ABSTRACT
The mechanisms governing the navigation of commissural axons during embryonic development have been extensively investigated in the past years, often using the drosophila ventral nerve cord and the spinal cord as model systems. Similarities but also specificities in the general strategies, the molecular signals as well as in the regulatory pathways controlling the response of commissural axons to the guidance cues have been found between species. Whether the semaphorin signaling contributes to midline crossing in the fly nervous system remains unknown, while in contrast, it does play a prominent contribution in vertebrates. In this review we discuss the functions of the semaphorins during commissural axon guidance in the developing spinal cord, focusing on the family member semaphorin 3B (Sema3B) in the context of midline crossing in the spinal cord.
Introduction to the semaphorin family
The semaphorins form a large family of secreted and membrane-associated molecules present from virus to human.Citation32 By activating a range of holoreceptors and downtream intracellular cascades, the semaphorins regulate the molecular machinery controlling actin and microtubule dynamics, thus contributing to a variety of processes implicating cell movements, from cell migration and axon migration in the nervous system, organ morphogenesis, to immunology and tumor metastasis.Citation16,29,32 Many contributions of the semaphorins have been discovered in the developing and adult spinal cord, both in physiological and pathological contexts such as spinal cord injury, which exemplify the diversity of functional properties that these cues exert (O'Malley et al., 2014). In this review, we concentrate on the role of the secreted semaphorin 3B (Sema3B) during the formation of the spinal commissures.
Crossing the midline of the central nervous system: An obligatory step for all commissural axons
In bilateral organisms, multiple reciprocal neuronal projections interconnect the 2 halves of the central nervous system (CNS), forming a dense network of commissures that allow integration and coordination of left-right neuronal activities.Citation9,36 During embryonic and early postnatal developmental periods, commissural axons navigate through the central midline at all axial levels, crossing from one side of the CNS to the other one at specific time points and positions.Citation9 Commissural axons can be surrounded by or mixed with ipsilateral axons that are committed to build circuits between neurons of the same side, and that never cross the midline. For instance, in the visual circuits of organisms with binocular vision, ipsilateral and contralateral ganglion cell axons exit the retina and navigate together, segregating at the optic chiasm when the contralateral axon tract achieves midline crossing.Citation18 Similarly, tracts of ipsilateral and contralateral axons navigate in close proximity in the developing spinal cordCitation41 ().
Figure 1. (A) Schematic representations of various commissural projections. In mammals, neocortical callosal axons (in red) turn medially and navigate toward the midline. They are guided by cues emanating from various sources: the midline zipper glia (in purple), the glial wedges (in light blue), the indusium griseum glia (in dark blue), and migrating neurons (in green). Ganglion cell axons exiting the retina connect both sides of the brain, forming ipsilateral (in red) and contralateral (in blue) tracts, the latter crossing the midline at the optic chiasm (LE: left eye; RE: right eye; LT: left thalamus; RT: right thalamus). In the Drosophila ventral nerve cord, ipsilateral (in red) and contralateral (in blue) neurons extend their axons medially toward the midline, but ipsilateral axons turn before crossing while contralateral ones turn after the crossing. In vertebrates, spinal commissural axons navigate first ventrally toward the floor plate (FP) lying at the ventral edge of the central canal (cc), cross the midline and then turn rostrally. (B) Temporal sequence of dl1 commissural interneuron generation and axon navigation. The first spinal commissural neurons are born from a dorsal territory around E9.5. Some of them already extend their axons across the midline at E10.5. By E12.5, most of them have completed midline crossing and all navigate post-crossing longitudinal routes by E.13.5. (C) Temporal sequence of guidance signaling controlling the navigation of the midline. As they navigate toward the FP, the sensitivity to midline repellents is silenced in spinal commissural growth cones. During FP crossing, commissural growth cones gain responsiveness to these FP repulsive cues, which prevent them from re-crossing and drive them out of the FP. At the exit, they follow rostro-caudal gradients of guidance cues, turning rostrally in the ventral (VF) or lateral (LF) funiculus. (D) Representation of the principal signaling implicated in the midline repulsion. Slit/Robo in the Drosophila, Robo1-2/SlitN, PlexA1/SlitC, and Sema3B/Nrp2/PlexA1 in the mouse.
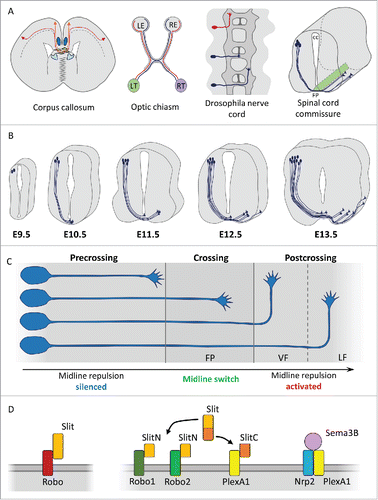
Specialized groups of local cells lying at the CNS midline are instrumental in segregating the ipsilateral and contralateral axon populations, allowing only the latter to cross the midline (Evans and Bashaw, 2009Citation9,36). In the developing spinal cord, midline crossing takes place ventrally through the floor plate (FP), a crucial patterning center composed of glial cells, which contribute to the specification of the neuronal lineages of the neural tube and adjacent territories by secreting the morphogen SHH.Citation17,24 The drosophila midline glia plays equivalent roles, secreting a TGF homolog to direct ventral cell fates of the CNS, the ectoderm and the mesoderm.Citation5 The FP also starts expressing guidance molecules at these early stages. It was recently discovered that semaphorin 3B (Sema3B) already has an instructive role prior to the stage of axon navigation. Sema3B is already detected at E9 and is secreted in the cerebrospinal fluid. Collected by receptors of neuroepithelial progenitors that undergo mitosis at the apical border lining the central canal, Sema3B triggers an intracellular signaling which regulates microtubule stability and promotes planar orientation of the cell divisions at the onset of the neuro-genesis.Citation2 Beyond this function, the FP source of Sema3B has been well characterized for its contribution to commissural axon guidance, a role that we will discuss in detail below.
