ABSTRACT
The semaphorins were initially characterized as axon guidance factors, but have subsequently been implicated also in the regulation of immune responses, angiogenesis, organ formation, and a variety of additional physiological and developmental functions. The semaphorin family contains more then 20 genes divided into 7 subfamilies, all of which contain the signature sema domain. The semaphorins transduce signals by binding to receptors belonging to the neuropilin or plexin families. Additional receptors which form complexes with these primary semaphorin receptors are also frequently involved in semaphorin signaling. Recent evidence suggests that semaphorins also fulfill important roles in the etiology of multiple forms of cancer. Some semaphorins have been found to function as bona-fide tumor suppressors and to inhibit tumor progression by various mechanisms while other semaphorins function as inducers and promoters of tumor progression.
The semaphorin family
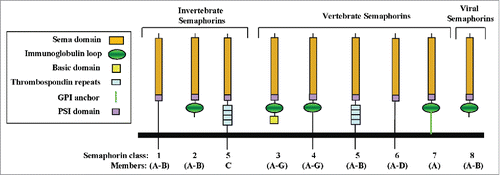
The semaphorin family members are divided into 8 subclasses of which subclasses 1 and 2 contain invertebrate semaphorins while subclasses 3-7 contain the 22 vertebrate semaphorins. The 8th subclass contains viral semaphorins. In early publications, semaphorins were assigned confusing names. This situation was rectified by the adoption of a unified nomenclature in which sema is followed by the subclass number and by alphabetic designation within the subclass.Citation1 Semaphorins are characterized by the presence of a ∼500 amino-acids long sema domain located close to their N-termini which is also present in semaphorin receptors of the plexin family, and by a plexin-semaphorin-integrin (PSI) domain located downstream to the sema domain. The sema domain is essential for semaphorin activity and plays a role in the determination of the receptor binding specificity.Citation2 The sema domains of several different semaphorins were characterized by X-ray crystallography revealing a β propeller topology.Citation3-5 Different semaphorin subclasses are characterized by class specific structural motifs. Thus, the vertebrate semaphorins belonging to classes 3, 4 and 7 contain immunoglobulin like domains, class-5 semaphorins contain thrombospondin repeats and class-3 semaphorins contain a basic domain. Class-3 semaphorins are the only vertebrate semaphorins produced as secreted proteins while other vertebrate semaphorins are membrane anchored or trans-membrane proteins that can be further processed into soluble forms by proteolytic cleavage (). Some membrane anchored semaphorins may themselves be able to function as signal transducing proteinsCitation6-9 although more proof for that may still be required. The active forms of several class-3 and class-6 semaphorins are homodimers,Citation5,10-12 suggesting that all active semaphorins are homodimeric.
Figure 1. The structure of the semaphorins and their receptors. (A) The structural elements of semaphorin subclasses are shown. All feature the signature N-terminal sema domain. A conserved stretch of amino-acid residues near the C-terminal of the sema domain bears homology to the N-terminal of β-integrins and is designated as the PSI domain. Class-3 semaphorins are distinguished by a conserved basic domain at their c-termini. Class 4–7 semaphorins are membrane anchored. Class 5 semaphorins are distinguished by thrombospondin repeats. All the vertebrate semaphorins except for the class-5 and 6 semaphorins also contain an immunoglobulin like domain.
Semaphorin receptors
Plexins
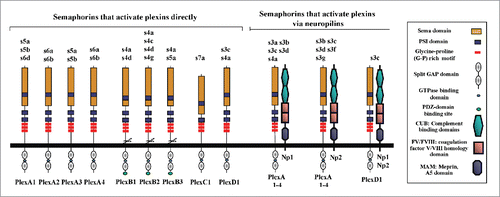
Most semaphorins bind to one or to several of the 9 receptors that constitute the plexin gene family.Citation13 The 9 receptors of the plexin family are segregated into 4 groups consisting of 4 Type-A plexins, 3 Type-B plexins, and single C and D plexins.Citation14,15 Plexins serve as direct binding receptors for most semaphorins. Thus, plexins-B1 is a receptor for sema4D,Citation16 plexin-B3 is a receptor for sema5A,Citation17 plexin-A1 is a binding receptor for sema6D, sema5A and sema5B,Citation18 plexin-A2 and plexin-A4 function as sema6A and sema6B receptors,Citation19,20 plexin-A3 functions as a receptor for sema5A and sema5B,Citation21 plexin-C1 is the receptor for sema7ACitation5 and plexin-D1 is a receptor for sema3E and sema4ACitation22,23 to name but a few examples (). The extracellular domains of all plexins contain a sema domain which serves as an auto-inhibitory domain in the basal, non-activated state of the receptor.Citation24 Plexins contain a split cytoplasmic SP (sex-plexin) domain (also known as the C1 and C2 domains). The intracellular domain contains putative tyrosine phosphorylation sites but no tyrosine kinase domain. The intracellular parts of the plexins are characterized by the presence of a GTPase activating protein (GAP) domain. This GAP domain is conserved quite highly throughout the plexin family.Citation25-27 In the cases of plexin-D1 and plexin-B1 it was demonstrated that most of the developmental effects of these plexins are lost if the function of this GAP domain is compromised.Citation28 Type-A plexins associate spontaneously to form homodimersCitation11,12 or heterodimers.Citation29 Recent data indicates that activation of plexin signaling by semaphorins that bind directly to plexins such as sema6A is likely to be associated with a change in the spatial organization of plexin dimers, shifting the conformation from the inactive to the active form.Citation5,12 In the case of the class-3 semaphorin sema3A there is functional and structural evidence suggesting that the receptor for sema3A is a tetramer composed of 2 plexin-A2 receptors and 2 neuropilin-1 receptors.Citation24,30 However, another study suggests that functional sema3A receptors may consist of complexes containing neuropilin-1, plexin-A1 and plexin-A4.Citation29 This observation is supported by studies in which it was observed that sema3A signaling is impaired in mice lacking functional plexin-A4 or plexin-A1 receptors.Citation31-34 In another study it was also found that plexin-A4 receptors transduce sema3A guidance signals that affect the organization of the cytoskeleton at growth cones. Interestingly, in this study it was fond that plexin-A3 also transduces sema3A signals. However, unlike plexin-A4, plexin-A3 was required specifically for the transduction of sema3A apoptosis promoting signals.Citation35 Further studies will be required in order to better characterize the signaling cascades induced by different plexins in response to semaphorin so as to clarify the reason for these differences. Lastly, plexins have also been found to form complexes with other receptors such as tyrosine-kinase receptors, and to activate them “in-trans” following the binding of specific semaphorins such as sema4D and sema6D.Citation18,36,37
Figure 2. The interaction of the various vertebrate semaphorins with their neuropilins and plexin receptors. The different semaphorins are described using a 3 letter code in which the S stands for semaphorin, the number designates the subfamily, and the following letter designates the specific sub-family member. Thus, s3a stands for sema3A. The specific interactions between individual semaphorins and either single plexins or specific neuropilins are shown.
Activation of plexin signaling by semaphorins such as sema4D activates the GAP domain of the sema4D receptor plexin-B1 leading to the inactivation of R-ras, resulting in the subsequent inactivation of beta1-integrin, and finally reduced adhesion.Citation38 Similar effects on cell adhesion and integrin function are also associated with the activation of type-A plexins and plexin-D1.Citation27,39,40 The activation of type-A plexins also leads to the activation of enzymes of the Mical family. These enzymes perform reduction-oxidation (redox) enzymatic reactions and oxidize actin subunits leading to the disassembly of actin fibers and to the localized collapse of the actin cytoskeleton of axonal growth cones, thereby contributing to growth cone guidance.Citation41-44 Activation of plexin signaling by semaphorins also results in the activation of various intracellular tyrosine-kinasesCitation45 and to the inactivation of small GTPases that control the polymerization of the actin cytoskeleton such as Rho as a result of the activation of regulators of Rho activity such as the p190 Rho-GTPase and Rho guanine nucleotide exchange factors.Citation28,46 However, semaphorin induced signal transduction is far from being completely understood and a thorough description of it is beyond the scope of the present review.
Neuropilins
Six of the 7 class-3 semaphorins are unable to bind to plexins directly but instead bind one or both of the 2 receptors that constitute the neuropilin receptor family.Citation22,47 The neuropilins subsequently associate with type-A plexins or with plexin-D1 to transduce class-3 semaphorin signals since their short intracellular domains render them unable to transduce semaphorin signals independently.Citation16,48,49 Recent studies indicate that functional class-3 semaphorin receptors consist of a tetramer containing a neuropilin homodimer and a plexin homodimer that are linked together by the binding of a class-3 semaphorin homodimer.Citation30 However, such complexes may also contain plexin heterodimers as it was recently observed that inhibition of the expression of either neuropilin-1 or plexin-A1 or plexin-A4 is sufficient to completely abrogate sema3A signal transduction suggesting that the receptor complex in these cells contains plexin-A4 as well as plexin-A1 in addition to neuropilin-1, and that all of these receptors are required for sema3A signaling.Citation29 It was similarly observed that sema3B induced signal transduction requires one of the 2 neuropilins as well as both plexin-A2 and plexin-A4 suggesting that functional sema3B receptors may also contain more than one type of plexin.Citation50
The neuropilins can perhaps be best described as “scaffold receptors” since they seem to bind to and modulate the activities of diverse types of receptors and ligands but do not seem to transduce signals independently. In addition to several class-3 semaphorin the neuropilins were also observed to bind several types of growth factors such as some heparin binding splice forms of vascular endothelial growth factor-A such as VEGF165 but not VEGF121,Citation51-53 VEGF-B, and VEGF-C,Citation51,52,54,55 placental growth factor (PLGF)Citation56 basic fibroblast growth factor (bFGF)Citation57 and hepatocyte growth factor (HGF)Citation58 to name but a few. The VEGF-A binding domain of neuropilin-1 seems to be distinct from its semaphorin binding domainCitation59 and it was indeed observed that sema3A and sema3F inhibit VEGF induced activation of ERK1/2 without inhibition of VEGF induced auto-phosphorylation of the VEGFR2 receptor which associates with neuropilin-1Citation52,60 lending support to the structural observations. However, there is also evidence suggesting that some class-3 semaphorins may compete with VEGF family members for binding to neuropilinsCitation61 and that post-translational modifications of semaphorins such as cleavage by furin like pro-protein convertasesCitation62 may modulate their neuropilin binding ability and their ability to complete with VEGFs for binding to neuropilins.Citation63 Interestingly, it is not clear if the binding of VFGF to neuropilins is required for the enhancement of VEGF induced signal transduction. Thus it was observed that neuropilins are also able to enhance signal transduction induced by VEGF121, a VEGF-A form that does not bind to neuropilinsCitation64 and in a more recent manuscript it was found that the vasculature of a mouse expressing a neuropilin-1 mutant that cannot bind VEGF develops normally.Citation65
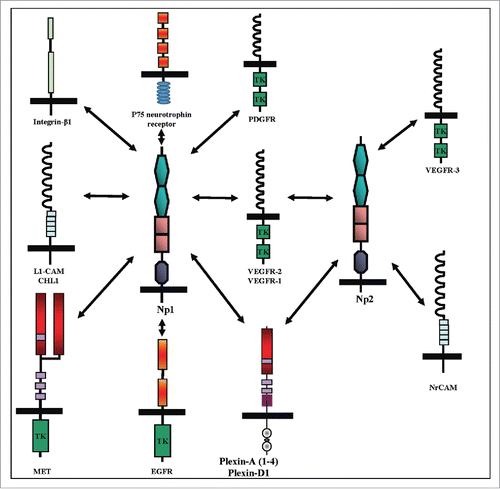
In addition to binding several types of diverse ligands, the neuropilins are also able to bind and form complexes with a diverse array of membrane anchored receptors in addition to plexins. The best studied such interaction is with the VEGF-A tyrosine-kinase receptor VEGFR-2 in which neuropilin-1 functions as an amplifier that enhances VEGF pro-angiogenic signaling mediated by this receptor.Citation52 Similarly, neuropilin-2 interacts with the VEGF-C tyrosine-kinase receptor VEGFR-3 and is crucial for the transduction of VEGF-C induced pro-lymphangiogenic signals.Citation55,66,67 The neuropilins were found to form complexes and modulate signal transduction mediated by several additional receptors including TGF-β receptors,Citation68 platelet derived growth factor (PDGF) receptors,Citation69 Neurotrophin receptors,Citation70 the epidermal growth factor (EGF) receptorCitation71 and the hepatocyte growth factor receptorCitation72 (). Mice lacking functional neuropilin-1 display, in addition to defects in the organization of their nervous systems, major defects in the organization of their blood vessels which result in embryonic lethality. Mice lacking functional neuropilin-2 developed almost normally but have defects in lymph vessels.Citation73 These defects were even more severe in mice lacking both neuropilin-1 and neuropilin-2.Citation74,75 Mice in which full length neuropilin-1 was replaced with a neuropilin-1 lacking the intracellular domain were viable and displayed only minor changes in the organization of their blood vessels as compared with mice completely lacking functional neuropilin-1.Citation76 Recent experiments however reveal that the short intracellular domain of neuropilin-1 is nevertheless essential for some functions since mice in which the neuropilin-1 gene was replaced with neuropilin-1 lacking the intracellular domain have a defect in arteriogenesis.Citation77 The intracellular domain of neuropilin-1 was also found to be important for the interaction of myofibroblasts with soluble fibronectin, an interaction that promotes alpha5/beta1 integrin dependent fibronectin fibril assembly.Citation78 The intracellular domain of neuropilin-1 contains a PDZ binding domain which binds synectin (also known as GIPC or NIP) and this interaction is important for the formation of complexes with VEGFR-2.Citation79,80 Lastly, the neuropilins can also form complexes with adhesion receptors such as L1-CAM which associates with neuropilin-1 or with Nr-CAM which associates with neuropilin-2, and these interactions were found to modulate signal transduction induced by class-3 semaphorins such as sema3A and sema3F.Citation81,82
Figure 3. Cell surface receptors that form complexes with neuropilins. Cell surface receptors that form complexes with np1 include the vascular endothelial growth factor (VEGF-A) tyrosine-kinase receptors VEGFR-1 and VEGFR-2, the hepatocyte growth factor/scatter factor (HGF/SF) tyrosine-kinase receptor MET, and the epidermal growth factor (EGF) receptor EGFR. In addition np1 can associate with the Ig adhesion receptors L1-CAM and CHL1, with integrin-β1, with the 4 type-A plexins, and with plexin-D1. Np2 forms complexes with the 3 tyrosine-kinases receptors belonging to the VEGF receptor family, VEGFR-(1–3), with type-A plexins and plexin-D1, and with the Ig adhesion receptor NrCAM.
