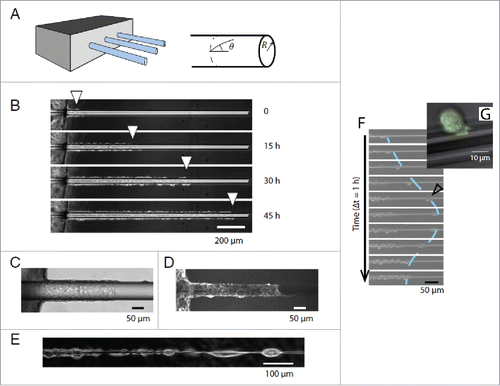
However, nanotopographic surfaces do not take into account an out-of-plane component which is relevant for certain cell types e.g. epithelial cells, which form curved assemblies e.g., tubular vessels. In vivo investigations of melanoma invasion have yielded important insights into the relevance of topographical details on cancer cell invasion.Citation30 Two types of topographic track systems were defined which result in either individual or collective migration of invasive cells. The mechanisms of this topographic cue were investigated using an in vitro experiment as seen in , and it was revealed that collective migration along a cylindrical wire is due to lateral confinement of cells, mimicking in vivo collective migration along blood vessels.Citation31 However, as seen from , topographic curvature can induce front edge cell detachment which is similar to the Epithelial-Mesenchymal Transition, and thus out-of-plane topographical properties may be a cue of this transition.
Figure 1. (A) Experimental setup to investigate migration of epithelial cells along glass wires. (B) Cells invade wire collectively at radii = 20 µm (C) Phase contrast and (D) Fluorescence images of the collective migration of cells. (E) For thin wires (radius< 5 µm) cells migrate individually in the form of a chain of cells. (F) For radius < 40 µm cells occasionally detached and migrated individually before returning to the monolayer. (G) Zoom of detached cell in fastest migration mode. Reproduced from ref. Citation31 with permission.
Effect of substrate stiffness
Huge progress has been made using synthetic biology approaches on achieving a better understanding of the underlying molecular mechanisms governing cell response to substrate stiffness. Almost 2 decades ago, a ground-breaking study acknowledged that fibroblasts and epithelial cells responded to the stiffness of their substrate,Citation32 where a reduction in cell spreading and increased motility on more flexible substrates was shown. Afterwards, many aspects of cell behavior were found to be regulated by the stiffness of the cell adhering substrate.Citation10,33 MSCs have been shown to differentiate into a lineage specific to the stiffness of their substrate.Citation4 Also, tuning the elastic moduli of hydrogels had an effect on the spreading, self-renewal and differentiation of adult neural stem cells.Citation34 Substrate stiffness is also known to affect the morphology, cytoskeletal structure, migration, proliferation and adhesion of fibroblasts, endothelial cells and neutrophils.Citation35 Lamin levels – an intermediate filament in the nucleus responsible for nuclear integrity and modulating transcription – in stem cells were also shown to be directly influenced by substrate stiffness.Citation36 The stiffness of the cellular environment can also have global physiological implications, as shown by recent results which demonstrate striation and contractile strains in cardiac muscle cells are optimized by substrate stiffness.Citation37,38
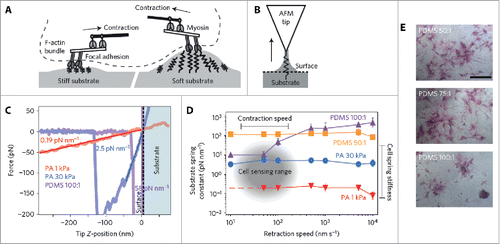
Determining a complete picture of the underlying mechanisms of cell response to substrate stiffness, however, has proven to be elusive. But recent research efforts have challenged the view that stem cell differentiation can be directed due to the cell responding to changes in only bulk matrix elasticity. Huebsch et al. reported a feedback mechanism of stem-cell fate where the cell itself interprets changes in the microenvironment through the presentation of adhesion-ligand complexes.Citation39 This finding was supported by a subsequent study which showed that collagen attached to porous substrates of varying stiffnesses leads to differences in collagen-coupled anchorage density, and the authors conclude it is this parameter which determines cell fate.Citation40 Strikingly, the authors reported that the differentiation of 2 stem cell lines from different tissues were both influenced by the stiffness of polyacrylamide (PAAm) but not polydimethylsiloxane (PDMS) substrates – indicating for the first time that bulk stiffness might not be not responsible for cell differentiation stimulus. The authors describe a mechanism whereby cells sense their environment by exerting a force on the ECM which provides the cell with the information to make cell-fate decisions. However, these insights were recently partly challenged when it was demonstrated that substrate porosity and protein tethering had no effect on stem cell differentiation.Citation41 In the latter study indications are provided that cells sense substrate stiffness using a mechanical feedback mechanism based on the magnitude of surface deformation, as shown in . These experiments confirm previous observations using FRET, which showed that cell traction forces influence the proliferation and differentiation of preosteoblasts.Citation42 Furthermore, mechanosensitive membrane ion channels can be triggered by cell traction forces which induce ionic currents relevant for signaling,Citation43 and recently the stretch-activated ion channel Piezo1 was shown to be a determinant of lineage choice in human neural stem cells.Citation44 These studies highlight the growing evidence of an electromechanical signaling mechanism of stem cell fate in response to its microenvironment. While these studies converge upon a force feedback mechanism of cell mechanotransduction, a very recent study has highlighted a potential oversight of using purely elastic materials as substrates. Chauduri et al. argue that the ECM in vivo is viscoelastic in nature and provide evidence of increased cell spreading on materials exhibiting stress relaxation, or higher viscoelasticity.Citation45
Figure 2. Cells sense their surrounding environment by contracting against the substrate. (A) Schematic illustrating dynamic deformation of stiff and soft substrates via myosin contraction in adhered cells. (B) AFM schematic illustrating the interaction between an AFM tip and the substrate surface. Arrow indicates pulling direction during experiments. (C) Retraction curves for substrates of different stiffnesses. (D) Spring constant of substrates as a function of retraction speed. The sensing range of cells is highlighted in the graph and is based on previous measurements of myosin contraction speeds. (E) ALP staining on PDMS substrates after 7 d. Reproduced from ref. Citation41 with permission. © 2014 Nature Publishing Group.
