ABSTRACT
Semaphorins constitute a large family of membrane-bound and secreted proteins that provide guidance cues for axon pathfinding and cell migration. Although initially discovered as repelling cues for axons in nervous system, they have been found to regulate cell adhesion and motility, angiogenesis, immune function and tumor progression. Notably, semaphorins are bifunctional cues and for instance can mediate both repulsive and attractive functions in different contexts. While many studies focused so far on the function of secreted family members, class 1 semaphorins in invertebrates and class 4, 5 and 6 in vertebrate species comprise around 14 transmembrane semaphorin molecules with emerging functional relevance. These can signal in juxtacrine, paracrine and autocrine fashion, hence mediating long and short range repulsive and attractive guidance cues which have a profound impact on cellular morphology and functions. Importantly, transmembrane semaphorins are capable of bidirectional signaling, acting both in “forward” mode via plexins (sometimes in association with receptor tyrosine kinases), and in “reverse” manner through their cytoplasmic domains. In this review, we will survey known molecular mechanisms underlying the functions of transmembrane semaphorins in development and cancer.
Semaphorins and their receptors
Semaphorins are secreted, transmembrane and GPI-linked glycoproteins that have been grouped into 8 classes, based on structural features and amino acid sequence similarity. There are around 20 semaphorins in humans, Drosophila has 5, and 2 are known from viral genomes. Semaphorins found in invertebrates are grouped in classes 1–2, vertebrate ones in classes 3–7, and a final group contains those encoded by viruses. Notably, class 1, 4, 5 and 6 comprise transmembrane molecules, which include a cytoplasmic domain. All members contain a conserved extracellular domain of about 500 amino acids known as the Sema-PSI domain, located at the N-terminal of the molecule. The size of transmembrane semaphorins may range from 400 to 1000 amino acid residues. In addition, downstream to the sema domain, class 4 semaphorins include an immunoglobulin(IG)-like domain, while class 5 semaphorins contain 7 thrombospondin motifs. Intracellular domains of class 4 semaphorins have a PDZ-domain binding motif at the C-terminus. Transmembrane semaphorins of class 6 have the longest cytoplasmic domain of about 400 amino acids, which also contains proline-rich motifs.
High-affinity receptors for transmembrane semaphorins are essentially represented by plexin family members.Citation1-3 Neuropilins, which are important co-receptors for secreted semaphorins, do not seem to have a role in the signaling cascade of transmembrane family members (with the reported exception of an interaction between Sema4A and Neuropilin-1).Citation4 Invertebrates bear 2 plexin genes, while there are 9 plexins in vertebrates. The latter are divided into 4 subfamilies: PlexinA(1–4), PlexinB(1–3), PlexinC1 and PlexinD1.The extracellular moiety of plexins contains one sema domain and 2–3 PSI motifs, similar to those of semaphorins; moreover, they include 3–4 IPT domains (shared by plexins, integrins and certain transcriptional factors). All plexins have very similar cytoplasmic structures, comprising a RasGTPase-activating protein(GAP) domain with an inserted Rho GTPase-binding domain(RBD).Citation5
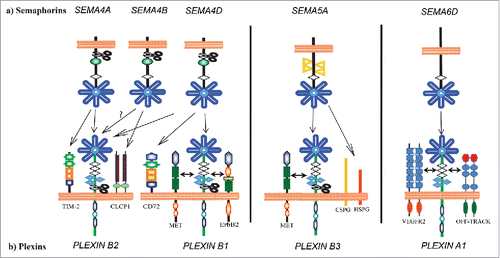
Different transmembrane semaphorins have been found to interact at lower affinity with additional cell surface receptors beyond plexins (see ). For example, Sema4A expressed in dendritic and B cells enhances the activation and differentiation of T cells and the generation of antigen specific T cells in vivo also via the receptor TIM-2.Citation6 In highly metastatic lung cancer cells, Sema4B interacts with CLCP1(CUB,LCCL-homology, coagulation factor V/VIII homology domains protein), a protein with similarity to neuropilins. Here, Sema4B acts as one of the ligands of CLCP1, and enhances its ubiquination and proteosome degradation, in turn regulating the motility of lung cancer cells.Citation7 A further member of the class 4, Sema4D, interacts with CD72, a negative regulator of B cell responsiveness; Sema4D stimulation induces tyrosine dephosphorylation of CD72 intracellular tail and its dissociation from the effector SHP-1, turning off CD72 inhibitory signaling.Citation8 Moreover, Sema5A exerts both attractive and inhibitory effects on developing axons of the fasciculus retroflexus by physically interacting with glycosaminoglycan chains of chondroitin sulfate proteoglycans(CSPGs) or heparin sulfate proteoglycans(HSPGs), expressed by different neuronal populations. In particular, CSPGs function as precisely localized extrinsic cues that convert Sema5A from an attractive to an inhibitory guidance cue, whereas axonal HSPGs mediate Sema5A mediated attraction.Citation9
Figure 1. Representative transmembrane semaphorins and their receptor complexes. A number of transmembrane semaphorins signal through diverse receptor complexes. Notable examples are illustrated in this figure. Sema4A can bind to Tim-2, a protein expressed on T cells, in addition to plexins. In lymphocytes, Sema4D can associate with CD72, a member of the C-type lectin family. In cancer cells, Sema4D can signal through complexes including PlexinB1 and ErbB2 or Met depending on the cell type. Sema5A can signal through PlexinB3 and Met in epithelial cancer cells. However, in neurons, proteoglycans such as HSPG and CSPG modulate Sema5A signaling, independent of PlexinB3. PlexinA1 is alternatively associated with OTK or VEGFR2 receptor tyrosine kinases in different cells of the developing heart, and these signaling complexes have distinct functions in cardiac development.
Signaling mode paradigms used by transmembrane semaphorins
Transmembrane semaphorins can act by multiple signaling modes. Clearly, when exposed on the cell surface, they can engage short-range cell-to-cell interactions with neighboring cells, either of the same type, or belonging to a different cell population in the tissue environment. Moreover, while they are synthesized as single-pass membrane-spanning molecules, in many cases their extracellular moiety can be shed in soluble form, and potentially act as a secreted diffusible signal. Unlike what is known for secreted class 3 semaphorins (which are processed by furin-like convertases), transmembrane semaphorin cleavage is mediated by diverse metalloproteases e.g. MT1-MMP mediates tumor angiogenesis through the release of Sema4D,Citation10 most of which have not been clearly identified; moreover, the targeted cleavage sites generally need elucidation.
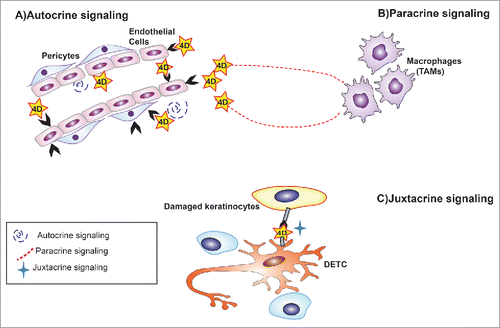
Thus transmembrane semaphorins can function by 3 different signaling paradigms: in juxtacrine mode (when membrane-bound), and in autocrine or paracrine mode (upon ectodomain release) (see ). Sema4D is a good example of this signaling versatility, and its proteolytically shed isoform has been characterized even better than its membrane-bound counterpart.Citation11 For instance, Sema4D autocrine signals in endothelial cells promote sprouting and angiogenesis;Citation12 however, Sema4D can also act in paracrine manner on the endothelium when released by other cells in the microenvironment.Citation13 As an example of juxtacrine signaling, the ligation of Sema4D/CD100 in γδ T cells to the receptor PlexinB2 exposed by damaged keratinocytes induces cell rounding via signals through ERK kinase and cofilin, contributing to the skin wounding process.Citation14
Figure 2. Various signaling mode paradigms used by Sema4D transmembrane semaphorin. Sema4D is taken as an example of diverse signaling paradigms of transmembrane semaphorins. In particular, Sema4D produced by endothelial cells can function in autocrine manner on its surface receptor such a PlexinB1. In addition, Sema4D released by other cells in the tumor microenvironment (e.g., Tumor Associated Macrophages) can signal in paracrine fashion to endothelial cells. Moreover, during wound healing, Sema4D expressed by dendritic epidermal T cells can bind to PlexinB2 expressed on the surface of damaged keratinocytes, acting in juxtacrine mode.
Bidirectional signaling of transmembrane semaphorins
All semaphorins are known to act through the intracellular domain of the plexins, by a so-called “forward” signaling pathway, which negatively regulates integrin-mediated adhesion and induces cytoskeletal remodeling. Moreover, exclusively transmembrane semaphorins can also mediate a “reverse” signaling mode, by acting as receptors rather than ligands, and signal through their own cytoplasmic domains.