Other semaphorin receptors
Some semaphorins bind to additional types of receptors besides plexins and neuropilins. Thus sema4A also signals using the Tim-2 receptor, a member of the family of T-cell immunoglobulin domain and mucin domain (Tim) proteins that is expressed on activated T cells.Citation83 Sema4D was also found to bind to the lymphocyte receptor CD-72Citation84 and sema5A was observed to interact with chondroitin sulfate proteoglycans, an interaction that can convert it from an attractive to an inhibitory guidance cue.Citation85 Finally, sema7A binds to α-1/β-1 integrin to modulate inflammatory responses mediated by these integrins.Citation86
Semaphorins as regulators of tumor progression
Overview
Some semaphorins such as sema3F and sema3B have been identified not as axon guidance factors but rather as tumor suppressors.Citation87-90 Since many types of tumor cells express semaphorin receptors it is not surprising that semaphorins have been found to inhibit the proliferation and metastatic spread of tumor cells by affecting tumor cells in a variety of ways.Citation71,91-93 In addition some semaphorins have been found to inhibit tumor progression as a result of their effects on the tumor microenvironment. Processes such as the recruitment of various stromal cells such as macrophages to the tumor microenvironment can be affected by various semaphorinsCitation94 but perhaps best studied are the effects of some semaphorins on tumor angiogenesis.
Vascular endothelial growth factor (VEGF-A) had been extensively characterized as a major angiogenesis promoting factor.Citation95-97 It is produced in several forms as a result of alternative splicing. VEGF-A signals are transduced by 2 tyrosine kinase receptors (VEGFR-1 and VEGFR-2) which bind all the VEGF-A splice forms. We hypothesized that receptors able to differentially recognize VEGF-A splice forms may also exist, and this resulted in the identification of such receptors in endothelial cells.Citation51 These were subsequently identified as the products of the neuropilin-1Citation52 and neuropilin-2 genes.Citation53 These findings suggested that class-3 semaphorins, which were already known to function as axon guidance factors that bind to neuropilins,Citation98-100 may modulate the behavior of endothelial cells and function as regulators of angiogenesis. Since angiogenesis is critical for tumor developmentCitation101 it was also clear that if the semaphorins modulate angiogenesis they will be likely to affect tumor progression. This logic led to the identification of several class-3 semaphorins such as sema3F as inhibitors of tumor angiogenesis and tumor progression and to their consideration as potential therapeutics for the treatment of cancer.Citation92,102,103
In contrast with the class-3 semaphorins, of which most have been characterized as anti-angiogenic and anti-tumorigenic factors, semaphorins such as sema4D,Citation104,105 sema5A,Citation106,107 sema6A,Citation108 sema4ACitation109 and sema7ACitation110 have been described as promoters of angiogenesis and as promoters of tumor progression. Such semaphorins are therefore considered as targets for the development of novel cancer therapeutics. Interestingly, some semaphorins such as sema3C, sema6A and sema3E display dual activities and have been characterized in some publications as inducers of tumor progressionCitation108,111-114 and in other publications as inhibitors of tumor progression.Citation61,92,115,116 The mechanisms responsible for this duality are not yet completely clear and are likely the result of post translational processing and the formation of complex associations between semaphorin receptors and other types of membrane bound receptors such as various tyrosine-kinase receptors and adhesion receptors.
The mechanisms by which some well studied semaphorins affect tumor progression
The mechanisms by which some semaphorins such as sema3A, sema3F, sema4D and sema3E affect tumor progression have been studied in depth while the mechanisms by which additional less well studied semaphorins affect tumor progression are assumed to be similar since they too transduce their signal using plexin receptors although differences may of course exist. The following paragraphs therefore focus on the description of the mechanisms by which the best studied semaphorins affect tumor progression.
Sema3A: Sema3A is the only class-3 semaphorin that transduces signals exclusively using the neuropilin-1 receptor.Citation98,99 In endothelial cells as well as in several additional cell types both plexin-A1 and plexin-A4 are required to transduce its signals but additional A- type plexins such as plexin-A2 seem to be able to compensate for the loss of plexin-A1 provided that they are expressed at high enough concentrations.Citation50,117
Sema3A functions as an inhibitor of developmental angiogenesis. It inhibits angiogenesis in chick embryo forelimbsCitation118 and vascular branching in the developing chick brain.Citation119 However, unlike other anti-angiogenic semaphorins, sema3A also functions as a vascular permeability factor.Citation120 Sema3A also functions as a potent inhibitor of angiogenesis and tumor progression in many types of solid tumors. Down-regulation of sema3A expression in tumor cells promotes tumor angiogenesis and tumor progression in many types of solid tumorsCitation121-125,125-127 suggesting that it functions as an endogenous negative regulator of the angiogenic switch.Citation128 Prolonged stimulation of endothelial cells with sema3A induces apoptosis of endothelial cells suggesting that induction of apoptosis is a part of the mechanism by which it inhibits angiogenesis.Citation60,129 Likewise, over-expression of sema3A in tumor cells or addition of exogenous sema3A can inhibit angiogenesis in-vivo and tumor progression.Citation120,130-134
Bone marrow derived cells can be recruited to sites of active angiogenesis by factors such as SDF-1 which are produced at sites of active angiogenesis, and these cells then promote angiogenesis by the secretion of angiogenic factors such as VEGF.Citation135 Interestingly, sema3A produced by tumor cells is also able to recruit bone marrow derived cells to tumors. These recruited bone marrow cells consist of a special sub-population of monocytes that express neuropilin-1. Interestingly, these cells were found to contribute to the stabilization and normalization of tumor vessels by promoting mural cell coverage of tumor vessels and by decreasing vascular leakiness, resulting in smaller but better perfused and less hypoxic tumors.Citation136,137 In addition, it was found that sema3A expression is up-regulated in hypoxic areas of tumors. Sema3A induces the phosphorylation of the VEGFR-1 tyrosine-kinase receptor in a neuropilin-1, plexin-A1 and plexin-A4 dependent manner resulting in the recruitment of macrophages to the hypoxic areas of tumors. These macrophages in turn secrete angiogenic factors that promote tumorigenesis.Citation117 Thus, sema3A seems to affect tumor angiogenesis and tumor progression by several concomitant mechanisms.
Sema3A was also observed to affect directly the behavior of tumor cells. Thus, sema3A inhibited the migration and spreading of MDA-MB-231 breast cancer cells as well as their ability to form colonies in soft agar, and also inhibited the invasiveness of prostate cancer cells in in-vitro assays.Citation130,138,139 In breast cancer cells sema3A functions as a regulator of the phosphorylation and nuclear translocation of phosphatase and tensin homolog (PTEN) and of FOXO-3a and the activation of FOXO-3a. Overexpression of PTEN and FOXO-3a enhances sema3A expression resulting in inhibition of breast cancer cells migration.Citation140 In agreement, it was reported that down regulation of sema3A expression by High mobility group box 1 (HMGB1), a chromatin-associated protein that aids in transcription and DNA repair that binds to the semaphorin 3A genomic locus and inhibits its expression, and as a result increases the migration of tumor cells.Citation141 Sema3A was also found to enhance the anti-angiogenic effects of VEGF receptor inhibitors such as DC101 and sunitinib. Interestingly, sema3A also counteracted the pro-metastatic side effects of these VEGF receptor inhibitors and drove sunitinib or DC101 treated tumors back from a pro-metastatic phenotype to a benign phenotype.Citation142
In contrast with all these above mentioned observations, in glioblastoma multiforme and in pancreatic cancer an opposite role was reported for sema3A, suggesting that sema3A promotes rather than inhibits the metastatic dissemination of tumor cells.Citation143,144
Sema3B:The sema3B gene was identified along with sema3F as a tumor suppressor gene whose function is lost in small cell lung carcinoma cells by a variety of mechanisms that include promoter methylation and loss of heterozygosity.Citation91,145-150 Sema3B also functions as an endogenous inhibitor of endometrial cancer,Citation151 and in oral squamous cell carcinoma in which its expression is inhibited as a result of promoter methylation.Citation152 In agreement with these observations single nucleotide polymorphisms in the sema3B gene were also found to be associated with poor prognosis of prostate cancer.Citation153 Furthermore, a single nucleotide alteration in the sema3B gene (T415I) resulted in decreased sema3B function and was associated with increased susceptibility to lung cancer in African-Americans and Latino-Americans indicating that sema3B plays a role in the determination of predisposition to lung cancer.Citation154 In addition, in stage-3 ovarian tumors and in breast cancer tumors sema3B expression is decreased suggesting a role in the development of these types of cancer as well.Citation155,156
Unlike sema3A which only binds to neuropilin-1, sema3B binds to both neuropilins.Citation157,158 In endothelial cells and in U87MG glioblastoma cells sema3B signal transduction also depends on the simultaneous presence of both plexin-A2 and plexin-A4 receptors.Citation50 Sema3B inhibits the anchorage independent growth of responsive lung cancer cells and induces apoptosis, indicating that it exerts direct inhibitory effects on tumor cells.Citation91 The pro-apoptotic effects of sema3B were inhibited by VEGF165 but not by VEGF121.Citation159 Since both neuropilins bind the VEGF165 splice form of VEGF but not the VEGF121 formCitation52,53 these results indicate that the pro-apoptotic effects of sema3B are mediated by neuropilins and that sema3B may compete with VEGF165 for binding to neuropilins. The pro-apoptotic and anti-proliferative effects of sema3B were linked to decreased Akt phosphorylation, increased cytochrome-c release, caspase-3 activation, as well as phosphorylation of several additional pro-apoptotic proteins including glycogen synthase kinase-3-β (GSKβ3), FKHR, and MDM-2.Citation160 Like other class-3 semaphorins, Sema3B functions as an inhibitor of angiogenesis.Citation157 Interestingly, it was also observed that sema3B can indirectly induce opposite effects and potentiate tumor metastasis as well as tumor angiogenesis in many types of tumors as a result of sema3B induced expression of interleukin-8, which in turn, induces the recruitment of tumor-associated macrophages and metastatic dissemination to lungs.Citation161 Since interleukin-8 is a well characterized angiogenic factorCitation162 it is also likely that when expressed it may counteract the direct anti-angiogenic effects of sema3B.
Sema3C: Sema3C was identified as the product of a gene that confers non-MDR drug resistance in human cancers.Citation163 It utilizes both np1 and np2 as receptors and transduces its signals using either plexin-A1, plexin-A2 or plexin-D1.Citation49,61,164,165 Contrary to other class-3 semaphorins its expression in tumor cells is associated with tumor progression rather than with inhibition of tumor progression in several types of tumors.Citation113,114,166,167 In some manuscripts it was also characterized as an inducer of angiogenesisCitation114,168,169 but recent publications suggest that sema3C, like other class-3 semaphorins, functions as an inhibitor of angiogenesis.Citation61,116 Like the other class-3 semaphorins, sema3C too is cleaved in conserved sites by furin like pro-protein convertases as well as by ADAMTS1.Citation170 The major cleavage product generated by furin like pro-protein convertases (p65-Sema3C) is inactive as an inducers of cell contraction but is still able to support survival of tumor cells in cell cultureCitation61 suggesting that it may perhaps contribute to tumor progression when generated in the tumor microenvironment which is usually enriched with these furins.Citation171 Likewise, the sema3C cleavage products generated by cleavage with ADAMTS1 promotes the migration of breast cancer cells.Citation170 These observations may merit further investigation in order to determine the mechanism responsible for the pro-tumorigenic effects of sema3C.