Polarity of charged substrate influences cell behavior
Electrical charge plays a very important role in cell proliferation and has been shown to be crucial in the bone remodeling process in conjunction with Wolff's Law (whereby bone adapts itself under load).Citation46 Strain-generated potentials observed in bone are considered to play a critical role in bone deposition and resorption.Citation47 Indeed, pulsed electromagnetic field therapy has been used in a clinical environment to heal bone fractures dating back to as early as 1841, yet the molecular origin of this healing mechanism remains elusive. In addition, the use of polarized hydroxyapatite and electrically active piezoelectric materials (generate charge under an applied stress) as implants is increasing due to their ability to increase osseointegration.Citation48 Therefore, the role of charge on cellular behavior is significantly important from both a fundamental and clinical viewpoint. In fact, piezoelectric materials are being widely adapted for use as scaffolds for tissue regeneration and repair applications and have even been shown to influence gene expression in the case of cartilaginous implants utilizing the piezoelectric polymer polyvinylidene fluoride (PVDF).Citation49,50 Fibroblasts grown on piezoelectrically-activated (presenting charge on surface) implants show enhanced migration, adhesion and secretion and the implants themselves showed higher fibrosis levels in rats in vivo, illustrating wound healing capabilities.Citation51
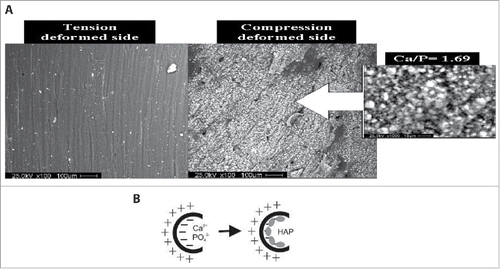
However, in order to elucidate the fundamental basis for the observed improved cellular function due to charge, synthetic biology techniques can be employed. The ferroelectric properties of certain crystals such as Lithium Niobate have been exploited recently to investigate the role of charge on cell behavior, and show great promise.Citation52 Ferroelectric materials have a spontaneous polarization which can be switched by an externally applied electric field. Therefore modulating polarization domains of user-specified size and shape are possible and this combined with the possibility of optically transparent samples yields a substrate ideal for cell-charge experiments. An in vitro investigation of the role of piezoelectrically-induced charges in deformed cortical bone collagen revealed that mineralization can occur in the presence of simulated body fluid, even in the absence of osteoblasts, as shown in .Citation53 Oppositely charged surfaces appear on the compressed and tensed faces of deformed bone, and these charges alone were sufficient to deposit calcium and phosphorus ions which led to hydroxyapatite precipitation purely from an electrochemical mechanism, as shown in . Therefore, these studies highlight the important role that charge plays in many biological processes and yet, this effect is often overlooked when considering replacement tissues.Citation54 Given the growing evidence that electromechanical cues are the most relevant for tissue engineering applications,Citation55 there is significant interest in producing active rather than conventional passive biomaterials.
Figure 3. In vitro deposition on hydroxyapatite on deformed cortical bone collagen via piezoelectric effect. (A) Tensed deformed face of cortical bone collagen subjected to simulated body fluid for 4 weeks shows no hydroxyapatite precipitation whereas compressed side shows significant mineralization. (B) Schematic illustrating the electrochemical biomineralization model of deformed bone leading to hydroxyapatite deposition. Reproduced from ref. Citation53 with permission. © 2007 American Chemical Society.
Effect of surface chemistry
Determining cell fate is a prerequisite for successful synthetic biology approaches. For example, in the context of regenerative medicine, metals (typically titanium) and metallic alloys are widely used for implants and the cytotoxic nature of the added constituents, e.g. aluminum, is a cause of concern regarding long term biocompatibility. Typically these alloys undergo surface treatments such as polishing, sterilization, machining and acid etching before implantation, in order to improve osseointegration.Citation56 However, surface treatments can alter the oxide layer and the surface chemical properties of the implant which subsequently affects the integration of the implant in the body.Citation57 This effect is also relevant for cases other than inorganic implants, as the type of sterilization method used for bioabsorbable polymers has been shown to influence adipose stem cell behavior.Citation58 The surface properties will also determine the adsorbed species from the surrounding medium and will thus influence the conformation and activity of biological adsorbents. The conformation of adsorbed fibronectin was shown to be influenced by the presentation of monolayers on the surface comprising different chemical functional groups (CH3, OH, COOH and NH2) which positively affected cell adhesion via cell transmembrane integrin binding and increased focal adhesions,Citation59,60 and even influenced differentiation (in the case of OH and COOH only, rather than CH3 and NH2 monolayers).Citation61 Considering that conventional approaches to engineer implants utilize bioactive coatings such as fibronectin,Citation62,63 the addition of an underlying monolayer of COOH or OH could greatly improve biocompatibility and function. In addition, synthetic materials that display adhesive motifs, e.g., Arg-Gly-Asp (RGD) peptides, can be used to influence cell behavior as they have a high binding affinity to integrins. Hydrogel surfaces with a controlled density of RGD containing peptides increase cell spreading by ligating integrins and can also be used as a model system to study cell traction forces using traction force microscopy.Citation64 Controlling ligand density was also shown to dictate growth factor activity.Citation65 Integrins have been well-studied as their vital role in cell adhesion and bi-directional signaling has been demonstrated numerous times.Citation66,67 Integrin clustering has also been shown to play a huge role in adhesion-based cell signaling,Citation68 and a seminal study using a synthetic surface with controlled separation of RGD-coated nanodots showed that a separation range of 58–73 nm between adhesive dots allowed for integrin clustering and activation.Citation69 Furthermore, different classes of integrins have recently been demonstrated to induce different signaling pathways but it was shown that they also cooperatively regulate cells to sense the rigidity of fibronectin environments.Citation70 The differences in integrin regulation was subsequently determined to be due to different bond dynamics between the integrin classes.Citation71 Micro- and nanopatterned surfaces functionalized with integrin ligands have been used to investigated integrin-based adhesion of fibroblasts.Citation72 This study revealed that the spatial organization of ligands plays a crucial role in cellular adhesion, with local, rather than global, ligand density promoting focal adhesion formation. This dependency on the interligand spacing is critical for designing biocompatible biomedical implants.