In fruit fly Drosophila melanogaster, Sema1a is a repulsive ligand controlling motor axon guidance during development. Sema1a interaction in trans with PlexinA exposed by adjacent cells is crucial for defasciculation of nerve bundles. This forward signaling cascade is modulated by perlecan, an extracellular matrix component, which enhances semaphorin-induced downregulation of integrin adhesive function and FAK dephosphorylation, leading to motor axon defasciculation.Citation15 Notably, Sema1a can also mediate motor axon defasciculation through reverse signaling mechanisms, whereby its cytoplasmic domain can interact with 2 major antagonistic regulators of the GTPase Pebble and the inhibitor RhoGAP p190. The first activates Rho1 and promotes axon-axon repulsion and defasciculation, while p190-RhoGAP antagonizes this mechanism allowing axonal attraction;Citation16,17 the extracellular Sema1a-binding molecule triggering this cascade is still unclear.
The signaling cascade elicited downstream of semaphorin/plexin interactions in vertebrates has been studied in a variety of cell types and models. Certain forward signaling mechanisms are shared by most plexins or family members of the same subclass. For instance, many plexins have been found to regulate the activity of GTPases of the Ras/Rho family. In particular, plexin cytoplasmic domain carries intrinsic GTPase Activating Protein (GAP) activity against R-Ras, M-Ras and/or Rap-1 GTPases. In different studies, this has been shown to inhibit beta1 integrin-dependent adhesion and cell detachment from the extracellular matrix;Citation18,19 hinder the activity of phosphoinositide 3-kinase, leading to AKT dephosphorylation and activation of GSK-3beta;Citation20 and derepress p120-Ras-GAP activity, leading to downregulation of RAS-MAPK signaling.Citation21 The final outcome of this signaling cascade typically is the inhibition of cell migration. Moreover, Rho GTPases, such as RhoA, Rac and Cdc42, known to control cell motility by regulating actin and microtubule dynamics, are considered important downstream effectors of plexin receptors. For instance, it was reported that Sema4D activated PlexinB1 can regulate RhoA activity via p190-RhoGAP protein,Citation22 or inhibit RAC-dependent PAK activation.Citation23 In addition, PlexinB1 and PlexinB2, by means of leukemia associated Rho-GEF(LARG) and p190-PDZ-RhoGEF tethered to their C-terminus consensus sequences, can upregulate GTP-bound active RhoA levels, impinging on cytoskeletal reorganization and growth cone morphology.Citation24,25
Notably, many forward semaphorin signals are mediated by multimeric receptor complexes, containing plexins in association with additional transmembrane subunits. For transmembrane semaphorins, these often implicate plexin-associated tyrosine kinase receptors (RTK) (see ). For example, semaphorin-dependent stimulation of PlexinB1, PlexinB2 or PlexinB3 can activate and induce the phosphorylation of ERBB2, MET and RON receptor tyrosine kinases in different cell types.Citation12,26-29 Furthermore, Sema6D-PlexinA1 forward signaling, required for the ventricular chamber morphogenesis during chick embryo heart development, depends on the differential involvement of 2 plexin-associated RTKs. In cells of the conotruncal segment, Sema6D binding to a PlexinA1-VEGF-R2 kinase complex mediates cell migration and invasive growth. By contrast, Sema6D inhibits the migration of cardiac muscle cells of ventricle region, which express PlexinA1 in association with another (kinase-dead) RTK, named OTK (off-track kinase).Citation30,31
On the other side of the street, the intracellular domain of transmembrane semaphorins, including Sema6D, has been found to interact with putative signaling effectors, potentially mediating reverse signaling cascades. In particular, the cytoplasmic portion of Sema6D can bind to both Abl kinase and Mena/Enabled. During cardiac chamber formation, upon Sema6D engagement in trans with PlexinA1, Abl kinase gets activated, resulting in the phosphorylation of Mena. This leads to the dissociation of Mena from Sema6D cytoplasmic tail, thereby promoting cell migration and trabeculation of the myocardial layer.Citation31
Other class 6 semaphorins have been found in association with intracellular effectors. For example, Sema6A can interact with EVL (Ena/VASP-like protein) via its zyxin-like C-terminal domain suggesting a possible role in retrograde signaling during neuronal development.Citation32 Furthermore, the intracellular domain of Sema6B was found to bind to the SH3 domain of the oncogenic tyrosine kinase c-Src ().Citation33
Figure 3. Forward and reverse signaling effectors of transmembrane semaphorins. The general paradigm of forward and reverse signaling of transmembrane semaphorins is depicted on the left. On the right, a table summarizes various effectors implicated in these distinctive signaling modes for different family members.
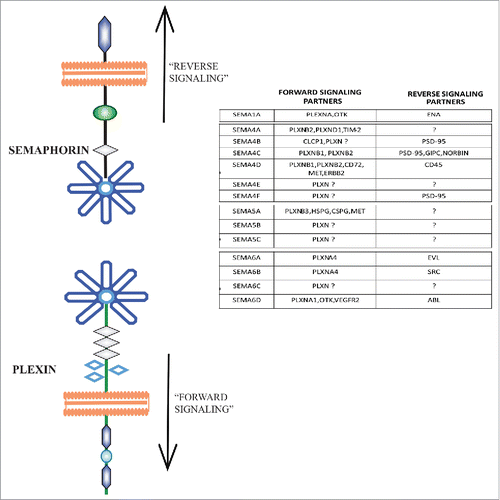
Interestingly, the cytoplasmic domain of many class 4 semaphorins terminates with a consensus sequence anchoring PDZ domains.Citation34-36 These protein-protein interaction domains mediate receptor clustering in neuronal post-synaptic membranes, and in general serve as scaffolds for the assembly of multi-molecular signaling complexes. Indeed, 3 different class-4 semaphorins have been shown to co-localize and interact with PSD-95/SAP90, e.g., Sema4C in cerebral cortical neurons,Citation37 and Sema4B and Sema4F in hippocampal neurons.Citation35,36 During muscle development, knocking down Sema4C or blocking its PDZ domain-binding motif resulted in inhibition of myogenic differentiation;Citation38 these data suggested a putative role of reverse signaling, though the plexin counterpart responsible for triggering this process has not been identified.
Finally, as mentioned above, the cytoplasmic domain of fly Sema1a can mediate opposite reverse signaling effects by interacting with the 2 major antagonistic regulators of RhoA: the GTPase exchanger Pebble and the inhibitor p190RhoGAP.Citation16,17
In cis versus in trans signaling functions of transmembrane semaphorins
In addition to their interaction in trans between adjacent cells, transmembrane semaphorins and plexins can also associate in cis on the surface of the same cell, resulting in the functional regulation of other signaling cascades. Notably, the association of a semaphorin with its co-expressed plexin receptor in cis can inhibit the signaling function of either of the 2 molecules in trans with adjacent cells. For example, in cis Sema6A-PlexinA4 association in dorsal root ganglion neurons hinders Plexin interactions in trans with Sema6A molecules expressed by adjacent cells.Citation39 Moreover, while Sema6A is widely expressed in the developing hippocampus, where it acts as repelling signal for extending axons (mossy fibers), its association in cis with PlexinA2 co-expressed in certain areas hinders Sema6A activity in trans there by establishing a permissive corridor for layer-restricted axonal innervations.Citation40 In other settings, in cis interaction between a semaphorin/plexin pair can instead activate plexin signaling, as shown in C.elegans for transmembrane semaphorin SMP-1 and class A plexin homolog PLX-1, leading to repelling signals inhibiting moto neuron synapse formation.Citation41
Transmembrane semaphorins in embryo development
The development of complex tissues and organs depends on cell proliferation, migration and differentiation. While semaphorins have been shown to regulate many of these processes, the best characterized feature of semaphorin/plexin signals is to provide repulsive or attractive cues for migrating cells and growing neurites.Citation42 Thus, semaphorin-deficient mouse models have been widely used to study the physiological role of these molecules in the developing nervous system (). Among mutants deficient for transmembrane semaphorins, Sema4B−/−mice displayed reduced proliferation of astrocytes after CNS injury.Citation43 On the other hand, Sema4C and Sema4G deficient mice showed severe defects in cerebellar development: in particular, Sema4C−/− mutants show exencephaly and neonatal lethality, a phenotype less prominent in Sema4G deficient mice.Citation44 Sema4D−/− mutants resulted in increased oligodendrocyte number in basal conditions and upon injury.Citation45 Gross defects in the early development were seen in Sema5A KO mice, leading to embryonic lethality, although the implicated deficient mechanism was not elucidated.Citation46 Recent studies also reported aberrant projections of thalamo-cortical axons in Sema6A null mice.Citation47 Moreover, Sema6A is expressed by tangentially migrating granule cells in the developing cerebellum, where it controls the switch from tangential to radial migration.Citation48 Studies of PlexinA4 and PlexinA3/A4 double mutants have shown that these plexins regulate the patterning of spinal sensory axons and cranial nerve projections.Citation49,50 In a recent study, double deletion mutants of PlexinB1 and PlexinB2 displayed impaired corticogenesis with cortical thinning. These homologous plexins seem to play redundant/compensatory roles during forebrain development, in order to ensure proper neuronal proliferation and neocortical expansion.Citation51 In most cases the absence of dramatic neuronal phenotypes in transmembrane semaphorin mutants may be explained by redundancy among family members or the existence of corrective mechanisms by which early axons which are misguided are eliminated.
