ABSTRACT
During the last 10 years, exosomes, which are small vesicles of 50–200 nm diameter of endosomal origin, have aroused a great interest in the scientific and clinical community for their roles in intercellular communication in almost all physiological and pathological processes. Most cells can potentially release these nanovesicles that share with the parent cell a similar lipid bilayer with transmembrane proteins and a panel of enclosed soluble proteins such as heat shock proteins and genetic material, thus acting as potential nanoshuttles of biomarkers. Exosomes surface proteins allow their targeting and capture by recipient cells, while the exosomes' content can modify the physiological state of recipient cells. Tumor derived exosomes by interacting with other cells of the tumor microenvironment modulate tumor progression, angiogenic switch, metastasis, and immune escape. Targeting tumor-derived exosomes might be an interesting approach in cancer therapy. Furthermore, because a key issue to improve cancer patients' outcome relies on earlier cancer diagnosis (metastases, as opposed to the primary tumor, are responsible for most cancer deaths) exosomes have been put forward as promising biomarker candidates for cancer diagnosis and prognosis. This review summarizes the roles of exosomes in cancer and clinical interest, focusing on the importance of exosomal heat shock proteins (HSP). The challenges of clinical translation of HSP-exosomes as therapeutic targets and biomarkers for early cancer detection are also discussed.
Introduction
For many years, researchers thought that intercellular communications were ensured only by hormones, cytokines or neurotransmitters. However, it is now well established that cells can communicate by means of extracellular vesicles (EVs). EVs are a generic name for all vesicles that are small spherical structures surrounded by a lipid bilayer (of similar structure to that of cell membranes) and that contain hydrophilic soluble components. Through the extracellular vesicles, cells can transfer information from the plasma membrane or internal compartments.Citation1 There are different EVs: (i) directly formed and released from the cells' plasma membrane e.g microparticles,Citation2 microvesicles,Citation3 or ectosomes,Citation4 (ii) with an endocytic origin and release in the extracellular media by exocytosis called exosomes.Citation5 (iii) that present several characteristics of exosomes but differ by certain biophysical properties, i.e. exosomes-like vesicles,Citation6,7 (iv) release by cells in apoptosis and called apoptotic vesicles.Citation8 Recently, a new type of EVs have been described in gastrointestinal stromal tumors called spheresomes.Citation9 All these vesicles types differ in their subcellular origin, their biophysical and/or biochemical properties, their receptors composition, and their content in soluble proteins and genetic material. As they contain some nanoliters of cytosol and expose at the outer space the same proteins than the parental cell, they are also considered as nanosized cells with a functional role in many biological processes. Among the different EVs, exosomes have been particularly studied since they have been shown to play a role in many physiological and pathological processes.Citation10-12 Exosomes are cup-shaped nanovesicles that represent a distinct class of membrane vesicles, with a density of 1.13–1.19 g/ml and a diameter of 50–200 nm. These vesicles form a bioactive cargo since they carry genetic material including DNA, mRNA and miRNA, and numerous proteins, notably heat shock proteins, known to play important roles in immunity and cancer.Citation10,13,14 The exosome, thanks to its a lipid bilayer, act like a nanoshuttle protecting these molecules from their degradation in the extracellular medium. In this review we provide a comprehensive overview of the interest of heat shock proteins contained in exosomes in cancer diagnosis and therapy.
Discovery of exosomes
The term “exosomes” was first used in 1981 by Trams et al. to appoint small vesicles secreted by several cell types in the extracellular media.Citation5 In 1983, Johnstone et Pan discovered with the help of electron microscopy that these vesicles derived from multivesicular bodies (MVBs) and have an endocytic origin.Citation15 At the time, exosomes generated a poor interest since they were considered as a mean to eliminate obsolete proteins.Citation16 But in 1996, Raposo et al. discovered for the first time that these nanovesicles secreted by antigen-presenting cells (APCs) bore functional peptide–MHC complexes.Citation17 This article opened a new field in the study of these interesting nanovesicles. Two years later, it was demonstrated the release of exosomes by dendritic cells (DCs) and the ability of tumor peptide-pulsed DC-derived exosomes to suppress growth tumor in vivo.Citation18 Following pioneer studies showing the potential role of exosomes in the regulation of immune responses, myriad of articles have been published related to the immune function of exosomesCitation10 and their role in cancer.Citation11 Furthermore, in addition to immune cells, many other cell types have been described as exosome secretory cells such as epithelial cells,Citation19 neuronsCitation20 and tumor cells.Citation21
Exosomes can be isolated from cell culture supernatants and can be found in numerous body fluids such as blood,Citation22,23 urine,Citation24 saliva,Citation25 bronchoalveolar fluid,Citation26 seminal fluid,Citation27 amniotic fluid,Citation28 breast milk,Citation29 tumor effusionsCitation30 and cerebrospinal fluid.Citation31
Biogenesis