Sema3D:Sema3D binds to both neuropilins like sema3C but unlike sema3C does not transduce signals utilizing the plexin-D1 receptor.Citation172 Over-expression of sema3D in breast cancer cells inhibits the development of tumors from breast cancer cells and from glioblastoma cells and the inhibition was accompanied by inhibition of angiogenesis.Citation130,133 However, sema3D was also recently reported to have a role in the induction of metastasis in pancreatic cancer suggesting that in its case too, as in the cases of sema3C and sema3E, the effects on tumor progression may involve several mechanisms that affect tumor progression differently.Citation173
Sema3E: Sema3E was initially identified as a pro-metastatic semaphorin.Citation111 It is the only class-3 semaphorin that does not bind to a neuropilin and utilizes instead the plexin-D1 receptor as a binding and signal transducing receptor.Citation22 It should be noted however, that plexin-D1 can associate with neuropilins to transduce signals of other class-3 semaphorin such as sema3A and sema3CCitation49 and that this association can change responses of cells to sema3E.Citation174 Like other class-3 semaphorins, sema3E functions as a repulsive factor for endothelial cells and as an inhibitor of angiogenesis.Citation27,40 Opposing sema3E gradients originating from the lateral plate mesoderm and the notochord repulse endothelial progenitor cells during early development, inducing them to concentrate and enabling the subsequent formation of the early dorsal aorta.Citation175 Sema3E is also highly expressed in somites in the early embryo and inhibits, possibly as a result of sema3E induced repulsion of blood vessels, the growth of blood vessels into somites.Citation22
The development of the retinal vasculature serves as a major model in which to study developmental angiogenesis as well as eye diseases associated with abnormal angiogenesis, because the whole network of blood vessels can be easily observed in retinal whole-mounts. During the development of the retina, the growth of the vascular network is driven by VEGF that is produced by astrocytes in response to local hypoxia.Citation176 Tip cells are endothelial cells located at the tips of the growing angiogenic sprouts. These cells send out filopodia and lamellopodia to guide the growing sprout while the stalk cells which are the endothelial cells that form the main body of the growing sprout do not extend such filopodia.Citation177,178 VEGF signals via the VEGFR-2 receptor of the tip cells and its activation by VEGF induces the expression of the notch ligand Dll4. Dll4 activates notch receptors in adjacent stalk cells which in response down regulate the expression of VEGFR-2 resulting in the maintenance of their stalk cell identity.Citation179,180 Interestingly, VEGF also induces the expression of plexin-D1 in tip cells of sprouting angiogenic retinal blood vessels. Sema3E produced by retinal ganglion cells acts specifically on the plexin-D1 expressing tip cells to inhibit the VEGF induced expression of Dll4 causing a cell fate shift that favors tip cells identity. Thus, sema3E expression is part of a feedback mechanism by which neuronal cells of the retina regulate the formation of the developing vascular network.Citation181 Sema4A, a membrane anchored semaphorin that also utilizes plexin-D1 as its receptor also functions as an anti-angiogenic factorCitation23 although it is not known if it is also part of a similar feedback mechanism.
Newborn babies as well as newborn mouse pups exposed to high partial oxygen pressure develop blindness when shifted back to normoxia (retinopathy of prematurity (ROP)), because of wild growth of new blood vessels that is driven by the acute hypoxia felt by astrocytes following the sudden drop in oxygen partial pressure which induces the astrocytes to express high levels of VEGF.Citation182 Interestingly, these new vessels are misdirected toward the vitreous and fail to vascularize the developing retina because they are repelled by sema3A expressed by hypoxic neuronal cells.Citation183 However, these abnormalities can be partially remedied by intra-vitreal injection of Sema3E which was observed to suppress the extra-retinal vascular outgrowth without affecting the desired regeneration of the retinal vasculature.Citation184 In addition it was recently observed that the avascular characteristic of the outer layers of the retina is due to the expression of sema3F in cells in these outer layers,Citation185 suggesting that several different semaphorins acting in concert regulate the distribution of blood vessels in the normal and diseased retina.
In the context of cancer it was observed that ectopic overexpression of sema3E in a variety of tumor cell types inhibits tumor development from such cells.Citation130,133 However, inhibition of tumor growth which occurs primarily because of the anti-angiogenic effects of sema3E is accompanied by sema3E induced induction of tumor metastasis.Citation111 A recent report suggested that sema3E can actually inhibit apoptosis that is induced by the sema3E receptor plexin-D1 in the absence of sema3E, suggesting that sema3E can contribute to tumor progression in this way too.Citation186 Furthermore, it was recently found that sema3E can induce inflammation that is mediated by macrophages.Citation187 Inflammation is recognized as a major contributor to tumor progression as are macrophages that are recruited to the tumor microenvironment,Citation187,188 suggesting that semaphorins such as sema3E may be able to influence tumor progression by modulation of the chronic inflammation that is a hallmark of many types of tumors.
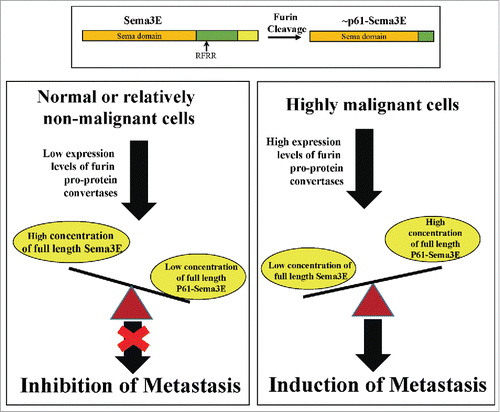
Like other class-3 semaphorins, sema3E contains conserved cleavage sites for furin like pro-protein convertases. Interestingly, it was found that the major furin cleavage product, p61-Sema3E, is responsible for the induction of tumor metastasis rather then full length sema3E.Citation112 It was observed that p61-Sema3E induces the association of plexin-D1 with the ErbB2 tyrosine-kinase receptor and induces “in-trans” the auto-phosphorylation of ErbB2 which in turn enhances the invasiveness of plexin-D1 and ErbB2 expressing tumor cells.Citation189 Since furins are upregulated in most metastatic cells,Citation171 the balance between the full length and cleaved forms of sema3E is tilted toward the cleaved form in most types of tumor cells thus promoting tumor metastasis (). A point mutated sema3E that resists cleavage by furin like pro-protein convertases is still able to bind to plexin-D1 and to activate plexin-D1 mediated inhibition of angiogenesis, but fails to induce tumor metastasis or phosphorylation of ErbB2. Furthermore, this cleavage resistant point mutated sema3E inhibits the pro-metastatic activity of p61-Sema3E because it competes with p61-Sema3E for binding to plexin-D1.Citation92 However, in cases in which tumor cells do not express plexin-D1 or tyrosine-kinase receptors that associates with plexin-D1 it is possible that wild type sema3E which in the tumor microenvironment may contain a high proportion of p61-Sema3E, may also display anti-metastatic properties due to its anti-angiogenic effects. Over-expression of wild type sema3E which is susceptible to furin like pro-protein convertases, nevertheless inhibited VEGF induced metastasis of melanoma cells, possibly due to inhibition of VEGF induced angiogenesis.Citation190 It is not known at this point in time if sema3E activities such as the induction of inflammationCitation187,191 or inhibition of tumor cells apoptosisCitation186 can be induced by both the full length form of sema3E, the p61 cleaved form, or by both.
Figure 4. The dual role of sema3E in tumor progression. Like all class-3 semaphorins, sema3E contains a conserved cleavage site for furin like pro-protein convertases (FPPC) which when cleaved generates the active N-terminal fragment p61-Sema3E (Upper Panel). In contrast with uncleaved full length sema3E, P61-Sema3E can induce the association of the sema3E plexin-D1 receptor with ErbB2 on tumor cells, thus activating “in-trans” ErbB2 mediated signal transduction that promotes tumor metastasis. Full length sema3E has anti-metastatic and anti-angiogenic properties. However, FPPC expression is strongly up-regulated in malignant tumor cells causing most of the sema3E produced by the tumor cells to be cleaved to p61-Sema3E and as a result to promote rather than to inhibit tumor metastasis.
Sema3F: The sema3F gene was initially identified as a tumor suppressor gene of lung cancer.Citation87,88,192 When recombinant sema3F is ectopically expressed in sema3F receptor expressing lung cancer cells it inhibits their anchorage free proliferation and invasiveness.Citation89,130 Similarly it can inhibit the proliferation and invasiveness of colorectalCitation193 and breast cancer cells,Citation194 and was recently also found to suppress the stemness of colorectal cancer cells.Citation195 In prostate cancer too, single nucleotide polymorphisms of sema3F were associated with increased prostate cancer risk and poor prognosis.Citation153 Taken together, it can be concluded that sema3F plays an inhibitory function in many types of tumors, and that the inhibition is due, at least in part, to sema3F induced inhibition of the migration and proliferation of responsive tumor cell types.
The effects of sema3F are primarily mediated by the neuropilin-2 and the plexin-A3 receptors although additional plexins such as plexin-A4 have also been implicated.Citation31,50,196 Sema3F was found to affect several signaling pathways in target tumor cells. In H157 lung cancer cells sema3F inhibited multiple signaling pathways including AKT/STAT3 signaling resulting in the loss of activated αvβ3-integrin.Citation197,198 The transcription factor retinoid orphan nuclear receptor α (RORα) functions in breast cancer cells as a tumor suppressor and this inhibitory activity is mediated at least in part through its control of sema3F expression.Citation199 Sema3F also inhibited the attachment and spreading of MCF-7 breast cancer cells and inhibited the expression of E-cadherin thus contributing to epithelial to mesenchymal transition (EMT),Citation200 a process of cardinal importance for the conversion of tumor cells into metastatic cells.Citation194,201 In addition, Sema3F inhibited integrin-β1 mediated attachment of A375 melanoma cells by a neuropilin-2 mediated mechanism and suppressed the metastatic spread of cells from tumors derived from these cells.Citation103 SEMA3F inhibits phosphatidyl inositol-3 kinase (PI3K) and Akt activity in a variety of target cells including endothelial cells and several tumor cell types. These, and responses were associated with the disruption of mTOR/rictor assembly and mTOR-dependent activation of the RhoA GTPase.Citation202
In addition, sema3F also has effects on the tumor microenvironment which contribute to its tumor progression inhibiting ability. It functions as a potent inhibitor of tumor angiogenesisCitation102,103 and as a result can inhibit tumor progression even when the tumor cells themselves do not express sema3F receptors.Citation102 Indeed, sema3F promoted apoptosis of endothelial cells and acted additively with sema3A to inhibit the proliferation of endothelial cells.Citation60 Sema3F also functions as a potent inhibitor of lymphangiogenesis, a process that is critical for the metastatic spread of some types of solid tumors such as head and neck tumors or breast cancer tumors.Citation103,203 Recently it was suggested that sema3F may also be able to inhibit angiogenesis through competition with VEGF-A for binding to neuropilin-1, a receptor to which full length sema3F binds with low affinity as compared to its affinity to neuropilin-2 and which seems unable to convey sema3F signals.Citation100,204 However, cleavage with furin like pro-protein convertases at the sema3F C-terminus was found to result in an increase in its affinity to neuropilin-1 enabling it to compete effectively with VEGF-A for binding to neuropilin-1.Citation63,205
The down regulation of endogenous sema3F expression in tumor cells which accompanies tumor progression in several types of cancer may enable the transition to the angiogenic phase in tumor development.Citation128 Indeed, sema3F inhibits the expression of HIF-1α and consequently VEGF expression, resulting in the inhibition of hypoxia induced angiogenesis.Citation197,198 The mechanisms by which sema3F expression is regulated are therefore of interest. The expression of sema3F is induced by wild type p53, and loss of functional p53 in tumor cells can thus result in reduced sema3F expression and consequently result in the induction of tumor angiogenesis.Citation206 Similarly, in Neurofibromatosis type 2 (NF2), an autosomal-dominant multiple neoplasia resulting from mutations in the NF2 tumor suppressor gene, the expression of sema3F is down-regulated. Reintroduction of SEMA3F into schwannoma cells lacking a functional NF2 gene resulted in the normalization of tumor blood vessels, reduced tumor burden, and extended survival suggesting that the product of the NF2 gene regulates angiogenesis via sema3F.Citation207 Sema3F expression is downregulated by the transcription repressor ZEB-1 which is highly active in lung cancer cells.Citation208 In metastatic tumor cells, myc driven expression of the transcription factor Id2 was also found to down-regulate sema3F expression resulting in the induction of tumor metastasis.Citation209 In endometrial cancer it was found that progesterone and 1,25-dihydroxyvitamin D(3) inhibit endometrial cancer cell growth by up-regulating the expression of sema3B and sema3F.Citation151 The progression of ovarian cancer was also associated with the downregulation of sema3F and its neuropilin-2 receptor whose expression in these cells was regulated by estrogen.Citation155,210 Sema3F inhibited integrin-β1 mediated attachment of A375 melanoma cells by a neuropilin-2 mediated mechanism and suppressed the metastatic spread of cells from tumors derived from these cells.Citation103 Taken together, it can be concluded that sema3F plays an inhibitory function in many types of tumors, and that the inhibition is due, at least in part, to sema3F induced inhibition of the migration and proliferation of responsive tumor cell types.