Notably, Sema4D/PlexinB1 signaling is a typical example mediating either attractive or repelling cues for different neurons. In hippocampal development, Sema4D inhibits axonal extension by suppressing R-Ras activity, leading to Akt dephoshorylation and activation of GSK-3β.Citation52 Opposite effects are seen in the hypothalamus, where gonadotropin-releasing hormone expressing neurons (GnRH neurons) control the release of reproductive hormones by the pituitary. Indeed, failure to stimulate the pituitary with GnRH causes reproductive disorders and lack of initiation of puberty, and PlexinB1 deficient mice revealed a migratory effect in GnRH-1 neurons, leading to smaller neuronal population in adult brains, and consequent fertility defects. Notably, in this context, Sema4D promotes directional migration of GnRH-1 cells by coupling PlexinB1 with MET kinase activation.Citation53
Oligodendrocytes are a type of neuroglia found in CNS, which is responsible for the formation of a myelin sheath surrounding neuronal projections. Several semaphorins, including Sema4D, Sema4F, Sema5A and Sema6A are known to be major modulators of oligodendrocyte development, and this is a particularly interesting model of short range cell-to-cell and bidirectional semaphorin signaling. For instance, Sema4D knockout mice display an increased number of oligodendrocytes in the adult cerebral cortex, which is due to reduced oligodendrocyte apoptosis; this effect could be reversed by adding soluble Sema4D, which suggests its role as a ligand in this process.Citation45,54 Another class-4 Semaphorin, Sema4F, is widely expressed by neuronal precursors, mature neurons and glial cells. Sema4F is reported to inhibit the migration of oligodendrocyte progenitor cells and promote their differentiation.Citation55 Sema5A expression is restricted to oligodendrocytes and their precursors, among optic nerve glial cells; and it was demonstrated that Sema5A induces growth cone collapse and inhibits axon growth of retinal ganglion cells (RGC).Citation56 Sema6A is also expressed at high levels during oligodendrocyte development, peaking during myelination. Sema6A knock-out mice show delayed oligodendrocyte differentiation both in vivo and in vitro and interestingly, this delayed differentiation of Sema6A-deficient oligodendrocytes is not rescued by the addition of exogenous Sema6A ex vivo, suggesting a possible reverse signaling mechanism, to be further elucidated.Citation57
As mentioned above, during chick embryo heart development, knockdown of Sema6D or its receptor PlexinA1 results in lesser expansion of the primitive ventricle and poor trabeculation of the muscular layer. In this context, the interaction between endocardial and myocardial cells (expressing both Sema6D and PlexinA1) can trigger both forward and reverse signaling cascades controlling cell migration, morphogenic patterning of the cardiac chambers and muscle layer trabeculation. In particular, (endocardial-expressed) Sema6D forward signals to myocardial cells of the conotruncal segment expressing PlexinA1-VEGFR2 receptor complexes to promote cell migration and invasive growth. By contrast, Sema6D inhibits the migration of cardiac muscle cells of ventricle region, which express PlexinA1 in association with the catalytic inactive off-track kinase.Citation30,31 On the other hand, trabecular formation is promoted by Sema6D reverse signaling into myocardial cells of the compact layer.Citation31
Transmembrane semaphorins implicated in cancer
Accumulating evidence indicates that semaphorin signals can play a major role in the tumor context, beyond their established role in development. Various cancer cells express both semaphorins and their receptor, and experimental evidence shows that these signals can either promote or impede the various hallmarks of cancer, like tumor cell proliferation and survival, tumor angiogenesis and evasion from immune response, to name a few. Notably, the expression of various semaphorins and their receptors has been found to be either up-regulated or down-regulated compared to normal tissues, consistent with their potential role as tumor promoters or suppressors ().Citation58
Also in the cancer context, while considerably more attention has been devoted to the role of semaphorins of the secreted type, scattered reports started to highlight the potential relevant role of transmembrane semaphorins, and their peculiar signaling modes. Especially semaphorins belonging to class 4 have been found to regulate the behavior of cancer cells, as well as tumor angiogenesis. Germline variants of Sema4A have been associated with increased risk for a type of familial non-polyposis colorectal cancer; Sema4A-V78M mutation in particular caused increased MAPK/Erk and PI3K/Akt signaling in HCT-116 colorectal cancer cells in vitroCitation59 and more studies are required to validate its tumorigenic activity in vivo.
In lung cancer, the role of Sema4B seems rather controversial. Sema4B expression is suppressed by hypoxiaCitation60 and it may inhibit growth of non-small lung cancer cells by suppressing PI3K/Akt signaling pathwayCitation61 and metastasis by down regulating expression of MMP9.Citation62 Other data showed that Sema4B interacts with CLCP1 and may drive its degradation and enhance cell motility; CLCP1 is a protein similar to neuropilins overexpressed in lung cancer metastatic cells.Citation7
Aberrant expression of Sema4C has been reported in esophageal, gastric and colorectal cancer.Citation63 In paclitaxel-resistant lung and breast cancer cells Sema4C levels is regulated by miR-125b, and its overexpression not only resensitizes these cells to the drug, but also reverts a mesenchymal to epithelial phenotype.Citation64,65 In glioblastoma, the activation of PlexinB2 receptor by the ligand Sema4C, induces actin-based cytoskeletal dynamics and cell migration by RhoA and Rac1 activity.Citation66 The expression of Sema4C was up regulated both at the transcriptional and the translational levels in lymphatic endothelial cells of breast cancer tissues.Citation67
Sema4D is widely expressed in cancer cells and it is the most studied transmembrane semaphorin in cancer. High expression of Sema4D was associated with poor survival in pancreatic ductal adenocarcinoma, where it enhances tumor cell motility,Citation68 and its higher expression was correlated with poorer overall and disease free survival in soft tissue sarcoma.Citation69 In breast carcinoma cells, PlexinB1 and PlexinB2 form complexes with ErbB2 tyrosine kinase, which elicits a pro-migratory effect in response to Sema4D. In these cells, Sema4D-PlexinB1 signaling can instead mediate an anti-migratory effect when associated with MET receptor.Citation26,70 In addition,Sema4D production by head and neck carcinoma cells elicits the expression of Platelet Derived Growth Factor-B and Angiopoietin-like-protein-4 by endothelial cells (in a PlexinB1/RhoA dependent manner) inducing proliferation and differentiation of pericytes, and vascular permeability. These data suggest that targeting Sema4D along with VEGF could be a better therapeutic option for the treatment of solid tumors.Citation71 Recent studies have identified Sema4D as an oncogene in osteosarcoma by forward genetic screening, where by Sema4D was demonstrated to be highly expressed in large fraction of human osteosarcoma tumors and cell lines associated, and overexpression of Sema4D is these cells lines activated AKT and/or MAPK pathways.Citation72 In addition to cancer cells, Tumor Associated Macrophages (TAM) may be a major source of Sema4D in the tumor microenvironment;Citation13 this was found to enhance angiogenesis and tumor cell invasiveness by transactivating oncogenic receptor tyrosine kinase MET, associated with PlexinB1.Citation28,73 In general, effective silencing of Sema4D in cancer cells inhibits tumor vasculature and tumor burden.Citation10,74-80 Moreover, Sema4D activity in cancer can be targeted with monoclonal antibodies, such as VX15/2503,Citation81-84 currently in clinical trials for treating solid tumors. Notably, blocking Sema4D with monoclonal antibodies in tumors may promote immune cell infiltration and enhance response to immunomodulatory drugs such as anti-CTLA-4.Citation85 Another member of this subclass, Sema4F, is a critical regulator of neuroepithelial interactions and considered as a biomarker in prostate cancer, as its cytoplasmic expression also correlates with nerve density and perineural invasion.Citation86
Also Sema5A-receptor PlexinB3 was found to interact with MET and promote tumor cell invasiveness.Citation29 Sema5A regulates cell motility and morphology of human glioma cells via RhoGDIalpha-mediated inactivation of Rac1 GTPase and the functional regulation of fascin-1 actin-binding protein.Citation87,88 In renal cell carcinoma cells, Sema5A downregulation significantly reduced viability.Citation89 On the other hand, lower expression of Sema5A was associated with poor survival among non-smoking women bearing non-small cell lung carcinomas (NSCLC).Citation90
A recent report pointed to the requirement of Sema6A for the survival of BRAF V600E human melanoma cells, whereby depletion of Sema6A causes loss of anchorage-independent growth and inhibition of migration and invasion.Citation91 Sema6B could have a pro-proliferative effect on U87MG cells as silencing it inhibited tumor formation.Citation92
Conclusion and future perspectives
Consistent evidence indicates that transmembrane semaphorins are major guidance cues for axon pathfinding and the wiring of the neural network, and emerging regulators of angiogenesis and tumor progression. They can act as versatile, short or long range signals, in either membrane bound or secreted form, respectively. Moreover, they can mediate downstream “forward” and “reverse” signaling cascades, which implicate a variety of potential effector molecules, beyond plexin receptors. In sum, our knowledge of transmembrane semaphorin functions and signaling pathways is still far from complete and further studies will be required to understand their relevance in development and cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Table 1. Transmembrane semaphorin functions in development and pathophysiology.