The biogenesis of the exosome starts with the invagination of the plasma membrane leading to the endosome formation. Endosomes can differentiate in multi-vesicular bodies (MVBs), which are endocytic structures formed by the budding of an endosomal membrane into the lumen of the compartment.Citation32 This leads to the formation of small vesicles called intraluminal vesicles (ILVs), future exosomes. Then, the fusion of these MVBs with the plasma membrane provokes the release of the ILVs in extracellular space, and become exosomes (). Although the biological function of MVBs was interpreted for many years to be a late step in the degradation pathway toward lysosomes, we now know that MVBs have an alternative fate participating in the exocytic fusion of their external membrane with the plasma membrane. This phenomenon allows the excretion of exosomes by exocytosis into the extracellular space. The mechanisms underlying the sorting of the intraluminal vesicles are not yet fully understood, but 2 ways of exosome sorting have been proposed, dependent or independent on Endosomal Sorting Complex Required for Transport (ESCRT) signals.Citation33 This complex consist of 4 soluble protein ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III. ESCRT is involved in the process of membrane invagination to the formation of ILVs and in the selection of proteins integrating these vesicles.Citation34 Concerning ESCRT-independent signals that regulate exosomes secretion we can name the ceramide pathway,Citation35 intracellular Ca2+ levels,Citation36 p53 status,Citation37 Rab protein family,Citation38,39 Syndecan-Syntenin-ALIX proteins,Citation40 high level of heparanaseCitation41 and pH.Citation42
Figure 1. Scheme of exosomes biogenesis, composition and internalization. Biogenesis: the biogenesis of exosomes involves 4 different steps: (1) the membrane invagination; (2) endosome formation; (3) generation of the exosomes precursors, called intraluminal vesicles (ILVs), by inward budding of endosomes. These accumulations of ILVs is termed as multivesicular bodies (MVBs); (4) the fusion of MVBs with the plasma membrane release the ILVs in the extracellular space by exocytosis and become exosomes. Composition: Exosome are composed by different types of enzymes and proteins involved in: adhesion, traffic, intracellular signaling, immunostimulatory molecules, multivesicular body (MVB) formation and heat shock proteins (HSPs). Exosomes contain lipids such as (I) saturated phospholipids (phosphatidyl-ethanolamine, glycero-phospholipids, phosphatidyl-choline and phosphatidyl-serine) (ii) sphingolipids (ceramides), (iii) cholesterol. Finally, exosomes contain nucleic acids, including miRNA, mRNA, DNA and small non coding RNA (snRNA, tRNA). Internalization : The exosome may, (i) elicit transduction of the signal via intracellular signaling pathways by direct contact through adhesion molecules like integrin or through a ligand-receptor interaction, (ii) be endocytosed via phagocytosis, macropinocytosis or receptor-mediated endocytosis, or (iii) fusion with the plasma membrane and transfer its content into the cytoplasm of the recipient cell.
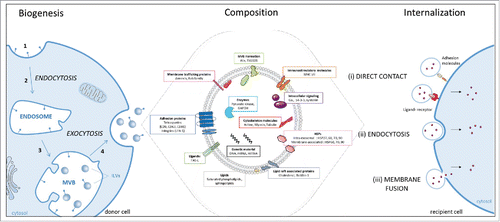
How the exosome penetrates into the recipient cell is still a debated issue. Three mechanisms have been proposed based on indirect evidences and in vitro studies: (i) direct contact between surface molecules of vesicles and cells, (ii) endocytosis of exosomes, and (iii) fusion between the membranes of the cell and the exosome.Citation10 Once the exosome penetrates into the host cell, its content is released in the plasma membrane or in the cytoplasm ().
Composition
The composition of exosomes allows their discrimination from other EVs' family members.Citation43,44 The exosome membrane composition is the same than that of the mother cell but present specific enrichments and they contain proteins, lipid and genetic material (). All exosome components described are listed in Exocarta website. Proteins present in exosomes include adhesion proteins such as tetraspanins (CD9, CD63, CD81) and integrins (LFA-1), immunostimulatory molecules (MHC I/II), cytoskeleton molecules (actin, myosin, tubulin), membrane trafficking proteins (Rab GTPases such as Rab 5 and annexin), proteins involved in MVB formation (ALIX, TSG101), intracellular signaling proteins (Gα, 14-3-3, syntenin), lipid raft associated proteins (flotillin-1), enzymes (pyruvate kinase, GAPDH), certain ligands such as FAS-L. Finally, several HSPs have been retrieved in exosomes lumen (HSP27, HSP60, HSP70, HSP90) and in exosome membrane (HSP70, HSP60 and HSP90). Some of these proteins are specifically enriched in exosomes compared to cell lysate and are classically used as exosome markers (CD9, CD63, CD81, ALIX, TSG101).Citation45 Nevertheless, very recently, Kowal et al. compared the composition of EVs subtypes and revealed that although exosomes are enriched in CD9, CD63 and CD81, only TSG101 allows to distinguish exosomes from other EVs subtypes.Citation46 Finally, numerous studies have revealed that some proteins within the exosomes are dependent on the cell type secreting them while others are independent from the parental cell.Citation47 These different types of proteins become incorporated into exosomes during exosome formation and serve as cargo for cell–cell communication. Besides, protein exosomes are enriched in lipids such as saturated phospholipids (i.e., phosphatidyl-ethanolamin, phosphatidyl-serin, phosphatidyl-choline), sphingolipids (e.g. ceramids), and cholesterol. These lipid compositions confer to exosomes an exceptional rigidity compared to a plasma membrane.Citation48 Additional components are found in exosomes including genetic materials such as mRNA (mRNA), transcripts, microRNA (miRNA), and small non coding RNA (snRNA, tRNA).Citation49
Clinical interest of exosomes in cancer
Researches on exosomes have considerably increased over the past decade. Although different areas of research are interested in exosomes, most scientific publications are related to cancer. Exosomes have been reported to be involved in all stages in cancer development: (i) tumorigenic transformation, (ii) tumor growth, (iii) angiogenesis, (iv) modulation of immune responses, and (v) induction of mechanisms to acquire therapy resistance.Citation50-53 The impact of exosomes in clinical research is demonstrated by the fact that there are already 19 clinical trials ongoing (web site https://clinicaltrials.gov/). Among them, 13 involve the study of exosomes as cancer diagnosis biomarkers whereas the others use the exosomes for cancer therapy purposes. Thus, exosomes has emerged as potential biomarkers and therapeutic targets in cancer.