Sema4D: Sema4D (also referred to frequently as CD100) is a membrane bound class-4 semaphorin that binds to plexin-B1, plexin-B2 and to the CD-72 receptors.Citation16,84 The extracellular domain of sema4D can be cleaved and released from producing cells by membrane type-1 matrix metalloproteinase (MT1-MMP) and by the metalloprotease ADAM17 (TACE). These two metalloproteases are up-regulated in many types of malignant cells.Citation211-213 Sema4D is stored in platelets and its extracellular domain can be released from platelets.Citation212 The soluble cleaved extracellular domain of sema4D retains the biological activity of full length sema4DCitation214 and was used extensively to study the role of sema4D in immune reactions, tumor progression and the control of angiogenesis.
The tyrosine-kinase receptor Met is the receptor for hepatocyte growth factor/scatter factor (HGF/SF), a potent inducer of tumor cells invasiveness and angiogenesis.Citation215,216 The soluble extracellular domain of sema4D was found to bind to plexin-B1 and to induce the association of the sema4D receptor plexin-B1 with Met. This association then promotes “in-trans” auto-phosphorylation of the Met receptor and induction of tumor cells invasiveness.Citation217 It was subsequently observed that sema4D can also trans activate the related macrophage stimulating protein (MSP) receptor Ron,Citation218 and that all 3 type-B plexins are able form complexes with the Met and Ron receptors.Citation105,219 Furthermore, sema5A, a semaphorin that binds to the plexin-B3 receptor, can also trans activate Met similarly to sema4D.Citation17 The “in-trans” activation of Met by sema4D is also of importance for the regulation of developmental processes such as the migration of GnRH-1 neurons during brain development.Citation220 Since HGF is also a potent inducer of angiogenesis, these observations suggested that sema4D may also function as a pro-angiogenic factor and thereby further promote tumor progression as is indeed the case.Citation105
There are also observations suggesting that activation of Met may not be required for the pro-angiogenic activity of sema4D. Sema4D was found to induce angiogenesis independently of Met utilizing plexin-B1 induced Rho dependent mechanisms.Citation104 This mechanism involves the activation of the PI3K/Akt pathway following the binding of sema4D to plexin-B1. Activated plexin-B1 activates in turn an intracellular tyrosine kinase cascade that involves the sequential activation of PYK2 and Src which results in the tyrosine phosphorylation of Plexin-B1, recruitment of a multimeric signaling complex that includes PYK2, Src, and PI3K to Plexin-B1 and the activation of the Akt signaling pathway.Citation221,222 It was recently reported that this Met independent activity is mediated in addition by Rho/Rho Kinase (ROK) dependent generation of PI(4,5)P(2) upon treatment of endothelial cells with Sema4D.Citation223,224 In addition, activation of plexin-B1 by sema4D in endothelial cells can results in the activation of NF-kappaB and subsequently in the induction of the expression of the angiogenesis inducing factor IL-8.Citation225,226 Because sema4D activates angiogenesis using the plexin-B1 receptor and not the VEGFR-2 receptor used by VEGF, it follows that sema4D can act additively with VEGF and that inhibition of sema4D signaling can represent an alternative anti-angiogenic treatment strategy.Citation227 Sema4D enhances the expression of PDGF-B and angiopoietin like protein-4 in endothelial cells which in turn inhibits the association between pericytes and endothelial cells thereby influencing proliferation and differentiation of pericytes and vascular permeability, whereas VEGF lacks these effects.Citation228
Sema4D also has immunoregulatory functions (reviewed in refs. Citation229, 230). This can have implications for tumor progression. Sema4D enhances the recruitment of monocytes and promotes the differentiation of monocytes toward the M2 phenotype of macrophages, and in ovarian cancer is a marker of poor prognosis.Citation231 In another study it was observed that inhibitory sema4D antibodies enhance the recruitment of activated monocytes and lymphocytes into tumors in murine Colon26 and ErbB2+ mammary carcinoma models, and that inhibition of sema4D shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the tumor microenvironment.Citation232
Regardless of the mechanisms involved, there are numerous observations suggesting that sema4D plays a stimulatory role in the progression of several types of tumors including head and neck tumors, breast carcinomas, ovarian cancinomas, pancreatic carcinoma, prostate cancer and soft tissue sarcomas such as osteosarcoma.Citation233-239 These observations lead to the development of inhibitory humanized antibodies that are developed as anti-tumorigenic drugs that target sema4D.Citation240 However, there are also opposite observations. In melanocytes and in malignant melanoma cells sema4D inhibits HGF induced activation of Met and the inhibition of plexin-B1 expression in these cells leads to the activation rather than to the inactivation of Met.Citation241-243 In breast carcinoma cells, plexin-B1 and plexins-B2 also form complexes with the ErbB2 tyrosine-kinase receptor, and sema4D as well as sema4C are both able to induce ErbB2 phosphorylation “in-trans” following their binding to plexins-B1 or plexins-B2 receptors.Citation244 In these cells, the binding of sema4D to plexin-B1 associated with ErbB2 induces cell migration and metastasis while the binding of sema4D to plexin-B1 associated with Met inhibits cell migration, indicating that the exchange of the 2 receptor tyrosine kinases is sufficient to convert the cellular response of Sema4D from pro- to anti-migratory and vice versa.Citation36 Similar observations were also reported in prostate cancer cells.Citation238 It was recently also observed that the activation of plexin-B1 by Sema4D in breast carcinoma cells results in tyrosine phosphorylation of plexin-B1 by Met, thus creating a docking site for the SH2 domain of growth factor receptor bound-2 (Grb2). Grb2 is thereby recruited into the plexin-B1 receptor complex and through its SH3 domain, interacts with p190 RhoGAP and mediates RhoA deactivation and leads to the subsequent inhibition of breast carcinoma cells motility.Citation245
Additional, less studied semaphorins that modulate tumor progression
Class-4 semaphorins: Sema4A is a membrane anchored semaphorin that utilizes the plexin-D1 receptor for signaling, and like sema3E, the other plexin-D1 agonist, functions as an inhibitor of developmental angiogenesis.Citation23 However, it was also found to produce an opposite effect and enhance angiogenesis by enhancing VEGF expression.Citation109 Unlike sema3E, sema4A also utilizes type-B plexins,Citation246,247 neuropilin-1Citation248 and Tim-2Citation83 as signal transducing receptors. Sema4A is a well characterized modulator of immune responsesCitation229 and may also affect tumor progression via the immune system. However, it is as yet unclear whether it has a role as a modulator of tumor angiogenesis and tumor progression.
Another class-4 semaphorin, sema4F was found to be down-regulated in a panel of human neurofibromas suggesting that it functions as an inhibitor of tumor progression.Citation249 However, there is also evidence suggesting that it promotes the progression of prostate cancerCitation250,251 and it was reported to contribute the progression of neurofibromatosis type 1 (NF1).Citation249
Class-5 semaphorins: Sema5A was recently found to promote tumor metastasis in gastric cancerCitation252 and in pancreatic cancerCitation107 but inhibited the motility of glioma cells.Citation253 Sema5B may play a role in renal cell carcinoma since down-regulation of sema5B expression in renal cell carcinoma cells significantly compromises their viability.Citation254
Class-6 semaphorins: Sema6A is a membrane bound semaphorin that signals via the plexin-A2 and plexin-A4 receptors.Citation19 A soluble extracellular domain derived from sema6A was found to function as an inhibitor of angiogenesisCitation29,115 suggesting that it may function as an inhibitor of tumor progression. Indeed, glioblastoma patients expressing high sema6A protein levels had a significantly longer overall survival.Citation255 However, sema6A may also be required for the survival of endothelial cells. Silencing its expression in endothelial cells leads to apoptosis and in-vivo to reduced tumor angiogenesis and tumor development due to inhibition of VEGF induced VEGFR-2 mediated signal transduction which can be rescued by the addition of the exogenous soluble extracellular domain of sema6A,Citation108 suggesting that it can affect tumor angiogenesis and tumor progression utilizing diverse mechanisms of action. In addition, inhibition of sema6A expression as well as MICAL-1 expression in melanoma cells containing mutated B-RAF inhibited tumor progression while overexpression of sema6A in these cells promoted tumor progression,Citation256 suggesting that the effects of sema6A on tumor progression may vary depending on tumor type.
Sema6D was found to activate the VEGFR-2 VEGF receptor “in-trans” following its binding to the plexin-A1 receptor.Citation18 Indeed, sema6D functions as a survival and metastasis inducing gene in mesothelioma in a plexin-A1 and VEGFR-2 dependent manner.Citation37 In addition, sema6D is important for tumor progression in a subtype of triple negative breast cancer.Citation257 Another family member, sema6B, was recently described as an inducer of metastasis in gastric cancerCitation258 but was also reported as a possible inhibitor of breast cancer progression.Citation259
Sema7A: Sema7A utilizes the plexin-C1 receptor for signal transduction. Sema7A functions as a modulator of immune responses and as an initiator of inflammatory responsesCitation229 by which it may also influence tumor progression.Citation260 In metastatic melanomas, sema7A was reported to contribute to the metastasis of melanoma cells to lungsCitation261 and to function as a pro-angiogenic factor.Citation262 Likewise, it was reported to contribute to the metastasis of breast cancer cells by promoting epithelial to mesenchymal transition.Citation263 Recently, sema7A was found to be upregulated in oral squamous cell carcinoma, to promote their proliferation of these tumor cells, and to enhance their invasiveness.Citation264
Conclusions
Initially it was thought that the semaphorins would function primarily as inhibitors of tumor progression and tumor angiogenesis. This turned out not to be the case and by now several semaphorins have been found to promote tumor progression and to enhance angiogenesis. Furthermore, several semaphorins were reported both to induce and to inhibit tumor progression. These different activities seem context dependent and there is evidence suggesting that interactions between semaphorin receptors and apparently unrelated receptors such as various tyrosine-kinase receptors as well as post translational modifications of the semaphorins and their receptors can profoundly affect their biological activities as exemplified in the case of sema3E.Citation71,174,186,189 These interactions and modifications can in turn profoundly affect the course of diseases such as cancer, and a better understanding of these interactions and post translational modifications is required if one considers the development of anti-tumorigenic and anti-angiogenic therapeutic agents that target or utilize semaphorin signal transduction. Thus, research aimed at a better understanding of the processing of semaphorins and their receptors and better characterization of the cross-talk between semaphorins and their receptors and other signal transduction systems is likely to be a focus of research for some time to come. In addition to cancer, it seems that semaphorins play major regulatory roles in the development and maintenance of the vascular and neuronal networks of organs such as the retina and the kidney, and it is likely that the study of the role of the semaphorins in the development of vascular diseases such as complications of diabetes such as diabetic retinopathy or diabetic nephropathy will also become a focus of intensive research in the near future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Israel Science Foundation (ISF) and by a grant from the Rappaport Family Institute for Research in the Medical Sciences of Technion.
References
- Goodman CS, Kolodkin AL, Luo Y, Pueschel AW, Raper JA. Unified nomenclature for the semaphorins collapsins. Cell 1999; 97:551-2; PMID:10367884; http://dx.doi.org/10.1016/S0092-8674(00)80766-7
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron 1997; 19:539-45; PMID:9331347; http://dx.doi.org/10.1016/S0896-6273(00)80370-0
- Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol 2003; 10:843-8; PMID:12958590; http://dx.doi.org/10.1038/nsb977
- Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, Rajashankar KR, Lu M, Hoemme C, Puschel AW, and others. Structure of the semaphorin-3A receptor binding module. Neuron 2003; 39:589-98; PMID:12925274; http://dx.doi.org/10.1016/S0896-6273(03)00502-6
- Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from sema7A and A39R complexes with PlexinC1. Cell 2010; 142:749-61; PMID:20727575; http://dx.doi.org/10.1016/j.cell.2010.07.040
- Toyofuku T, Nojima S, Ishikawa T, Takamatsu H, Tsujimura T, Uemura A, Matsuda J, Seki T, Kumanogoh A. Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev 2012; 26:816-29; PMID:22465952; http://dx.doi.org/10.1101/gad.184481.111
- Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, Verdino P, Wilson IA, Kumanogoh A, Kikutani H, and others. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal gammadelta T cell function. Immunity 2012; 37:314-25; PMID:22902232; http://dx.doi.org/10.1016/j.immuni.2012.05.026
- Ben-Gigi L, Sweetat S, Besser E, Fellig Y, Wiederhold T, Polakiewicz RD, Behar O. Astrogliosis induced by brain injury is regulated by Sema4B Phosphorylation(123). eNeuro 2015; e0078-14:1-15.
- Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem 2000; 275:39647-53; PMID:10993894; http://dx.doi.org/10.1074/jbc.M006316200
- Klostermann A, Lohrum M, Adams RH, Puschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem 1998; 273:7326-31; PMID:9516427; http://dx.doi.org/10.1074/jbc.273.13.7326
- Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature 2010; 467:1118-22; PMID:20877282; http://dx.doi.org/10.1038/nature09468
- Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, and others. Structural basis for semaphorin signalling through the plexin receptor. Nature 2010; 467:1123-7; PMID:20881961; http://dx.doi.org/10.1038/nature09473
- Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci 2012; 69:3765-805; PMID:22744749; http://dx.doi.org/10.1007/s00018-012-1019-0
- Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci 2005; 62(12):1363-71:1363-1371; PMID:15818466; http://dx.doi.org/10.1007/s00018-005-5018-2
- Gutmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The Neuropilins and their roll in tumorigenesis and tumor progression. Cancer Lett 2006; 231:1-11; PMID:16356825; http://dx.doi.org/10.1016/j.canlet.2004.12.047
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, and others. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999; 99:71-80; PMID:10520995; http://dx.doi.org/10.1016/S0092-8674(00)80063-X
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep 2004; 5:710-4; PMID:15218527; http://dx.doi.org/10.1038/sj.embor.7400189
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, and others. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 2004; 18:435-47; PMID:14977921; http://dx.doi.org/10.1101/gad.1167304
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, and others. Interactions between Plexin-A2, Plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 2007; 53:535-47; PMID:17296555; http://dx.doi.org/10.1016/j.neuron.2007.01.028
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, and others. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 2005; 25:3628-37; PMID:15814794; http://dx.doi.org/10.1523/JNEUROSCI.4480-04.2005
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, and others. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 2011; 71:460-73; PMID:21835343; http://dx.doi.org/10.1016/j.neuron.2011.06.009
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 2005; 307:265-8; PMID:15550623; http://dx.doi.org/10.1126/science.1105416
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J 2007; 26:1373-84; PMID:17318185; http://dx.doi.org/10.1038/sj.emboj.7601589
- Takahashi T, Strittmatter SM. PlexinA1 autoinhibition by the plexin sema domain. Neuron 2001; 29:429-39; PMID:11239433; http://dx.doi.org/10.1016/S0896-6273(01)00216-1
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 2004; 305:862-5; PMID:15297673; http://dx.doi.org/10.1126/science.1097545
- He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci U S A 2009; 106:15610-5; PMID:19717441; http://dx.doi.org/10.1073/pnas.0906923106
- Sakurai A, Gavard J, nnas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, and others. Semaphorin 3E initiates anti-angiogenic signaling through Plexin-D1 by regulating Arf6 and R-Ras. Mol Cell Biol 2010; 30:3086-98; PMID:20385769; http://dx.doi.org/10.1128/MCB.01652-09
- Worzfeld T, Swiercz JM, Senturk A, Genz B, Korostylev A, Deng S, Xia J, Hoshino M, Epstein JA, Chan AM, and others. Genetic dissection of plexin signaling in vivo. Proc Natl Acad Sci U S A 2014; 111:2194-9; PMID:24469813; http://dx.doi.org/10.1073/pnas.1308418111
- Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood 2011; 118:4285-96; PMID:21832283; http://dx.doi.org/10.1182/blood-2011-03-341388
- Janssen BJ, Malinauskas T, Weir GA, Cader MZ, Siebold C, Jones EY. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat Struct Mol Biol 2012; 19:1293-9; PMID:23104057; http://dx.doi.org/10.1038/nsmb.2416
- Schwarz Q, Waimey KE, Golding M, Takamatsu H, Kumanogoh A, Fujisawa H, Cheng HJ, Ruhrberg C. Plexin A3 and plexin A4 convey semaphorin signals during facial nerve development. Dev Biol 2008; 324:1-9; PMID:18804103; http://dx.doi.org/10.1016/j.ydbio.2008.08.020
- Wen H, Lei Y, Eun S-Y, Ting JP. Plexin-A4 semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J Exp Med 2010; 207:2943-57; PMID:21098092; http://dx.doi.org/10.1084/jem.20101138
- Bouvree K, Brunet I, Del TR, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, and others. Semaphorin3A, neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ Res 2012; 111:437-45; PMID:22723296; http://dx.doi.org/10.1161/CIRCRESAHA.112.269316
- Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood 2007; 111:2290-9; PMID:18056484; http://dx.doi.org/10.1182/blood-2007-06-096529
- Ben-Zvi A, Manor O, Schachner M, Yaron A, Tessier-Lavigne M, Behar O. The semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J Neurosci 2008; 28:12427-32; PMID:19020035; http://dx.doi.org/10.1523/JNEUROSCI.3573-08.2008
- Swiercz JM, Worzfeld T, Offermanns S. ERBB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem 2008; 283:1893-901; PMID:18025083; http://dx.doi.org/10.1074/jbc.M706822200
- Catalano A, Lazzarini R, Di NS, Orciari S, Procopio A. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-{kappa}B to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res 2009; 69:1485-93; PMID:19176370; http://dx.doi.org/10.1158/0008-5472.CAN-08-3659
- Negishi M, Oinuma I, Katoh H. R-ras as a key player for signaling pathway of plexins. Mol Neurobiol 2005; 32:217-22; PMID:16385138; http://dx.doi.org/10.1385/MN:32:3:217
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 2005; 8:1712-9; PMID:16286926; http://dx.doi.org/10.1038/nn1596
- Sakurai A, Jian X, Lee CJ, Manavski Y, Chavakis E, Donaldson J, Randazzo PA, Gutkind JS. Phosphatidylinositol-4-phosphate 5-kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-Plexin-D1 through Arf6 Protein. J Biol Chem 2011; 286:34335-45; PMID:21795701; http://dx.doi.org/10.1074/jbc.M111.259499
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 2002; 109:887-900; PMID:12110185; http://dx.doi.org/10.1016/S0092-8674(02)00794-8
- Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. Mical links semaphorins to F-actin disassembly. Nature 2010; 463:823-7; PMID:20148037; http://dx.doi.org/10.1038/nature08724
- Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by mical. Science 2011; 334:1710-3; PMID:22116028; http://dx.doi.org/10.1126/science.1211956
- Hung RJ, Spaeth CS, Yesilyurt HG, Terman JR. SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics. Nat Cell Biol 2013; 15:1445-54; PMID:24212093; http://dx.doi.org/10.1038/ncb2871
- Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep 2008; 9:865-71; PMID:18660749; http://dx.doi.org/10.1038/embor.2008.139
- Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol 2007; 600:12-23; PMID:17607943; http://dx.doi.org/10.1007/978-0-387-70956-7_2
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008; 8:632-45; PMID:18580951; http://dx.doi.org/10.1038/nrc2404
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999; 99:59-69; PMID:10520994; http://dx.doi.org/10.1016/S0092-8674(00)80062-8
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell 2004; 7:107-16; PMID:15239958; http://dx.doi.org/10.1016/j.devcel.2004.06.002
- Sabag AD, Smolkin T, Mumblat Y, Ueffing M, Kessler O, Gloeckner CJ, Neufeld G. The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction. J Cell Sci 2014; 127:5240-52; PMID:25335892; http://dx.doi.org/10.1242/jcs.155960
- Gitay-Goren H, Cohen T, Tessler S, Soker S, Gengrinovitch S, Rockwell P, Klagsbrun M, Levi B-Z, Neufeld G. Selective binding of VEGF121 to one of the three VEGF receptors of vascular endothelial cells. J Biol Chem 1996; 271:5519-23; PMID:8621410; http://dx.doi.org/10.1074/jbc.271.10.5519
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform specific receptor for vascular endothelial growth factor. Cell 1998; 92:735-45; PMID:9529250; http://dx.doi.org/10.1016/S0092-8674(00)81402-6
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 and Neuropilin-1 are receptors for 165-amino acid long form of vascular endothelial growth factor (VEGF) and of placenta growth factor-2, but only neuropilin-2 functions as a receptor for the 145 amino acid form of VEGF. J Biol Chem 2000; 275:18040-5; PMID:10748121; http://dx.doi.org/10.1074/jbc.M909259199
- Makinen T, Olofsson B, Karpanen T, Hellman U, Soker S, Klagsbrun M, Eriksson U, Alitalo K. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem 1999; 274:21217-22; PMID:10409677; http://dx.doi.org/10.1074/jbc.274.30.21217
- Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J 2006; 20:1462-72; PMID:16816121; http://dx.doi.org/10.1096/fj.05-5646com
- Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem 1998; 273:22272-8; PMID:9712843; http://dx.doi.org/10.1074/jbc.273.35.22272
- West DC, Chris RG, Duchesne L, Patey SJ, Terry CJ, Turnbull JE, Delehedde M, Heegaard CW, Allain F, Vanpouille C, and others. Interactions of multiple heparin-binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem 2005; 280:13457-64; PMID:15695515; http://dx.doi.org/10.1074/jbc.M410924200
- Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2007; 111:2036-45; PMID:18065694; http://dx.doi.org/10.1182/blood-2007-04-084269
- Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, and others. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J 2007; 26:4902-12; PMID:17989695; http://dx.doi.org/10.1038/sj.emboj.7601906
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem 2007; 282:26294-305; PMID:17569671; http://dx.doi.org/10.1074/jbc.M609711200
- Mumblat Y, Kessler O, Ilan N, Neufeld G. Full length semaphorin-3C functions as an inhibitor of tumor lymphangiogenesis and tumor metastasis. Cancer Res 2015; 75:2177-86; PMID:25808871; http://dx.doi.org/10.1158/0008-5472.CAN-14-2464
- Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J 1997; 16:6077-86; PMID:9321387; http://dx.doi.org/10.1093/emboj/16.20.6077
- Guo HF, Li X, Parker MW, Waltenberger J, Becker PM, Vander Kooi CW. Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry 2013; 52:7551-8; PMID:24079887; http://dx.doi.org/10.1021/bi401034q
- Shraga-Heled N, Kessler O, Prahst C, Kroll J, Augustin HG, Neufeld G. Neuropilin-1 and neuropilin-2 enhance VEGF121 stimulated signal transduction by the VEGFR-2 receptor. FASEB J 2007; 21:915-26; PMID:17185751; http://dx.doi.org/10.1096/fj.06-6277com
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, Gu C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. Elife 2014; 3:e03720; PMID:25244320; http://dx.doi.org/10.7554/eLife.03720
- Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del TR, Suchting S, Medvinsky A, and others. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 2010; 188:115-30; PMID:20065093; http://dx.doi.org/10.1083/jcb.200903137
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, and others. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 2006; 108:1243-50; PMID:16621967; http://dx.doi.org/10.1182/blood-2005-11-4447
- Glinka Y, Stoilova S, Mohammed N, Prud'homme GJ. Neuropilin-1 exerts coreceptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis 2011; 32:613-21; PMID:21186301; http://dx.doi.org/10.1093/carcin/bgq281
- Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, and others. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest 2010; 120:2379-94; PMID:20577048; http://dx.doi.org/10.1172/JCI41203
- Ben-Zvi A, Ben-Gigi L, Klein H, Behar O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J Neurosci 2007; 27:13000-11; PMID:18032673; http://dx.doi.org/10.1523/JNEUROSCI.3373-07.2007
- Rizzolio S, Rabinoviz N, Rainero E, Lanzetti L, Serini G, Norman JC, Neufeld G, Tamagnone L. Neuropilin-1 dependent regulation of EGF-Receptor signaling. Cancer Res 2012; 72:5801-11; PMID:22986738; http://dx.doi.org/10.1158/0008-5472.CAN-12-0995
- Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res 2007; 67:10309-16; PMID:17974973; http://dx.doi.org/10.1158/0008-5472.CAN-07-3256
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002; 129:4797-806; PMID:12361971.
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999; 126:4895-902; PMID:10518505.
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki JJ, Hirota S, Kitamura Y, and others. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A 2002; 99:3657-62; PMID:11891274; http://dx.doi.org/10.1073/pnas.022017899
- Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development 2011; 138:4185-91; PMID:21852397; http://dx.doi.org/10.1242/dev.070037
- Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, and others. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev Cell 2013; 25:156-68; PMID:23639442; http://dx.doi.org/10.1016/j.devcel.2013.03.019
- Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, de Assuncao TM, Cao Y, Szabolcs A, Thorgeirsson S, and others. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012; 72:4047-59; PMID:22738912; http://dx.doi.org/10.1158/0008-5472.CAN-11-3907
- Cai HB, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: A PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci 1999; 19:6519-27; PMID:10414980.
