ABSTRACT
The nutritional compound capsaicin inhibits the invasion of many types of human cancers. The clinical development of capsaicin as an anti-cancer drug is limited due to its unfavorable side effects like burning sensation, stomach cramps, gut pain and nausea. This study compared the anti-invasive activity of capsaicin to non-pungent long chain capsaicin analogs, namely arvanil and olvanil, in human small cell lung cancer cells. Boyden chamber invasion assays revealed that arvanil and olvanil displayed improved anti-invasive activity relative to capsaicin in human SCLC cells. The results of the Boyden chamber assay were confirmed by the spherical invasion assay, and similar results were obtained. The anti-invasive activity of arvanil, olvanil and capsaicin were independent of TRPV and CB1 receptors. Furthermore, the anti-invasive activity of arvanil, olvanil and capsaicin was mediated by the AMPK pathway. Depletion of AMPK levels by siRNA methodology abrogated the anti-invasive activity of arvanil, olvanil and capsaicin. The non-pungent capsaicin analogs arvanil and olvanil display improved anti-invasive activity relative to capsaicin in human SCLC cells. These agents may represent the second generation of capsaicin-like compounds which are more potent than the parent molecule and have a better side effect profile.
Introduction
Capsaicin is the spicy ingredient of chili peppers.Citation1 Recent evidence has shown that capsaicin displays potent chemopreventive and anti-tumor activity in many types of human cancers.Citation1 Apart from its growth-inhibitory activity, capsaicin has also been found to suppress invasion and migration of multiple human cancers like prostate cancer, melanoma, cholangiocarcinoma and fibrosarcoma.Citation2-5 Small cell lung cancer (SCLC) is a highly invasive malignancy; limited stage SCLC patients show invasion of the tumor into in the submucosa and peribronchial connective tissue at the time of presentation.Citation6 Extended stage SCLC (which affects a majority of the patients) is characterized by the invasion of malignant cells beyond the hemithorax, in the pleural or pericardial effusion, blood and lymph (NCCC, UK). Agents like capsaicin which suppress the invasion of tumor cells may be of value in human SCLC therapy.Citation1
The clinical application of capsaicin is restricted by its unfavorable side-effect profile. Several convergent studies have shown that systemic administration of capsaicin in humans leads to intense gut pain, hyperalgesia, stomach cramps and nausea.Citation7-10 These adverse side effects of capsaicin have led patients to abandon taking the drug.Citation8-10 Such observations emphasize the need for novel capsaicin analogs, which retain the biological activity of capsaicin but do not produce the “heat-sensation” of capsaicin.
The analgesic activity of capsaicin has led to intense research focused on the design of novel capsaicin analogs. Structure activity relationship (SAR) studies have shown that long chain capsaicin compounds (: with chain length R ≥ C16) are non-pungent, display better oral bioavailability and have lower “heat-sensation” than capsaicin.Citation11-13 After examining SAR studies, we selected two capsaicin-like compounds, arvanil and olvanil (). Arvanil and olvanil were selected because they are orally available, non-pungent, and have comparable pharmacological activity profile, relative to capsaicin.
Figure 1. A diagram representing the structure of capsaicinoids and capsaicin-analogs. (A) General structure of capsaicinoids. R = alkyl group. (B) Molecular structure of arvanil, olvanil and capsaicin.
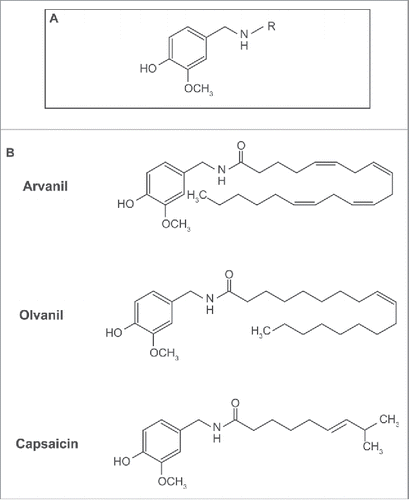
The analgesic activity of capsaicin is mediated by the transient receptor potential vanilloid (TRPV) superfamily of ion channel receptors.Citation14 Capsaicin functions as an agonist of the TRPV1 receptor.Citation14 Arvanil and olvanil bind to TRPV1 with higher affinity than capsaicin.Citation12,15,16 A unique feature of arvanil and olvanil is that they are agonists of cannabinoid receptor (CB1). Although, the analgesic activity of capsaicin is mediated by TRPV1, its anti-tumor activities have been found to be independent of TRPV1.Citation1 Similarly, the biological activity of arvanil and olvanil have been shown to be independent of TRPV1 or CB1 or both in various experimental systems.Citation17,18 For example, the apoptotic activity of arvanil in human leukemia cell lines was found to be independent of both TRPV1 and CB1,Citation17,18 whereas its apoptotic effect on glioma cells required TRPV1 but not CB1.Citation19
Previous studies show that capsaicin suppressed the invasion of human cancer cells in both cell culture and transgenic mouse models.Citation2-5 The 5′ AMP-activated protein kinase (AMPK) pathway has been shown to mediate the anti-invasive activity of capsaicin in cholangiocarcinoma cells in vitro.Citation5 Furthermore, the AMP kinase pathway has been implicated in the pro-apoptotic, pro-autophagic and anti-inflammatory effects of capsaicin in several experimental model systems.Citation20-22 Emerging data also show that dietary capsaicin stimulates glucose uptake in muscles and attenuates metabolic dysfunction in genetically obese mice via the AMPK pathway.Citation23,24 Therefore the AMPK signaling network seems to an important converging point mediating the biochemical activities of capsaicin.
The effect of capsaicin on the invasion of human SCLC cells has not been studied. There are no published reports which have described the impact of olvanil and arvanil on invasion of human cancer cells. The main objective of the present studies was to compare the anti-invasive activity of arvanil, olvanil and capsaicin in human SCLC cells. We show that the non-pungent long chain capsaicin analogs arvanil and olvanil display better anti-invasive activity relative to capsaicin in human SCLC cell lines. We also wanted to analyze the role of TRPV1 and CB1 receptors in the anti-invasive activity of capsaicin olvanil and arvanil. We observed that the anti-invasive activity of arvanil, olvanil and capsaicin was independent of the TRPV1 and CB1 pathway. Most interestingly, arvanil, olvanil and capsaicin inhibited invasion of human SCLC cells by activation of the AMPK pathway. Our results suggest that arvanil and olvanil may represent the next-generation of capsaicin compounds with better anti-invasive activity and an improved side effect profile.
Materials and methods
Materials
Arvanil, olvanil, AM281 and dorsomorphine dichloride were obtained from Tocris Biosciences (Bristol, United Kingdom). Capsaicin and ruthenium red were purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines and culture
The human SCLC cell lines DMS 53 and DMS 114 were obtained from American Type Culture Collection (Manassas, VA). DMS 114 was cultured in RPMI-1640 medium supplemented with 2 mM glutamine, 25 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, 100 units/ml penicillin, 50 µg/ml streptomycin and 10% FBS.Citation25 DMS 53 was cultured in RPMI-1640 containing with 2 mM glutamine, 1 mM sodium pyruvate, 1X non-essential amino acids, 1X insulin-transferrin-selenium (ITS; Invitrogen, ThermoFisher, Carlsbad, CA) supplement 100 units/ml penicillin, 50 µg/ml streptomycin and 5% FBS.Citation26
Boyden chamber invasion assay
Boyden chamber assays were used to analyze the effect of arvanil, olvanil and capsaicin on the invasion of DMS 114 and DMS 53 cells.Citation27 Corning® BioCoat™ Matrigel® invasion chambers containing 8.0 µm pore size transwell filters (precoated with Matrigel) and companion 24 well plates were ordered from Corning (Corning, NY). The filters were rehydrated by incubating them in warm RPMI basal media according to manufacturer's instructions. An aliquot of approximately 5×105 DMS 114 cells were seeded in the apical chamber in RPMI containing 0.1% FBS, in the presence or absence of the indicated compounds. RPMI media containing 20% FBS was used as the chemoattractant and added to the lower basolateral chamber. The plates were incubated at 37°C for 24 hours.Citation27 After 24 hours, the invasion chambers were removed and non-invading cells on the upper surface of the filters were removed by wiping with cotton swabs. The cells that had invaded to the lower surface of the filters were quantified by staining with 0.5% crystal violet for 20 minutes.Citation28,29 Cells which had invaded to the lower surface of the filters were extracted with DMSO. An aliquot of 100 µl of cell lysate was placed in a 96 well plate and the absorbance was read at 560 nm. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control.