Commissural axon navigation in the spinal cord: The prototypical dl1 tract
The dorsal interneuron lineages in the developing spinal cord are specified by transcriptional programs, according to their position, birthdate and pattern of connections. Various classes of commissural interneurons have been identified among which the commissural component of the dl1 population, born from a Math1+ progenitor pool that lies close to the roof plate and which also generates an ipsilateral component.Citation22 dl1 commissural neurons, specified by Lhx2/9 transcription factors,Citation44 elaborate a typical pattern of axonal projections, whose navigation has been widely investigated. The axons extend ventrally toward the FP, cross the midline, exit the FP and turn rostrally to ascend toward supraspinal levels, to convey proprioceptive information to the cerebellum.Citation1 The generation of transgenic mice expressing LacZ or GFP under the control of the Math1 promotor has allowed a precise spatial and temporal mapping of commissural dl1 axon navigation in the mouse embryo. Born from around E10, dl1 commissural neurons extend waves of axons toward the FP starting at this stage. Some of them already cross the midline as early as E10.5. It is widely accepted that most of them have crossed the midline by E12.5, and are already navigating distant longitudinal routes at E13Citation11,25,26,27,30,44 ().
Repulsive signaling controlling midline crossing in the vertebrate spinal cord and invertebrate nerve cord
The FP and midline glia are sources of both attractive and repulsive cues for commissural axons. A temporal sequence has been proposed which orchestrates the different steps of commissural axon navigation by controlling axon responsiveness to the FP attractive and repulsive cues, thus preventing conflict of guidance information. First, the commissural growth cones perceive chemoattractants, including Netrin, which orient their trajectory toward the midline. Commissural growth cones then interact with local cells to navigate the FP/midline glia. Next, upon crossing, they gain responsiveness to FP/midline glia-derived repulsive cues, which prevent them from turning back and re-crossing the midline, and also push them out of the FP toward the contralateral side. Finally, after FP exit, rostro-caudal gradients of guidance cues elicit a longitudinal turning of commissural axons, accompanied by a sorting of the axons into the ventral and lateral funiculi in which they navigate rostrally ().
Such a temporal sequence of guidance programs relies on a tight control of growth cone sensitivity to the guidance cues. In particular, the sensitivity to the midline repellents must be first silenced before crossing to be triggered only after the crossing. The premature action of the repellents would prevent the axons from entering the FP.
The repulsive signaling found to regulate midline crossing in vertebrates and invertebrates present some degrees of conservation, although vertebrates evolved significant differences.Citation36 In drosophila, midline repulsion was shown to be ensured by SLIT, a gene encoding a protein which acts through binding to Roundabout (Robo) Robo 1 and Robo2 receptors on commissural axons. Similarly, in vertebrates, 3 SLIT genes (Slit1,2,3) were shown to act in synergy to control midline crossing, mediating their effects through Robo1 and Robo2 receptors.Citation6
The N-terminal (140 kDa) and C-terminal products (55-60 kDa) resulting from Slit protein cleavage were recently found to both contribute to the FP navigation in vertebrates. Until this recent work, Slit-Ns, which contain the binding sequence for Robo receptors were logically considered as the bioactive protein fragments. In contrast, the Slit-C fragments, for which no receptor was identified, were thought to be inactive. From initial analysis of mutant mouse models, deletion of Slit1-3 or Robo1/2 genes in mice were both known to disturb commissural navigation. However unexpectedly, phenotypic differences were noted between the 2 deletion contexts. Midline re-crossing was observed after Slit1-3 but not Robo1/2 deletion.Citation27,33 These studies raised the idea that Slits exhibit some Robo-independent functions that might be mediated by an as yet-unknown receptor. This receptor turned out to be PlexinA1,Citation15 a receptor shared by members of another prominent axon guidance family, the semaphorins.Citation32 Indeed, PlexinA1 deletion was found to induce the re-crossing phenotype, and also to confer the re-crossing phenotype to Robo1/2 mutants. Various combinations of PlexinA1 and Slit1/2/3 allelic deletions also confirmed that Slits and PlexinA1 both participate in preventing midline re-crossing during commissural axon guidance. Moreover, biochemical analysis revealed that PlexinA1 binds Slit full-length and Slit-Cs, but not Slit-Ns, and mediates a repulsive action of the Slit-C fragments.Citation15 Slit processing also occurs in invertebrates. However, in transgenic rescue assays in drosophila, expression of a Slit mutant that resists proteolytic cleavage in the midline glia lineage was shown to rescue midline crossing defects resulting from general Slit loss equally as well as the wild type Slit, thus supporting that Slit processing is dispensable for midline navigation.Citation13
PlexinA1 was identified in previous work as the Plexin A member which associates with Neuropilin2 (Nrp2). Nrp2/PlexinA1 complex forms a functional commissural receptor for Sema3B, a semaphorin that was demonstrated to act as a FP repellent for post-crossing commissural axons in the mouse.Citation34,38 Mouse embryos lacking Nrp2 were shown to present FP crossing defects consistent with a role of semaphorin ligands as repellents for commissural axons after midline crossing. Among the class3 semaphorins, Sema3B expressed by FP cells, exhibits the expected profile for exerting this role. In contrast, Sema3F, another prominent Nrp2 ligand, instead of being expressed by the FP, localizes in a domain adjacent to the FP.Citation34,38 Consistently, Sema3B deletion was reported to result in alterations of FP crossing and these defects were phenocopied in PlexinA1 null mutant embryos.Citation34 Implication of semaphorins in the FP navigation of spinal commissural axons also came from studies in the chick model. Over-expression of a dominant negative PlexinA, abolishing the signaling by all PlexinAs, resulted in strong alterations of crossing and post-crossing axon trajectories.Citation15,34 Similarly, specific knock-down of individual PlexinAs such as PlexinA1, PlexinA2 and PlexinA4, all induced stalling at the FP exit and failure of rostral turning.Citation1
Altogether, these studies suggest that several repulsive signaling mechanisms operate during FP crossing in the mouse, such as Slit-N/Robo, Slit-C/PlexinA1 and Sema3B/PlexinA1-Nrp2 (). How these signals are acting and which specific aspects of commissural growth cone behavior they control remains unclear. Mouse models allowing the investigation of the specific contributions of Slit-Cs and Slit-Ns still lack, in part because the nature of the protease(s) responsible for Slit cleavage remains unknown.