Table 2. Transmembrane semaphorins implicated in cancer development.
Acknowledgments
We are grateful to all Tamagnone lab members, Chiara Battistini in particular, for advice and discussion.
Funding
The work was supported by grants from Italian Association for Cancer Research (AIRC) (IG #2014-15179) and the Fondazione Piemontese per la Ricerca sul Cancro (FPRC-ONLUS) (Grant “MIUR 2010 Vaschetto-5 per mille 2010 MIUR”).
References
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999; 99:71-80; PMID:10520995; http://dx.doi.org/10.1016/S0092-8674(00)80063-X
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell 1997; 90:753-62; PMID:9288754; http://dx.doi.org/10.1016/S0092-8674(00)80535-8
- Kolodkin AL, Ginty DD. Steering clear of semaphorins: neuropilins sound the retreat. Neuron 1997; 19:1159-62; PMID:9427240; http://dx.doi.org/10.1016/S0896-6273(00)80408-0
- Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013; 501:252-6; PMID:23913274; http://dx.doi.org/10.1038/nature12428
- Tong Y, Hota PK, Penachioni JY, Hamaneh MB, Kim SJ, Alviani RS, Shen L, He H, Tempel W, Tamagnone L, et al. Structure and function of the intracellular region of the Plexin-B1 transmembrane receptor. J Biol Chem 2009; 284:35962-72; PMID:19843518; http://dx.doi.org/10.1074/jbc.M109.056275
- Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 2002; 419:629-33; PMID:12374982; http://dx.doi.org/10.1038/nature01037
- Nagai H, Sugito N, Matsubara H, Tatematsu Y, Hida T, Sekido Y, Nagino M, Nimura Y, Takahashi T, Osada H. CLCP1 interacts with semaphorin 4B and regulates motility of lung cancer cells. Oncogene 2007; 26:4025-31; PMID:17213806; http://dx.doi.org/10.1038/sj.onc.1210183
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 2000; 13:621-31; PMID:11114375; http://dx.doi.org/10.1016/S1074-7613(00)00062-5
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004; 44:961-75; PMID:15603739; http://dx.doi.org/10.1016/j.neuron.2004.12.002
- Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 2007; 282:6899-905; PMID:17204469; http://dx.doi.org/10.1074/jbc.M609570200
- Mou P, Zeng Z, Li Q, Liu X, Xin X, Wannemacher KM, Ruan C, Li R, Brass LF, Zhu L. Identification of a calmodulin-binding domain in Sema4D that regulates its exodomain shedding in platelets. Blood 2013; 121:4221-30; PMID:23564909; http://dx.doi.org/10.1182/blood-2012-11-470609
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 2005; 105:4321-9; PMID:15632204; http://dx.doi.org/10.1182/blood-2004-07-2885
- Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 2008; 205:1673-85; PMID:18559453; http://dx.doi.org/10.1084/jem.20072602
- Bonneville M. Semaphorins: new cues for skin healing by gammadelta T cells. Immunity 2012; 37:194-6; PMID:22921116; http://dx.doi.org/10.1016/j.immuni.2012.08.003
- Cho JY, Chak K, Andreone BJ, Wooley JR, Kolodkin AL. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev 2012; 26:2222-35; PMID:23028146; http://dx.doi.org/10.1101/gad.193136.112
- Cafferty P, Yu L, Rao Y. The receptor tyrosine kinase Off-track is required for layer-specific neuronal connectivity in Drosophila. Development 2004; 131:5287-95; PMID:15456725; http://dx.doi.org/10.1242/dev.01406
- Jeong S, Juhaszova K, Kolodkin AL. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron 2012; 76:721-34; PMID:23177958; http://dx.doi.org/10.1016/j.neuron.2012.09.018
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 2004; 305:862-5; PMID:15297673; http://dx.doi.org/10.1126/science.1097545
- Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem 2007; 282:37215-24; PMID:17916560; http://dx.doi.org/10.1074/jbc.M703800200
- Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M. Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep 2006; 7:704-9; PMID:16799460; http://dx.doi.org/10.1038/sj.embor.7400737
- Okada T, Sinha S, Esposito I, Schiavon G, López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M, et al. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nat Cell Biol 2015; 17:81-94; PMID:25531777; http://dx.doi.org/10.1038/ncb3082
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci 2005; 118:4689-700; PMID:16188938; http://dx.doi.org/10.1242/jcs.02590
- Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev 2002; 16:836-45; PMID:11937491; http://dx.doi.org/10.1101/gad.966402
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 2002; 35:51-63; PMID:12123608; http://dx.doi.org/10.1016/S0896-6273(02)00750-X
- Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem 2002; 277:43115-20; PMID:12183458; http://dx.doi.org/10.1074/jbc.M206005200
- Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem 2008; 283:1893-901; PMID:18025083; http://dx.doi.org/10.1074/jbc.M706822200
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 2004; 165:869-80; PMID:15210733; http://dx.doi.org/10.1083/jcb.200312094
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene 2004; 23:5131-7; PMID:15184888; http://dx.doi.org/10.1038/sj.onc.1207650
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep 2004; 5:710-4; PMID:15218527; http://dx.doi.org/10.1038/sj.embor.7400189
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 2004; 18:435-47; PMID:14977921; http://dx.doi.org/10.1101/gad.1167304
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol 2004; 6:1204-11; PMID:15543137; http://dx.doi.org/10.1038/ncb1193
- Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem 2000; 275:39647-53; PMID:10993894; http://dx.doi.org/10.1074/jbc.M006316200
- Eckhardt F, Behar O, Calautti E, Yonezawa K, Nishimoto I, Fishman MC. A novel transmembrane semaphorin can bind c-src. Mol Cell Neurosci 1997; 9:409-19; PMID:9361278; http://dx.doi.org/10.1006/mcne.1997.0644
- Inagaki S, Ohoka Y, Sugimoto H, Fujioka S, Amazaki M, Kurinami H, Miyazaki N, Tohyama M, Furuyama T. Sema4C, a transmembrane semaphorin, interacts with a post-synaptic density protein, PSD-95. J Biol Chem 2001; 276:9174-81; PMID:11134026; http://dx.doi.org/10.1074/jbc.M009051200
- Schultze W, Eulenburg V, Lessmann V, Herrmann L, Dittmar T, Gundelfinger ED, Heumann R, Erdmann KS. Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95. J Neurochem 2001; 78:482-9; PMID:11483650; http://dx.doi.org/10.1046/j.1471-4159.2001.00447.x
- Burkhardt C, Muller M, Badde A, Garner CC, Gundelfinger ED, Puschel AW. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS Lett 2005; 579:3821-8; PMID:15978582; http://dx.doi.org/10.1016/j.febslet.2005.05.079
- Inagaki S, Ohoka Y, Sugimoto H, Fujioka S, Amazaki M, Kurinami H, Miyazaki N, Tohyama M, Furuyama T. Sema4c, a transmembrane semaphorin, interacts with a post-synaptic density protein, PSD-95. J Biol Chem 2001; 276:9174-81; PMID:11134026; http://dx.doi.org/10.1074/jbc.M009051200
- Ko JA, Gondo T, Inagaki S, Inui M. Requirement of the transmembrane semaphorin Sema4C for myogenic differentiation. Febs Letters 2005; 579:2236-42; PMID:15811348; http://dx.doi.org/10.1016/j.febslet.2005.03.022
- Haklai-Topper L, Mlechkovich G, Savariego D, Gokhman I, Yaron A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J 2010; 29:2635-45; PMID:20606624; http://dx.doi.org/10.1038/emboj.2010.147