Exosomes as biomarkers
It is well established that the earlier the cancer is diagnosed, the better the survival rate. Although numerous works have been consecrated to early cancer diagnosis, today there is not yet a reliable detection non-invasive method. The main reasons for this are: first of all, in general there is a poor patients compliance, which make difficult to draw any conclusions from the clinical studies. For example, in France, in 2014, the participation rate for breast cancer screening was only of 52.1%.Citation54 Secondly, actual detection methods, mainly based on medical imaging, have the limitation of tumor detection at an early stage. Finally, certain publications have shown that some imaging approaches can have undesirable side effects and favor the appearance of tumors. For instance, mammography has been related to cancer apparition -about 1 to 20 for 100 000 mammograms.Citation55 For all these reasons, it is necessary to develop more performing diagnosis methods. Exosomes appear to be powerful circulating biomarkers.Citation56-58 These vesicles, reported to be stable and biologically active in human blood plasma up to 3 months, can reveal potential diagnostic information through their examination in body fluids, known as liquid biopsies.Citation59 They are great potential tools for providing noninvasive, sensitive and economically justifiable new diagnosis methods in oncology.Citation60 The main advantage of quantifying tumor-derived exosomes compared to circulating tumor cells (CTCs) is that exosomes are found in large amounts compared to CTCs (e.g : 53.2 ± 1.6 × 108 exosomes per 106 cells in the 24 h period, determined by Nanoparticle Tracking Analysis, Nanosight LM10,Citation61 Furthermore, exosomes can be quantified non-invasively in urines and other human fluids.
During the last years, improvement in some techniques like mass spectrometry has allowed to better study exosome protein content. First, several studies indicate that tumor derived exosomes carry more proteins than healthy donors-derived exosomes, particularly when compared to patients with an advanced stage disease.Citation62,63 In 2012, Peinado's team defined a melanoma-specific exosome signature that included tyrosinase-related protein-2 (TYRP2), very late antigen 4 (VLA-4), heat-shock-protein 70 (HSP70), an HSP90 isoform and the MET oncoprotein.Citation62 Furthermore, TrkB (Tropomyosin receptor kinase B) expression was detected in exosomes isolated from plasma of glioblastoma patients, suggesting that this receptor may be considered also as a new biomarker for glioblastoma diagnosis.Citation64 It was latter on reported that certain proteins were differentially expressed dependently on the melanoma cells from which the exosomes were analyzed, revealing a specific signature for metastatic cell lines.Citation65 In this way, in exosomes from patients with metastatic melanoma, MIA (Melanoma Inhibitory Activity) and S100B can be detected, therefore their quantification presents diagnostic and prognostic utility.Citation66 In 2015, Hoshino et al. revealed that specific integrin expression in exosomes could be used to predict organ-specific metastasis.Citation67 In Non Small Cell Lung Cancer (NSCLC), leucine-rich α-2-glycoprotein (LRG1) was found to be expressed at higher levels in urinary exosomes of NSCLC patients suggesting that LRG1 may be a candidate biomarker for non-invasive diagnosis of NSCLC in urine. (Li et al.,Citation68 1) More recently, it has been determined a combination of several exosomal proteins (CD151, CD171 and tetraspanin 8) that could be used as a promising diagnostic tool of lung cancer independently of its stage and histology.Citation69 In acute myeloid leukemia, TGFβ1 expression seems to be useful to predict response to immunotherapy.Citation70 Finally, in urological malignancies, exosomes in the urine have been described as robust biomarkers and particularly those expressing survivin for early detection of prostate cancer.Citation71 Other proteins have been described as specific of cancer-derived exosomes compared to healthy donors and seem also candidates as cancer diagnosis tools. This is the case for Claudin, which is present only in exosomes derived from the plasma of women with ovarian cancer,Citation72 for Glypican-1 that appears to allow to distinguish an ovarian cancer with high specificity and sensitivityCitation56 or for CD9-CD147 that is embedded in colorectal cancer-derived exosomes.Citation73 It has also been reported that 80 percent of the exosomes isolated from NSCLC samples was positive for surface EGFR (epithelium growth factor receptor) by immune staining compared to only 2% of the exosomes in chronic inflammatory lung tissue.Citation74 Finally, as a general marker of cancer-derived exosomes, our team has recently proposed membrane HSP70 that is present in exosomes released by large panel of cancer cells but not by their normal counterpartsCitation50; see below.
New researches focus on miRNA potential because exosomes offer a miRNA protection from RNases contrary to free circulating miRNA. In 2007, Valadi et al. showed for the first time the transfer of functional miRNAs between 2 cells by means of exosomes.Citation75 miRNAs are a class of 21–25 small non coding but functional RNA that negatively regulates mRNA expression. These small non-coding RNAs plays important roles in cancer,Citation76 explaining why this discovery suggested a new regulatory role for exosomes in cancer. Today, numerous studies have identified different functional exosomal miRNAs and their role in cancer,Citation77,78 and proposed their use as diagnosis biomarkers.Citation79,80,81 For example, in lung cancer 2 miRNAs, miR-21 and miR-155 have been found to be significantly upregulated in recurrent tumors compared to primary tumors.
Exosomes as immunotherapy agents
Despite improvements in treatment and longer survival, cancer stays a principal cause of death in the world. Since the discovery of functional MHC-peptides complexes in DCs-derived exosomes,Citation18 many immune functions for exosomes have been described.Citation10 Researchers tend also to find a new way to modulate immune responses against cancer with the help of exosomes: it is called cell-free vaccines. A classical approach consists in loading exosomes derived from DCs with a tumor specific antigen to restore antitumor immunity. For example, André et al. isolated exosomes from DCs following tumor peptide pulse and their administration in murine tumor models resulted in rejection of established tumors, an action mediated by T-cell activity.Citation82 It was later shown that vaccination with exosomes containing modified IL-2 could induce a significant regression of a pre-established tumor by targeting the antigen-specific Th1-polarized immune response and cytotoxic T lymphocytes (CTL).Citation83 More recently, it has been described an alternative approach to prepare exosomes GPI-IL-12 from fusion gene-modified renal cancer cells and to use them for immunization. This modified exosomes-based vaccine can induce an antigen-specific immune response and CTL more efficiently, resulting in more significant cytotoxic effects in vitro.Citation84
Chaput et al. demonstrated that isolated DCs-derived exosomes pulsed with Mart1 (Melanoma antigen recognized by T-cells 1) peptides in vitro were able to activate CTL and in, combination with appropriate adjuvants, to induce an antitumor response.Citation85 In a sarcoma mice model, it was found that OVA (chicken egg ovalbumin) packaged-exosomes allowed a more efficient induction of antitumor immune responses than the native soluble OVA secreted form.Citation86
Another way to modulate antitumor immune responses is to combine vaccination by exosomes with other molecules. It was found that ascite-derived exosomes combined to GM-CSF in the immunotherapy of colorectal cancer could induce an antitumor cytotoxic T lymphocyte responseCitation87 whereas combined vaccination with tumor antigen loaded DC-derived exosomes with metronic cyclophosphamide, which inhibit Treg function and restore T and NK cell effector functions, could boost NK cell mediated antitumor immunity in lung cancer patients.Citation88 Finally, recent several studies have shown that HSP-exosomes can also modulate the immune system. This part is discussed in more detail below.