Silencing of the semaphorin signaling during FP navigation
Based on a variety of experimental set-ups, several studies provided evidence that the commissural axon responsiveness to Slits and Sema3B-derived FP repellents is silenced before the crossing. In early work, spinal cord open books were co-cultured with COS cell aggregates secreting Slits or Sema3B.Citation47 The behavior of commissural axons emerging from the explant border facing the cell aggregates was examined. In these spinal cord preparations, the endogenous FP was removed to mimic a pre-crossing context, so that commissural axons exiting the ventral side of the open-book had not experienced FP crossing in their native tissue before growing out of the explant. Under these conditions, similar growth toward control, Sema3B or Slit2-secreting cell aggregates was observed, indicating a lack of sensitivity of commissural axons to Sema3B and Slit2. These experiments were reproduced for Sema3B and the data were confirmed in more recent worksCitation10,15,38 ().
Figure 2. Experimental paradigms to assess commissural axon sensitivity to FP repellents. (A) Spinal cord open-book co-cultured with control (ctrl) COS cells or COS cells secreting Sema3B or Slit2. In the absence of endogenous FP, comparable axonal growth is observed toward ctrl and Sema3B or Slit2-secreting aggregates. (B) Ectopic graft of COS cells along the lateral side of the spinal cord open-book. Netrin1-secreting aggregate induces a re-routing of pre-crossing commissural fibers toward the ectopic cells. Sema3B and Slit2-secreting ectopic aggregates do not deflect pre-crossing commissural axons. Lateral grafting of roof-plate tissue (RP) re-routes commissural axons away from the ectopic tissue. (C) Collapse assay on dissociated neurons dissected from the dorsal spinal cord and grown in cultures. Growth cones responsive to repulsive cues collapse after short-term application of the cues, whereas unresponsive growth cones remain intact. (D) In the presence of the endogenous FP in the open-book, the growth of commissural axons emerging from the tissue toward the Sema3B or Slit2 sources is inhibited.
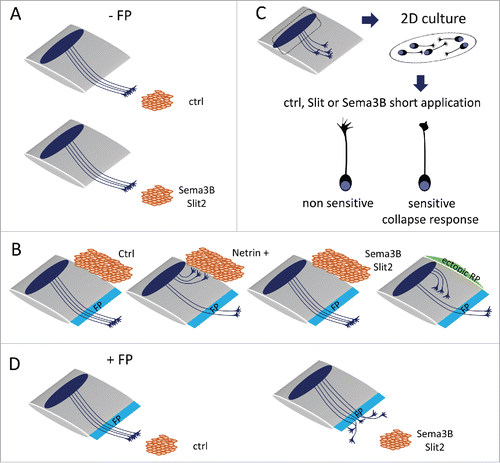
A second assay was used to demonstrate the lack of sensitivity to FP repellents at the pre-crossing stage which consisted in grafting FP and roof plate (RP) tissues as well as cell aggregates along the lateral side of an intact spinal cord. This assay challenges the ability of cues released by the ectopic tissue or the cell aggregate to re-route the trajectory of commissural axons toward or away from their natural path to the endogenous FP.Citation47 Such a model had been developed to show that the RP releases a repellent for pre-crossing commissural axons, whose action is to orient their initial trajectory toward the ventral side of the spinal cord.Citation3 Using this assay, commissural axons were found re-routed toward FP tissue, and Netrin1 expressing cell aggregates, reflecting that they are subjected to attractive cues. In contrast to ectopic RP, both Slit2 and Sema3B-secreting cell aggregates failed to deflect commissural axons away, confirming their lack of sensitivity to the FP repellents prior to crossing ().
Finally, a last paradigm was employed in several studies, to assess the individual response of commissural axons to Sema3B.Citation15,34,43 Growth cone behavior to Sema3B application was examined in dissociated commissural neuron cultures collected from E11 to E13, which revealed their inability to undergo the collapse response, normally observed when growth cones perceive repulsive cues (). Collapse assays were also conducted on dorsal spinal cords (lacking endogenous FP) collected from Atoh1-tauGFP and NeuroG2-tauGFP, transgenic mouse embryos, GFP identifying the dl1 and dl4 populations of commissural interneurons respectively. Both populations were found unresponsive to Sema3B.Citation43
Activation of the semaphorin signaling after midline crossing
Spinal cord open-books and commissural neuron cultures were used to establish that commissural axons acquire sensitivity to the FP repellents after the crossing. First, the behavior of commissural axons emerging in front of a Sema3B or Slit-secreting cell aggregate was examined in open-books in which the endogenous FP was left intact. In contrast to what was observed when the FP had been removed, the growth of commissural axons that experienced FP crossing was strongly prevented by Slit2 and Sema3B released from the cell aggregateCitation47 (). Second in collapse assays, commissural growth cones insensitive to Sema3B at basal condition acquired a strong collapse response induced by application of FP conditioned medium (FPcm). The sensitization was obtained in condition of co-application but also when FPcm was applied first and Sema3B treatment was applied after washing. This indicated that some FP signals prime commissural growth cones for Sema3B repulsion.Citation10,34 In collapse assays conducted on intact open-books from Atoh1-tauGFP and Neurog-tauGFP transgenic mouse embryos (having endogenous FP through which the axons navigate), dl1 but not dl4 commissural growth cones were reported to collapse in response to Sema3B. Thus, some cell-type specificities might exist in the responsiveness of post-crossing commissural axons to the FP repellents.Citation43
Mechanisms mediating pre-crossing silencing of repulsive signaling
The control of the sensitivity of commissural axons to midline repellents has been the topic of extensive investigations over the years, and pioneered by studies of midline crossing in the drosophila ventral nerve cord. This work uncovered key molecular mechanisms acting within commissural neurons to control guidance receptor trafficking and consequently responsiveness to midline repellents. Thereafter, in vertebrates, a panel of molecular mechanisms which regulate guidance receptors has been discovered, showing that, similar to the control of midline crossing in drosophila, strict control of receptor distribution and function is required for setting the temporal sequence of responsiveness to midline repellents.Citation36
Silencing by receptor degradation
In drosophila, the silencing of pre-crossing commissural axons to Slit repulsion was found to be achieved by active degradation of Robo receptors. The endosomal protein Comm plays a key role in preventing the presence of Robo at the growth cone surface, acting essentially but not exclusively by sorting the receptor to lysosomal degradation. After crossing, Comm is down-regulated, and as a consequence, Robos become available in commissural growth cones to transduce the Slit repulsive signal.Citation36 A key aspect of this regulatory pathway thus resides in the spatio-temporal control of Comm expression to restrict its action to the period of axon crossing. It was recently discovered that cleavage of Frazzled/DCC in commissural neurons, releases an intracellular fragment that acts as a transcriptional activator of Comm.Citation36
Silencing by receptor-receptor trans interactions
A second mechanism has been recently reported, which complements the Comm action by blocking the Robo receptors that start reaching the commissural growth cone surface before the crossing is completed. Such a situation is likely to occur, during crossing, after the onset of Comm down-regulation. This mechanism is mediated by trans interactions of this cell surface pool of Robo with Robo2 expressed by midline glial cells, which result in preventing Slit binding or activity. Such coupled mechanisms might ensure the robustness of the spatial and temporal control of Robo receptor availability during midline crossingCitation19 ().