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 2007; 53:535-47; PMID:17296555; http://dx.doi.org/10.1016/j.neuron.2007.01.028
- Mizumoto K, Shen K. Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron 2013; 77:655-66; PMID:23439119; http://dx.doi.org/10.1016/j.neuron.2012.12.031
- Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development. Development 2014; 141:3292-7; PMID:25139851; http://dx.doi.org/10.1242/dev.105544
- Ben-Gigi L, Sweetat S, Besser E, Fellig Y, Wiederhold T, Polakiewicz RD, Behar O. Astrogliosis induced by brain injury is regulated by Sema4B Phosphorylation(). eNeuro 2015; 2; PMID:26464987
- Maier V, Jolicoeur C, Rayburn H, Takegahara N, Kumanogoh A, Kikutani H, Tessier-Lavigne M, Wurst W, Friedel RH. Semaphorin 4C and 4G are ligands of Plexin-B2 required in cerebellar development. Mol Cell Neurosci 2011; 46:419-31; PMID:21122816; http://dx.doi.org/10.1016/j.mcn.2010.11.005
- Taniguchi Y, Amazaki M, Furuyama T, Yamaguchi W, Takahara M, Saino O, Wada T, Niwa H, Tashiro F, Miyazaki J, et al. Sema4D deficiency results in an increase in the number of oligodendrocytes in healthy and injured mouse brains. J Neurosci Res 2009; 87:2833-41; PMID:19472224; http://dx.doi.org/10.1002/jnr.22124
- Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol 2005; 25:2310-9; PMID:15743826; http://dx.doi.org/10.1128/MCB.25.6.2310-2319.2005
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature 2001; 410:174-9; PMID:11242070; http://dx.doi.org/10.1038/35065539
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chedotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci 2005; 8:1516-24; PMID:16205717; http://dx.doi.org/10.1038/nn1555
- Suto F, Murakami Y, Nakamura F, Goshima Y, Fujisawa H. Identification and characterization of a novel mouse plexin, plexin-A4. Mech Dev 2003; 120:385-96; PMID:12591607; http://dx.doi.org/10.1016/S0925-4773(02)00421-5
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 2005; 45:513-23; PMID:15721238; http://dx.doi.org/10.1016/j.neuron.2005.01.013
- Daviaud N, Chen K, Huang Y, Friedel RH, Zou H. Impaired cortical neurogenesis in Plexin-B1 and -B2 double deletion mutant. Dev Neurobiol 2015; PMID:26579598; http://dx.doi.org/10.1002/dneu.22364
- Oinuma I, Ito Y, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem 2010; 285:28200-9; PMID:20610402; http://dx.doi.org/10.1074/jbc.M110.147546
- Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, Giordano S, Tamagnone L, Fasolo A. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol 2008; 183:555-66; PMID:18981235; http://dx.doi.org/10.1083/jcb.200806160
- Yamaguchi W, Tamai R, Kageura M, Furuyama T, Inagaki S. Sema4D as an inhibitory regulator in oligodendrocyte development. Mol Cell Neurosci 2012; 49:290-9; PMID:22198439; http://dx.doi.org/10.1016/j.mcn.2011.12.004
- Armendariz BG, Bribian A, Perez-Martinez E, Martinez A, de Castro F, Soriano E, Burgaya F. Expression of Semaphorin 4F in neurons and brain oligodendrocytes and the regulation of oligodendrocyte precursor migration in the optic nerve. Mol Cell Neurosci 2012; 49:54-67; PMID:21945643; http://dx.doi.org/10.1016/j.mcn.2011.09.003
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci 2004; 24:4989-99; PMID:15163691; http://dx.doi.org/10.1523/JNEUROSCI.4390-03.2004
- Bernard F, Moreau-Fauvarque C, Heitz-Marchaland C, Zagar Y, Dumas L, Fouquet S, Lee X, Shao Z, Mi S, Chedotal A. Role of transmembrane semaphorin Sema6A in oligodendrocyte differentiation and myelination. Glia 2012; 60:1590-604; PMID:22777942; http://dx.doi.org/10.1002/glia.22378
- Rehman M, Tamagnone L. Semaphorins in cancer: biological mechanisms and therapeutic approaches. Semin Cell Dev Biol 2013; 24:179-89; PMID:23099250; http://dx.doi.org/10.1016/j.semcdb.2012.10.005
- Schulz E, Klampfl P, Holzapfel S, Janecke AR, Ulz P, Renner W, Kashofer K, Nojima S, Leitner A, Zebisch A, et al. Germline variants in the SEMA4A gene predispose to familial colorectal cancer type X. Nat Commun 2014; 5:5191; PMID:25307848; http://dx.doi.org/10.1038/ncomms6191
- Jian H, Liu B, Zhang J. Hypoxia and hypoxia-inducible factor 1 repress SEMA4B expression to promote non-small cell lung cancer invasion. Tumour Biol 2014; 35:4949-55; PMID:24474252; http://dx.doi.org/10.1007/s13277-014-1651-4
- Jian H, Zhao Y, Liu B, Lu S. SEMA4B inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signalling 2015; 27:1208-13; PMID:25746385; http://dx.doi.org/10.1016/j.cellsig.2015.02.027
- Jian H, Zhao Y, Liu B, Lu S. SEMA4b inhibits MMP9 to prevent metastasis of non-small cell lung cancer. Tumour Biol 2014; 35:11051-6; PMID:25095981; http://dx.doi.org/10.1007/s13277-014-2409-8
- Ye SM, Han M, Kan CY, Yang LL, Yang J, Ma QF, Wang SX. [Expression and clinical significance of Sema4C in esophageal cancer, gastric cancer and rectal cancer]. Zhonghua Yi Xue Za Zhi 2012; 92:1954-8; PMID:22944267
- Zhang Y, Huang S. Up-regulation of miR-125b reverses epithelial-mesenchymal transition in paclitaxel-resistant lung cancer cells. Biol Chem 2015; PMID:26351908; http://dx.doi.org/10.1515/hsz-2015-0153
- Yang Q, Wang Y, Lu X, Zhao Z, Zhu L, Chen S, Wu Q, Chen C, Wang Z. MiR-125b regulates epithelial-mesenchymal transition via targeting Sema4C in paclitaxel-resistant breast cancer cells. Oncotarget 2015; 6:3268-79; PMID:25605244; http://dx.doi.org/10.18632/oncotarget.3065
- Le AP, Huang Y, Pingle SC, Kesari S, Wang H, Yong RL, Zou H, Friedel RH. Plexin-B2 promotes invasive growth of malignant glioma. Oncotarget 2015; 6:7293-304; PMID:25762646; http://dx.doi.org/10.18632/oncotarget.3421
- Wu M, Han L, Shi Y, Xu G, Wei J, You L, Chen Y, Zhu T, Li Q, Li S, et al. Development and characterization of a novel method for the analysis of gene expression patterns in lymphatic endothelial cells derived from primary breast tissues. J Cancer Res Clin Oncol 2010; 136:863-72; PMID:19936789; http://dx.doi.org/10.1007/s00432-009-0727-9
- Kato S, Kubota K, Shimamura T, Shinohara Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F, Ishiguro H, et al. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci 2011; 102:2029-37; PMID:21812859; http://dx.doi.org/10.1111/j.1349-7006.2011.02053.x
- Ch'ng E, Tomita Y, Zhang B, He J, Hoshida Y, Qiu Y, Morii E, Nakamichi I, Hamada K, Ueda T, et al. Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer 2007; 110:164-72; PMID:17520683; http://dx.doi.org/10.1002/cncr.22764
- Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Investigat 2012; 122:1296-305; PMID:22378040; http://dx.doi.org/10.1172/JCI60568
- Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis 2014; 17:261-74; PMID:24114199; http://dx.doi.org/10.1007/s10456-013-9395-0
- Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, Manlove LA, LaRue RS, Temiz NA, Molyneux SD, et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet 2015; 47:615-24; PMID:25961939; http://dx.doi.org/10.1038/ng.3293
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002; 4:720-4; PMID:12198496; http://dx.doi.org/10.1038/ncb843
- Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res 2004; 64:5212-24; PMID:15289326; http://dx.doi.org/10.1158/0008-5472.CAN-04-0126