Investigation of in-vitro cytoskeletal constituents
The previous section reviewed the progress made in understanding cellular response by utilizing a defined synthetic environment; here, properties of intracellular components responsible for cell migration, adhesion, division and structural integrity, i.e., the cytoskeleton, is investigated in vitro using synthetic approaches. Given the crosstalk existing between each constituent of the cytoskeleton which yields its dynamic properties,Citation73 it is necessary to isolate and reconstitute individual components in order to determine their properties and role in global cell behavior. The cytoskeletal protein actin has many functions including in cell mechanical stability, motility, division and shape. Filamentous actin (F-actin) forms the actin cortex which is a dynamic 2D cross-linked network residing just underneath the cell membrane and is decorated with myosin molecular motors driving its contractibility. The cortex is attached to the cell membrane and connects to the surrounding microenvironment by transmembrane integrins. Thus, the actin cortex plays a central role in cell shape, adhesion and motility and yet, information is lacking on the physical properties of such a quasi-2D network, with many studies focusing on the mechanics of 3D actin networks and gels.Citation74 By defining an in vitro environment with reduced dimensionality and well-defined boundary conditions, the elastic properties of actin filaments can be determined as well as the interaction of actin with target proteins, such as molecular motors. These systems can also be extended to study other networks such as microtubules, which are responsible for intracellular transport and mitosis.
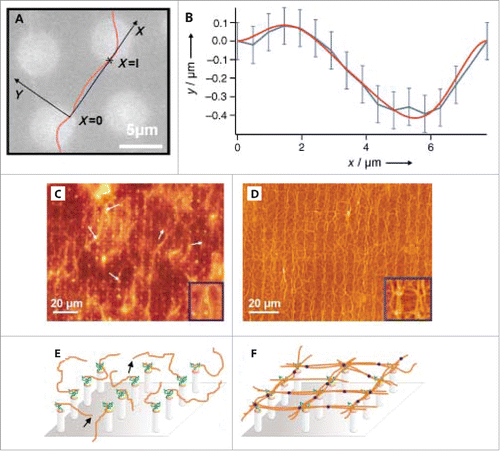
As described in section 2.1, the progress made in microfabrication techniques have provided biophysics researchers with the ability to mimic the native 3D environment of the ECM by using a combination of nano- and microscale topographical cues. This approach can be extended to better understand the dynamics of cytoskeletal proteins such as actin filaments.Citation75-77 For instance, in studies using microfabricated epoxy pillars to quantify the elastic properties of individual actin filaments (). Using a Fourier analysis of the filaments thermal fluctuations, the persistence length lp of individual actin filaments was measured. The reported value of lp ∼15 µm is in the same range as that reported for bulk solutions of actin lp ∼16 µm.Citation78 In addition, silicon pillars were tested for the capability of producing an artificial 2D cortex, as shown in . Filaments attached to the micropillar posts at one end are visualized using fluorescence microscopy, and a 2D actin network can be formed after introducing the actin cross-linker filamin. This study highlights the capabilities of 3-dimensional surfaces in determining physical properties of cytoskeletal filaments but also for constructing artificial 2D actin cortex models. One consequence of using pillars to create 2D networks under flow conditions is the complicated flow profile arising from the geometry of such a chamber. Since the flow field directly influences the structure of the formed network, understanding this aspect of the experiment is essential. Weiße et al. applied digital in-line holographic microscopy to investigate the trajectory of particles flowing through the micropillar flow cell and discovered the laminar flow profile is disrupted across the pillars. This leads to the highest flow velocity occurring near the center of the channel, i.e. the pillar heads.Citation79
Figure 4. Assembly of actin filaments on pillar substrates. (A) Thermally fluctuating actin filament extending between 2 epoxy pillars. (B) Fit of filament positions. (C) and (D) Fluorescence images of actin filaments on silicon pillars before and after the introduction of actin cross-linker filamin, respectively. (E) and (F) Schematics of actin filament orientations before and after filamin, respectively. Reproduced from ref. Citation75 with permission. © 2003 Wiley Publishing Group.