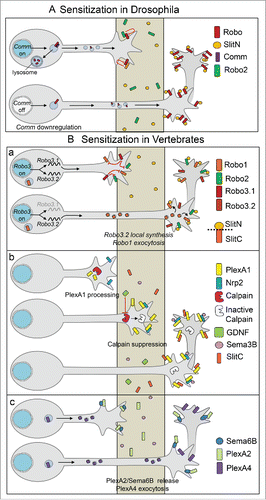
Figure 3. Mechanisms reported to mediate pre-crossing silencing to midline repellents. (A) In drosophila, endosomal protein Comm silences Slit responsiveness in pre-crossing axons, by sorting the majority of Robo receptors to the lysosomal degradation. Robos that escape the degradation and reach the growth cone surface are inhibited via a trans-interaction by Robo2 expressed by midline glial cells. (B) (a) In the mouse, the isoform Robo3.1 of the Robo3 gene is expressed in the pre-crossing axons and the resulting protein antagonizes Slit-Robo signaling. (b) In the chick, Robo1 is trafficked to vesicles to maintain it at low levels at the pre-crossing stage. (c) In the mouse, pre-crossing commissural axons express Nrp2 at their surface but only low levels of PlexinA1, whose cell surface expression is prevented through processing by Calpain proteases. (d) In the chick, PlexinA2 and Sema6B form cis complex in pre-crossing commissural axons and PlexinA4 traffics in vesicles and is excluded from the growth cones.
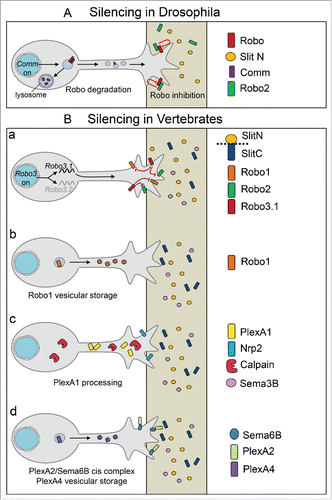
Notably, although the pre-crossing silencing of the midline repellents is conserved in vertebrates, the underlying mechanisms appears to differ sharply from those identified in the fly. First, the vertebrate genome was found to lack Comm. Second, active degradation of Robo receptors at the pre-crossing stage has not been reported yet. However, in the chick embryo, Robo1 was reported to distribute in intracellular vesicles, which might be the mechanism that maintains cell surface Robo at low levels before the crossingCitation39 (). In addition, the silencing of Slit signaling was proposed to be achieved by Robo3, a divergent Robo family member having several binding partners including DCC and neural epidermal growth factor-like-like 2 (NELL2).Citation28,46 An isoform of Robo3 gene, Robo3.1 was reported to have a distribution restricted to the pre-crossing commissural axons. In vivo manipulations in chick and mouse embryos resulted in alterations of FP navigation, consistent with a function in blocking Robo/Slit activity before the crossing.Citation11 How the silencing of Slit-Robo signaling by Robo3.1 is achieved remains to be understood.
Silencing by prevention of receptor cell surface sorting
Although distinct from degradation, the sensitivity of spinal commissural axons to Sema3B also appears to be controlled through regulation of the Sema3B receptor. The signaling moiety of the complex, PlexinA1, was found to be a target of calpains, proteases known to process rather than degrade targets, capable of modulating both their functions and binding partner interactions.Citation8,45 Active calpain was shown to cleave PlexinA1, as well as other PlexinAs, generating 2 distinct PlexinA1 fragments. Both PlexinA1 integral and cleaved fragments could be detected by immunoblotting of lysates of dorsal spinal cord tissue. Treatment of fresh dorsal spinal cord tissue with calpain inhibitor prior to immunoblotting induced an increase of full-length PlexinA1 at the expense of the cleaved forms.Citation34 In the developing spinal cord, Nrp2 and PlexinA1 transcripts are both detected in commissural neurons at stages of pre-crossing navigation. Nevertheless, at protein levels, differences between the 2 receptor distributions were observed in embryonic immunolabelled sections. Using an anti-PlexinA1 antibody directed against an extracellular epitope and likely recognizing the integral protein, very low labeling was found in pre-crossing axon segments in stark contrast labeling was very strong on crossing and post-crossing axon segments.Citation34 Interestingly, Nrp2 was detected in both pre-crossing and post crossing commissural axon segments. Ex vivo, immunolabeling of PlexinA1 in DCC+ commissural axons emerging from spinal cord open-books with and without endogenous FP revealed that integral PlexinA1 labeling was only observed in commissural axons that experienced FP crossing. Finally, in vivo experiments consisting of expressing PlexinA1 fused to the pH sensitive GFP pHLuo, a selective reporter of the cell surface protein pool, in commissural neurons of the chick embryo showed that in a very large majority of the cases, the green fluorescence was detected in commissural growth cones undergoing FP crossing.Citation34 The link between calpain-mediated PlexinA1 processing and commissural axon sensitivity to Sema3B was further investigated using in vitro and in vivo approaches. Inhibiting calpains was sufficient to confer a growth cone collapse response of commissural neurons to Sema3B. Administration of a pharmacological calpain inhibitor to pregnant mice resulted in strong alteration of FP crossing in the embryos. Commissural axons stalled at the FP entry, consistent with the acquisition of a premature sensitivity to Sema3B preventing them from entering the FP due to increased PlexinA1 cell surface levels. Similarly, overexpression of PlexinA1 leading to increased PlexinA1 levels at the pre-crossing stage also resulted in stalling at the FP entry.Citation34 Thus, post-translational regulation of PlexinA1 levels appears to be a first mechanism to prevent pre-crossing commissural axons from responding to Sema3B ().