The experiment was repeated using 5×105 DMS 53 cells using the protocol described above. Each sample was measured in duplicate and the entire assay was repeated two independent times.
Spherical invasion assay
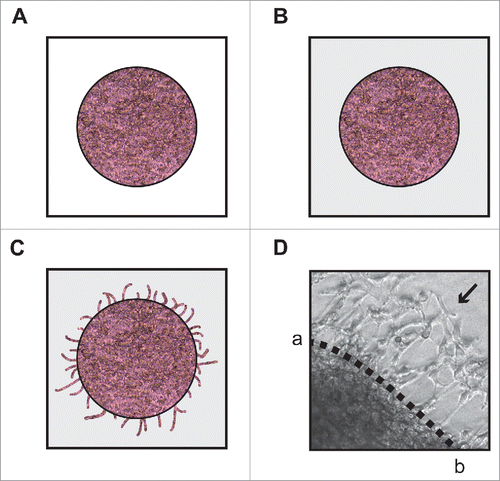
The spherical invasion assay used was a modification of the assay used by Evenssen et al.Citation30 DMS 114 cells were cultured to 70–80% confluence in RPMI containing 25 mM HEPES and 10% FBS. Subsequently, the cells were harvested and re-suspended in RPMI containing 25 mM HEPES and 20% FBS at a concentration of 5 × 107 cells/ml. The cell suspension was then treated with indicated doses of the test compounds and subsequently diluted 1:1 in Matrigel. A 6 µl aliquot of this mixture was pipetted as a drop onto the center of an 8-well chamber slide (). These spots of cells mixed with Matrigel were allowed to polymerize at 37°C for 1 hour, after which they were bathed in 300 µl RPMI containing 25 mM HEPES and 10% FBS and incubated for 18 hours at 37°C. At this stage, DMS 114 cells grew within and up to the edge of the Matrigel spot. After 18 hours, the media was then gently aspirated. A second solution of phenol-red free Matrigel was prepared by diluting phenol-red free Matrigel with phenol-red free RPMI containing 25 mM HEPES and 20% FBS at a ratio of 1:1. Two hundred microliters of this diluted phenol-red free Matrigel solution was pipetted over the drops and incubated for 1 hour at 37°C (). The cells were then bathed in 300 µl RPMI containing 25 mM HEPES and 10% FBS and incubated for 24 hours at 37°C.Citation30 Subsequently, each spot was observed by phase contrast microscopy (Leica DMIL LED, Bannockburn, IL). Under the phase contrast microscope, the dark line demarcating the first pink Matrigel layer and the second white Matrigel layer was clearly visible (). Three independent fields of these cultures were photographed by phase contrast microscopy (Leica DMIL LED). On the photographed image of these cultures, a dotted line was drawn to mark the interface between the primary and secondary Matrigel layers (marked ab, ). The NIH Image J software (version 1.47) was used to average the invading distance covered by the cells in the secondary Matrigel layer from the dotted line ab. In each photograph, seven distance measurements were recorded to recapitulate the variance in the invading distances of DMS 114 cells. This process was repeated in three independent photographic fields. The distance traveled by the invading cells (from the interface line ab) was counted in 21 (7 measurements X 3 fields) distinct areas, over three fields in a double blind fashion by three independent observers. The distance traveled by the control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. Each data point was performed in duplicate, and the whole experiment was repeated twice.
Figure 2. Diagrammatic illustration of the spherical invasion assay. (A) The first layer is comprised of DMS 114 cells mixed in a 1:1 suspension with phenol-red containing Matrigel (light pink area). After 18 hours, DMS 114 cells grow and extend up to the boundary of this first layer. (B) After 24 hours, a second layer of 1:1 solution phenol-red free Matrigel, in phenol-red free RPMI (gray area) is added on top of the first Matrigel spot. The cells are incubated for 24 hours at 37°C. (C) After 24 hours, DMS 114 cells invade into the secondary Matrigel layer. The chamber slides are observed by phase contrast microscopy. (D) A representative photograph of untreated DMS 114 cells is shown. The black arrow indicates the vessels in second layer. The dotted line ab is taken as the interface between the two layers. The distance to which the cells have traveled (into the secondary Matrigel layer) is measured at seven sites (for each photograph) in a randomized double blind fashion by three independent observers, using NIH Image J Version 1.47. This process is repeated for three separate photographic fields per sample.
MTT assay
MTT assays were performed as described by Brown et al.Citation31 DMS 114 and DMS 53 cells were plated in 96-well plates at a density of 5,000 cells/well. The plates were incubated overnight to allow complete reattachment of the cells. Subsequently, cells were treated with the indicated concentrations of arvanil, olvanil and capsaicin for 36 hours. After the indicated time points, 25 μl of sterile MTT solution (5 mg/ml in PBS) was added to each well, and the plates were incubated for 4 hours at 37°C.Citation31 Then, the media was aspirated, and 150 μl of DMSO was added to each well to solubilize the formazan crystals. The absorbance of the plates was measured on an ELISA reader (Benchmark, Bio-Rad, Hercules, CA) at a wavelength of 540 nm. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in cell viability were calculated as fold change from control. Each sample was performed in triplicate, and the entire experiment was repeated twice.
Lysates and western blotting
Cell lysates were made using the IGEPAL CA-630-based lysis protocol.Citation31,32 DMS 114 cells were grown in 100 mm diameter tissue culture dishes to approximately 70–80% confluence. Cells were harvested and washed three times with ice cold PBS. Cells were then lysed with M2 lysis buffer (20 mM Tris, pH 7.6, 0.5% IGEPAL CA-630, 250 mM NaCl, 3 mM EGTA, 3 mM EDTA, 4 µM DTT, 5 mM PMSF, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 25 µg/ml leupeptin, 5 µg/ml pepstatin, 5 µg/ml aprotinin and 25 µg/ml trypsin-chymotrypsin inhibitor). Seventy microliters of lysis buffer was added for every 20 µl of packed cell volume. The lysate was rotated at 4°C for 40 minutes and subsequently spun at 15,000 g for 15 minutes at 4°C. The supernatant was collected and stored in −80°C for further analysis.Citation31,32 The protein concentration of the lysate was measured using a Bradford Reagent (Bio-Rad). An aliquot of one hundred microgram of the protein was run on a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (Bio-Rad) using semi-dry transfer protocols.