Exosomes as drug delivery cargos
From their characteristics and properties, exosomes have been used as natural drug delivery cargos. Indeed, exosomes offers several advantages: (i) from their composition, exosomes are capable to avoid immune response and are less immunogenic than any other drug delivery system,Citation89 (ii) exosomes can naturally and easily penetrate in a host cell by several means,Citation10 (iii) their nanometric size (50–200 nm) allows them to avoid phagocytosis by the circulating mononuclear phagocytic systems, and the easy extravasation through hyper-permeable blood vessels surrounding tumors, in order to reach tumor tissuesCitation90,91 and (iv) exosomes' membrane protects their content from degradation and are very stable. Several means of modifying exosomes composition exists.Citation92
The vast majority of exosome-based drug delivery works and reviews describe the therapeutic transfer of interfering RNAs like synthetic siRNAs or miRNAs and therefore will not be discussed here. We will focus on chemical compound, drugs and proteins.
In a zebrafish brain cancer model, exosome-delivered anticancer drugs through the blood brain barrier decreased tumor growth markers and so could be potentially used as a carrier for brain delivery of anticancer drugs.Citation93 In 2014, Pascucci et al. showed that Mesenchymal Stem Cells (MSC) could incorporate and deliver Paclitaxel to recipient cells through exosomes with increased anti-tumor effects.Citation94 This study suggests that MSC-derived exosomes could be a new strategy for drug delivery in cancer treatment. Exosomes have also been used as cargos of paclitaxel to increase the effectiveness of the treatment in prostate cancer cells.Citation95 It has also been shown the effectiveness of targeted exosome-encapsulated doxorubicin for integrin-positive breast cancer cells in inhibition of tumor growth.Citation96 Recently, Fuhrmann et al. found that exosomes loaded with hydrophilic porphyrins induced a stronger phototoxic effect than the free drug in a cancer cell model (Integrin-positive cancer cells).Citation97
Zhang's research group used exosomes derived from different cell types to successfully delivered curcumin to activate myeloid cells, producing anti-inflammatory activity and apoptosis in monocytes.Citation98 Finally, genetically engineered exosomes expressing high levels of a suicide gene mRNA and protein-cytosine deaminase (CD) fused to uracil phosphoribosyltransferase (UPRT) have been used to treat pre-established nerve sheath tumors (schwannomas) in an orthotopic mouse model and led to tumor regression.Citation99
Exosomes seem to be new actors in theranostic oncology. In this context, recent studies have validated a major role for heat shock proteins in exosomes.
Heat shock proteins and exosomes
Heat shock proteins are stress proteins subdivided in several families according to their molecular weight: HSP110, HSP90, HSP70, HSP60 and small HSPs. These proteins, very well conserved during evolution, were first discovered in 1962. They represent about 2–3% of cellular proteins. In case of a cellular stress, several of these proteins are overexpressed. A wide variety of stress might induce HSPs expression such as hypoxia, infections, drugs and ischemia.Citation100 The induction of HSP genes require the activation and translocation to the nucleus of specific transcription factors called “Heat Shock Factors” (HSF). These HSF bind to DNA particular sequences named “Heat Shock Elements” (HSE) in the promoter of HSP genes allowing their expression.Citation101
HSPs have been retrieved in all cellular compartments including cytoplasm, nucleus, membrane, mitochondria or endoplasmic reticulum and act as molecular chaperones to maintain cellular homeostasis. HSPs allow the correct refolding of newly-synthesized proteins or incorrectly folded proteins, following physiological conditions or in response to stress.Citation102,103 If they can't refold correctly the abnormal protein, HSPs can facilitate their proteasomal degradation.Citation104 In case of a cell death stimulus, HSPs are overexpressed and have strong anti-apoptotic properties by associating to different key proteins of the apoptosis transduction signaling pathway.Citation105
HSPs can also be extracellular (membrane-bound or free after secretion).Citation106,107 HSP27,Citation108 HSP70Citation109 and HSP90Citation110 have been found secreted in the extracellular media, and some of them, as already mentioned above, have been shown to be present in extracellular vesicles, notably in exosomes.Citation111-113
HSPs have an important function in cancer by acting at different levels. First, they can promote tumor growth by stabilizing oncogenic proteins. For example, HSP90 can stabilize c-Src, STAT3, Raf-1 or HER2/neu.Citation114 Certain HSPs, mainly HSP70 and HSP27, can also increase the resistance to chemotherapy by inhibiting apoptosis.Citation115 Further, some HSPs can promote angiogenesis such as HSP70 and HSP90 that can sequester HIF-a, which is necessary for VEGF production.Citation116 HSP90 is also involved in the VEGF synthesis and may be a potential novel target for anti-angiogenic therapy.Citation117 Moreover, HSPs play a role in metastasis formation; some clinical studies have shown a correlation between the expression of HSP27 and/or HSP70 and the metastatic potential.Citation118-120 Finally, extracellular HSPs can have immunosuppressive functions. Indeed, HSP70 secreted by colorectal cancer cells can activate myeloid-derived suppressor cells and inhibit T cells activation.Citation121
During the last few years there have been a growing interest in extracellular HSPs because of the increasing evidences of their role in the induction of innate immune responses with immunostimulatory or immunosuppressive effects, depending on the nature of the HSP, its localization and cell type.Citation122 Among them, exosomal HSPs seems to modulate the immune response and play anti-tumor functions.Citation13 This is the rational for the use of exosomal HSPs in cancer therapy and diagnosis.