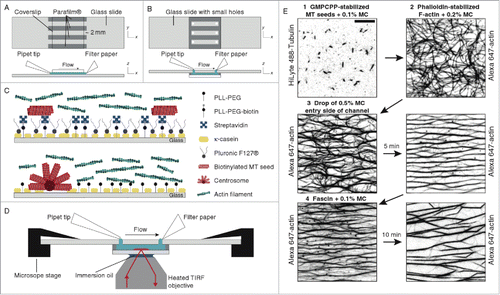
Microfabricated surfaces provide an excellent system to study the interactions between target proteins and assembled cytoskeletal structures, with only limited or no interaction with the synthetic surface, i.e., mimicking in vivo situations. This for instance allows studies of interactions between microtubules and kinesin molecular motors with only minimally perturbing environments.Citation80 Other examples include an assembled 2D network of microtubules on pillars, which was utilized in a combined fluorescence study to investigate the interaction between the kinesin motors themselves.Citation81 Using micropillars and super-resolution fluorescence microscopy it was discovered that there exists attractive interactions between kinesin motors, resulting in motor clustering along the microtubule as shown in . Understanding motor protein interactions is critical when considering intracellular transport mechanisms, and controlled in vitro conditions now allow for directly probing collective motor behavior. Motor proteins are also critical for the contractibility and dynamic properties of the cytoskeleton. An in vitro study using a 2D biomimetic actin-myosin cortex revealed how actin network contraction perfectly correlates with the degree of actin buckling due to myosin activity.Citation82 Myosin-mediated actin contraction was also shown to result in actin severing at a radius of curvature ∼300 nm. Furthermore, adhesion of the supporting membrane can constrain actin curvature and thus result in an increased likelihood of filament severing. Another similar study, utilizing a synthetic membrane-bound actin cortex, revealed that individual myofilaments can generate enough force to buckle and break actin filaments residing in the artificial cortex.Citation83 These studies highlight the possibility of a contraction and fragmentation mechanism during active actin processes. The dependence of myosin activity on actin architecture was investigated using micropatterned surfaces in a study by Reymann et al., where myosin-induced contraction and disassembly of actin filaments was found to be orientation specific.Citation84 Controlled 2D micropatterning of an actin-promoting factor led to the possibility of nucleating desired actin orientations.Citation85 The schematic in displays the architecture used to create regions of unordered, parallel and antiparallel actin filaments in the absence or presence of myosin. Remarkably, myosin VI was only effective at contracting and disassembling antiparallel filaments whereas parallel filaments tended to align and elongate, recruiting monomers from the disassembled antiparallel filaments. Using actin ring networks having variations in the number of antiparallel filament zones, the network contraction rate was also shown to depend of the proportion of antiparallel bundles and not on the ring size itself. These results shed light on the mechanism behind the observed increased contraction rates of the in vivo cytokinetic ring.Citation86,87 The same micropatterning method has recently been adapted and improved to grow controlled network geometries of microtubules,Citation88 and another complementary assay has been produced using an immobilized microtubule polymerase to study microtubule nucleation, transport and mesoscale organization.Citation89 Though it is imperative to isolate individual cytoskeletal filaments in order to measure their properties and interactions with other intracellular proteins, it is a different situation in vivo where there exists significant cytoskeletal crosstalk due to steric hindrance, for example. For this reason is it also important to study the interaction between cytoskeletal filaments, such as actin-microtubule coordination as seen in . The combination of a functionalized flow cell and total internal reflection microscopy (TIRF) was utilized and it was demonstrated that microtubules can be guided by actin and conversely, microtubules can define actin growth and organization.Citation90,91 Many other in vitro systems can be used to reveal fundamental biophysical cytoskeletal properties, such as the dynamics of single actin filaments by microfluidics,Citation92 templated assembly of actomyosin bundles,Citation93 microtubule self-organization by the motor protein Eg5,Citation80 and the adhesion-driven spreading dynamics of biomimetic actin cortices.Citation94
Figure 5. Kinesin motors on freely suspended MTs on micropillars. (A) Schematic of experiment showing MTs in green. (B) SEM image of the micropillar surface. (C) Fluorescence image of kinesin docking on MTs (kinesins labeled red). (D) Binding kinetics of kinesin recorded using super-resolution fluorescence imaging. Number of kinesins bound as a function of MT length is shown (top). Furthermore a calculated convoluted image is shown (bottom) for the same experiment, as would be obtained if epi-fluorescence microscopy was used, revealing the large increase in resolving power using super-resolution microscopy. Reproduced from ref. Citation81 with permission.
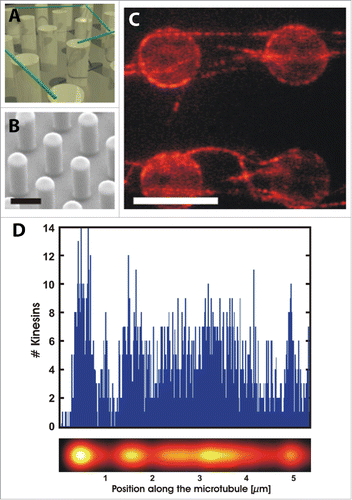
Figure 6. Selective contraction and disassembly of antiparallel actin filaments via myosin motor action. (A) Fluorescence images over time of actin network assembly on an 8-branch radial array. (B) Fluorescence time series of myosin VI-induced selective contraction and disassembly of actin. (C) Schematic of final architecture on an 8-branch actin array in absence and presence of myosin. Reproduced from ref. Citation84 with permission. © 2012 The American Association for the Advancement of Science.
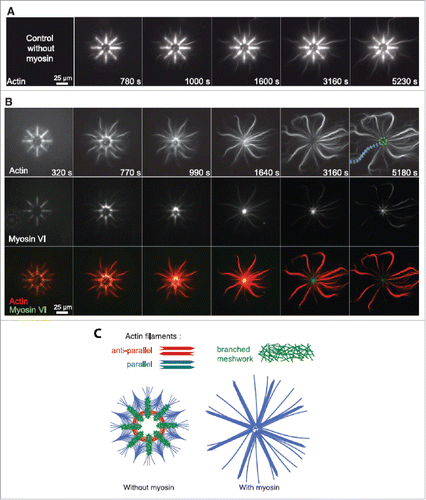
Figure 7. Functionalized glass flow-cell combined with TIRF microscopy. Flow cell schematic with (A) isotropic actin solution and radial microtubule arrays and (B) random microtubule arrays and aligned actin bundles. (C) Surface functionalization steps for actin bundle (top) and microtubules array (bottom) experiments. (D) Schematic of flow-cell combined with TIRF microscopy. (E) TIRF images displaying MT seeds (1) followed by actin filaments (2) and a MC solution (methylcellulose) to align and bundle actin filaments (3) and finally a fascin solution to exchange MC with fascin (4). Reproduced from ref. Citation90 with permission. © 2014 Elsevier Publishing Group.