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol 2005; 25:6889-98; PMID:16055703; http://dx.doi.org/10.1128/MCB.25.16.6889-6898.2005
- Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A 2006; 103:9017-22; PMID:16754882; http://dx.doi.org/10.1073/pnas.0508825103
- Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR. Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PloS One 2011; 6:e25826; PMID:22028792; http://dx.doi.org/10.1371/journal.pone.0025826
- Zhou H, Yang YH, Binmadi NO, Proia P, Basile JR. The hypoxia-inducible factor-responsive proteins semaphorin 4D and vascular endothelial growth factor promote tumor growth and angiogenesis in oral squamous cell carcinoma. Exp Cell Res 2012; 318:1685-98; PMID:22652457; http://dx.doi.org/10.1016/j.yexcr.2012.04.019
- Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis 2012; 15:391-407; PMID:22476930; http://dx.doi.org/10.1007/s10456-012-9268-y
- Binmadi NO, Yang YH, Zhou H, Proia P, Lin YL, De Paula AM, Guimaraes AL, Poswar FO, Sundararajan D, Basile JR. Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. Am J Pathol 2012; 180:1232-42; PMID:22252234; http://dx.doi.org/10.1016/j.ajpath.2011.12.009
- Patnaik A, Weiss GJ, Leonard JE, Rasco DW, Sachdev JC, Fisher TL, Winter LA, Reilly C, Parker RB, Mutz D, et al. Safety, pharmacokinetics, and pharmacodynamics of a humanized anti-semaphorin 4D antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res 2015; 22(4):827-36; PMID: 26446947; http://dx.doi.org/10.1158/1078-0432.CCR-15-0431
- Leonard JE, Fisher TL, Winter LA, Cornelius CA, Reilly C, Smith ES, Zauderer M. Nonclinical safety evaluation of VX15/2503, a humanized IgG4 anti-SEMA4D antibody. Mol Cancer Therap 2015; 14:964-72; PMID:25657333; http://dx.doi.org/10.1158/1535-7163.MCT-14-0924
- Fisher TL, Seils J, Reilly C, Litwin V, Green L, Salkowitz-Bokal J, Walsh R, Harville S, Leonard JE, Smith E, et al. Saturation monitoring of VX15/2503, a novel semaphorin 4D-specific antibody, in clinical trials. Cytometry B Clin Cytom 2015; PMID:26566052
- Fisher TL, Reilly CA, Winter LA, Pandina T, Jonason A, Scrivens M, Balch L, Bussler H, Torno S, Seils J, et al. Generation and preclinical characterization of an antibody specific for SEMA4D. mAbs 2016; 8:150-62; PMID:26431358; http://dx.doi.org/10.1080/19420862.2015.1102813
- Evans EE, Jonason AS Jr, Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, et al. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res 2015; 3:689-701; PMID:25614511; http://dx.doi.org/10.1158/2326-6066.CIR-14-0171
- Ding Y, He D, Florentin D, Frolov A, Hilsenbeck S, Ittmann M, Kadmon D, Miles B, Rowley D, Ayala G. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res 2013; 19:6101-11; PMID:24097862; http://dx.doi.org/10.1158/1078-0432.CCR-12-3669
- Li X, Law JW, Lee AY. Semaphorin 5A and plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the actin cytoskeleton. Oncogene 2012; 31:595-610; PMID:21706053
- Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. J Biol Chem 2010; 285:32436-45; PMID:20696765; http://dx.doi.org/10.1074/jbc.M110.120451
- Hirota E, Yan L, Tsunoda T, Ashida S, Fujime M, Shuin T, Miki T, Nakamura Y, Katagiri T. Genome-wide gene expression profiles of clear cell renal cell carcinoma: identification of molecular targets for treatment of renal cell carcinoma. Int J Oncol 2006; 29:799-827; PMID:16964377
- Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, et al. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers Prev 2010; 19:2590-7; PMID:20802022; http://dx.doi.org/10.1158/1055-9965.EPI-10-0332
- Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di Carlo S, Visca P, et al. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget 2015; 6:2779-93; PMID:25576923; http://dx.doi.org/10.18632/oncotarget.2995
- Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood 2011; 118:4285-96; PMID:21832283; http://dx.doi.org/10.1182/blood-2011-03-341388
- Rice DS, Huang W, Jones HA, Hansen G, Ye GL, Xu N, Wilson EA, Troughton K, Vaddi K, Newton RC, et al. Severe retinal degeneration associated with disruption of semaphorin 4A. Invest Ophthalmol Vis Sci 2004; 45:2767-77; PMID:15277503; http://dx.doi.org/10.1167/iovs.04-0020
- Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 2005; 22:305-16; PMID:15780988; http://dx.doi.org/10.1016/j.immuni.2005.01.014
- Yukawa K, Tanaka T, Bai T, Ueyama T, Owada-Makabe K, Tsubota Y, Maeda M, Suzuki K, Kikutani H, Kumanogoh A. Semaphorin 4A induces growth cone collapse of hippocampal neurons in a Rho/Rho-kinase-dependent manner. Int J Mol Med 2005; 16:115-8; PMID:15942687
- Abid A, Ismail M, Mehdi SQ, Khaliq S. Identification of novel mutations in the SEMA4A gene associated with retinal degenerative diseases. J Med Genet 2006; 43:378-81; PMID:16199541; http://dx.doi.org/10.1136/jmg.2005.035055
- Makino N, Toyofuku T, Takegahara N, Takamatsu H, Okuno T, Nakagawa Y, Kang S, Nojima S, Hori M, Kikutani H, et al. Involvement of Sema4A in the progression of experimental autoimmune myocarditis. FEBS Lett 2008; 582:3935-40; PMID:18977352; http://dx.doi.org/10.1016/j.febslet.2008.10.040
- Nkyimbeng-Takwi EH, Shanks K, Smith E, Iyer A, Lipsky MM, Detolla LJ, Kikutani H, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4A downregulates the severity of allergic response. Mucosal Immunol 2012; 5:409-19; PMID:22472774; http://dx.doi.org/10.1038/mi.2012.18
- Toyofuku T, Nojima S, Ishikawa T, Takamatsu H, Tsujimura T, Uemura A, Matsuda J, Seki T, Kumanogoh A. Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev 2012; 26:816-29; PMID:22465952; http://dx.doi.org/10.1101/gad.184481.111
- Morihana T, Goya S, Mizui M, Yasui T, Prasad DV, Kumanogoh A, Tamura M, Shikina T, Maeda Y, Iwamoto Y, et al. An inhibitory role for Sema4A in antigen-specific allergic asthma. J Clin Immunol 2013; 33:200-9; PMID:23007237; http://dx.doi.org/10.1007/s10875-012-9798-5
- Ito D, Nojima S, Nishide M, Okuno T, Takamatsu H, Kang S, Kimura T, Yoshida Y, Morimoto K, Maeda Y, et al. mTOR complex signaling through the SEMA4A-Plexin B2 axis is required for optimal activation and differentiation of CD8+ T cells. J Immunol 2015; 195:934-43; PMID:26116513; http://dx.doi.org/10.4049/jimmunol.1403038
- Wang L, Song G, Zheng Y, Tan W, Pan J, Zhao Y, Chang X. Expression of Semaphorin 4A and its potential role in rheumatoid arthritis. Arthritis Res Ther 2015; 17:227; PMID:26303122; http://dx.doi.org/10.1186/s13075-015-0734-y
- Nakagawa Y, Takamatsu H, Okuno T, Kang S, Nojima S, Kimura T, Kataoka TR, Ikawa M, Toyofuku T, Katayama I, et al. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J Immunol 2011; 186:2881-8; PMID:21270411; http://dx.doi.org/10.4049/jimmunol.1003485
- Ben-Gigi L, Sweetat S, Besser E, Fellig Y, Wiederhold T, Polakiewicz RD, Behar O. Astrogliosis Induced by Brain Injury Is Regulated by Sema4B Phosphorylation(123). eNeuro 2015; 2; PMID:26464987
- Fan JD, Zhu LL, Zhao T. [Sema4C expresses in neural stem cells]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2007; 23:153-4.
- Fan W, Wu H, Fan J, Wu Y, Fan M. SEMA4C expression in neural stem/progenitor cells and in adult neurogenesis induced by cerebral ischemia. J Physiol Sci 2009; 59:>442.