Silencing by ligand-receptor cis and trans complex
A variety of additional mechanisms have been characterized to control the semaphorin signaling during midline crossing. First, macro-complexes of receptors were shown to orchestrate midline crossing in the optic chiasm of vertebrates. NrCAM, which participates in the semaphorin signaling, and PlexinA1 from midline glia cells were found to interact with NrCAM and PlexinA1 from retinal commissural axons, temporarily switching repulsive effects of Sema6B at the midline into attraction, to allow the crossing.Citation31
Second in the chick embryo, a recent study investigated the PlexinA/Semaphorin signaling during spinal commissural axon guidance.Citation1 PlexinAs were noted to have dynamic spatio-temporal expression patterns, with some members being expressed by commissural axons and FP cells such as PlexinA1 and PlexinA2, and others expressed only by commissural axons such as PlexinA4. Knock-down of individual Plexin A1,-A2, and -A4 in commissural neurons were all found to result in commissural axon stalling at the FP exit and failure of post-crossing rostral turning. Specific knockdown of FP-PlexinA2 also resulted in stalling, as did so the specific knock-down of Sema6B, which is endogenously expressed in commissural neurons. Thus, this identified a first signaling for crossing and post-crossing commissural axons arising from Sema6B acting as a commissural receptor for FP-PlexinA2, acting non-cell autonomously as a ligand.Citation1 In addition, PlexinA2 over-expression in pre-crossing commissural neurons strongly altered their ability to reach the FP, a phenotype that was interpreted as resulting from oversensitivity to ventral spinal cord/FP repellents. The observation in cultured commissural neurons that Sema6B co-localized with PlexinA2 led the authors to propose a model whereby prior to the crossing, the Sema6B/PlexinA2 cis complex prevents commissural axons from sensing the FP repellents. The mechanism that silences the PlexinA4/Semaphorin signaling might be different. In cultured commissural neurons, while PlexinA2 was detected along axon shafts and growth cones together with Sema6B, PlexinA4 was reported to have in contrast a vesicular punctate pattern, and was excluded from the growth cones.Citation1
Overall, these mechanisms illustrate in various contexts that the silencing of semaphorin repellents is achieved through cell autonomous mechanisms desensitizing pre-crossing commissural axons, by controlling the cell surface sorting or the signaling activity of PlexinAs ().
Silencing by ligand trapping
Recently, long-term (24h) application of Sema3B to dorsal spinal cord explant cultures was reported to result in reduced axon growth, with strong inhibition at high dose.Citation23 These data suggested that commissural axons at the pre-crossing stage might be able to perceive Sema3B. Indeed, since several previous studies failed to detect any Sema3B repulsion and collapse on pre-crossing commissural axons using a panoply of different paradigmsCitation15,34,38,43,47 it could be that Sema3B exerts 2 distinct and independent effects on commissural axons, acting as a growth regulator at the pre-crossing stage and a repulsive cue at the post-crossing stage.
How the growth-inhibition effect is achieved and whether it is mediated by PlexinA1, as suggested by the authors, remains to be determined. It could be that the pre-crossing sensitivity of commissural axons to Sema3B reported by the authors is mediated by other PlexinAs, several being expressed by commissural growth conesCitation34 This would be consistent with previous findings that the repulsive post-crossing response is conferred by a dual mechanism which first prevents PlexinA1 to be available at the growth cone surface before the crossing and second triggers cell surface expression when commissural axons navigate the FP. It could also be that low levels of PlexinA1 present in pre-crossing commissural growth cones are sufficient for Sema3B to elicit a long-term growth response, but not to produce a repulsive effect. The implication of PlexinA1 was suggested by immunohistochemistry with home-made antibody directed against an C-terminal epitope of PlexinA1, which was observed to label pre-crossing commissural axons,Citation23 while a commercial antibody directed against the extracellular PlexinA1 domain only revealed substantial PlexinA1 levels in crossing and post-crossing axons.Citation10 Since PlexinA1 cleaved fragments were found present in lysates from dorsal commissural tissue,Citation34 it could be that these PlexinA1 forms are those recognized in the pre-crossing commissural axons. Whatever the case, PlexinA1 contribution could be addressed by blocking PlexinA1 in commissural explants to examine whether long term Sema3B exposure still elicits growth-inhibition.
Beyond, is this growth inhibition acting at the pre-crossing stage or is it silenced as is Sema3B repulsion? In their study, the authors reported that deletion of Nrp2 in the FP affects the commissure formation. Measures of the ventral part of the pre-crossing tract and the crossing tract in embryonic transverse sections showed reduced thickness in the mutants compared with wild-types, suggesting that FP-Nrp2 has a non-cell autonomous role. Whether this defect results from loss of fibers, growth delay or increased fasciculation remains to be determined. The reduction of commissure thickness was no longer observed when FP-Nrp2 deletion was combined with a general loss of PlexinA1. In the scenario proposed by the authors, Nrp2 would trap Sema3B in the FP, thus silencing Sema3B by making it inaccessible to commissural axons. FP-Nrp2 deletion would then result in Sema3B release, inducing pre-crossing growth inhibitory effect and subsequently reduction of the ventral commissure (). Additional ablation of PlexinA1 would then induce pre-crossing commissural axons to loose their sensitivity to Sema3B, thus rescuing the normal size of the commissure.