Conclusion and future prospects
Interfacing cells and purified cytoskeletal proteins with controlled microenvironments has vastly progressed our understanding of the molecular mechanisms governing both cell interactions with the ECM and engineering processes within the cell. The growing complexity of designed experimental methods have allowed biophysical researchers to relate in vitro experiments to in vivo conditions, as described throughout this review. It is clear the movement from the conventional 2D “flat glass” experimental setup to 3D microenvironments has led to the development of “smart” biosystems capable of producing user-defined biochemical and biophysical responses from cells. Fundamental knowledge has been obtained regarding cell transduction pathways through the use of synthetic biology techniques. A full interpretation of underlying molecular mechanisms involved in cell-ECM interactions is now an achievable goal, and will lead to artificially governing cellular function. The next steps will be to combine all the knowledge we have gained on the effect of each parameter of the ECM on cell function to produce synthetic materials which are tailored to elicit the desired cell response or fate. Major progress has already been made in this direction as illustrated here, and this represents a key advancement in several bioengineering fields including tissue engineering.
Reducing the complexity of the in vivo ECM to a synthetic environment for the purpose of studying cellular response has worked remarkably well. Researchers are now going a step further and beginning to create artificial cells in the form of liposomes containing lipid membrane-bound receptors, such as integrin.Citation95 These synthetic cells have already been utilized to study receptor-mediated spreading and adhesionCitation95,96 and actin-loaded liposomes were shown to drive actin polymerization to spontaneously form cytokinetic rings.Citation97 These synthetic “cells” have huge potential and could help to answer many complex biological questions in several fields of life sciences such as virology (in particular viral entry), and loaded liposomes have already been adapted for clinical applications in drug delivery.Citation98 The concept of creating a more complex artificial cell, however, is also now a realistic possibility given that its component parts such as an artificial actin cortex, microtubule networks and reconstituted vesicles can be synthesized in vitro.Citation99 In addition to these exciting applications, synthetic biology approaches will result in advancements spanning a broad range of areas, such as the production of biofuels or pharmaceuticals from engineered organisms, for example. As highlighted throughout this review, synthetic biology approaches have already garnered rich fundamental science on the behavior of cells and their counterparts. Indeed, synthetic biology is on course to changing our life and health in the coming years due to improvements in targeted therapies and the ability to design increasingly complex biological systems and constructs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
WHR would like to acknowledge support by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) through a VIDI grant and by the National Institutes of Health (NIH) through a R21 grant.
References
- Benner SA, Sismour AM. Synthetic biology. Nat Rev Genet 2005; 6:533-43; PMID:15995697; http://dx.doi.org/10.1038/nrg1637
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature 2009; 462:433-41; PMID:19940913.
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol 2003; 163:583-95; PMID:14610060; http://dx.doi.org/10.1083/jcb.200305010
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388; http://dx.doi.org/10.1016/j.cell.2006.06.044
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009; 5:17-26; PMID:19570510; http://dx.doi.org/10.1016/j.stem.2009.06.016
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Bio 2009; 10:21-33; PMID:19197329; http://dx.doi.org/10.1038/nrm2593
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Bio 2006; 7:211-24; PMID:19197329; http://dx.doi.org/10.1038/nrm1858
- Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surf Sci 2002; 500:28-60; http://dx.doi.org/10.1016/S0039-6028(01)01587-4
- Cowin S, Hegedus D. Bone remodeling I: theory of adaptive elasticity. J Elasticity 1976; 6:313-26; http://dx.doi.org/10.1007/BF00041724
- Discher DE, Janmey P, Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310:1139-43; PMID:16293750; http://dx.doi.org/10.1126/science.1116995
- Brunette D, Chehroudi B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J Biomech Eng 1999; 121:49-57; PMID:10080089; http://dx.doi.org/10.1115/1.2798042
- Bodhak S, Bose S, Bandyopadhyay A. Role of surface charge and wettability on early stage mineralization and bone cell–materials interactions of polarized hydroxyapatite. Acta Biomater 2009; 5:2178-88; PMID:19303377; http://dx.doi.org/10.1016/j.actbio.2009.02.023
- Greiner AM, Chen H, Spatz JP, Kemkemer R. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS One 2013; 8:e77328; PMID:24204809; http://dx.doi.org/10.1371/journal.pone.0077328
- Curtis A, Wilkinson C. Topographical control of cells. Biomaterials 1997; 18:1573-83; PMID:9613804; http://dx.doi.org/10.1016/S0142-9612(97)00144-0
- Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 2009; 184:481-90; PMID:19221195; http://dx.doi.org/10.1083/jcb.200810041
- Choi C-H, Hagvall SH, Wu BM, Dunn JC, Beygui RE. Cell interaction with three-dimensional sharp-tip nanotopography. Biomaterials 2007; 28:1672-9; PMID:17174392; http://dx.doi.org/10.1016/j.biomaterials.2006.11.031
- Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LYL. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A 2006; 79:456-63; PMID:16752400; http://dx.doi.org/10.1002/jbm.a.30870
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol 1984; 99:2024-33; PMID:6542105; http://dx.doi.org/10.1083/jcb.99.6.2024
- Elias KL, Price RL, Webster TJ. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002; 23:3279-87; PMID:12102199; http://dx.doi.org/10.1016/S0142-9612(02)00087-X
- Bigerelle M, Giljean S, Anselme K. Existence of a typical threshold in the response of human mesenchymal stem cells to a peak and valley topography. Acta Biomater 2011; 7:3302-11; PMID:21640206; http://dx.doi.org/10.1016/j.actbio.2011.05.013
- Clark P, Connolly P, Curtis A, Dow J, Wilkinson C. Cell guidance by ultrafine topography in vitro. J Cell Sci 1991; 99:73-7; PMID:1757503.