- Wu H, Fan J, Zhu L, Liu S, Wu Y, Zhao T, Wu Y, Ding X, Fan W, Fan M. Sema4C expression in neural stem/progenitor cells and in adult neurogenesis induced by cerebral ischemia. J Mol Neurosci 2009; 39:27-39; PMID:19189244; http://dx.doi.org/10.1007/s12031-009-9177-8
- Perala N, Jakobson M, Nymark M, Penachioni J, Tanninen T, Immonen T, Tamagnone L, Sariola H. Sema4C-plexin B2-signalling modulates morphogenesis of the ureteric epithelium. Mech Dev 2009; 126:S213-S4; PMID:21035938; http://dx.doi.org/10.1016/j.mod.2009.06.537
- Perala N, Jakobson M, Ola R, Fazzari P, Penachioni JY, Nymark M, Tanninen T, Immonen T, Tamagnone L, Sariola H. Sema4C-Plexin B2 signalling modulates ureteric branching in developing kidney. Differentiation 2010; 80:S55-S; PMID:21035938; http://dx.doi.org/10.1016/j.diff.2010.09.120
- Zeng R, Han M, Luo Y, Li C, Pei G, Liao W, Bai S, Ge S, Liu X, Xu G. Role of Sema4C in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. Nephrol Dial Transplant 2011; 26:1149-56; PMID:20959347; http://dx.doi.org/10.1093/ndt/gfq619
- Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity 2000; 13:633-42; PMID:11114376; http://dx.doi.org/10.1016/S1074-7613(00)00063-7
- Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood 2001; 97:3498-504; PMID:11369643; http://dx.doi.org/10.1182/blood.V97.11.3498
- Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 2003; 101:1962-9; PMID:12406905; http://dx.doi.org/10.1182/blood-2002-05-1339
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci 2003; 23:9229-39; PMID:14534257
- Masuda K, Furuyama T, Takahara M, Fujioka S, Kurinami H, Inagaki S. Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells 2004; 9:821-9; PMID:15330859; http://dx.doi.org/10.1111/j.1365-2443.2004.00766.x
- Duran-Struuck R, Tawara I, Lowler K, Clouthier SG, Weisiger E, Rogers C, Luker G, Kumanogoh A, Liu C, Ferrara JL, et al. A novel role for the semaphorin Sema4D in the induction of allo-responses. Biol Blood Marrow Transplant 2007; 13:1294-303; PMID:17950916; http://dx.doi.org/10.1016/j.bbmt.2006.12.237
- Lin X, Ogiya M, Takahara M, Yamaguchi W, Furuyama T, Tanaka H, Tohyama M, Inagaki S. Sema4D-plexin-B1 implicated in regulation of dendritic spine density through RhoA/ROCK pathway. Neurosci Lett 2007; 428:1-6; PMID:17950529; http://dx.doi.org/10.1016/j.neulet.2007.09.045
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A 2007; 104:1621-6; PMID:17244710; http://dx.doi.org/10.1073/pnas.0606344104
- Abe M, Inagaki S, Furuyama T, Iwamoto M, Wakisaka S. Semaphorin 4D inhibits collagen synthesis of rat pulp-derived cells. Arch Oral Biol 2008; 53:27-34; PMID:17920031; http://dx.doi.org/10.1016/j.archoralbio.2007.08.005
- Korostylev A, Worzfeld T, Deng S, Friedel RH, Swiercz JM, Vodrazka P, Maier V, Hirschberg A, Ohoka Y, Inagaki S, et al. A functional role for semaphorin 4D/plexin B1 interactions in epithelial branching morphogenesis during organogenesis. Dev (Cambridge, England) 2008; 135:3333-43; PMID:18799546; http://dx.doi.org/10.1242/dev.019760
- Fuchikawa T, Nakamura F, Fukuda N, Takei K, Goshima Y. Protein tyrosine phosphatase SHP2 is involved in Semaphorin 4D-induced axon repulsion. Biochem Biophys Res Commun 2009; 385:6-10; PMID:19433062; http://dx.doi.org/10.1016/j.bbrc.2009.05.024
- Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep 2009; 10:614-21; PMID:19444311; http://dx.doi.org/10.1038/embor.2009.63
- Toguchi M, Gonzalez D, Furukawa S, Inagaki S. Involvement of Sema4D in the control of microglia activation. Neurochem Int 2009; 55:573-80; PMID:19467284; http://dx.doi.org/10.1016/j.neuint.2009.05.013
- Yukawa K, Tanaka T, Takeuchi N, Iso H, Li L, Kohsaka A, Waki H, Miyajima M, Maeda M, Kikutani H, et al. Sema4D/CD100 deficiency leads to superior performance in mouse motor behavior. Can J Neurol Sci 2009; 36:349-55; PMID:19534337; http://dx.doi.org/10.1017/S0317167100007101
- Wannemacher KM, Zhu L, Jiang H, Fong KP, Stalker TJ, Lee D, Tran AN, Neeves KB, Maloney S, Kumanogoh A, et al. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood 2010; 116:5707-15; PMID:20855865; http://dx.doi.org/10.1182/blood-2010-04-279943
- Yukawa K, Tanaka T, Kishino M, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med 2010; 26:39-44; PMID:20514420; http://dx.doi.org/10.3892/ijmm_00000432
- Kuzirian MS, Moore AR, Staudenmaier EK, Friedel RH, Paradis S. The class 4 semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus. J Neurosci 2013; 33:8961-73; PMID:23699507; http://dx.doi.org/10.1523/JNEUROSCI.0989-13.2013
- Shanks K, Nkyimbeng-Takwi EH, Smith E, Lipsky MM, DeTolla LJ, Scott DW, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4D is necessary for optimal lung allergic inflammation. Mol Immunol 2013; 56:480-7; PMID:23911404; http://dx.doi.org/10.1016/j.molimm.2013.05.228
- Yang J, Zeng Z, Wei J, Jiang L, Ma Q, Wu M, Huang X, Ye S, Li Y, Ma D, et al. Sema4d is required for the development of the hindbrain boundary and skeletal muscle in zebrafish. Biochem Biophys Res Commun 2013; 433:213-9; PMID:23466355; http://dx.doi.org/10.1016/j.bbrc.2013.02.085
- Xiao T, Shoji W, Zhou W, Su F, Kuwada JY. Transmembrane sema4E guides branchiomotor axons to their targets in zebrafish. J Neurosci 2003; 23:4190-8; PMID:12764107
- Parrinello S, Noon LA, Harrisingh MC, Wingfield Digby P, Rosenberg LH, Cremona CA, Echave P, Flanagan AM, Parada LF, Lloyd AC. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev 2008; 22:3335-48; PMID:19056885; http://dx.doi.org/10.1101/gad.490608
- Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development 2003; 130:775-84; PMID:12506007; http://dx.doi.org/10.1242/dev.00299
- Hilario JD, Rodino-Klapac LR, Wang C, Beattie CE. Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Dev Biol 2009; 326:190-200; PMID:19059233; http://dx.doi.org/10.1016/j.ydbio.2008.11.007
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, et al. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 2011; 71:460-73; PMID:21835343; http://dx.doi.org/10.1016/j.neuron.2011.06.009
- Matsuoka RL, Sun LO, Katayama K, Yoshida Y, Kolodkin AL. Sema6B, Sema6C, and Sema6D expression and function during mammalian retinal development. PLoS One 2013; 8:e63207; PMID:23646199; http://dx.doi.org/10.1371/journal.pone.0063207
- Duan Y, Wang SH, Song J, Mironova Y, Ming GL, Kolodkin AL, Giger RJ. Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. Elife 2014; 3; PMID:25313870
- Masuda T, Sakuma C, Yaginuma H, Taniguchi M. Attractive and permissive activities of semaphorin 5A toward dorsal root ganglion axons in higher vertebrate embryos. Cell Adh Migr 2014; 8:603-6; PMID:25622099; http://dx.doi.org/10.4161/19336918.2014.972770
- Yu X, Wang F, Zhang JP. Meta analysis of the association of rs7702187 SNP in SEMA5A gene with risk of Parkinson's disease. Euro Rev Med Pharmacol Sci 2014; 18:900-4; PMID:24706317
- O'Connor TP, Cockburn K, Wang W, Tapia L, Currie E, Bamji SX. Semaphorin 5B mediates synapse elimination in hippocampal neurons. Neural Dev 2009; 4:18; PMID:19463192; http://dx.doi.org/10.1186/1749-8104-4-18
- Browne K, Wang W, Liu RQ, Piva M, O'Connor TP. Transmembrane semaphorin5B is proteolytically processed into a repulsive neural guidance cue. J Neurochem 2012; 123:135-46; PMID:22817385; http://dx.doi.org/10.1111/j.1471-4159.2012.07885.x
- Liu RQ, Wang W, Legg A, Abramyan J, O'Connor TP. Semaphorin 5B is a repellent cue for sensory afferents projecting into the developing spinal cord. Development 2014; 141:1940-9; PMID:24718987; http://dx.doi.org/10.1242/dev.103630
- Rollmann SM, Yamamoto A, Goossens T, Zwarts L, Callaerts-Vegh Z, Callaerts P, Norga K, Mackay TF, Anholt RR. The early developmental gene Semaphorin 5c contributes to olfactory behavior in adult Drosophila. Genetics 2007; 176:947-56; PMID:17435226; http://dx.doi.org/10.1534/genetics.106.069781
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci 2000; 20:2638-48; PMID:10729344
- Gautier G, de Saint-Vis B, Senechal B, Pin JJ, Bates EE, Caux C, Geissmann F, Garrone P. The class 6 semaphorin SEMA6A is induced by interferon-gamma and defines an activation status of langerhans cells observed in pathological situations. Am J Pathol 2006; 168:453-65; PMID:16436660; http://dx.doi.org/10.2353/ajpath.2006.050288
- Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev 2007; 2:28; PMID:18088409; http://dx.doi.org/10.1186/1749-8104-2-28
- Renaud J, Kerjan G, Sumita I, Zagar Y, Georget V, Kim D, Fouquet C, Suda K, Sanbo M, Suto F, et al. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci 2008; 11:440-9; PMID:18327254; http://dx.doi.org/10.1038/nn2064
- Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev 2008; 3:34; PMID:19063725; http://dx.doi.org/10.1186/1749-8104-3-34
- Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 2009; 61:359-72; PMID:19217374; http://dx.doi.org/10.1016/j.neuron.2008.12.022
- Rogalewski A, Dittgen T, Klugmann M, Kirsch F, Kruger C, Pitzer C, Minnerup J, Schabitz WR, Schneider A. Semaphorin 6A improves functional recovery in conjunction with motor training after cerebral ischemia. PLoS One 2010; 5:e10737; PMID:20505770
- Runker AE, O'Tuathaigh C, Dunleavy M, Morris DW, Little GE, Corvin AP, Gill M, Henshall DC, Waddington JL, Mitchell KJ. Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology. PLoS One 2011; 6:e26488; PMID:22132072; http://dx.doi.org/10.1371/journal.pone.0026488
- Ebert AM, Childs SJ, Hehr CL, Cechmanek PB, McFarlane S. Sema6a and Plxna2 mediate spatially regulated repulsion within the developing eye to promote eye vesicle cohesion. Development 2014; 141:2473-82; PMID:24917502; http://dx.doi.org/10.1242/dev.103499
- Tawarayama H, Yoshida Y, Suto F, Mitchell KJ, Fujisawa H. Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. J Neurosci 2010; 30:7049-60; PMID:20484647; http://dx.doi.org/10.1523/JNEUROSCI.0073-10.2010
- Andermatt I, Wilson NH, Bergmann T, Mauti O, Gesemann M, Sockanathan S, Stoeckli ET. Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance. Development (Cambridge, England) 2014; 141:3709-20; PMID:25209245; http://dx.doi.org/10.1242/dev.112185
- Burgaya F, Fontana X, Martinez A, Montolio M, Mingorance A, Simo S, del Rio JA, Soriano E. Semaphorin 6C leads to GSK-3-dependent growth cone collapse and redistributes after entorhino-hippocampal axotomy. Mol Cell Neurosci 2006; 33:321-34; PMID:17029982; http://dx.doi.org/10.1016/j.mcn.2006.08.008
- Svensson A, Libelius R, Tagerud S. Semaphorin 6C expression in innervated and denervated skeletal muscle. J Mol Histol 2008; 39:5-13; PMID:17605078; http://dx.doi.org/10.1007/s10735-007-9113-6
- Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y. Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development 2011; 138:4085-95; PMID:21831918; http://dx.doi.org/10.1242/dev.066076
- Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, Mason C. Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 2012; 74:676-90; PMID:22632726; http://dx.doi.org/10.1016/j.neuron.2012.03.025
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J 2007; 26:1373-84; PMID:17318185; http://dx.doi.org/10.1038/sj.emboj.7601589
- Donnard E, Asprino PF, Correa BR, Bettoni F, Koyama FC, Navarro FC, Perez RO, Mariadason J, Sieber OM, Strausberg RL, et al. Mutational analysis of genes coding for cell surface proteins in colorectal cancer cell lines reveal novel altered pathways, druggable mutations and mutated epitopes for targeted therapy. Oncotarget 2014; 5:9199-213; PMID:25193853; http://dx.doi.org/10.18632/oncotarget.2374
- Li J, Wang Q, Wen R, Liang J, Zhong X, Yang W, Su D, Tang J. MiR-138 inhibits cell proliferation and reverses epithelial-mesenchymal transition in non-small cell lung cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med 2015; 19:2793-805; PMID:26283050
- Liu H, Yang Y, Xiao J, Yang S, Liu Y, Kang W, Li X, Zhang F. Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients. Microvasc Res 2014; 93:1-8; PMID:24603190; http://dx.doi.org/10.1016/j.mvr.2014.02.007
- Mu L, Wang J, Chen Y, Li L, Guo X, Zheng S, Jing C. Hypoxia-inducible factor-1alpha and semaphorin4D genes involved with tumor-associated macrophage-induced metastatic behavior and clinical significance in colon cancer. Chin Med J (Engl) 2014; 127:3568-75; PMID:25316231
- Sun Q, Zhou H, Binmadi NO, Basile JR. Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem 2009; 284:32066-74; PMID:19762474; http://dx.doi.org/10.1074/jbc.M109.057166
- Qiang R, Wang F, Shi LY, Liu M, Chen S, Wan HY, Li YX, Li X, Gao SY, Sun BC, et al. Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int J Biochem Cell Biol 2011; 43:632-41; PMID:21216304; http://dx.doi.org/10.1016/j.biocel.2011.01.002
- Chen Y, Zhang L, Pan Y, Ren X, Hao Q. Over-expression of semaphorin4D, hypoxia-inducible factor-1alpha and vascular endothelial growth factor is related to poor prognosis in ovarian epithelial cancer. Int J Mol Sci 2012; 13:13264-74; PMID:23202951; http://dx.doi.org/10.3390/ijms131013264
- Soong J, Chen Y, Shustef EM, Scott GA. Sema4D, the ligand for Plexin B1, suppresses c-Met activation and migration and promotes melanocyte survival and growth. J Invest Dermatol 2012; 132:1230-8; PMID:22189792; http://dx.doi.org/10.1038/jid.2011.414
- Chen Y, Zhang L, Lv R, Zhang WQ. Overexpression of Semaphorin4D indicates poor prognosis and prompts monocyte differentiation toward M2 macrophages in epithelial ovarian cancer. Asian Pac J Cancer Prev 2013; 14:5883-90; PMID:24289594; http://dx.doi.org/10.7314/APJCP.2013.14.10.5883
- Ruan SS, Li RC, Han Q, Liu J, Li GL, Song YQ, Wu G. Expression and clinical significance of Semaphorin4D in non-small cell lung cancer and its impact on malignant behaviors of A549 lung cancer cells. J Huazhong Univ Sci Technolog Med Sci 2014; 34:491-6; PMID:25135716; http://dx.doi.org/10.1007/s11596-014-1304-2
- Younis RH, Han KL, Webb TJ. Human head and neck squamous cell carcinoma-associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J Immunol (Baltimore, Md: 1950) 2016; 196:1419-29; PMID:26740106
- Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 2008; 14:7593-603; PMID:19047084; http://dx.doi.org/10.1158/1078-0432.CCR-08-1164
- Wang X, Zbou C, Qiu G, Fan J, Tang H, Peng Z. Screening of new tumor suppressor genes in sporadic colorectal cancer patients. Hepatogastroenterology 2008; 55:2039-44; PMID:19260473
- Sadanandam A, Varney ML, Kinarsky L, Ali H, Mosley RL, Singh RK. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. Omics: J Integrat Biol 2007; 11:41-57; PMID:17411395; http://dx.doi.org/10.1089/omi.2006.0004
- Sadanandam A, Rosenbaugh EG, Singh S, Varney M, Singh RK. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc Res 2010; 79:1-9; PMID:19850054; http://dx.doi.org/10.1016/j.mvr.2009.10.005
- Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, Lele SM, Batra SK, Singh RK. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer J Int du Cancer 2010; 127:1373-83; PMID:20073063; http://dx.doi.org/10.1002/ijc.25166
- Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang CS, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Brit J Cancer 2012; 107:501-7; PMID:22782341; http://dx.doi.org/10.1038/bjc.2012.298
- Grundmann S, Lindmayer C, Hans FP, Hoefer I, Helbing T, Pasterkamp G, Bode C, de Kleijn D, Moser M. FoxP1 stimulates angiogenesis by repressing the inhibitory guidance protein semaphorin 5B in endothelial cells. PLoS One 2013; 8:e70873; PMID:24023716
- Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF 3rd, Liotta LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci U S A 2003; 100:11463-8; PMID:14500904; http://dx.doi.org/10.1073/pnas.2031202100
- Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A, Li X, Tosato G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood 2012; 120:4104-15; PMID:23007403; http://dx.doi.org/10.1182/blood-2012-02-410076
- Zhao J, Tang H, Zhao H, Che W, Zhang L, Liang P. SEMA6A is a prognostic biomarker in glioblastoma. Tumour Biol: J Int Soc Oncodev Biol Med 2015; 36:8333-40; PMID:26014517; http://dx.doi.org/10.1007/s13277-015-3584-y
- Correa RG, Sasahara RM, Bengtson MH, Katayama ML, Salim AC, Brentani MM, Sogayar MC, de Souza SJ, Simpson AJ. Human semaphorin 6B [(HSA)SEMA6B], a novel human class 6 semaphorin gene: alternative splicing and all-trans-retinoic acid-dependent downregulation in glioblastoma cell lines. Genomics 2001; 73:343-8; PMID:11350127; http://dx.doi.org/10.1006/geno.2001.6525
- Murad H, Collet P, Huin-Schohn C, Al-Makdissy N, Kerjan G, Chedotal A, Donner M, Devignes MD, Becuwe P, Schohn H, et al. Effects of PPAR and RXR ligands in semaphorin 6B gene expression of human MCF-7 breast cancer cells. Int J Oncol 2006; 28:977-84; PMID:16525649
- D'Apice L, Costa V, Valente C, Trovato M, Pagani A, Manera S, Regolo L, Zambelli A, Ciccodicola A, De Berardinis P. Analysis of SEMA6B gene expression in breast cancer: identification of a new isoform. Biochim Biophys Acta 2013; 1830:4543-53; PMID:23665584; http://dx.doi.org/10.1016/j.bbagen.2013.05.003
- Catalano A, Lazzarini R, Di Nuzzo S, Orciari S, Procopio A. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res 2009; 69:1485-93; PMID:19176370; http://dx.doi.org/10.1158/0008-5472.CAN-08-3659
- Chen D, Li Y, Wang L, Jiao K. SEMA6D expression and patient survival in breast invasive carcinoma. Int J Breast Cancer 2015; 2015:539721; PMID:25973277; http://dx.doi.org/10.1155/2015/539721