The equivalent loading of proteins on the SDS-PAGE gel was confirmed by staining of membranes with Ponceau S.Citation31,32 Membranes were blocked for 1 hour in blocking buffer (1X PBS, 5% milk, 0.10% Tween-20) and placed in primary antibody (usually at a 1:500 dilution in 1X PBS, 0.10% Tween-20) overnight at 4°C. Membranes were washed three times in wash buffer (1X PBS, 0.10% Tween-20) for 5 minutes each. Primary antibody was detected using horseradish peroxidase–linked goat anti-mouse or goat anti-rabbit IgG or rabbit anti-goat antibodies (ThermoFisher, Waltham, MA; usually at a 1:3000 dilution in 1X PBS, 5% milk, 0.10% Tween-20) for one hour at room temperature. The membranes were washed thrice with wash buffer for ten minutes each. The proteins of interest were visualized with the enhanced chemiluminescent detection system ECL (SuperSignal™ West Dura Extended Duration Substrate, ThermoFisher).Citation31,32 Rabbit polyclonal phospho-AMPK-α1/2- (dilution 1:1000 in 1X PBS, 0.10% Tween-20), goat polyclonal AMPK-α1 (dilution 1:500 in 1X PBS, 0.10% Tween-20) and goat polyclonal AMPK-α2 (dilution 1:500 in 1X PBS, 0.10% Tween-20; Santa Cruz Biotechnologies, Inc., Santa Cruz, CA) and GAPDH antibody (1:5000 in 1X PBS, 0.10% Tween-20; Trevigen Inc., Gaithersburg, MD) were used for the immunoblotting experiments. The results of the protein gel blotting assays were quantitated by densitometry by using NIH Image J Version 1.47.
siRNA transfection and assays
Chemically synthesized, double stranded AMPK-α1-siRNA was purchased from Santa Cruz Biotechnologies, Inc. The transfection experiments were performed in DMS 114 cells. The transfection of 75 nM of AMPK-α1-siRNA or control-siRNA in DMS 114 human SCLC cells was performed by using Oligofectamine reagent (Invitrogen, ThermoFisher, Carlsbad, CA), according to the manufacturers protocol. Twenty-four hours post-transfection, the cells were harvested, and a Boyden chamber invasion assay was performed.Citation27 A non-targeting siRNA sequence (Santa Cruz Biotechnologies, Inc.) was used as a control-siRNA for the transfection experiments.Citation31,32 Each transfection was performed in duplicate, and the entire assay was performed two independent times. The entire transfection experiment was repeated in a second human SCLC cell line, DMS 53.Citation31,32
Western blotting experiments were performed to assess the expression of proteins after siRNA transfection in DMS 114 and DMS 53 cells.Citation31,32 The results of the western blotting assays were quantitated by densitometry using NIH Image J Version 1.47.
Statistical analysis
All data was plotted using GraphPad Prism Software, Inc. (La Jolla, CA), and was represented as the mean ± standard error of the mean (SEM). Results from the control and treated samples were compared using an analysis of variance followed by a Tukey's post hoc test. All analyses were completed using a 95% confidence interval. Data was considered significant when P < 0.05.
Results
Capsaicin displays anti-invasive activity in human SCLC cells
Boyden chamber invasion assays were used to measure the anti-invasive activity of capsaicin in DMS 114 human SCLC cells. We observed that capsaicin inhibited the invasion of DMS 114 cells in a concentration-dependent manner (). The anti-invasive activity of capsaicin was significant from concentrations of 10 µM −100 µM (*P<0 .05). Next, we wanted to ascertain that the anti-invasive activity of capsaicin was not due to its growth-inhibitory activity. MTT assays show that capsaicin decreases the viability of DMS 114 human SCLC cells between 30 µM −100 µM (). Therefore, the anti-invasive activity of capsaicin displayed at 10 µM and 20 µM was independent of its growth-inhibitory activity. Since the magnitude of capsaicin-induced inhibition of DMS 114 cell invasion was greater at 20 µM, we decided to use this concentration of capsaicin for all our subsequent experiments.
Figure 3. Capsaicin suppresses invasion of DMS 114 human SCLC cells in a concentration-dependent and time-dependent manner. (A) Boyden chamber assays indicate that capsaicin inhibited the invasion of DMS 114 human SCLC cells in a concentration-dependent manner over 24 hours. (B) MTT assays show that capsaicin decreases the viability of DMS 114 cells from concentrations ranging from 30 μM-100 μM over 24 hours. (C) Time kinetics of the anti-invasive activity of 20 μM capsaicin from 24–48 hours. Values indicated by * are statistically significant relative to controls. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. (D) MTT assays show that 20 μM capsaicin does not impact the viability of DMS 114 cells at 24 hours or 36 hours. However, the treatment of 20 μM capsaicin decreases cell viability in DMS 114 cells over 48 hours. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in cell viability were calculated as fold change from control. Values indicated by * are statistically significant relative to controls. The figure represents the average of two independent experiments, and the data have been represented as mean ± SEM.
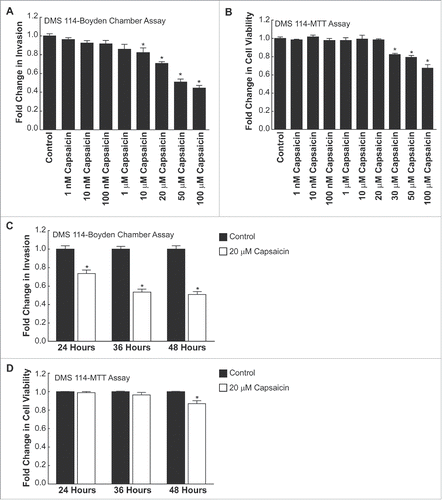
Subsequently, we analyzed the time-kinetics of the anti-invasive activity of capsaicin (). We found that the treatment of DMS 114 cells with 20 µM capsaicin caused approximately a 29 ± 0.015 % decrease in the invasion of DMS 114 cells over 24 hours. The maximal anti-invasive activity of capsaicin was observed at 36 hours () and remained constant thereafter (approximately 53 ± 0.03% relative to controls). MTT assays revealed that 20 µM capsaicin did not display any growth inhibitory activity over 24 and 36 hours (). Since our objective was to determine whether the anti-invasive of arvanil and olvanil was greater than capsaicin in human SCLCs, we chose the 24-hour time-point as a baseline for comparing the anti-invasive activity of all the three compounds.
Arvanil and olvanil display greater anti-invasive activity than capsaicin in human SCLC cells in Boyden chamber invasion assays
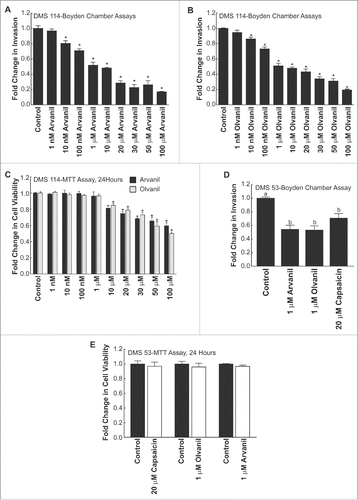
shows the chemical structures of arvanil, olvanil and capsaicin. The primary difference between these three compounds resides in the length of the aliphatic side chain R (). Boyden chamber assays were performed to analyze the anti-invasive activity of arvanil and olvanil. show that both arvanil and olvanil display significant anti-invasive activity in DMS 114 human SCLC cells from 10 nM-100 µM (*P < 0 .05). In contrast, capsaicin showed anti-invasive activity in DMS 114 from 10 µM −100 µM (). Such data suggests to us that the invasion-inhibitory ability of arvanil and olvanil may be greater than capsaicin. Next, we analyzed if the potent anti-invasive activity of arvanil and olvanil was due to their growth-inhibitory activity. MTT assays revealed that arvanil and olvanil decreased cell viability from 10 µM-100 µM (). Therefore, the anti-invasive activity of arvanil and olvanil were independent of their growth-inhibitory activity at a concentration range of 10 nM-1 µM. Arvanil and olvanil showed the maximal anti-invasive activity at a concentration of 1 µM, and therefore, this concentration of arvanil and olvanil were chosen for subsequent experiments. The magnitude of the anti-invasive activity of arvanil (and olvanil) was similar to capsaicin; however, arvanil (and olvanil) showed the maximal anti-invasive activity at a 20-fold lower concentration relative to capsaicin. The results obtained in DMS 114 human SCLC cell line was verified in a second human SCLC cell line, namely DMS 53. demonstrates that 1 µM arvanil, 1 µM olvanil and 20 µM capsaicin display potent anti-invasive activity in human DMS 53 cells over 24 hours, as measured by the Boyden chamber assay. MTT cell viability experiments reveal that 1 µM arvanil, 1 µM olvanil and 20 µM capsaicin did not cause decrease in cell viability in DMS 53 human SCLC cells ().