Published data about extracellular HSPs can be confusing as the term “extracellular HSPs” is generally employed for both soluble, membrane-bound and exosomal HSPs. EV-associated HSPs are still quite new in the field of extracellular HSPs and therefore most papers do not unambiguously differentiate between the different forms of extracellular HSPs. Moreover, very often, researchers write about HSPs in “extracellular vesicles” without given any precision about which subtypes of vesicles they are analyzing. To overcome this problem, in this review, we will summarize mainly data about clearly established exosomal HSPs (i.e. studies in which HSPs are determined from previously isolated exosomes -from human body fluids or culture supernatants).
HSP-exosomes in cancer therapy
Several studies have shown that certain exosomal HSPs could modulate the immune system (). HSP70-exosomes could stimulate natural killer cells (NK) reactivity.Citation123 When preincubated with HSP70 surface-positive exosomes, NK cells initiated colon tumor cells apoptosis through granzyme B release.Citation123 It was later on discovered that extracellular HSP70 could also activate macrophages and that this immune modulator effect depended on the ability of HSP70, present on the cell surface, to translocate into the plasma membrane.Citation124 It was suggested that HSP70, release through exosomes derived from stressed cells, constitute a form of intercellular communication in order to inform macrophages and to induce innate immune responses.Citation125 These studies suggested that exosomal HSPs could be used in cancer therapy. In 2006, Chen al. tested the vaccination with exosomes presenting HSP60 and HSP90, derived from lymphoma cells. Researchers found an increase in the antitumor immune response involving the induction of IFN production and the activation/maturation of dendritic cells.Citation126 Several years later, myeloma cell derived exosomes were genetically modified to express endogenous P1A tumor antigen and a transgenic form of membrane-bound HSP70. These HSP70-modified exosomes were able to stimulate in vitro DC maturation more efficiently. The researchers used them as a vaccine and found that they stimulate type 1 CD4(+) helper T (Th1) cell responses, P1A-specific CD8(+) CTL responses and antitumor immunity.Citation127 More recently, Li-Hong et al. demonstrated that exosomes derived from resistant anticancer drug-treated Hepatocellular Carcinoma (HCC) cells conferred a higher antitumor response by inducing HSP-specific NK cell responses in vitro and suggested HSP-bearing exosomes could be used as an efficient vaccine for hepatocellular carcinoma immunotherapy.Citation128
Figure 2. Exosomal heat shock proteins: theranostic oncology tools. HSP60 and HSP70 are proposed as potential cancer biomarkers because of their presence only in exosomes derived from cancer cells. Exosomal HSP70 can act in 2 different ways in the modulation of the immune system. Indeed, it can play an immunosuppressive role through the activation of MDSCs that block the anti-tumor response. But it can have an opposite effect by activating immune cells such as macrophages, dendritic cells (DC) or natural killer cells (NK) which may lead to anti-tumor response. Furthermore, HSP90 may represent a therapeutic target because of their ability to respectively increase tumor cell motility (activation of plasmin tPA).
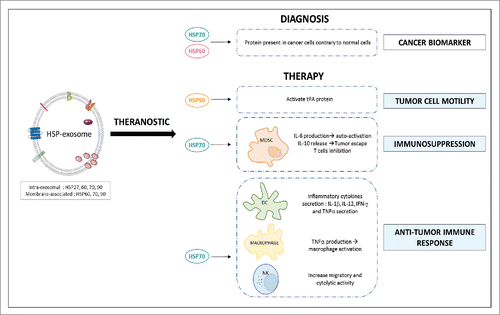
In apparent contrast with these results, we discovered an immunosuppressive function of HSP70 at the surface of exosomes. We have shown that all cancer cells analyzed so far have the ability to secrete exosomes with HSP70 in their membrane while normal “non cancerous” cells do not. These tumor-derived exosomes, through membrane-anchored HSP70, can activate Myeloid Derived Suppressor Cells (MDSCs),Citation121,50 which are abundant cells in a cancer context that restrain antitumor immunity and promote tumor expansion. At the molecular level, the extracellular domain of membrane HSP70 binds to the Toll-Like Receptor 2 at the surface of MDSCs thus activating them. This interaction triggers NF-kB signaling pathway allowing the expression of the inflammatory cytokine IL-6, which binds to its receptor IL-6R in an autocrine manner. This interaction leads to STAT3 phosphorylation via JAK2 pathway, activating survival genes in MDSCs that could exert their immunosuppressive functions.
Recently, our team has confirmed the release of HSP70-exosomes by cancer cells and their ability to activate MDSC in a small cohort of colon cancer patients. Further, we have developed a peptide aptamer (A8) that binds to the extracellular domain of membrane-bound HSP70, called “TKD.”Citation129 Membrane HSP70 binds with much higher affinity to A8 than to the TLR2 receptor in the MDSC. As a result, A8 block the capacity of these tumor-derived exosomes to activate MDSC. Thereby, in vivo and in vitro, A8 induce the development of an efficient anti-tumor immune response that was associated to an inhibition of MDSC.Citation50 In line with our results proposing an HSP70 inhibitor -A8- as an agent that can boost the anti-cancer immune response, HSP90-exosomes have been described as to be involved in the activation of plasmin and cancer cells' motility in several cancer models. Thus, targeting HSP90 could also represent a way to limit tumor invasion by inhibiting a growing number of proteins that are involved in tumor cell motility.Citation130
HSP-exosomes in cancer diagnosis
The detection and quantification of exosomal HSPs can provide useful information for establishing new circulating and non-invasive biomarkers (). Our team suggests the use of HSP70-exosomes as a cancer marker because they seem a general feature of cancer cells (but not of “normal” non-cancerous cells) and we have demonstrated that they can be measured in large amounts in biological fluids from cancer patients but not from healthy individuals where they are hardly detected.Citation50 We have patented an interference biolayer protocol to easily capture HSP70-exosomes isolated from human fluids using as a high affinity ligand our peptide aptamer A8 (WO2015/189395Citation131). To move beyond the proof of principle that these tumor-derived exosomes (HSP70-exosomes) can be quantified and might be interesting to follow up cancer patients, we have started a prospective study with the anticancer Center Georges-François Leclerc (CGFL, Dijon, France) in breast, ovarian and lung cancer aiming at determining whether the presence of HSP70-exosomes is predictive of the patients' outcome and whether their detection precedes CTCs and the apparition of metastases.