Figure 4. Models. for the regulation of the semaphorin signaling from pre-crossing to post-crossing. (A) In this model, PlexinA1 and Nrp2 are both expressed at the growth cone surface of pre-crossing commissural axons. Their sensitivity to Sema3B is prevented by trapping of Sema3B by FP-Nrp2. After the crossing, Nrp2 is transcriptionnaly downregulated in the FP at E13, which releases Sema3B and allows repulsion. (B) In this model, cell surface PlexinA1 is kept at low levels in pre-crossing commissural axons, to desensitize them to Sema3B. Upon crossing, calpain activity is suppressed by FP GDNF, PlexinA1 reaches the growth cone surface and can associate with Nrp2. The receptor complex activity is blocked by FP Nrp2 and PlexinA1, until the crossing is achieved. After the crossing, the complex is functional for Sema3B repulsion.
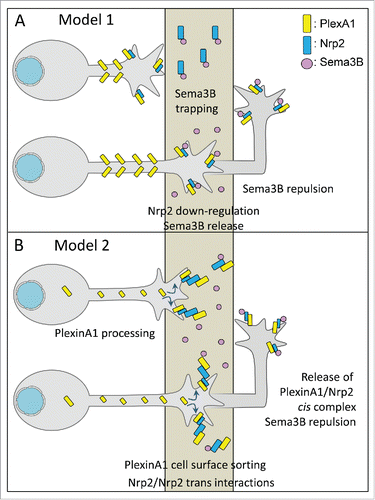
According to this model, Sema3B-mediated growth inhibition would not play an instructive role at the pre-crossing stage and needs to be suppressed. The transition toward sensitivity to Sema3B would not be triggered by changing of Sema3B responsiveness between the pre-crossing and the post-crossing stages. Rather it would be achieved through unmasking of Sema3B after the crossing, proposed by the authors to result from downregulation of Nrp2 transcripts. Nevertheless, Nrp2 is detected in the FP over the entire period of FP navigation (from E10 to E13.5 on the least;Citation7,19,34 Hernandez-Enriques et al, 2015), during which asynchronous waves of commissural axons navigate the FP. Thus without any changing between pre-crossing and post-crossing stages, it is difficult to understand how this sole mechanism would be responsible for switching on Sema3B repulsion. Moreover, interpretations of the mouse phenotypes are particularly complex. Indeed, first, not only Nrp2 but also PlexinA1 is expressed by both commissural axons and FP glial cells. Second as shown in the chick embryo for PlexinA2,Citation1 FP-PlexinA1 could have non cell autonomous functions. Thus, a key experiment would be to test which of commissural- or FP-specific PlexinA1 deletion rescues the ventral commissure thickness in context of FP-Nrp2 deletion.
An alternative scenario can be proposed, which would fully mirror the mechanisms of Slit silencing by Robo/Robo2 interactions in the drosophila context.Citation19 Rather than trapping Sema3B, FP-Nrp2 (complexed or not with PlexinA1) could interact in trans with Nrp2 on commissural axons approaching and entering the FP. This receptor trapping would silence Sema3B responsiveness until the crossing is accomplished by preventing axonal Nrp2 from forming cis complexes with the PlexinA1 receptor pool accumulating at the cell surface (). FP-Nrp2 ablation would prevent this effect, resulting in premature action of Sema3B. Loss of PlexinA1 would desensitize commissural axons to Sema3B, thus rescuing the commissure. In this scenario, both cell autonomous (axonal PlexinA1 processing) and non-cell autonomous (via FP-Nrp2 and possibility FP-PlexinA1) would act in synergy to accurately control the silencing of pre-crossing/crossing commissural axon responsiveness to Sema3B.
Finally, the outcome of Sema3B trapping by Nrp2 might not be to mask Sema3B but rather to control its spatial distribution, restricting the cue to the FP, where it could be active to slow down commissural axon growth. Previous work already established that cues released by the FP regulate the outgrowth of commissural axons. Such a property was reported for the Stem Cell Factor (SCF), which promotes the growth of post-crossing commissural axons.Citation21 Thus a balance of growth-promoting and growth-inhibitory effects could set a precise temporal pattern of FP navigation, adapting growth cone motility to the guidance decisions that have to be made.
Mechanisms controlling the transition from pre-crossing silencing to post-crossing sensitization to Slit and Sema3B repellents
The mechanisms controlling the switch of sensitivity to midline repellents after the crossing also appear to be highly diverse, depending on the signaling and the species (). In drosophila, down-regulation of COMM allows Robo to accumulate at the cell surface of commissural growth cones, resulting in the gain of sensitivity to Slit.Citation20 Studies conducted in the chick model revealed that Robo1 cell surface expression is up-regulated between the pre-crossing and post-crossing navigation, through transcriptional control of RabGDI, a key component of the exocytosis machinery.Citation39 Although different, these 2 mechanisms have in common that they control the temporal activity of Slit-Robo signaling at the midline by regulating guidance receptor cell surface levels. In the mouse, transition from Robo3.1 at the pre-crossing stage to Robo3.2 at the post-crossing stage was proposed to switch on the sensitivity to Slit repellents.Citation11 How this transition is accomplished has been partially resolved by the findings that Robo3.2 mRNA is locally translated in crossing commissural axons, under the action of FP signals.Citation12
Figure 5. Mechanisms releasing the pre-crossing silencing and mediating sensitization to midline repellents. (A) In drosophila, after the crossing, Comm protein is down-regulated and Robo proteins are sorted at the growth cone surface to transduce the Slit repulsive signal. (B) (a) In the mouse, upon crossing, Robo3.1 is replaced by Robo3.2, locally synthesized under local FP triggers, which acts as an agonist of the Slit-Robo signaling. In the chick, exocytosis of Robo1 is activated by transcriptional up-regulation of RABgdi, and the receptor is sorted at the cell surface. (b) In the mouse, upon the crossing, GDNF secreted by FP glial cells inhibits Calpain activity and allows PlexinA1 to reach the surface, to associate with Nrp2 and to mediate Sema3B repulsive response. Gain of cell surface PlexinA1 also switches on repulsion by Slit-C fragments. (c) In the chick, the pre-crossing PlexinA2/Sema6B cis complex is released, and replaced by a Sema6B/PlexinA2 trans interaction, releasing commissural PlexinA2 which become available for semaphorin repulsion. PlexinA4 might be sorted at the cell surface to also mediate semaphorin repulsion.