Figure 4. (see previous page) Arvanil and olvanil decrease the invasion of DMS 114 human SCLC cells (A) Boyden chamber assays indicate that arvanil inhibited the invasion of DMS 114 human SCLC cells in a concentration-dependent manner over 24 hours. Arvanil displayed significant anti-invasive activity at 10 nM-100 μM (P < 0 .05) (B) Olvanil suppressed the invasion of DMS 114 human SCLC cells in a concentration-dependent manner. Olvanil decreased the invasion of DMS 114 cells between 10 nM-100 μM. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. (C) MTT assays reveal that arvanil and olvanil decreased the viability of DMS 114 cells at concentrations ranging from 10 μM-100 μM. Values indicated by * are statistically significant relative to controls. Based on the combined results of the Boyden chamber assay and MTT assay, the concentrations of 1 μM arvanil, 1 μM olvanil and 20 μM capsaicin were chosen for subsequent experiments. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in cell viability were calculated as fold change from control. (D) The Boyden chamber assays were repeated in a second human SCLC cell line DMS 53 and similar results were obtained. (E) MTT experiments indicate that 1 μM arvanil, 1 μM olvanil and 20 μM capsaicin do not cause any change in the viability of DMS 53 cells over 24 hours. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. Values indicated by same letter are not statistically significant. The figure represents the average of two independent experiments, and the data have been represented as mean ± SEM.
We also performed Boyden Chamber assays where the crystal-violet-stained cells were visualized by phase contrast brightfield microscopy. Untreated DMS114 cells showed robust invasion across the Matrigel-coated membrane in 24 hours (Supplementary Fig. 1, top left). When the cells were treated with 1 µM arvanil (Supplementary Fig. 1, top right) or 1 µM olvanil (Supplementary Fig. 1, bottom left), we observed a decrease in the number of invading cells. Similarly, the presence of 20 µM capsaicin (Supplementary Fig. 1, bottom right) suppressed the invasion of DMS 114 cells over 24 hours.
Arvanil and olvanil show greater anti-invasive activity than capsaicin in human SCLC cells in spherical invasion assays
The anti-invasive activity of arvanil, olvanil and capsaicin was confirmed by a second invasion assay, the spherical invasion assay (). In this assay, the invasive activity of cells was measured by their ability to invade into the secondary Matrigel layer.Citation30 Untreated DMS 114 cells showed substantial invasive activity and migrated robustly across the interface (dotted line ab) into the secondary Matrigel layer (; left column, top, middle and bottom picture). The invading cells are indicated by black arrows in . The treatment of DMS 114 cells with 1 µM arvanil caused a substantial reduction in the distance to which these cells invaded the secondary Matrigel layer, across the interface line ab (; right column, top picture). The treatment of DMS 114 cells with 1 µM olvanil yielded similar results (; right column, middle picture). The anti-invasive activity of 20 µM capsaicin in DMS 114 cells is depicted in (right column, bottom picture).
Figure 5. (see previous page) Arvanil, olvanil and capsaicin suppress invasion of DMS 114 cells in spherical invasion assay. (A) Spherical invasion assays indicate that untreated DMS 114 showed robust invasion of cells (indicated by black arrows) into the secondary Matrigel layer across the interface ab (Left column, top, middle and bottom pictures). The treatment of DMS 114 cells with 1 μM arvanil (Right column, top picture) or 1 μM olvanil (Right column, middle picture) or 20 μM capsaicin (Right column, bottom picture) caused a substantial inhibition of invading DMS 114 cells (indicated by black arrows) across the line ab in the secondary Matrigel layer. (B) Quantification of the anti-invasive activity of 1 μM arvanil, 1 μM olvanil and 20 μM capsaicin in DMS 114 cells, as measured by the spherical invasion assay. (C) The experiment was repeated in DMS 53 human SCLC cells, and similar results were obtained. The distance traveled by the control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. Values indicated by the same letter are not statistically significant. The figure represents the average of two independent experiments and the data have been represented as mean ± SEM. (D) MTT assays demonstrate that 1 μM arvanil or 1 μM olvanil or 20 μM capsaicin do not decrease the viability of DMS 114 cells (black bars) or DMS 53 cells (white bars) over 36 hours. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in cell viability were calculated as fold change from control. The figure represents the average of two independent experiments, and the data have been represented as mean ± SEM.
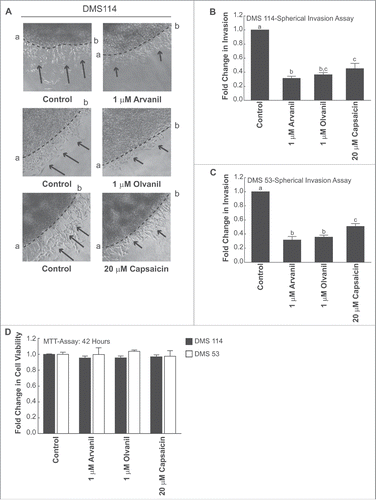
The amount of the invasion was calculated by measuring the distance invaded by the cells from the dotted interface line ab, as described in Methods. The results are graphically depicted in . We noticed that the trend observed in the Boyden chamber assays () was maintained in the spherical invasion assay. Arvanil and olvanil displayed greater anti-invasive activity than capsaicin in DMS 114 cells. The results of the spherical invasion assay were repeated in DMS 53 cells and similar results were obtained (). The time point for the spherical invasion assay was 36 hours. MTT experiments showed that 1 µM arvanil, 1 µM olvanil and 20 µM capsaicin did not reduce the viability of DMS 53 cells over 36 hours ().
The anti-invasive activity of arvanil, olvanil and capsaicin were independent of the TRPV pathway
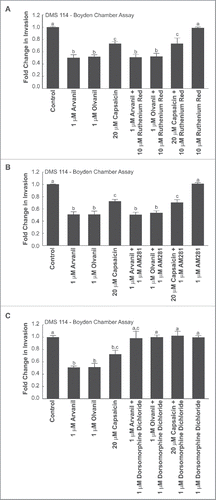
The biological activity of capsaicin is mediated by the TRPV superfamily of transmembrane receptors.Citation1 Specifically, capsaicin, arvanil and olvanil are high-affinity agonists for the TRPV1 receptor.Citation12,15,16 The role of TRPV receptors in the anti-invasive activity of arvanil, olvanil and capsaicin was examined by using the generalized TRPV receptor antagonist ruthenium red.Citation32 Boyden chamber assays showed that 10 µM ruthenium red did not reverse the anti-invasive activity of arvanil, olvanil or capsaicin (), indicating the anti-invasive activity of these compounds were independent of the TRPV receptor pathway in DMS 114 human SCLC cells. We repeated these experiments in a second human SCLC cell line, namely DMS 53 (Supplemental Fig. 2A) and obtained similar results.
Figure 6. The anti-invasive activity of arvanil, olvanil and capsaicin were independent of TRPV pathway, CB1 pathway and required AMPK activation in DMS 114 cells. (A) Boyden chamber assays reveal that the anti-invasive activity of arvanil, olvanil and capsaicin were unaffected by the generalized TRPV antagonist ruthenium red in DMS 114 cells. (B) The invasion-inhibitory activity of arvanil, olvanil and capsaicin is not influenced by the CB1 antagonist AM281 in DMS 114 cells, as measured by Boyden chamber assays. (C) The AMPK inhibitor dorsomorphine dichloride reverses the anti-invasive activity of arvanil, olvanil and capsaicin in DMS 114 cells, as analyzed by Boyden chamber invasion assays. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. Values indicated by the same letter are not statistically significant. The figure represents the average of two independent experiments and the data have been represented as mean ± SEM.