- Prahst C, Heroult M, Lanahan AA, Uziel N, Kessler O, Shraga-Heled N, Simons M, Neufeld G, Augustin HG. Neuropilin-1/VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J Biol Chem 2008; 283:25110-4; PMID:18628209; http://dx.doi.org/10.1074/jbc.C800137200
- Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 2002; 21:6348-57; PMID:12456642; http://dx.doi.org/10.1093/emboj/cdf645
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, and others. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 2005; 48:63-75; PMID:16202709; http://dx.doi.org/10.1016/j.neuron.2005.10.024
- Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, and others. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 2002; 419:629-33; PMID:12374982; http://dx.doi.org/10.1038/nature01037
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, and others. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 2000; 13:621-31; PMID:11114375; http://dx.doi.org/10.1016/S1074-7613(00)00062-5
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, and others. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004; 44:961-75; PMID:15603739; http://dx.doi.org/10.1016/j.neuron.2004.12.002
- Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, and others. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature 2007; 446:680-4; PMID:17377534; http://dx.doi.org/10.1038/nature05652
- Xiang RH, Hensel CH, Garcia DK, Carlson HC, Kok K, Daly MC, Kerbacher K, van den BA, Veldhuis P, Buys CH, and others. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics 1996; 32:39-48; PMID:8786119; http://dx.doi.org/10.1006/geno.1996.0074
- Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, and others. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene 1996; 12:1289-97; PMID:8649831.
- Xiang R, Davalos AR, Hensel CH, Zhou XJ, Tse C, Naylor SL. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res 2002; 62:2637-43; PMID:11980661.
- Tse C, Xiang RH, Bracht T, Naylor SL. Human semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res 2002; 62:542-6; PMID:11809707.
- Tomizawa Y, Sekido Y, Kondo M, Gao B, Yokota J, Roche J, Drabkin H, Lerman MI, Gazdar AF, Minna JD. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci U S A 2001; 98:13954-9; PMID:11717452; http://dx.doi.org/10.1073/pnas.231490898
- Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol Med 2012; 4:234-50; PMID:22247010; http://dx.doi.org/10.1002/emmm.201100205
- Li X, Law JW, Lee AY. Semaphorin 5A and plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the actin cytoskeleton. Oncogene 2011; 31:595-610; PMID:21706053.
- Casazza A, Mazzone M. Altering the intratumoral localization of macrophages to inhibit cancer progression. Oncoimmunology 2014; 3:e27872; PMID:24800172; http://dx.doi.org/10.4161/onci.27872
- Gospodarowicz D, Abraham JA, Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc Natl Acad Sci USA 1989; 86:7311-5; PMID:2798412; http://dx.doi.org/10.1073/pnas.86.19.7311
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246:1306-9; PMID:2479986; http://dx.doi.org/10.1126/science.2479986
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989; 246:1309-12; PMID:2479987; http://dx.doi.org/10.1126/science.2479987
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell 1997; 90:753-62; PMID:9288754; http://dx.doi.org/10.1016/S0092-8674(00)80535-8
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997; 90:739-51; PMID:9288753; http://dx.doi.org/10.1016/S0092-8674(00)80534-6
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 1997; 19:547-59; PMID:9331348; http://dx.doi.org/10.1016/S0896-6273(00)80371-2
- Folkman J. What is the evidence that tumors are angiogenesis dependent. J Nat Cancer Inst 1990; 82:4-7; PMID:1688381; http://dx.doi.org/10.1093/jnci/82.1.4
- Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res 2004; 64:1008-15; PMID:14871832; http://dx.doi.org/10.1158/0008-5472.CAN-03-3090
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest 2004; 114:1260-71; PMID:15520858; http://dx.doi.org/10.1172/JCI21378
- Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating rho-initiated pathways through Plexin-B. Cancer Res 2004; 64:5212-24; PMID:15289326; http://dx.doi.org/10.1158/0008-5472.CAN-04-0126
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 2005; 105:4321-9; PMID:15632204; http://dx.doi.org/10.1182/blood-2004-07-2885
- Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, Lele SM, Batra SK, Singh RK. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer 2010; 127:1373-83; PMID:20073063; http://dx.doi.org/10.1002/ijc.25166
- Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Br J Cancer 2012; 107:501-7; PMID:22782341; http://dx.doi.org/10.1038/bjc.2012.298
- Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A, Li X, Tosato G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood 2012; 120:4104-15; PMID:23007403; http://dx.doi.org/10.1182/blood-2012-02-410076
- Meda C, Molla F, De PM, Regano D, Maione F, Capano S, Locati M, Mantovani A, Latini R, Bussolino F, and others. Semaphorin 4A exerts a proangiogenic effect by enhancing vascular endothelial growth factor-a expression in macrophages. J Immunol 2012; 188:4081-92; PMID:22442441; http://dx.doi.org/10.4049/jimmunol.1101435
- Ghanem RC, Han KY, Rojas J, Ozturk O, Kim DJ, Jain S, Chang JH, Azar DT. Semaphorin 7A promotes angiogenesis in an Experimental corneal neovascularization model. Curr Eye Res 2011; 36:989-96; PMID:21999225; http://dx.doi.org/10.3109/02713683.2011.593730
- Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res 1998; 58:1238-44; PMID:9515811.
- Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal sema3E into an inducer of invasive growth and lung metastasis. Cancer Res 2005; 65:6167-77; PMID:16024618; http://dx.doi.org/10.1158/0008-5472.CAN-04-4309
- Vaitkiene P, Skiriute D, Steponaitis G, Skauminas K, Tamasauskas A, Kazlauskas A. High level of Sema3C is associated with glioma malignancy. Diagn Pathol 2015; 10:58; PMID:26032848; http://dx.doi.org/10.1186/s13000-015-0298-9
- Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci 2012; 103:1961-6; PMID:22924992; http://dx.doi.org/10.1111/cas.12003
- Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, Macdougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, and others. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther 2005; 4:659-68; PMID:15917651; http://dx.doi.org/10.4161/cbt.4.6.1733
- Yang WJ, Hu J, Uemura A, Tetzlaff F, Augustin HG, Fischer A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med 2015; e201404922
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013; 24:695-709; PMID:24332039; http://dx.doi.org/10.1016/j.ccr.2013.11.007
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol 2003; 255:77-98; PMID:12618135; http://dx.doi.org/10.1016/S0012-1606(02)00045-3
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, and others. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003; 424:391-7; PMID:12879061; http://dx.doi.org/10.1038/nature01784
- Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 2008; 111:2674-80; PMID:18180379; http://dx.doi.org/10.1182/blood-2007-08-110205
- Vacca A, Scavelli C, Serini G, Di PG, Cirulli T, Merchionne F, Ribatti D, Bussolino F, Guidolin D, Piaggio G, and others. Loss of inhibitory semaphorin 3A (SEMA3A) autocrine loops in bone marrow endothelial cells of patients with multiple myeloma. Blood 2006; 108:1661-7; PMID:16684957; http://dx.doi.org/10.1182/blood-2006-04-014563
- Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest 2009; 119:3356-72; PMID:19809158.
- Barresi V, Tuccari G. Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology 2010; 30:537-46; PMID:20337947.
- Song X, Zhang W, Zhang Y, Zhang H, Fu Z, Ye J, Liu L, Song X, Wu Y. Expression of semaphorin 3A and neuropilin 1 with clinicopathological features and survival in human tongue cancer. Med Oral Patol Oral Cir Bucal 2012; 17:e962-968; PMID:22926477; http://dx.doi.org/10.4317/medoral.18168
- Jiang H, Qi L, Wang F, Sun Z, Huang Z, Xi Q. Decreased semaphorin 3A expression is associated with a poor prognosis in patients with epithelial ovarian carcinoma. Int J Mol Med 2015; 35:1374-80; PMID:25812535.
- Tang C, Gao X, Liu H, Jiang T, Zhai X. Decreased expression of SEMA3A is associated with poor prognosis in gastric carcinoma. Int J Clin Exp Pathol 2014; 7:4782-94; PMID:25197349.
- Zhou H, Wu A, Fu W, Lv Z, Zhang Z. Significance of semaphorin-3A and MMP-14 protein expression in non-small cell lung cancer. Oncol Lett 2014; 7:1395-400; PMID:24765144.
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86:353-64; PMID:8756718; http://dx.doi.org/10.1016/S0092-8674(00)80108-7
- Shirvan A, Ziv I, Fleminger G, Shina R, He ZG, Brudo I, Melamed E, Barzilai A. Semaphorins as mediators of neuronal apoptosis. J Neurochem 1999; 73:961-71; PMID:10461885; http://dx.doi.org/10.1046/j.1471-4159.1999.0730961.x
- Kigel B, Varshavsky A, Kessler O, Neufeld G. Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE 2008; 3:e3287; PMID:18818766; http://dx.doi.org/10.1371/journal.pone.0003287
- Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, Squadrito ML, Venneri MA, Mazzone M, Larsson E, and others. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol 2011; 741-9; PMID:21205984; http://dx.doi.org/10.1161/ATVBAHA.110.211920
- Chakraborty G, Kumar S, Mishra R, Patil TV, Kundu GC. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS ONE 2012; 7:e33633; PMID:22448259; http://dx.doi.org/10.1371/journal.pone.0033633
- Sabag AD, Bode J, Fink D, Kigel B, Kugler W, Neufeld G. Semaphorin-3D and semaphorin-3E inhibit the development of tumors from glioblastoma cells implanted in the cortex of the brain. PLoS One 2012; 7:e42912; PMID:22936999; http://dx.doi.org/10.1371/journal.pone.0042912
- Huang C, Wang Y, Huang JH, Zhang J, Wang Z, Liu W. Lentiviral mediated overexpression of Sema3A in oral cancer cells decreases tumor growth by inhibiting angiogenesis. Biochem Biophys Res Commun 2015; 10; PMID:25634693.
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 2006; 124:175-89; PMID:16413490; http://dx.doi.org/10.1016/j.cell.2005.10.036
- Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, Arsic N, Tafuro S, Sinagra G, Giacca M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest 2008; 118:2062-75; PMID:18483621.
- Carrer A, Moimas S, Zacchigna S, Pattarini L, Zentilin L, Ruozi G, Mano M, Sinigaglia M, Maione F, Serini G, and others. Neuropilin-1 identifies a subset of bone marrow Gr1- monocytes that can induce tumor vessel normalization and inhibit tumor growth. Cancer Res 2012; 15:6371-81; http://dx.doi.org/10.1158/0008-5472.CAN-12-0762
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res 2003; 63:5230-3; PMID:14500350.
- Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol 2007; 30:1231-8; PMID:17390026.
- Mishra R, Thorat D, Soundararajan G, Pradhan SJ, Chakraborty G, Lohite K, Karnik S, Kundu GC. Semaphorin 3A upregulates FOXO 3a-dependent MelCAM expression leading to attenuation of breast tumor growth and angiogenesis. Oncogene 2015; 34:1584-95; PMID:24727891; http://dx.doi.org/10.1038/onc.2014.79
- Nehil M, Paquette J, Tokuyasu T, McCormick F. High mobility group box 1 promotes tumor cell migration through epigenetic silencing of semaphorin 3A. Oncogene 2013; 33:5151-62; PMID:24213571; http://dx.doi.org/10.1038/onc.2013.459
- Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, Bussolino F, Serini G, Giraudo E. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest 2012; 122:1832-48; PMID:22484816; http://dx.doi.org/10.1172/JCI58976
- Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene 2009; 28:3537-50; PMID:19684614; http://dx.doi.org/10.1038/onc.2009.204
- Muller MW, Giese NA, Swiercz JM, Ceyhan GO, Esposito I, Hinz U, Buchler P, Giese T, Buchler MW, Offermanns S, and others. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer 2007; 121:2421-33; PMID:17631638; http://dx.doi.org/10.1002/ijc.22949
- Kuroki T, Trapasso F, Yendamuri S, Matsuyama A, Alder H, Williams NN, Kaiser LR, Croce CM. Allelic loss on chromosome 3p21.3 and promoter hypermethylation of semaphorin 3B in non-small cell lung cancer. Cancer Res 2003; 63:3352-5; PMID:12810670.
- Tischoff I, Markwarth A, Witzigmann H, Uhlmann D, Hauss J, Mirmohammadsadegh A, Wittekind C, Hengge UR, Tannapfel A. Allele loss and epigenetic inactivation of 3p21.3 in malignant liver tumors. Int J Cancer 2005; 115:684-9; PMID:15704097; http://dx.doi.org/10.1002/ijc.20944
- Nair PN, McArdle L, Cornell J, Cohn SL, Stallings RL. High-resolution analysis of 3p deletion in neuroblastoma and differential methylation of the SEMA3B tumor suppressor gene. Cancer Genet Cytogenet 2007; 174:100-10; PMID:17452250; http://dx.doi.org/10.1016/j.cancergencyto.2006.11.017
- Campioni M, Ambrogi V, Pompeo E, Citro G, Castelli M, Spugnini EP, Gatti A, Cardelli P, Lorenzon L, Baldi A, and others. Identification of genes down-regulated during lung cancer progression: a cDNA array study. J Exp Clin Cancer Res 2008; 27:38; PMID:18793406; http://dx.doi.org/10.1186/1756-9966-27-38
- Chen R, Zhuge X, Huang Z, Lu D, Ye X, Chen C, Yu J, Lu G. Analysis of SEMA3B methylation and expression patterns in gastric cancer tissue and cell lines. Oncol Rep 2014; 31:1211-8; PMID:24402303.