The release of Sema3B silencing has been investigated in the mouse model, with the goal to identify cues present in the FP conditioned medium which conferred a collapse response of commissural growth cones to Sema3B. Two FP cues were identified acting in synergy, the Ig SuperFamily Cell Adhesion Molecule NrCAM, probably released by ectodomain shedding, and the neurotrophic factor GDNF, which was found to provide the major contribution.Citation10 Double GDNF/NrCAM deletion in mice resulted in strong alteration of PlexinA1 levels in crossing/post-crossing commissural axons, with synergistic effects compared with the single knockouts. Reductions of PlexinA1 levels were also correlated with FP crossing defects. In co-cultures of dorsal spinal cord explants with COS cell aggregates, commissural axons were found to gain repulsion to Sema3B when the cell aggregate secreted both Sema3B and GDNF, compared to aggregate only secreting Sema3B. Both GDNF and NrCAM increased PlexinA1 levels in the growth cones of cultured commissural neurons. GDNF acting independently from RET via the NCAM receptor and GFRα1, both expressed by commissural axons, was able to inhibit calpain activity, switching off the mechanism ensuring Sema3B silencing. An additional FP cue, SHH, was reported to trigger gain of sensitivity to Sema3B, by down-regulating the activity of cAMP-dependent protein kinase A (PKA) in commissural growth cones.Citation38 Thus overall, these studies support that release from the pre-crossing Sema3B silencing is triggered by FP cues, through changes of the guidance machinery of commissural axons.Citation10,34
In the chick, the model proposed is that the PlexinA2/Sema6B cis complex formed in pre-crossing commissural axons would be released, allowing FP-PlexinA2 to bind to Sema6B in trans, and commissural PlexinA2 to bind to FP semaphorin repellents. In addition, as is the case for PlexinA1 in the mouse, PlexinA4 might be sorted to the growth cones during FP crossing to allow them to sense the semaphorin repellents.Citation1
Thus in conclusion, given the diversity of possibilities by which the semaphorin signaling can be modulated, significant issues remain unclear. In particular, it will be important to better characterize the dynamics of Plexins, Neuropilin receptors and their semaphorin ligands as well as their cell-autonomous and non-cell autonomous functions. Addressing these questions is required to obtain a clear picture of how the silencing of midline repellents is achieved and released during spinal commissural axon navigation of the FP.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Pr. Edmund Derrington, Dr. Servane Tauszig-delamasure and Dr. Céline Delloye-Bourgeois for proofreading the manuscript and helpful comments.
Funding
VC is supported by funding from the LABEX CORTEX and Labex DevWeCAN of Université de Lyon, within the program “Investissements d'Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
References
- Andermatt I, Wilson NH, Bergmann T, Mauti O, Gesemann M, Sockanathan S, Stoecssli ET. Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance. Development 2014; 141(19):3709-20; PMID:25209245; http://dx.doi.org/10.1242/dev.112185
- Arbeille E, Reynaud F, Sanyas I, Bozon M, Kindbeiter K, Causeret F, Pierani A, Falk J, Moret F, Castellani V. Cerebrospinal fluid-derived Semaphorin3B orients neuroepithelial cell divisions in the apicobasal axis. Nat Commun 2015; 6:6366; PMID:25721514; http://dx.doi.org/10.1038/ncomms7366
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron 1999; 24(1):127-41; PMID:10677032; http://dx.doi.org/10.1016/S0896-6273(00)80827-2
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron 2001; 30(2):411-22; PMID:11395003; http://dx.doi.org/10.1016/S0896-6273(01)00305-1
- Bossing T, Brand AH. Determination of cell fate along the anteroposterior axis of the Drosophila ventral midline. Development 2006; 133(6):1001-12; PMID:16467357; http://dx.doi.org/10.1242/dev.02288
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999; 96(6):795-806; PMID:10102268; http://dx.doi.org/10.1016/S0092-8674(00)80590-5
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 2001; 128(16):3071-80; PMID:11688557
- Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol 2002; 34(12):1539-43; PMID:12379276; http://dx.doi.org/10.1016/S1357-2725(02)00069-9
- Castellani V. Building spinal and brain commissures: axon guidance at the midline ISRN Cell Biology. 2013; 315387
- Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, Falk J, Helmbacher F, Kindbeiter K, Castellani V. gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron 2012; 75:1051-66; PMID:22998873; http://dx.doi.org/10.1016/j.neuron.2012.08.021
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 2008; 58(3):325-32; PMID:18466743; http://dx.doi.org/10.1016/j.neuron.2008.02.016
- Colak D, Ji SJ, Porse BT, Jaffrey SR. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 2013; 153(6):1252-65; PMID:23746841; http://dx.doi.org/10.1016/j.cell.2013.04.056
- Coleman HA, Labrador JP, Chance RK, Bashaw GJ. The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline (2010). Development 2010; 137(14):2417-26; PMID:20570941; http://dx.doi.org/10.1242/dev.047993
- Delloye-Bourgeois C, Jacquier A, Falk J, Castellani V. Use of pHluorin to assess the dynamics of axon guidance receptors in cell culture and in the chick embryo. J Vis Exp 2014; 12(83):e50883
- Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci 2015; 18(1):36-45. Epub 2014; PMID:25485759; http://dx.doi.org/10.1038/nn.3893
- Drabkin H, Nasarre P, Gemmill R. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. OncoTargets Ther 2014; 7:1663-87; http://dx.doi.org/10.2147/OTT.S37744