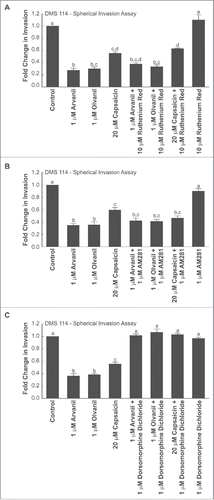
The results obtained from the Boyden chamber assay were repeated using the spherical invasion assay. shows that the invasion-inhibitory activity of arvanil, olvanil and capsaicin were not altered by 10 µM ruthenium red.Citation32 These findings confirm that the ability of arvanil, olvanil and capsaicin to suppress the inhibition of human SCLC cells is not mediated by the TRPV signaling pathway.
Figure 7. Spherical invasion assays reveal that the anti-invasive activity of arvanil, olvanil and capsaicin did not involve the TRPV or CB1 pathway but were mediated by the AMPK pathway, in DMS 114 cells (A) Spherical invasion assays demonstrate that the anti-invasive activity of arvanil, olvanil or capsaicin were unaffected by the generalized TRPV antagonist ruthenium red in DMS 114 cells. (B) Spherical invasion assays reveal that invasion-inhibitory activity of arvanil, olvanil and capsaicin was not abrogated by the CB1 antagonist AM281 in DMS 114 cells. (C) The AMPK inhibitor dorsomorphine dichloride reverses the anti-invasive activity of arvanil, olvanil and capsaicin in DMS 114 cells, as measured by spherical invasion assays. The distance traveled by the control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. Values indicated by the same letter are not statistically significant. The figure represents the average of two independent experiments and the data have been represented as mean ± SEM.
The anti-invasive activity of arvanil olvanil and capsaicin were independent of the CB1 pathway
Arvanil and olvanil are agonists for the cannabinoid receptor 1 (CB1). Therefore, the next series of experiments aimed to investigate if the CB1 receptors were mediating the anti-invasive activity of arvanil, olvanil and capsaicin. We used the CB1 receptor antagonist AM281 for our experiments.Citation33 AM281 did not reverse the anti-invasive effects of arvanil, olvanil or capsaicin (), in DMS 114 cells, as measured by Boyden chamber assays. These experiments were repeated in DMS 53 human SCLC cells and similar results were obtained (Supplemental Fig. 2B).
The results from the Boyden Chamber assay were repeated using the spherical invasion assay in DMS 114 cells and comparable results were obtained. The CB1 antagonist AM281Citation33 did not affect the anti-invasive activity of arvanil, olvanil and capsaicin () in human DMS 114 cells.
The anti-invasive activity of arvanil, olvanil and capsaicin were mediated by the AMPK pathway
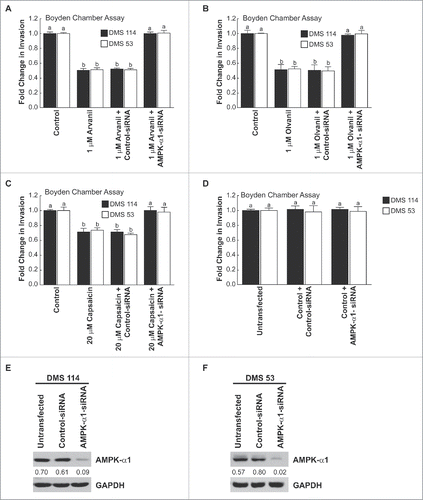
Data from Lee et al. (2014) have shown that capsaicin suppresses the migration of human cholangiocarcinoma cells via the 5′ AMP-activated protein kinase-α (AMPK-α) kinase pathway.Citation5 Similarly, cannabinoid agonists have been shown to exert anti-tumoral effects in pancreatic adenocarcinoma and hepatocellular carcinoma cells via the AMPK pathway.Citation20,34 Since the AMPK pathway seemed to be involved in the bioactivity of capsaicin and cannabinoid ligands, we conjectured that the AMPK-α pathway may be responsible for the anti-invasive activity of arvanil, olvanil and capsaicin. Boyden chamber invasion assays revealed that the AMPK-α inhibitor dorsomorphine dichloride (also called compound C) abrogated the anti-invasive activity of arvanil, olvanil and capsaicin in DMS 114 and DMS 53 cells ().Citation5 The results obtained from the Boyden chamber experiments were verified by the spherical invasion assay in DMS 114 cells and similar results were obtained ().
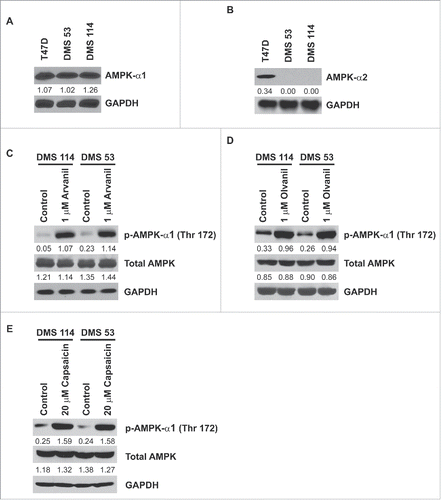
AMPK-α exists in two isoforms, AMPK-α1 and AMPK-α2.Citation35 Immunoblotting experiments showed that DMS 114 and DMS 53 human SCLC cells expressed robust amounts of AMPK-α1 ( topmost lane). However, AMPK-α2 was not detected in DMS 114 and DMS 53 (). T47D human breast cancer cells were used as the positive controls for the immunoblotting experiments.Citation36 Western blotting experiments were performed to test whether arvanil, olvanil and capsaicin induced activation of AMPK-α1 over 24 hours. Untreated DMS 114 cells had low amounts of phospho-AMPK-α (Thr172) ( top row, leftmost lane). The treatment of DMS 114 cells with 1µM arvanil (, top row, second lane from the left) or 1µM olvanil (, top row, second lane from the left) caused robust activation and phosphorylation of AMPK-α1-kinase.Citation35 Similarly, the treatment of DMS 114 with 20µM capsaicin increased the levels of phosphorylated AMPK-α (Thr172) ( top row, lanes 1 and 2 from the left). The results of these experiments were repeated in DMS 53 human SCLC cells and similar results were obtained ( top panel, lanes 3 and 4 from the left).
Figure 8. Human SCLC cells express AMPK-α1 but not AMPK-α2. Arvanil, olvanil and capsaicin block activation of AMPK-α1 in human SCLC cells. (A) Immunoblotting experiments show that AMPK-α1 is robustly expressed in DMS 114 and DMS 53 cells. T47D human breast cancer cells were used as the positive controls for the experiment. GAPDH was used as the loading control for the protein gel blotting experiments, and the results were quantitated by densitometric analysis. (B) Western blotting experiments demonstrate that AMPK-α2 is not expressed by DMS 53 and DMS 114 human SCLC cells. T47D human breast cancer cells were used as the positive controls for the experiment. GAPDH was used as the loading control for the western blotting experiments, and the results were quantitated by densitometric analysis. (C) The treatment of 1 μM arvanil caused robust phosphorylation (and activation) of AMPK-α1 at threonine residue 172, in DMS 114 and DMS 53 cells over 24 hours (top panel). Total AMPK levels remained constant (middle panel). GAPDH was used as the loading control (bottom panel) for the protein gel blotting experiments, and the results were quantitated by densitometric analysis. The experiments were repeated using 1 μM olvanil (D) and 20 μM capsaicin (E) and similar results were obtained.
The role of AMPK-α1 in the anti-invasive activity of arvanil, olvanil and capsaicin was confirmed by siRNA methodology. The transfection of AMPK-α1-siRNA in DMS 114 human SCLC cells (, black bars) and DMS 53 cells (, white bars) reversed the anti-invasive activity of arvanil. The AMPK-siRNA experiment was repeated using olvanil () and capsaicin (), and similar results were obtained. The transfection of a non-targeting control-siRNA did not have any effect on the invasion-inhibitory effects of arvanil, olvanil and capsaicin ( third set of bars from the left). Finally, the transfection of AMPK-α1-siRNA did not influence the invasion of untreated DMS 114 ( black bars) and DMS 53 cells ( white bars). Western blotting experiments show that the transfection of AMPK-α1-siRNA suppressed the levels of AMPK-α1 in both DMS 114 () and DMS 53 cells ().