- Loginov VI, Dmitriev AA, Senchenko VN, Pronina IV, Khodyrev DS, Kudryavtseva AV, Krasnov GS, Gerashchenko GV, Chashchina LI, Kazubskaya TP, and others. Tumor suppressor function of the SEMA3B gene in human lung and renal cancers. PLoS ONE 2015; 10:e0123369; PMID:25961819; http://dx.doi.org/10.1371/journal.pone.0123369
- Nguyen H, Ivanova VS, Kavandi L, Rodriguez GC, Maxwell G, Syed V. Progesterone and 1,25-dihydroxyvitamin D3 inhibit endometrial cancer cell growth by upregulating semaphorin 3B and semaphorin 3F. Mol Cancer Res 2011; 9:1479-92; PMID:21933904; http://dx.doi.org/10.1158/1541-7786.MCR-11-0213
- Wang K, Ling T, Wu H, Zhang J. Screening of candidate tumor-suppressor genes in 3p21.3 and investigation of the methylation of gene promoters in oral squamous cell carcinoma. Oncol Rep 2013; 29:1175-82; PMID:23292452.
- Beuten J, Garcia D, Brand TC, He X, Balic I, Canby-Hagino E, Troyer DA, Baillargeon J, Hernandez J, Thompson IM, and others. Semaphorin 3B and 3F single nucleotide polymorphisms are associated with prostate cancer risk and poor prognosis. J Urol 2009; 182:1614-20; PMID:19683737; http://dx.doi.org/10.1016/j.juro.2009.06.016
- Marsit CJ, Wiencke JK, Liu M, Kelsey KT. The race associated allele of semaphorin 3B (SEMA3B) T415I and its role in lung cancer in African-Americans and Latino-Americans. Carcinogenesis 2005; 26:1446-9; PMID:15831529; http://dx.doi.org/10.1093/carcin/bgi098
- Joseph D, Ho SM, Syed V. Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Mol Cancer Ther 2010; 9:499-509; PMID:20124444; http://dx.doi.org/10.1158/1535-7163.MCT-09-0664
- Staton CA, Shaw LA, Valluru M, Hoh L, Koay I, Cross SS, Reed MW, Brown NJ. Expression of class 3 semaphorins and their receptors in human breast neoplasia. Histopathology 2011; 59:274-82; PMID:21884206; http://dx.doi.org/10.1111/j.1365-2559.2011.03922.x
- Varshavsky A, Kessler O, Abramovitch S, Kigel B, Zaffryar S, Akiri G, Neufeld G. Semaphorin-3B Is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res 2008; 68:6922-31; PMID:18757406; http://dx.doi.org/10.1158/0008-5472.CAN-07-5408
- Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci 1998; 1:487-93; PMID:10196546; http://dx.doi.org/10.1038/2203
- Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci U S A 2004; 101:11432-7; PMID:15273288; http://dx.doi.org/10.1073/pnas.0403969101
- Castro-Rivera E, Ran S, Brekken RA, Minna JD. Semaphorin 3B inhibits the phosphatidylinositol 3-kinase/Akt pathway through neuropilin-1 in lung and breast cancer cells. Cancer Res 2008; 68:8295-303; PMID:18922901; http://dx.doi.org/10.1158/0008-5472.CAN-07-6601
- Rolny C, Capparuccia L, Casazza A, Mazzone M, Vallario A, Cignetti A, Medico E, Carmeliet P, Comoglio PM, Tamagnone L. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med 2008; 205:1155-71; PMID:18458115; http://dx.doi.org/10.1084/jem.20072509
- Huang S, Mills L, Mian B, Tellez C, Mccarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 2002; 161:125-34; PMID:12107097; http://dx.doi.org/10.1016/S0002-9440(10)64164-8
- Yamada T, Endo R, Gotoh M, Hirohashi S. Identification of semaphorin E as a non-MDR drug resistance gene of human cancers. Proc Natl Acad Sci USA 1997; 94:14713-8; PMID:9405678; http://dx.doi.org/10.1073/pnas.94.26.14713
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 2001; 128:3071-80; PMID:11688557.
- Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci U S A 2009; 106:13933-8; PMID:19666519; http://dx.doi.org/10.1073/pnas.0904744106
- Man J, Shoemake J, Zhou W, Fang X, Wu Q, Rizzo A, Prayson R, Bao S, Rich JN, Yu JS. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep 2014; 9:1812-26; PMID:25464848; http://dx.doi.org/10.1016/j.celrep.2014.10.055
- Blanc V, Nariculam J, Munson P, Freeman A, Klocker H, Masters J, Williamson M. A role for class 3 semaphorins in prostate cancer. Prostate 2010; 71:649-58; PMID:20949546; http://dx.doi.org/10.1002/pros.21281
- Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J 2006; 20:2150-2; PMID:16940438; http://dx.doi.org/10.1096/fj.05-5698fje
- Cole-Healy Z, Vergani P, Hunter K, Brown NJ, Reed MW, Staton CA. The relationship between semaphorin 3C and microvessel density in the progression of breast and oral neoplasia. Exp Mol Pathol 2015; 99:19-24; PMID:25910410; http://dx.doi.org/10.1016/j.yexmp.2015.03.041
- Esselens C, Malapeira J, Colome N, Casal C, Rodriguez-Manzaneque JC, Canals F, Arribas J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J Biol Chem 2009; 285:2463-73; PMID:19915008; http://dx.doi.org/10.1074/jbc.M109.055129
- Bassi DE, Fu J, Lopez de CR, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog 2005; 44:151-61; PMID:16167351; http://dx.doi.org/10.1002/mc.20134
- Aghajanian H, Choi C, Ho VC, Gupta M, Singh MK, Epstein JA. Sema3d and Sema3e direct endothelial motility through distinct molecular signaling pathways. J Biol Chem 2014; 289:17971-9; PMID:24825896; http://dx.doi.org/10.1074/jbc.M113.544833
- Foley K, Rucki AA, Xiao Q, Zhou D, Leubner A, Mo G, Kleponis J, Wu AA, Sharma R, Jiang Q, and others. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signal 2015; 8:ra77
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, and others. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 2007; 56:807-22; PMID:18054858; http://dx.doi.org/10.1016/j.neuron.2007.10.019
- Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape Mammalian blood vessels. Circ Res 2012; 110:34-46; PMID:22076636; http://dx.doi.org/10.1161/CIRCRESAHA.111.249847
- Stone J, Itin A, Alon T, Peer J, Gnessin H, Chanling T, Keshet E. Development of retinal vasculature is mediated by hypoxia- induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995; 15:4738-47; PMID:7623107.
- Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays 2008; 30:303-13; PMID:18348190; http://dx.doi.org/10.1002/bies.20736
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol 2010; 22:617-25; PMID:20817428; http://dx.doi.org/10.1016/j.ceb.2010.08.010
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, and others. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007; 445:776-80; PMID:17259973; http://dx.doi.org/10.1038/nature05571
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A 2007; 104:3219-24; PMID:17296940; http://dx.doi.org/10.1073/pnas.0611206104
- Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev 2011; 25:1399-411; PMID:21724832; http://dx.doi.org/10.1101/gad.2042011
- Alon T, Hemo I, Itin A, Peer J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med 1995; 1:1024-8; PMID:7489357; http://dx.doi.org/10.1038/nm1095-1024
- Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, and others. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 2011; 117:6024-35; PMID:21355092; http://dx.doi.org/10.1182/blood-2010-10-311589
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa SI, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 2011; 121:1974-85; PMID:21505259; http://dx.doi.org/10.1172/JCI44900
- Buehler A, Sitaras N, Favret S, Bucher F, Berger S, Pielen A, Joyal JS, Juan AM, Martin G, Schlunck G, and others. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett 2013; 587:1650-5; PMID:23603393; http://dx.doi.org/10.1016/j.febslet.2013.04.008
- Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lecine P, Borg JP, Mehlen P, and others. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 2013; 24:673-85; PMID:24139859; http://dx.doi.org/10.1016/j.ccr.2013.09.010
- Schmidt AM, Moore KJ. The semaphorin 3E/PlexinD1 axis regulates macrophage inflammation in obesity. Cell Metab 2013; 18:461-2; PMID:24093672; http://dx.doi.org/10.1016/j.cmet.2013.09.011
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431:461-6; PMID:15329734; http://dx.doi.org/10.1038/nature02924
- Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, and others. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest 2010; 120:2684-98; PMID:20664171; http://dx.doi.org/10.1172/JCI42118
- Roodink I, Kats G, van KL, Grunberg M, Maass C, Verrijp K, Raats J, Leenders W. Semaphorin 3E expression correlates inversely with plexin D1 during tumor progression. Am J Pathol 2008; 173:1873-81; PMID:18974298; http://dx.doi.org/10.2353/ajpath.2008.080136
- Shimizu I, Yoshida Y, Moriya J, Nojima A, Uemura A, Kobayashi Y, Minamino T. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab 2013; 18:491-504; PMID:24093674; http://dx.doi.org/10.1016/j.cmet.2013.09.001
- Sekido Y, Bader S, Latif F, Chen JY, Duh FM, Wei MH, Albanesi JP, Lee CC, Lerman MI, Minna JD. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A 1996; 93:4120-5; PMID:8633026; http://dx.doi.org/10.1073/pnas.93.9.4120
- Wu F, Zhou Q, Yang J, Duan G, Ou J, Zhang R, Pan F, Peng Q, Tan H, Ping YF, and others. Axon guiding chemorepulsant semaphorin-3F inhibits tumor growth and metastasis of colorectal carcinoma. Clin Cancer Res 2011; 17:2702-11; PMID:21349996; http://dx.doi.org/10.1158/1078-0432.CCR-10-0839
- Nasarre P, Kusy S, Constantin B, Castellani V, Drabkin HA, Bagnard D, Roche J. Semaphorin SEMA3F Has a repulsing activity on breast cancer cells and inhibits e-cadherin-mediated cell adhesion. Neoplasia 2005; 7:180-9; PMID:15802023; http://dx.doi.org/10.1593/neo.04481
- Rao J, Zhou Z, Yang J, Shi Y, Xu S, Wang B, Ping Y, Chen L, Cui Y, Zhang X, and others. Semaphorin-3F suppresses the stemness of colorectal cancer cells by inactivating Rac1. Cancer Lett 2014; 10
- Xiang X, Zhang X, Huang QL. Plexin A3 is involved in semaphorin 3F-mediated oligodendrocyte precursor cell migration. Neurosci Lett 2012; 530:127-32; PMID:23063687; http://dx.doi.org/10.1016/j.neulet.2012.09.058
- Potiron VA, Sharma G, Nasarre P, Clarhaut JA, Augustin HG, Gemmill RM, Roche J, Drabkin HA. Semaphorin SEMA3F affects multiple signaling pathways in lung cancer cells. Cancer Res 2007; 67:8708-15; PMID:17875711; http://dx.doi.org/10.1158/0008-5472.CAN-06-3612
- Kusy S, Nasarre P, Chan D, Potiron V, Meyronet D, Gemmill RM, Constantin B, Drabkin HA, Roche J. Selective suppression of in vivo tumorigenicity by semaphorin SEMA3F in lung cancer cells. Neoplasia 2005; 7:457-65; PMID:15967098; http://dx.doi.org/10.1593/neo.04721
- Xiong G, Wang C, Evers BM, Zhou BP, Xu R. RORalpha suppresses breast tumor invasion by inducing SEMA3F expression. Cancer Res 2012; 72:1728-39; PMID:22350413; http://dx.doi.org/10.1158/0008-5472.CAN-11-2762
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998; 392:190-3; PMID:9515965; http://dx.doi.org/10.1038/32433
- Nasarre P, Constantin B, Rouhaud L, Harnois T, Raymond G, Drabkin HA, Bourmeyster N, Roche J. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia 2003; 5:83-92; PMID:12659673; http://dx.doi.org/10.1016/S1476-5586(03)80020-9
- Nakayama H, Bruneau S, Kochupurakkal N, Coma S, Briscoe DM, Klagsbrun M. Regulation of mTOR signaling by semaphorin 3F-neuropilin 2 interactions in vitro and in vivo. Sci Rep 2015; 5:11789; PMID:26156437; http://dx.doi.org/10.1038/srep11789
- Doci CL, Mikelis CM, Lionakis MS, Molinolo AA, Gutkind JS. Genetic identification of SEMA3F as an anti-lymphangiogenic metastasis suppressor gene in head and neck squamous carcinoma. Cancer Res 2015; 75:2937-48; PMID:25952650; http://dx.doi.org/10.1158/0008-5472.CAN-14-3121
- Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 1998; 21:1079-92; PMID:9856463; http://dx.doi.org/10.1016/S0896-6273(00)80625-X
- Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry 2010; 18:4068-75; http://dx.doi.org/10.1021/bi100327r
- Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, Masuda Y, Arakawa H. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res 2007; 67:1451-60; PMID:17308083; http://dx.doi.org/10.1158/0008-5472.CAN-06-2485
- Wong HK, Shimizu A, Kirkpatrick ND, Garkavtsev I, Chan AW, di TE, Klagsbrun M, Jain RK. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/Semaphorin 3F-dependent mechanism. Neoplasia 2012; 14:84-94; PMID:22431917; http://dx.doi.org/10.1593/neo.111600
- Clarhaut J, Gemmill RM, Potiron VA, it-Si-Ali S, Imbert J, Drabkin HA, Roche J. ZEB-1, a repressor of the semaphorin 3F tumor suppressor gene in lung cancer cells. Neoplasia 2009; 11:157-66; PMID:19177200; http://dx.doi.org/10.1593/neo.81074
- Coma S, Amin DN, Shimizu A, Lasorella A, Iavarone A, Klagsbrun M. Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res 2010; 70:3823-32; PMID:20388805; http://dx.doi.org/10.1158/0008-5472.CAN-09-3048
- Drenberg CD, Livingston S, Chen R, Kruk PA, Nicosia SV. Expression of semaphorin 3F and its receptors in epithelial ovarian cancer, fallopian tubes, and secondary mullerian tissues. Obstet Gynecol Int 2009; 2009:730739; PMID:20041133.
- Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta 2010; 1803:133-41; PMID:19406172; http://dx.doi.org/10.1016/j.bbamcr.2009.04.009
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, and others. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A 2007; 104:1621-6; PMID:17244710; http://dx.doi.org/10.1073/pnas.0606344104
- Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des 2009; 15:2319-35; PMID:19601834; http://dx.doi.org/10.2174/138161209788682398
- Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 2007; 282:6899-905; PMID:17204469; http://dx.doi.org/10.1074/jbc.M609570200
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 1992; 119:629-41; PMID:1383237; http://dx.doi.org/10.1083/jcb.119.3.629
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010; 11:834-48; PMID:21102609; http://dx.doi.org/10.1038/nrm3012
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The Semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002; 4:720-4; PMID:12198496; http://dx.doi.org/10.1038/ncb843
- Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer 2013; 13:466-81; PMID:23792360; http://dx.doi.org/10.1038/nrc3545
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene 2004; 23:5131-7; PMID:15184888; http://dx.doi.org/10.1038/sj.onc.1207650
- Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, Giordano S, Tamagnone L, Fasolo A. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol 2008; 183:555-66; PMID:18981235; http://dx.doi.org/10.1083/jcb.200806160
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/Plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol 2005; 25:6889-98; PMID:16055703; http://dx.doi.org/10.1128/MCB.25.16.6889-6898.2005
- Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem 2007; 282:34888-95; PMID:17855350; http://dx.doi.org/10.1074/jbc.M705467200
- Binmadi NO, Proia P, Zhou H, Yang YH, Basile JR. Rho-mediated activation of PI(4)P5K and lipid second messengers is necessary for promotion of angiogenesis by Semaphorin 4D. Angiogenesis 2011; 14:309-19; PMID:21538148; http://dx.doi.org/10.1007/s10456-011-9214-4
- Binmadi NO, Yang YH, Zhou H, Proia P, Lin YL, Batista De Paula AM, Sena Guimaraes AL, Poswar FO, Sundararajan D, Basile JR. Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. Am J Pathol 2012; 180:1232-42; PMID:22252234; http://dx.doi.org/10.1016/j.ajpath.2011.12.009
- Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR. Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE 2011; 6:e25826; PMID:22028792; http://dx.doi.org/10.1371/journal.pone.0025826
- Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8 - a corneal factor that induces neovascularization. Am J Pathol 1992; 141:1279-84; PMID:1281615.
- Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis 2012; 15:391-407; PMID:22476930; http://dx.doi.org/10.1007/s10456-012-9268-y
- Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis 2013; 17:261-74; PMID:24114199; http://dx.doi.org/10.1007/s10456-013-9395-0
- Vadasz Z, Toubi E. Semaphorins: their dual role in regulating immune-mediated diseases. Clin Rev Allergy Immunol 2014; 47:17-25; PMID:23397481; http://dx.doi.org/10.1007/s12016-013-8360-4
- Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol 2012; 33:127-35; PMID:22325954; http://dx.doi.org/10.1016/j.it.2012.01.008
- Chen Y, Zhang L, Lv R, Zhang WQ. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer. Asian Pac J Cancer Prev 2013; 14:5883-90; PMID:24289594; http://dx.doi.org/10.7314/APJCP.2013.14.10.5883
- Evans EE, Jonason AS Jr., Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, and others. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res 2015; 3:689-701; PMID:25614511; http://dx.doi.org/10.1158/2326-6066.CIR-14-0171
- Valente G, Nicotra G, Arrondini M, Castino R, Capparuccia L, Prat M, Kerim S, Tamagnone L, Isidoro C. Co-expression of plexin-B1 and Met in human breast and ovary tumours enhances the risk of progression. Cell Oncol 2009; 31:423-36; PMID:19940359.
- Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A 2006; 103:9017-22; PMID:16754882; http://dx.doi.org/10.1073/pnas.0508825103
- Campos M, DE Campos SG, Ribeiro GG, Eguchi FC, Silva SR, DE Oliveira CZ, DA Costa AM, Curcelli EC, Nunes MC, Penna V, and others. Ki-67 and CD100 immunohistochemical expression is associated with local recurrence and poor prognosis in soft tissue sarcomas, respectively. Oncol Lett 2013; 5:1527-35; PMID:23759874.
- Kato S, Kubota K, Shimamura T, Shinohara Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F, Ishiguro H, and others. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci 2011; 102:2029-37; PMID:21812859; http://dx.doi.org/10.1111/j.1349-7006.2011.02053.x
- Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, and others. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 2008; 205:1673-85; PMID:18559453; http://dx.doi.org/10.1084/jem.20072602
- Damola A, Legendre A, Ball S, Masters JR, Williamson M. Function of mutant and wild-type plexinb1 in prostate cancer cells. Prostate 2013; 73:1326-35; PMID:23775445; http://dx.doi.org/10.1002/pros.22678
- Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, Manlove LA, LaRue RS, Temiz NA, Molyneux SD, and others. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet 2015; 47:615-24; PMID:25961939; http://dx.doi.org/10.1038/ng.3293
- Patnaik A, Weiss GJ, Leonard JE, Rasco D, Sachdev JC, Fisher TL, Winter LA, Reilly C, Parker RB, Mutz D, and others. Safety, pharmacokinetics and pharmacodynamics of a humanized anti-semaphorin 4D antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res 2016; 22:824-36; clincanres
- McClelland L, Chen Y, Soong J, Kuo I, Scott G. Plexin B1 inhibits integrin-dependent pp125(FAK) and Rho activity in melanoma. Pigment Cell Melanoma Res 2011; 24:165-74; PMID:21029396; http://dx.doi.org/10.1111/j.1755-148X.2010.00797.x
- Soong J, Chen Y, Shustef EM, Scott GA. Sema4D, the ligand for Plexin B1, suppresses c-Met activation and migration and promotes melanocyte survival and growth. J Invest Dermatol 2012; 132:1230-8; PMID:22189792; http://dx.doi.org/10.1038/jid.2011.414
- Soong J, Scott G. Plexin B1 inhibits MET through direct association and regulates Shp2 expression in melanocytes. J Cell Sci 2012; 126:688-95; PMID:23203808; http://dx.doi.org/10.1242/jcs.119487
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 2004; 165:869-80; PMID:15210733; http://dx.doi.org/10.1083/jcb.200312094
- Sun T, Krishnan R, Swiercz JM. Grb2 mediates semaphorin-4D-dependent RhoA inactivation. J Cell Sci 2012; 125:3557-567; PMID:22505611; http://dx.doi.org/10.1242/jcs.101063
- Ito D, Nojima S, Nishide M, Okuno T, Takamatsu H, Kang S, Kimura T, Yoshida Y, Morimoto K, Maeda Y, and others. mTOR complex signaling through the SEMA4A-Plexin B2 axis is required for optimal activation and differentiation of CD8+ T Cells. J Immunol 2015; 1403038
- Yukawa K, Tanaka T, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Sema4A induces cell morphological changes through B-type plexin-mediated signaling. Int J Mol Med 2010; 25:225-30; PMID:20043131.
- Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, and others. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013; 501:252-6; PMID:23913274; http://dx.doi.org/10.1038/nature12428
- Parrinello S, Noon LA, Harrisingh MC, Digby PW, Rosenberg LH, Cremona CA, Echave P, Flanagan AM, Parada LF, Lloyd AC. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev 2008; 22:3335-48; PMID:19056885; http://dx.doi.org/10.1101/gad.490608
- Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, and others. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 2008; 14:7593-603; PMID:19047084; http://dx.doi.org/10.1158/1078-0432.CCR-08-1164
- Ding Y, He D, Florentin D, Frolov A, Hilsenbeck SG, Ittmann MM, Kadmon D, Miles B, Rowley DR, Ayala GE. Semaphorin 4F as a critical regulator of neuro-epithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res 2013; 19:6101-11.
- Pan G, Zhang X, Ren J, Lu J, Li W, Fu H, Zhang S, Li J. Semaphorin 5A, an axon guidance molecule, enhances the invasion and metastasis of human gastric cancer through activation of MMP9. Pathol Oncol Res 2012; 19:11-8; PMID:22821546; http://dx.doi.org/10.1007/s12253-012-9550-8
- Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDI{alpha}-mediated inactivation of Rac1 GTPase. J Biol Chem 2010; 285:32436-45; PMID:20696765; http://dx.doi.org/10.1074/jbc.M110.120451
- Hirota E, Yan L, Tsunoda T, Ashida S, Fujime M, Shuin T, Miki T, Nakamura Y, Katagiri T. Genome-wide gene expression profiles of clear cell renal cell carcinoma: Identification of molecular targets for treatment of renal cell carcinoma. Int J Oncol 2006; 29:799-827; PMID:16964377.
- Zhao J, Tang H, Zhao H, Che W, Zhang L, Liang P. SEMA6A is a prognostic biomarker in glioblastoma. Tumour Biol 2015; 36:8333-40.
- Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di CS, Visca P, and others. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget 2015; 6:2779-93; PMID:25576923; http://dx.doi.org/10.18632/oncotarget.2995
- Chen D, Li Y, Wang L, Jiao K. SEMA6D expression and patient survival in breast invasive carcinoma. Int J Breast Cancer 2015; 2015:article 539721; PMID:25973277.
- Ge C, Li Q, Wang L, Xu X. The role of axon guidance factor semaphorin 6B in the invasion and metastasis of gastric cancer. J Int Med Res 2013; 41:284-92; PMID:23781008; http://dx.doi.org/10.1177/0300060513476436
- D'Apice L, Costa V, Valente C, Trovato M, Pagani A, Manera S, Regolo L, Zambelli A, Ciccodicola A, De BP. Analysis of SEMA6B gene expression in breast cancer: identification of a new isoform. Biochim Biophys Acta 2013; 1830:4543-53; PMID:23665584; http://dx.doi.org/10.1016/j.bbagen.2013.05.003
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 2011; 12:715-23; PMID:21772280; http://dx.doi.org/10.1038/ni.2060
- Ma B, Herzog EL, Lee CG, Peng X, Lee CM, Chen X, Rockwell S, Koo JS, Kluger H, Herbst RS, and others. Role of chitinase 3-like-1 and semaphorin 7A in pulmonary melanoma metastasis. Cancer Res 2014; 75:487-96; PMID:25511377; http://dx.doi.org/10.1158/0008-5472.CAN-13-3339
- Garcia-Areas R, Libreros S, Amat S, Keating P, Carrio R, Robinson P, Blieden C, Iragavarapu-Charyulu V. Semaphorin7A promotes tumor growth and exerts a pro-angiogenic effect in macrophages of mammary tumor-bearing mice. Front Physiol 2014; 5:17; PMID:24550834; http://dx.doi.org/10.3389/fphys.2014.00017
- Allegra M, Zaragkoulias A, Vorgia E, Ioannou M, Litos G, Beug H, Mavrothalassitis G. Semaphorin-7a reverses the ERF-induced inhibition of EMT in Ras-dependent mouse mammary epithelial cells. Mol Biol Cell 2012; 23:3873-3881; PMID:22875994; http://dx.doi.org/10.1091/mbc.E12-04-0276
- Saito T, Kasamatsu A, Ogawara K, Miyamoto I, Saito K, Iyoda M, Suzuki T, Endo-Sakamoto Y, Shiiba M, Tanzawa H, and others. Semaphorin7A promotion of tumoral growth and metastasis in human oral cancer by regulation of G1 cell cycle and matrix metalloproteases: possible contribution to tumoral angiogenesis. PLoS ONE 2015; 10:e0137923; PMID:26378920; http://dx.doi.org/10.1371/journal.pone.0137923