Finally, HSP60 have also been localized at the membrane of exosomes isolated from blood patients suffering from large bowel cancer, before surgery, but was absent after surgery and in healthy controls. Because of its presence in tumor cells but not in healthy cells, HSP60-exosomes seem to be also interesting biomarkers in cancer, at least for large bowel cancer diagnosis.Citation132
Concluding remarks
In conclusion, there are no doubts that even if there are still many questions remaining to be answered, the relatively young field of exosomes in cancer is gaining greater interest within the scientific and medical communities. There are 2 main limitations in the discussion of the works presented in this review. The first and most important is the lack of standardized protocols for isolation of tumor-derived exosomes; the second is the still partial understanding of the mechanisms involving exosomes functions in cancer. Indeed, there is a Janus faced implication of exosomes in cancer biology explained by the fact that exosomes can transfer both tumor-promoting molecules (e.g.,: oncoproteins) and tumor suppressors and can either induce or suppress an immune response. We believed these debated issues could be solved with more precise protocols to isolate cancer exosomes and taking into account the in vivo cancer environmental context.
The available data on exosomes strongly suggest that these diamonds in the rough might represent a revolution in cancer diagnosis and toward a more personalized medicine. Exosomes can be a fingerprint of the parental cell type and of its status. Moreover, they are abundant in body fluids such as blood and urine, therefore representing a precious biomedical tool for non-invasive approaches in cancer diagnosis and cancer patients' follow up. Furthermore, as nanoshuttles of biomarkers and/or anti-tumor drugs, exosomes open new avenues for the clinical management of cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by the “Investissements d'Avenir” (ANR-11-LABX-0021), the Fondation pour la Recherche Médicale (FRM grant number ECO20160736090 to GC), the Ligue Nationale Contre le Cancer, the Association pour la Recherche sur le Cancer, l'Institut National du Cancer, Centre Georges-François Leclerc, Cancéropole Grand-Est and FEDER.
References
- Hurley JH, Boura E, Carlson L-A, Różycki B Membrane budding. Cell 2010; 143:875-87; PMID:21145455; http://dx.doi.org/10.1016/j.cell.2010.11.030
- Marzesco A-M, Janich P, Wilsch-Bräuninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 2005; 118:2849-58; PMID:15976444; http://dx.doi.org/10.1242/jcs.02439
- Borges FT, Reis LA, Schor N Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res Rev Bras Pesqui Médicas E Biológicas Soc Bras Biofísica 2013; Al 46:824-830.
- Gasser O, Hess C, Miot S, Deon C, Sanchez J-C, Schifferli JA Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res 2003; 285:243-57; PMID:12706119; http://dx.doi.org/10.1016/S0014-4827(03)00055-7
- Trams EG, Lauter CJ, Salem N, Heine U Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981; 645:63-70; PMID:6266476; http://dx.doi.org/10.1016/0005-2736(81)90512-5
- Hawari FI, Rouhani FN, Cui X, Yu Z-X, Buckley C, Kaler M, Levine SJ Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A 2004; 101:1297-302; PMID:14745008; http://dx.doi.org/10.1073/pnas.0307981100
- Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, Lombardo D, Verine A Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J Off Publ Fed Am Soc Exp Biol 2008; 22:3358-69.
- Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol Baltim Md 1950 2001; 166:7309-18.
- Junquera C, Castiella T, Muñoz G, Fernández-Pacheco R, Luesma MJ, Monzón M Biogenesis of a new type of extracellular vesicles in gastrointestinal stromal tumors: ultrastructural profiles of spheresomes. Histochem. Cell Biol 2016
- Théry C, Ostrowski M, Segura E Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009a; 9:581-93; http://dx.doi.org/10.1038/nri2567
- Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W Exosomes in cancer: small particle, big player. J Hematol Oncol J Hematol Oncol 2015; 8:83; http://dx.doi.org/10.1186/s13045-015-0181-x
- Soung YH, Nguyen T, Cao H, Lee J, Chung J. Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep 2016; 49:18–25.
- Campanella C, Bavisotto CC, Gammazza AM, Nikolic D, Rappa F, David S, Cappello F, Bucchieri F, Fais S Exosomal Heat Shock Proteins as New Players in Tumour Cell-to-cell Comm unication. J Circ Biomark 2014; 1; http://dx.doi.org/10.5772/58721
- De Maio A, Vazquez D Extracellular heat shock proteins: a new location, a new function. Shock Augusta Ga 2013a; 40:239-246; http://dx.doi.org/10.1097/SHK.0b013e3182a185ab
- Pan BT, Johnstone RM Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983; 33:967-78; PMID:6307529; http://dx.doi.org/10.1016/0092-8674(83)90040-5
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987; 262:9412-20; PMID:3597417
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161-72; PMID:8642258; http://dx.doi.org/10.1084/jem.183.3.1161
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4:594-600; PMID:9585234; http://dx.doi.org/10.1038/nm0598-594
- van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001; 121:337-49; PMID:11487543; http://dx.doi.org/10.1053/gast.2001.26263
- Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 2006; 31:642-8; http://dx.doi.org/10.1016/j.mcn.2005.12.003
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7:297-303; PMID:11231627; http://dx.doi.org/10.1038/85438
- Taylor DD, Akyol S, Gercel-Taylor C Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol Baltim Md 2006; 1950 176:1534-42.
- Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol 2005; 17:879–887.
- Pisitkun T, Shen R-F, Knepper MA Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004; 101:13368-73; PMID:15326289; http://dx.doi.org/10.1073/pnas.0403453101
- Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 2008; 31:1059-62; PMID:18520029; http://dx.doi.org/10.1248/bpb.31.1059
- Admyre C, Grunewald J, Thyberg J, Gripenbäck S, Tornling G, Eklund A, Scheynius A, Gabrielsson S Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 2003; 22:578-83; PMID:14582906; http://dx.doi.org/10.1183/09031936.03.00041703
- Gatti J-L, Métayer S, Belghazi M, Dacheux F, Dacheux J-L Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod 2005; 72:1452-65; PMID:15635128; http://dx.doi.org/10.1095/biolreprod.104.036426
- Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager H-D, Abdel-Bakky MS, Gutwein P, Altevogt P CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int 2007; 72:1095-102; PMID:17700640; http://dx.doi.org/10.1038/sj.ki.5002486
- Admyre C, Johansson SM, Qazi KR, Filén J-J, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S Exosomes with immune modulatory features are present in human breast milk. J Immunol Baltim Md 1950 2007; 179:1969-78.
- Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet Lond Engl 2002; 360:295-305; http://dx.doi.org/10.1016/S0140-6736(02)09552-1
- Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RTA, Webb DJ, Dear JW Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 2012; 10:5; PMID:22221959; http://dx.doi.org/10.1186/1479-5876-10-5
- Duijvesz D, Luider T, Bangma CH, Jenster G Exosomes as biomarker treasure chests for prostate cancer. Eur Urol 2011; 59:823-31; PMID:21196075; http://dx.doi.org/10.1016/j.eururo.2010.12.031
- Hurley JH ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 2008; 20:4-11; PMID:18222686; http://dx.doi.org/10.1016/j.ceb.2007.12.002
- Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 2007; 8:355–368.
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319:1244-7; PMID:18309083; http://dx.doi.org/10.1126/science.1153124
- Savina A, Furlán M, Vidal M, Colombo MI Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003; 278:20083-90; PMID:12639953; http://dx.doi.org/10.1074/jbc.M301642200
- Feng Z p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb Perspect Biol 2010; 2:a001057; PMID:20182617; http://dx.doi.org/10.1101/cshperspect.a001057
- Hsu C, Morohashi Y, Yoshimura S-I, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, Barr FA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189:223-32; PMID:20404108; http://dx.doi.org/10.1083/jcb.200911018
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19-30-13; PMID:19966785; http://dx.doi.org/10.1038/ncb2000
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14:677-85; PMID:22660413; http://dx.doi.org/10.1038/ncb2502
- Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 2013; 288:10093-9; PMID:23430739; http://dx.doi.org/10.1074/jbc.C112.444562
- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 2009; 284:34211-22; PMID:19801663; http://dx.doi.org/10.1074/jbc.M109.041152
- Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2013; 2; PMID:24223256; http://dx.doi.org/10.3402/jev.v2i0.20677
- Raposo G, Stoorvogel W Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373-83; PMID:23420871; http://dx.doi.org/10.1083/jcb.201211138
- Logozzi, M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PloS One 2009; 4:e5219.
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci 2016; 113:E968-77; http://dx.doi.org/10.1073/pnas.1521230113
- Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics 2012; MCP 11:863–885.
- Ibrahim A, Marban E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol 2016; 78:67-83; PMID:26667071
- Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 2012; 13:328–335.
- Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse V, et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. J Natl Cancer Inst 2016; 108:; PMID:26598503; http://dx.doi.org/10.1093/jnci/djv330
- Crunkhorn S. Cancer: Cancer exosomes promote tumorigenesis. Nat Rev Drug Discov 2015; 14:16–16.
- Raimondo S, Corrado C, Raimondi L, De Leo G, Alessandro R. Role of Extracellular Vesicles in Hematological Malignancies. BioMed Res Int 2015; 2015:821613.
- Yu, D. et al. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci 2015; 106:959–964.
- Santé publique France - InVS / Accueil. Available at http://invs.santepubliquefrance.fr/ (Accessed: 4th November 2016)
- Institut National Du Cancer - Accueil. Available at http://www.e-cancer.fr/ (Accessed: 4th November 2016)
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015; 523:177-82; PMID:26106858; http://dx.doi.org/10.1038/nature14581
- Gumireddy K, Li A, Chang DH, Liu Q, Kossenkov AV, Yan J, Korst RJ, Nam BT, Xu H, Zhang L, et al. AKAP4 is a circulating biomarker for non-small cell lung cancer. Oncotarget 2015; 6:17637-47; PMID:26160834; http://dx.doi.org/10.18632/oncotarget.3946
- Madhavan B, Yue S, Galli U, Rana S, Gross W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer 2015; 136:2616-27.
- Kalra H, Adda CG, Liem M, Ang C-S, Mechler A, Simpson RJ, Hulett MD, Mathivanan S Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013; 13:3354-64; PMID:24115447; http://dx.doi.org/10.1002/pmic.201300282
- Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F, Carreca AP, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta 2014; 1846:539-46.