Figure 9. The AMPK-pathway mediated the anti-invasive activity of arvanil, olvanil and capsaicin in human SCLC cells. (A) Boyden chamber assays indicated that the transfection of AMPK-α1-siRNA significantly abrogated the anti-invasive activity of arvanil in DMS 114 cells (black bars). The experiment was repeated in human DMS 53 cells and comparable results were obtained (white bars). The transfection of a non-targeting control-siRNA did not have any effect on the anti-invasive activity of arvanil in DMS 114 and DMS 53 cells. (B) Depletion of AMPK-α1 levels by siRNA techniques ablated the anti-invasive activity of olvanil in DMS 114 (black bars) and DMS 53 cells (white bars). The anti-invasive activity of olvanil was not influenced by transfection of a control non-targeting siRNA in DMS 114 and DMS 53 cells. (C) AMPK-α1-siRNA reversed the anti-invasive activity of capsaicin in DMS 114 (black bars) and DMS 53 cells (white bars), whereas the control-siRNA have not effect on the anti-invasive activity of capsaicin in these two cell lines. The absorbance of control cells was assumed to be 1, and arvanil-, olvanil-, capsaicin-induced decreases in invasion were calculated as fold change from control. (D) The transfection of AMPK-α1-siRNA did not have any effect on the invasion of untreated DMS 114 (black bars) or DMS 53 cells (white bars). (E) Western blotting analysis showed that AMPK-α1 expression in DMS 114 cells and DMS 53 cells (F) was suppressed upon siRNA transfection. GAPDH was used as the loading control for the western blotting experiments, and the results were quantitated by densitometric analysis.
The structures of arvanil and olvanil closely resemble capsaicin. However, these compounds are non-pungent and do not produce the unpleasant side effects of capsaicin. Therefore, it may be envisaged that these compounds represent the second-generation of capsaicin-like compounds with better anti-invasive activity and a better side effect profile. SCLC is a highly invasive tumor with a propensity for early metastasis. Long chain capsaicin compounds like arvanil and olvanil foster the hope of improved therapies in the treatment and management of SCLC.
Discussion
The invasion of tumor cells into the surrounding stroma, neighboring blood vessels and lymph nodes is an essential step for metastasis.Citation37 It is well established that metastasis is the major cause of death in cancer patients. Among the many steps of metastasis, tumor invasion has been targeted by many therapeutic agents to suppress the distant spread of tumors.Citation38 These agents include tyrosine kinase inhibitors, transmembrane receptor antagonist, small synthetic molecules against intracellular signaling agents and humanized antibodies. Recent evidence shows that nutritional compounds like capsaicin can suppress the invasion and migration of many types of tumor cells.Citation38 The present study investigates the anti-invasive effect of capsaicin in human SCLC, which is a highly invasive and metastatic cancer. We find that capsaicin decreases the invasion of two human SCLC cell lines in both Boyden chamber assays and spherical invasion assays. Our data agrees with that of other researchers who found that capsaicin suppresses the invasion and migration of prostate cancer, melanoma, fibrosarcoma and cholangiocarcinoma cells. Controversy exists on the effect of capsaicin on metastasis. Whereas data from Venier et al., (2015) show that capsaicin decreases invasion and metastasis burden in TRAMP model of prostate cancer,Citation2 other published reports have shown that capsaicin increases breast cancer and colon cancer metastasis.Citation39-41 Erin et al., (2004 and 2006) used a high concentration of capsaicin to deactivate sensory nerves and showed that such deactivation increased breast cancer metastasis.Citation40,41 Similarly, Yang et al., (2013) have treated human colon cancer cell lines with high concentration of capsaicin (as high as 200 µM) to examine the pro-metastatic effect of capsaicin.Citation39 However, it must be remembered that metastasis is a complex phenomenon and consists of many other steps apart from invasion. Our results show that capsaicin exerts anti-invasive activity at much lower concentrations (about 20 µM). At such low concentrations, capsaicin does not display any growth-inhibitory activity on human SCLC cell lines. Our published data show that the growth-inhibitory activity of capsaicin is displayed at about 50 µM.Citation32 Therefore, the anti-invasive activity of capsaicin is independent of its growth inhibitory effects and occurs at lower concentrations.
A survey of literature shows that arvanil, olvanil and capsaicin display some similarities in their pharmacological profile. All the three compounds are high-affinity agonists of TRPV1 receptors. The affinity of arvanil and olvanil for TRPV1 is greater than that of capsaicin.Citation7 Arvanil and olvanil upregulate the levels of intracellular calcium in a manner analogous to capsaicin.Citation12 However, arvanil and olvanil are high affinity agonists of the cannabinoid receptor 1 (CB1), unlike capsaicin.Citation15,16,42 Data from several laboratories also suggest interesting parallels in the growth-inhibitory activity of capsaicin, arvanil and olvanil. Although capsaicin is a TRPV1 ligand, its growth-inhibitory activity in most types of cancer cells has been shown to be independent of TRPV1.Citation43 Similarly, published reports indicate that the growth-inhibitory activity of arvanil and olvanil (and related compounds) may be independent of TRPV1, CB1 or both.Citation17-19
The growth-inhibitory activity of arvanil and olvanil has been studied in several cancers; however, there are no reports of their effect on the invasion of cancer cells. Our studies show for the first time that the non-pungent long chain capsaicin compounds olvanil and arvanil inhibit the invasion of human SCLC cells. The magnitude of the anti-invasive activity of olvanil and arvanil is similar to capsaicin but it is displayed at a 20-fold lower concentration relative to capsaicin. The anti-invasive activity of arvanil, capsaicin and olvanil was found to be independent of the TRPV and CB1 pathway. This is in agreement with data from several research laboratories which have shown that the growth-inhibitory effect of capsaicin in several human cancers is independent of TRPV receptors.Citation44 Similarly, the apoptotic effects of olvanil and arvanil have been found to be autonomous of TRPV and CB1 pathways.Citation18 Our data shows that the invasion-inhibitory activity of these compounds is yet another example of the TRPV- and CB1-independent effect of arvanil, olvanil and capsaicin.
The anti-invasive activity of arvanil, olvanil and capsaicin was mediated by activation of AMP kinase. Originally characterized as a regulator of fatty acid levels, cholesterol levels and lipid metabolism, the AMPK pathway has been recently recognized as a vital controller of cellular energy homeostasis.Citation35 AMPK acts as a metabolic sensor which is activated by cellular stress conditions like ischemia, hypoxia and glucose deprivation.Citation45 Several convergent studies show that AMPK controls intracellular energy pathways and maintains cell growth at normal levels via its downstream targets namely mTORC1, p53 and fatty acid synthase (FASN), and their associated metabolic processes.Citation45 The tumor suppressor activity of AMPK is also due to its ability to regulate the classical tumor suppressor protein LKB1.Citation45 Emerging evidence shows that the AMPK signaling network links metabolic syndrome to cancer.Citation45 Clinical studies show that AMPK activation is decreased in lung cancer tumors isolated from patients who are active smokers compared to lung cancer tumors isolated from never smokers.Citation46 This trend in also observed in SCLCs whose incidence is 90% correlated with smoking habits.Citation46 The treatment of human SCLC cells with cisplatin or Titanocene Y caused robust phosphorylation of AMPK-α1 at Thr172.Citation47 Therefore, agents that cause the activation of AMPK-α1 may be useful for SCLC therapy. Capsaicin activates AMPK during cell migration, adipocyte differentiation, metabolic balance and glucose uptake processes.Citation23,48,49 Similarly CB1 agonists regulate mitochondrial biogenesis, energy uptake and skeletal muscle metabolism via activation of the AMPK pathway.Citation50,51 Although cannabinoid receptor ligands like R-methanandamide, arachidonoyl cyclopropamide (ACPA) or GW 405833 have been shown to induce activation of AMPK,Citation34,52 our data is the first to report the activation of AMPK by arvanil and olvanil in human SCLC cells.