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. PNAS 2003; 100:1484-9; PMID:12552122; http://dx.doi.org/10.1073/pnas.0235407100
- Du Roure O, Dequidt C, Richert A, Austin RH, Buguin A, Chavrier P, et al. Microfabricated arrays of elastomeric posts to study cellular mechanics. Micromachining and Microfabrication: International Society for Optics and Photonics, 2004: 26-34; http://dx.doi.org/10.1117/12.530688
- Matsuzaka K, Walboomers X, De Ruijter J, Jansen J. The effect of poly-L-lactic acid with parallel surface micro groove on osteoblast-like cells in vitro. Biomaterials 1999; 20:1293-301; PMID:10403047; http://dx.doi.org/10.1016/S0142-9612(99)00029-0
- Teixeira AI, Nealey PF, Murphy CJ. Responses of human keratocytes to micro‐and nanostructured substrates. J biomed Mater Res A 2004; 71:369-76; PMID:15470741; http://dx.doi.org/10.1002/jbm.a.30089
- Curtis A, Gadegaard N, Dalby M, Riehle M, Wilkinson C, Aitchison G. Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans Nanobioscience 2004; 3:61-5; PMID:15382646; http://dx.doi.org/10.1109/TNB.2004.824276
- Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005; 26:5405-13; PMID:15814139; http://dx.doi.org/10.1016/j.biomaterials.2005.01.058
- Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly (lactic acid) substrates. Biomaterials 2006; 27:596-606; PMID:16023716; http://dx.doi.org/10.1016/j.biomaterials.2005.05.084
- van Hoorn H, Harkes R, Spiesz EM, Storm C, van Noort D, Ladoux B, Schmidt T. The nanoscale architecture of force-bearing focal adhesions. Nano Lett 2014; 14:4257-62; PMID:24998447; http://dx.doi.org/10.1021/nl5008773.
- Weigelin B, Bakker G-J, Friedl P. Intravital third harmonic generation microscopy of collective melanoma cell invasion: principles of interface guidance and microvesicle dynamics. IntraVital 2012; 1:32-43; http://dx.doi.org/10.4161/intv.21223
- Yevick HG, Duclos G, Bonnet I, Silberzan P. Architecture and migration of an epithelium on a cylindrical wire. PNAS 2015; 112:5944-9; PMID:25922533; http://dx.doi.org/10.1073/pnas.1418857112
- Pelham RJ, Wang Y-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. PNAS 1997; 94:13661-5; PMID:9391082
- Lo C-M, Wang H-B, Dembo M, Wang Y-l. Cell movement is guided by the rigidity of the substrate. Biophys J 2000; 79:144-52; PMID:10866943; http://dx.doi.org/10.1016/S0006-3495(00)76279-5
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J 2008; 95:4426-38; PMID:18658232; http://dx.doi.org/10.1529/biophysj.108.132217
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskel 2005; 60:24-34; PMID:15573414; http://dx.doi.org/10.1002/cm.20041
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PDP, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013; 341:1240104; PMID:23990565; http://dx.doi.org/10.1126/science.1240104
- Dasbiswas K, Majkut S, Discher D, Safran SA. Substrate stiffness-modulated registry phase correlations in cardiomyocytes map structural order to coherent beating. Nat Commun 2015; 6:6085; PMID:25597833; http://dx.doi.org/10.1038/ncomms7085
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol 2013; 23:2434-9; PMID:24268417; http://dx.doi.org/10.1016/j.cub.2013.10.057
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010; 9:518-26; PMID:20418863; http://dx.doi.org/10.1038/nmat2732
- Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 2012; 11:642-9; PMID:22635042; http://dx.doi.org/10.1038/nmat3339
- Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater 2014; 13:979-87; PMID:25108614.
- Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. PNAS 2005; 102:4300-5; PMID:15767572; http://dx.doi.org/10.1073/pnas.0405873102
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010; 330:55-60; PMID:20813920; http://dx.doi.org/10.1126/science.1193270
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. PNAS 2014; 111:16148-53; PMID:25349416; http://dx.doi.org/10.1073/pnas.1409802111
- Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nat Commun 2015; 6:6364; PMID:25695512.
- Wolff J. The law of bone remodelling. Springer Science & Business Media, 2012.
- Ahn AC, Grodzinsky AJ. Relevance of collagen piezoelectricity to “Wolff's Law”: A critical review. Med Eng Phys 2009; 31:733-41; PMID:19286413; http://dx.doi.org/10.1016/j.medengphy.2009.02.006
- Ohgaki M, Kizuki T, Katsura M, Yamashita K. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res 2001; 57:366-73; PMID:11523031; http://dx.doi.org/10.1002/1097-4636(20011205)57:3%3c366::AID-JBM1179%3e3.0.CO;2-X
- Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric Materials for Tissue Regeneration: A Review. Acta Biomater 2015; 24:12-23 PMID:26162587.
- Mitani G, Sato M, Lee JI, Kaneshiro N, Ishihara M, Ota N, Kokubo M, Sakai H, Kikuchi T, Mochida J. The properties of bioengineered chondrocyte sheets for cartilage regeneration. BMC Biotechnol 2009; 9:17; PMID:19267909; http://dx.doi.org/10.1186/1472-6750-9-17
- Guo H-F, Li Z-S, Dong S-W, Chen W-J, Deng L, Wang Y-F, Ying DJ. Piezoelectric PU/PVDF electrospun scaffolds for wound healing applications. Colloids Surfaces B 2012; 96:29-36; PMID:22503631; http://dx.doi.org/10.1016/j.colsurfb.2012.03.014
- Carville NC, Collins L, Manzo M, Gallo K, Lukasz BI, McKayed KK, Simpson JC, Rodriguez BJ. Biocompatibility of ferroelectric lithium niobate and the influence of polarization charge on osteoblast proliferation and function. J Biomed Mater Res A 2014; 103:2540-8; PMID:25504748.