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic hedgehog signaling required for the specification of motorneuron identity. Cell 1996; 87:661-73; PMID:8929535; http://dx.doi.org/10.1016/S0092-8674(00)81386-0
- Erskine L, Herrera E. Connecting the retina to the brain. ASN Neuro 2014; 6(6). pii: 1759091414562107 Print 2014; PMID:25504540; http://dx.doi.org/10.1177/1759091414-562107
- Evans TA, Santiago C, Arbeille E, Bashaw GJ. Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. Elife 2015; 4:e08407; PMID:26-186-094
- Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol 2010; 20:79-85; PMID:20074930; http://dx.doi.org/10.1016/j.conb.2009.12.006
- Gore BB, Wong KG, Tessier-Lavigne M. Stem cell factor functions as an outgrowth-promoting factor to enable axon exit from the midline intermediate target. Neuron 2008; 57(4):501-10; PMID:18304480; http://dx.doi.org/10.1016/j.neuron.2008.01.006
- Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development 2005; 132(12):2709-19; PMID:15901662; http://dx.doi.org/10.1242/dev.01859
- Hernandez-Enriquez B, Wu Z, Martinez E, Olsen O, Kaprielian Z, Maness PF, Yoshida Y, Tessier-Lavigne M, Tran TS. Floor plate-derived neuropilin-2 functions as a secreted semaphorin sink to facilitate commissural axon midline crossing. Genes Dev 2015; 29(24):2617-32; PMID:26680304
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 1995; 15(1):35-44; PMID:7619528; http://dx.doi.org/10.1016/0896-6273(95)90062-4
- Imondi R, Kaprielian Z. Commissural axon pathfinding on the contralateral side of the floor plate: a role for B-class ephrins in specifying the dorsoventral position of longitudinally projecting commissural axons. Development 2001; 128(23):4859-71; PMID:11731465
- Imondi R, Jevince AR, Helms AW, Johnson JE, Kaprielian Z. Mis-expression of L1 on pre-crossing spinal commissural axons disrupts pathfinding at the ventral midline. Mol Cell Neurosci 2007; 36(4):462-71. Epub 2007; PMID:17884558; http://dx.doi.org/10.1016/j.mcn.2007.08.003
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci 2010; 30(28):9445-53; PMID:20631173; http://dx.doi.org/10.1523/JNEUROS-CI.6290-09.2010
- Jaworski A, Tom I, Tong RK, Gildea HK, Koch AW, Gonzalez LC, Tessier-Lavigne M. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science 2015; 350(6263):961-5; PMID:26586761; http://dx.doi.org/10.1126/science.aad2615
- Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development. Development 2014; 141:3292-7; PMID:25139851; http://dx.doi.org/10.1242/dev.105544
- Kadison SR, Kaprielian Z. Diversity of contralateral commissural projections in the embryonic rodent spinal cord. J Comp Neurol 2004; 472(4):411-22; PMID:15065116; http://dx.doi.org/10.1002/cne.20086
- Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, Mason C. optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 2012; 74:676-90; PMID:22632726; http://dx.doi.org/10.1016/j.neuron.2012.03.025
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol 2005; 6(10):789-800; PMID:16314868; http://dx.doi.org/10.1038/nrm1740
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 2004; 42(2):213-23; PMID:15091338; http://dx.doi.org/10.1016/S0896-6273(04)00179-5
- Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev 2010; 24:396-410; PMID:20159958; http://dx.doi.org/10.1101/gad.542510
- Nawabi H, Castellani V. Axonal commissures in the central nervous system: how to cross the midline? Cell Mol Life Sci 2011; 68:2539-53; PMID:21538161; http://dx.doi.org/10.1007/s00018-011-0691-9
- Neuhaus-Follini A, Bashaw GJ. Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target. Wiley Interdiscip Rev Dev Biol 2015; 4(4):377-89. Epub 2015
- O'Malley AM, Shanley DK, Kelly AT, Barry DS. Towards an understanding of semaphorin signalling in the spinal cord. Gene 2014; 553(2):69-74; PMID:25300255; http://dx.doi.org/10.1016/j.gene.2014.10.005
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci 2010; 13(1):29-35; PMID:19946319; http://dx.doi.org/10.1038/nn.2457
- Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev 2012; 7:36; PMID:23140504; http://dx.doi.org/10.1186/1749-8104-7-36
- Sabag AD, Smolkin T, Mumblat Y, Ueffing M, Kessler O, Gloeckner CJ, Neufeld G. The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction. J Cell Sci 2014; 127(Pt 24):5240-52. Epub 2014; PMID:25335892; http://dx.doi.org/10.1242/jcs.155960
- Sakai N, Kaprielian Z. Guidance of longitudinally projecting axons in the developing central nervous system. Front Mol Neurosci 2012; 5:59; PMID:22586366; http://dx.doi.org/10.3389/fnmol.2012.00059
- Suárez R, Gobius I, Richards LJ. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci 2014; 8:497
- Tran TS, Carlin E, Lin R, Martinez E, Johnson JE, Kaprielian Z. Neuropilin2 regulates the guidance of post-crossing spinal commissural axons in a subtype-specific manner. Neural Dev 2013; 8:15; PMID:23902858; http://dx.doi.org/10.1186/1749-8104-8-15
- Wilson SI, Shafer B, Lee KJ, Dodd J. A molecular program for contralateral trajectory: Rig-1 control by LIM homeodomain transcription factors. Neuron 2008; 59(3):413-24; PMID:18701067; http://dx.doi.org/10.1016/j.neuron.2008.07.020
- Wu HY, Lynch DR. Calpain and synaptic function. Mol Neurobiol 2006; 33(3):215-36; PMID:16954597; http://dx.doi.org/10.1385/MN:33:3:215
- Zelina P, Blockus H, Zagar Y, Péres A, Friocourt F, Wu Z, Rama N, Fouquet C, Hohenester E, Tessier-Lavigne M, et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 2014; 84(6):1258-72; PMID:25433640; http://dx.doi.org/10.1016/j.neuron.2014.11.004
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 2000; 102(3):363-75; PMID:10975526; http://dx.doi.org/10.1016/S0092-8674(00)00041-6