The α-subunit of AMPK is the catalytic subunit, and its phosphorylation at threonine residue 172 is essential for AMP activation.Citation45 The α-subunit of AMPK exists in two isoforms, α1 and α2. We found that the AMPK-α1 subunit was robustly expressed in human SCLC cells whereas the AMPK-α2 subunit was not detected. Our data agrees with the results of other researchers who have not detected AMPK-α2 in the lung.Citation53,54 The onset of extracellular stress causes the induction of AMPK-α2 specifically in the endothelial cells and smooth muscle cells of pulmonary vasculature.Citation55 Our ongoing and future studies aim to explore the mechanisms by which olvanil, arvanil and capsaicin cause AMPK activation. However, the emphasis of this manuscript is to describe two novel non-pungent long chain capsaicin compounds which have better anti-invasive activity than capsaicin. Combination therapies involving capsaicin along with standard chemotherapeutic drugs and nutritional compounds have been investigated for several cancers.Citation56,57 Recent studies have shown that capsaicin enhances the anti-invasive activity of the natural compound brassinin in human prostate cancer.Citation58 Similarly, the combination of arvanil and temozolomide show better anti-tumor activity than either agent alone in mouse models of glioblastoma.Citation19 This report describes two non-pungent long-chain capsaicin compounds namely arvanil and olvanil which display anti-invasive activity comparable to capsaicin at lower concentrations. Arvanil and olvanil are structurally similar to capsaicin, recruit similar signaling pathways as capsaicin and display better pharmacological activity.Citation12 Taken together, long-chain capsaicin compounds like arvanil and olvanil may improve the efficacy and therapeutic index of capsaicin-based therapies in human small cell lung cancer.
Abbreviations
| AMPK | = | 5′ AMP-activated protein kinase |
| CB1 | = | cannabinoid receptor1 |
| MTT | = | [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] |
| RPMI | = | Rosewell Park Memorial Institute |
| SAR | = | Structure activity relationship |
| SCLC | = | small cell lung cancer |
| siRNA | = | small interfering RNA |
| TRPV | = | Transient receptor potential vanilloid |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Data.docx
Download MS Word (4.5 MB)Acknowledgments
We thank Dr. Srikumar Chellappan and his laboratory for continuous support.
Funding
This work was supported by the grants American Institute of Cancer Research (AICR) and the NIH R15 AREA grant (1R15CA161491-01A1 and 2R15CA161491-02) to PDG. NAN and ATA are recipients of an undergraduate fellowship from the WV NASA Space Grant Consortium.
References
- Srinivasan K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit Rev Food Sci Nutr 2015 [Epub ahead of print].
- Venier NA, Yamamoto T, Sugar LM, Adomat H, Fleshner NE, Klotz LH, Venkateswaran V. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate 2015; 75:1300-11; PMID:26047020; http://dx.doi.org/10.1002/pros.23013
- Hwang YP, Yun HJ, Choi JH, Han EH, KIm HG, Song GY, Kwon K, Jeong TC, Jeong HG. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res 2011; 55:594-605; PMID:21462327
- Shin DH, Kim OH, Jun HS, Kang MK. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp Mol Med 2008; 40:486-94; PMID:18985006.
- Lee GR, Jang SH, Kim CJ, Kim AR, Yoon DJ, Park NH, Han IS. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK-NF-kappaB signaling pathway. Clin Exp Metastasis 2014; 31:897-907; PMID:25217963; http://dx.doi.org/10.1007/s10585-014-9678-x
- Chou PC, Lin SM, Lo CY, Chen HC, Wang CW, Chou CL, Yu CT, Lin HC, Wang CH, Kuo HP. Endobronchial mucosa invasion predicts survival in patients with small cell lung cancer. PLoS One 2012; 7:e47613.
- O'Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev 2012; 64:939-71; PMID:23023032; http://dx.doi.org/10.1124/pr.112.006163
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 1992; 448:749-64; PMID:1593488; http://dx.doi.org/10.1113/jphysiol.1992.sp019068
- Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, Arendt-Nielsen L. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain 2003; 104:333-41; PMID:12855343; http://dx.doi.org/10.1016/S0304-3959(03)00039-3
- Hammer J. Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation. Aliment Pharmacol Ther 2006; 24:679-86.
- Thomas KC, Ethirajan M, Shahrokh K, Sun H, Lee J, Cheatham TE 3rd, Yost GS, Reilly CA. Structure-activity relationship of capsaicin analogs and transient receptor potential vanilloid 1-mediated human lung epithelial cell toxicity. J Pharmacol Exp Ther 2011; 337:400-10; PMID:21343315; http://dx.doi.org/10.1124/jpet.110.178491
- Huang XF, Xue JY, Jiang AQ, Zhu HL. Capsaicin and its analogues: structure-activity relationship study. Curr Med Chem 2013; 20:2661-72; PMID:23627937; http://dx.doi.org/10.2174/0929867311320210004
- Tuoya Baba N, Shimoishi Y, Murata Y, Tada M, Koseki M, Takahata K. Apoptosis induction by dohevanil, a DHA substitutive analog of capsaicin, in MCF-7 cells. Life Sci 2006; 78:1515-9; PMID:16260002; http://dx.doi.org/10.1016/j.lfs.2005.07.019
- Chen J, Luan Y, Yu R, Zhang Z, Zhang J, Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Bioscience trends 2014; 8:1-10; PMID:24647107; http://dx.doi.org/10.5582/bst.8.1
- Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, Gazzieri D, Ligresti A, Campi B, Fontana G, et al. Development of the first ultra-potent “capsaicinoid” agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther 2005; 312:561-70; PMID:15356216
- Di Marzo V, Griffin G, De Petrocellis L, Brandi I, Bisogno T, Williams W, Grier MC, Kulasegram S, Mahadevan A, Razdan RK, et al. A structure/activity relationship study on arvanil, an endocannabinoid and vanilloid hybrid. J Pharmacol Exp Ther 2002; 300:984-91; PMID:11861807; http://dx.doi.org/10.1124/jpet.300.3.984
- Sanchez-Sanchez L, Alvarado-Sansininea JJ, Escobar ML, Lopez-Munoz H, Hernandez-Vazquez JM, Monsalvo-Montiel I, Demare P, Regla I, Weiss-Steider B. Evaluation of the antitumour activity of Rinvanil and Phenylacetylrinvanil on the cervical cancer tumour cell lines HeLa, CaSKi and ViBo. Eur J Pharmacol 2015; 758:129-36; PMID:25864613; http://dx.doi.org/10.1016/j.ejphar.2015.04.003
- Sancho R, de la Vega L, Appendino G, Di Marzo V, Macho A, Munoz E. The CB1/VR1 agonist arvanil induces apoptosis through an FADD/caspase-8-dependent pathway. Br J Pharmacol 2003; 140:1035-44; PMID:14530215; http://dx.doi.org/10.1038/sj.bjp.0705532
- Stock K, Kumar J, Synowitz M, Petrosino S, Imperatore R, Smith ES, Wend P, Purfurst B, Nuber UA, Gurok U, et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med 2012; 18:1232-8; PMID:22820645; http://dx.doi.org/10.1038/nm.2827
- Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ 2011; 18:1099-111; PMID:21475304; http://dx.doi.org/10.1038/cdd.2011.32
- Hwang JT, Lee YK, Shin JI, Park OJ. Anti-inflammatory and anticarcinogenic effect of genistein alone or in combination with capsaicin in TPA-treated rat mammary glands or mammary cancer cell line. Ann N Y Acad Sci 2009; 1171:415-20; PMID:19723084; http://dx.doi.org/10.1111/j.1749-6632.2009.04696.x
- Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci 2007; 1095:496-503; PMID:17404062; http://dx.doi.org/10.1196/annals.1397.053
- Kim SH, Hwang JT, Park HS, Kwon DY, Kim MS. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem Biophys Res Communications 2013; 439:66-70; http://dx.doi.org/10.1016/j.bbrc.2013.08.027
- Kang JH, Tsuyoshi G, Le Ngoc H, Kim HM, Tu TH, Noh HJ, Kim CS, Choe SY, Kawada T, Yoo H, et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J Med Food 2011; 14:310-5; PMID:21332406; http://dx.doi.org/10.1089/jmf.2010.1367
- Stovold R, Meredith SL, Bryant JL, Babur M, Williams KJ, Dean EJ, Dive C, Blackhall FH, White A. Neuroendocrine and epithelial phenotypes in small-cell lung cancer: implications for metastasis and survival in patients. Br J Cancer 2013; 108:1704-11; PMID:23519056; http://dx.doi.org/10.1038/bjc.2013.112
- Improgo MR, Johnson CW, Tapper AR, Gardner PD. Bioluminescence-based high-throughput screen identifies pharmacological agents that target neurotransmitter signaling in small cell lung carcinoma. PLoS One 2011; 6:e24132; PMID:21931655
- Demelash A, Rudrabhatla P, Pant HC, Wang X, Amin ND, McWhite CD, Naizhen X, Linnoila RI. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol Biol Cell 2012; 23:2856-66; PMID:22696682; http://dx.doi.org/10.1091/mbc.E10-12-1010
- Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, Chin L, Kim M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene 2010; 29:6222-32; PMID:20711233; http://dx.doi.org/10.1038/onc.2010.349
- Ogasawara M, Matsubara T, Suzuki H. Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol Pharm Bull 2001; 24:917-20; PMID:11510485; http://dx.doi.org/10.1248/bpb.24.917
- Evensen NA, Li J, Yang J, Yu X, Sampson NS, Zucker S, Cao J. Development of a high-throughput three-dimensional invasion assay for anti-cancer drug discovery. PLoS One 2013; 8:e82811; http://dx.doi.org/10.1371/journal.pone.0082811
- Brown KC, Witte TR, Hardman WE, Luo H, Chen YC, Carpenter AB, Lau JK, Dasgupta P. Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS One 2010; 5:e10243.