- Noris-Suárez K, Lira-Olivares J, Ferreira AM, Feijoo JL, Suárez N, Hernández MC, Barrios E. In vitro deposition of hydroxyapatite on cortical bone collagen stimulated by deformation-induced piezoelectricity. Biomacromolecules 2007; 8:941-8; PMID:17261065; http://dx.doi.org/10.1021/bm060828z
- Denning D, Abu-Rub M, Zeugolis D, Habelitz S, Pandit A, Fertala A, Rodriguez BJ. Electromechanical properties of dried tendon and isoelectrically focused collagen hydrogels. Acta Biomater 2012; 8:3073-9; PMID:22522132; http://dx.doi.org/10.1016/j.actbio.2012.04.017
- Ribeiro C, Sencadas V, Correia DM, Lanceros-Méndez S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloid Surface B 2015; 136:46-55; PMID:26355812.
- Larsson C, Thomsen P, Aronsson B-O, Rodahl M, Lausmaa J, Kasemo B, Ericson LE. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials 1996; 17:605-16; PMID:8652779; http://dx.doi.org/10.1016/0142-9612(96)88711-4
- Anselme K, Linez P, Bigerelle M, Le Maguer D, Le Maguer A, Hardouin P, Hildebrand HF, Iost A, Leroy JM. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 2000; 21:1567-77; PMID:10885729; http://dx.doi.org/10.1016/S0142-9612(00)00042-9
- Kroeze RJ, Helder MN, Roos WH, Wuite GJL, Bank RA, Smit TH. The effect of ethylene oxide, glow discharge and electron beam on the surface characteristics of poly (L-lactide-co-caprolactone) and the corresponding cellular response of adipose stem cells. Acta Biomater 2010; 6:2060-5; PMID:19944190; http://dx.doi.org/10.1016/j.actbio.2009.11.022
- Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A 2003; 66:247-59; PMID:12888994; http://dx.doi.org/10.1002/jbm.a.10537
- Keselowsky BG, Collard DM, Garcıa AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004; 25:5947-54; PMID:15183609; http://dx.doi.org/10.1016/j.biomaterials.2004.01.062
- Keselowsky BG, Collard DM, García AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. PNAS 2005; 102:5953-7; PMID:15827122; http://dx.doi.org/10.1073/pnas.0407356102
- Cowles EA, Brailey LL, Gronowicz GA. Integrin‐mediated signaling regulates AP‐1 transcription factors and proliferation in osteoblasts. J Biomed Mater Res 2000; 52:725-37; PMID:11033556; http://dx.doi.org/10.1002/1097-4636(20001215)52:4%3c725::AID-JBM18%3e3.0.CO;2-O
- Krause A, Cowles EA, Gronowicz G. Integrin‐mediated signaling in osteoblasts on titanium implant materials. J Biomed Mater Res 2000; 52:738-47; PMID:11033557; http://dx.doi.org/10.1002/1097-4636(20001215)52:4%3c738::AID-JBM19%3e3.0.CO;2-F
- Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir 2003; 19:1573-9; http://dx.doi.org/10.1021/la026142j
- Li L, Klim JR, Derda R, Courtney AH, Kiessling LL. Spatial control of cell fate using synthetic surfaces to potentiate TGF-β signaling. PNAS 2011; 108:11745-50; PMID:21719709; http://dx.doi.org/10.1073/pnas.1101454108
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science 1987; 238:491-7; PMID:2821619; http://dx.doi.org/10.1126/science.2821619
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992; 69:11-25; PMID:1555235; http://dx.doi.org/10.1016/0092-8674(92)90115-S
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 1995; 267:883-5; PMID:7846531; http://dx.doi.org/10.1126/science.7846531
- Arnold M, Cavalcanti‐Adam EA, Glass R, Blümmel J, Eck W, Kantlehner M, Kessler H, Spatz JP. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 2004; 5:383-8; PMID:15067875; http://dx.doi.org/10.1002/cphc.200301014
- Schiller HB, Hermann M-R, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Théry M, et al. β1-and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 2013; 15:625-36; PMID:23708002; http://dx.doi.org/10.1038/ncb2747
- Elosegui-Artola A, Bazellières E, Allen MD, Andreu I, Oria R, Sunyer R, Gomm JJ, Marshall JF, Jones JL, Trepat X, et al. Rigidity sensing and adaptation through regulation of integrin types. Nat Mater 2014; 13:631-7; PMID:24793358; http://dx.doi.org/10.1038/nmat3960
- Deeg JA, Louban I, Aydin D, Selhuber-Unkel C, Kessler H, Spatz JP. Impact of local versus global ligand density on cellular adhesion. Nano Lett 2011; 11:1469-76; PMID:21425841; http://dx.doi.org/10.1021/nl104079r
- Huber F, Boire A, López MP, Koenderink GH. Cytoskeletal crosstalk: when three different personalities team up. Curr Opin Cell Biol 2015; 32:39-47; PMID:25460780; http://dx.doi.org/10.1016/j.ceb.2014.10.005
- MacKintosh FC, Janmey PA. Actin gels. Curr Opin Solid St M 1997; 2:350-7; http://dx.doi.org/10.1016/S1359-0286(97)80127-1
- Roos WH, Roth A, Konle J, Presting H, Sackmann E, Spatz JP. Freely suspended actin cortex models on arrays of microfabricated pillars. ChemPhysChem 2003; 4:872-7; PMID:12961988; http://dx.doi.org/10.1002/cphc.200300712
- Mohrdieck C, Wanner A, Roos W, Roth A, Sackmann E, Spatz JP, Arzt E. A theoretical description of elastic pillar substrates in biophysical experiments. ChemPhysChem 2005; 6:1492-8; PMID:16082672; http://dx.doi.org/10.1002/cphc.200500109
- Ayadi R, Roos W. Building an artificial actin cortex on microscopic pillar arrays. Building a Cell from its Component Parts: Methods of Cell Biology, Elsevier. 2015:105-24.