- Lau JK, Brown KC, Dom AM, Witte TR, Thornhill BA, Crabtree CM, Perry HE, Brown JM, Ball JG, Creel RG, et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014; 19:1190-201; PMID:24878626; http://dx.doi.org/10.1007/s10495-014-1007-y
- Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 2007; 50:528-36; PMID:17678736; http://dx.doi.org/10.1016/j.jacc.2007.03.057
- Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D'Alessandro A, Zolla L, Palmieri M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis 2013; 4:e664; PMID:23764845
- Dasgupta B, Chhipa RR. Evolving Lessons on the Complex Role of AMPK in Normal Physiology and Cancer. Trends Pharmacol Sci 2015; 37(3):192-206; PMID:26711141.
- Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-Activated Protein Kinase α 2 Isoform Suppression in Primary Breast Cancer Alters AMPK Growth Control and Apoptotic Signaling. Genes Cancer 2013; 4:3-14; PMID:23946867; http://dx.doi.org/10.1177/1947601913486346
- Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol 2015; 35:S244-75; PMID:25865774; http://dx.doi.org/10.1016/j.semcancer.2015.03.008
- Jones RJ, Green TP, Elvin P. Discovery and development of Drugs Targeting Tumor Invasion and Metastasis. New York, NY: Cambridge University Press, 2011
- Yang J, Li TZ, Xu GH, Luo BB, Chen YX, Zhang T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013; 60:364-72; PMID:23581408; http://dx.doi.org/10.4149/neo_2013_048
- Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res 2004; 24:1003-9; PMID:15161056
- Erin N, Zhao W, Bylander J, Chase G, Clawson G. Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat 2006; 99:351-64; PMID:16583263; http://dx.doi.org/10.1007/s10549-006-9219-7
- Janusz JM, Buckwalter BL, Young PA, LaHann TR, Farmer RW, Kasting GB, Loomans ME, Kerckaert GA, Maddin CS, Berman EF, et al. Vanilloids. One. Analogs of capsaicin with antinociceptive and antiinflammatory activity. J Med Chem 1993; 36:2595-604; PMID:8410971
- Diaz-Laviada I, Rodriguez-Henche N. The potential antitumor effects of capsaicin. Prog Drug Res 2014; 68:181-208; PMID:24941670
- Lau JK, Brown KC, Dom AM, Dasgupta P. Capsaicin: Potential Applications in Cancer Therapy. London, United Kingdom: Bentham Press Inc. 2012.
- Li N, Huang D, Lu N, Luo L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review). Oncol Rep 2015; 34:2821-6; PMID:26398719.
- William WJ, Jin Q, Kim E, Feng L, Prudkin L, Lee J, Behrens C, Wistuba I, Lee H. Expression of phospho(p)-AMP-activated protein kinase (AMPK) in lung cancer. J Clin Oncol 2008; 25:Suppl 11085.
- Olszewski U, Deally A, Tacke M, Hamilton G. Alterations of phosphoproteins in NCI-H526 small cell lung cancer cells involved in cytotoxicity of cisplatin and titanocene Y. Neoplasia 2012; 14:813-22; PMID:23019413; http://dx.doi.org/10.1593/neo.12962
- Chen S, Zhu P, Guo HM, Solis RS, Wang Y, Ma Y, Wang J, Gao J, Chen JM, Ge Y, et al. Alpha1 catalytic subunit of AMPK modulates contractile function of cardiomyocytes through phosphorylation of troponin I. Life Sci 2014; 98:75-82; PMID:24447627; http://dx.doi.org/10.1016/j.lfs.2014.01.006
- Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 2005; 338:694-9; PMID:16236247; http://dx.doi.org/10.1016/j.bbrc.2005.09.195
- Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol 2010; 44:87-97; PMID:19625456; http://dx.doi.org/10.1677/JME-09-0063
- Shah M, Kola B, Bataveljic A, Arnett TR, Viollet B, Saxon L, Korbonits M, Chenu C. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone 2010; 47:309-19; PMID:20399918; http://dx.doi.org/10.1016/j.bone.2010.04.596
- Lu Y, Akinwumi BC, Shao Z, Anderson HD. Ligand activation of cannabinoid receptors attenuates hypertrophy of neonatal rat cardiomyocytes. J Cardiovasc Pharmacol 2014; 64:420-30; PMID:24979612; http://dx.doi.org/10.1097/FJC.0000000000000134
- Creighton J, Insel P. Adenosine monophosphate kinase (AMPK) expression and activity in the lung. FASEB J 2008; 22:928.4.
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 1996; 271:611-4; PMID:8557660; http://dx.doi.org/10.1074/jbc.271.2.611
- Creighton J, Sayner S, Alexeyev M, Insel P. Adenosine monophosphate kinase (AMPK) and cell-cell adhesion: a role for AMPK in pulmonary microvascular endothelial barrier function. FASEB J 2009; 23:Suppl 964.4.
- Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP, Chi XQ, Zhang S, Wang XM. Capsaicin Enhances the Drug Sensitivity of Cholangiocarcinoma through the Inhibition of Chemotherapeutic-Induced Autophagy. PLoS One 2015; 10:e0121538.
- Arzuman L, Beale P, Chan C, Yu JQ, Huq F. Synergism from combinations of tris(benzimidazole) monochloroplatinum(II) chloride with capsaicin, quercetin, curcumin and cisplatin in human ovarian cancer cell lines. Anti Cancer Res 2014; 34:5453-64; PMID:25275041
- Kim SM, Oh EY, Lee JH, Nam D, Lee SG, Lee J, Kim SH, Shim BS, Ahn KS. Brassinin Combined with Capsaicin Enhances Apoptotic and Anti-metastatic Effects in PC-3 Human Prostate Cancer Cells. Phytother Res 2015; 29:1828-36; PMID:26426257; http://dx.doi.org/10.1002/ptr.5478