- Ott A, Magnasco M, Simon A, Libchaber A. Measurement of the persistence length of polymerized actin using fluorescence microscopy. Phys Rev E 1993; 48:R1642; PMID:9960868; http://dx.doi.org/10.1103/PhysRevE.48.R1642
- Weiße S, Heydt M, Maier T, Schulz S, Spatz JP, Grunze M, Haraszti T, Rosenhahn A. Flow conditions in the vicinity of microstructured interfaces studied by holography and implications for the assembly of artificial actin networks. Phys Chem Chem Phys 2011; 13:13395-402; PMID:21698333; http://dx.doi.org/10.1039/c1cp20153k
- Roos W, Ulmer J, Gräter S, Surrey T, Spatz JP. Microtubule gliding and cross-linked microtubule networks on micropillar interfaces. Nano letters 2005; 5:2630-4; PMID:16351227; http://dx.doi.org/10.1021/nl051865j
- Roos WH, Campàs O, Montel F, Woehlke G, Spatz JP, Bassereau P, Cappello G. Dynamic kinesin-1 clustering on microtubules due to mutually attractive interactions. Phys Biol 2008; 5:046004; PMID:19029597; http://dx.doi.org/10.1088/1478-3975/5/4/046004
- Murrell MP, Gardel ML. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. PNAS 2012; 109:20820-5; PMID:23213249; http://dx.doi.org/10.1073/pnas.1214753109
- Vogel SK, Petrasek Z, Heinemann F, Schwille P. Myosin motors fragment and compact membrane-bound actin filaments. Elife 2013; 2:e00116; PMID:23326639; http://dx.doi.org/10.7554/eLife.00116
- Reymann A-C, Boujemaa-Paterski R, Martiel J-L, Guérin C, Cao W, Chin HF, De La Cruz EM, Théry M, Blanchoin L. Actin network architecture can determine myosin motor activity. Science 2012; 336:1310-4; PMID:22679097; http://dx.doi.org/10.1126/science.1221708
- Reymann A-C, Martiel J-L, Cambier T, Blanchoin L, Boujemaa-Paterski R, Théry M. Nucleation geometry governs ordered actin networks structures. Nat Mater 2010; 9:827-32; PMID:20852617; http://dx.doi.org/10.1038/nmat2855
- Calvert ME, Wright GD, Leong FY, Chiam K-H, Chen Y, Jedd G, Balasubramanian MK. Myosin concentration underlies cell size–dependent scalability of actomyosin ring constriction. J Cell Biol 2011; 195:799-813; PMID:22123864; http://dx.doi.org/10.1083/jcb.201101055
- Carvalho A, Desai A, Oegema K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell 2009; 137:926-37; PMID:19490897; http://dx.doi.org/10.1016/j.cell.2009.03.021
- Portran D, Gaillard J, Vantard M, Thery M. Quantification of MAP and molecular motor activities on geometrically controlled microtubule networks. Cytoskeleton 2013; 70:12-23; PMID:23027541; http://dx.doi.org/10.1002/cm.21081
- Ghosh S, Hentrich C, Surrey T. Micropattern-controlled local microtubule nucleation, transport, and mesoscale organization. ACS Chem Biol 2013; 8:673-8; PMID:23294267; http://dx.doi.org/10.1021/cb300583p
- López MP, Huber F, Grigoriev I, Steinmetz MO, Akhmanova A, Dogterom M, et al. In vitro reconstitution of dynamic microtubules interacting with actin filament networks. Reconstituting the cytoskeleton 2014; 540:301-20; http://dx.doi.org/10.1016/B978-0-12-397924-7.00017-0
- López MP, Huber F, Grigoriev I, Steinmetz MO, Akhmanova A, Koenderink GH, Dogterom M.. Actin–microtubule coordination at growing microtubule ends. Nat Commun 2014; 5:4778; PMID:25159196.
- Jégou A, Niedermayer T, Orbán J, Didry D, Lipowsky R, Carlier M-F, et al. Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol 2011; 9:e1001161; PMID:21980262; http://dx.doi.org/10.1371/journal.pbio.1001161
- Thoresen T, Lenz M, Gardel ML. Reconstitution of contractile actomyosin bundles. Biophys J 2011; 100:2698-705; PMID:21641315; http://dx.doi.org/10.1016/j.bpj.2011.04.031
- Murrell M, Pontani L-L, Guevorkian K, Cuvelier D, Nassoy P, Sykes C. Spreading dynamics of biomimetic actin cortices. Biophys J 2011; 100:1400-9; PMID:21402021; http://dx.doi.org/10.1016/j.bpj.2011.01.038
- Streicher P, Nassoy P, Bärmann M, Dif A, Marchi-Artzner V, Brochard-Wyart F, Spatz J, Bassereau P. Integrin reconstituted in GUVs: a biomimetic system to study initial steps of cell spreading. BBA-Biomembranes 2009; 1788:2291-300; PMID:19665445; http://dx.doi.org/10.1016/j.bbamem.2009.07.025
- Frohnmayer JP, Brüggemann D, Eberhard C, Neubauer S, Mollenhauer C, Boehm H, Kessler H, Geiger B, Spatz JP. Minimal Synthetic Cells to Study Integrin‐Mediated Adhesion. Angew Chem Int Edit 2015; 54:12472-8; PMID:26257266.
- Miyazaki M, Chiba M, Eguchi H, Ohki T, Ishiwata Si. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat Cell Biol 2015; 17:480-89; PMID:25799060; http://dx.doi.org/10.1038/ncb3142
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliver Rev 2013; 65:36-48; PMID:23036225; http://dx.doi.org/10.1016/j.addr.2012.09.037
- Ross JMW. Building a cell from its component parts. Methods Cell Biol 2015; 128:xvii-xviii; PMID:26193711.