- Riches A, Campbell E, Borger E, Powis S Regulation of exosome release from mammary epithelial and breast cancer cells – A new regulatory pathway. Eur J Cancer 2014; 50(5):1025-34.; PMID:24462375; http://dx.doi.org/10.1016/j.ejca.2013.12.019
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18:883-91; PMID:22635005; http://dx.doi.org/10.1038/nm.2753
- Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, Drzewiecka H, Szpurek D, Nowakowski A, Spaczynski M, Baranowski W, et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol Obstet Sunnyvale Calif Suppl 2013; 4:3
- Pinet S, Bessette B, Vedrenne N, Lacroix A, Richard L, Jauberteau M-O, Battu S & Lalloué F. TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget 2016; 7:6349-50364; PMID:27385098
- Lazar I, Clement E, Ducoux-Petit M, Denat L, Soldan V, Dauvillier S, Balor S, Burlet-Schiltz O, Larue L, Muller C, et al. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment Cell Melanoma Res 2015; 28:464-75; PMID:25950383; http://dx.doi.org/10.1111/pcmr.12380
- Alegre E, Zubiri L, Perez-Gracia JL, González-Cao M, Soria L, Martín-Algarra S, González A Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin Chim Acta 2016; 454:28-32; PMID:26724367; http://dx.doi.org/10.1016/j.cca.2015.12.031
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527:329-35; PMID:26524530; http://dx.doi.org/10.1038/nature15756
- Li Y, Zhang Y, Qiu F, Qiu Z Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis 2011; 32:1976-83; PMID:21557262; http://dx.doi.org/10.1002/elps.201000598
- Sandfeld-Paulsen B, Jakobsen KR, Bæk R, Folkersen BH, Rasmussen TR, Meldgaard P, Varming K, Jørgensen MM, Sorensen BS. Exosomal Proteins as a Diagnostic Biomarkers in Lung Cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 2016; 11:1701-1710; http://dx.doi.org/10.1016/j.jtho.2016.05.034
- Hong C-S, Muller L, Whiteside TL, Boyiadzis M Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol 2014; 5:160; PMID:24782865; http://dx.doi.org/10.3389/fimmu.2014.00160
- Khan S, Jutzy JMS, Valenzuela MMA, Turay D, Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB, Wall NR Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PloS One 2012; 7:e46737; PMID:23091600; http://dx.doi.org/10.1371/journal.pone.0046737
- Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer 2009; 9:244; PMID:19619303; http://dx.doi.org/10.1186/1471-2407-9-244
- Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun 2014; 5:3591; PMID:24710016; http://dx.doi.org/10.1038/ncomms4591
- Huang S-H, Li Y, Zhang J, Rong J, Ye S Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest 2013; 31:330-5; PMID:23614656; http://dx.doi.org/10.3109/07357907.2013.789905
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Esquela-Kerscher A, Slack FJ Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006; 6:259-69; PMID:16557279; http://dx.doi.org/10.1038/nrc1840
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014; 26:707-21; PMID:25446899; http://dx.doi.org/10.1016/j.ccell.2014.09.005
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor STF, Chin AR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25:501-15; PMID:24735924; http://dx.doi.org/10.1016/j.ccr.2014.03.007
- Chiam K, Wang T, Watson DI, Mayne GC, Irvine TS, Bright T, Smith L, White IA, Bowen JM, Keefe D, et al. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J Gastrointest Surg Off J Soc Surg Aliment Tract 2015; 19:1208-15; http://dx.doi.org/10.1007/s11605-015-2829-9
- Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med 2016; http://dx.doi.org/10.1007/s13277-016-4939-8
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008; 110:13–21.
- André F, Chaput N, Schartz NEC, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu D-H, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol Baltim Md 1950 2004; 172:2126-36.
- Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, Cao X Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol 2007; 133:389-99; PMID:17219198; http://dx.doi.org/10.1007/s00432-006-0184-7
- Zhang Y, Luo C-L, He B-C, Zhang J-M, Cheng G, Wu X-H Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int J Oncol 2010; 36:133-40; PMID:19956842
- Chaput N, Schartz NEC, André F, Taïeb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol Baltim Md 1950 2004; 172:2137-46
- Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq J-B, Combadière B, Amigorena S, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 2008; 68:1228-35; PMID:18281500; http://dx.doi.org/10.1158/0008-5472.CAN-07-3163
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther J Am Soc Gene Ther 2008; 16:782-90; http://dx.doi.org/10.1038/mt.2008.1
- Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016; 5:e1071008; PMID:27141373; http://dx.doi.org/10.1080/2162402X.2015.1071008
- van den Boorn JG, Schlee M, Coch C, Hartmann G SiRNA delivery with exosome nanoparticles. Nat Biotechnol 2011; 29:325-6; PMID:21478846; http://dx.doi.org/10.1038/nbt.1830
- Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H, Kim B, Park J-H Liposome-Based Engineering of Cells To Package Hydrophobic Compounds in Membrane Vesicles for Tumor Penetration. Nano Lett 2015; 15:2938-44; PMID:25806671; http://dx.doi.org/10.1021/nl5047494
- Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, Sood AK, McMeekin S, Ramesh R Exploitation of Exosomes as Nanocarriers for Gene-, Chemo-, and Immune-Therapy of Cancer. J Biomed Nanotechnol 2016; 12:1159-73; PMID:27319211; http://dx.doi.org/10.1166/jbn.2016.2205
- Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M A comprehensive overview of exosomes as drug delivery vehicles — Endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta BBA - Rev Cancer 2014; 1846:75-87; http://dx.doi.org/10.1016/j.bbcan.2014.04.005
- Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 2015; 32:2003-14; PMID:25609010; http://dx.doi.org/10.1007/s11095-014-1593-y
- Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigan∫ L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G & Pessina A Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release Off J Control Release Soc 2014; 192:262-70; http://dx.doi.org/10.1016/j.jconrel.2014.07.042
- Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release Off J Control Release Soc 2015; 220:727-37; http://dx.doi.org/10.1016/j.jconrel.2015.09.031
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014; 35:2383-90; PMID:24345736; http://dx.doi.org/10.1016/j.biomaterials.2013.11.083
- Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release Off J Control Release Soc 2015; 205:35-44; http://dx.doi.org/10.1016/j.jconrel.2014.11.029
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther J Am Soc Gene Ther 2010; 18:1606-14; http://dx.doi.org/10.1038/mt.2010.105
- Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO, Saydam O Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther J Am Soc Gene Ther 2013; 21:101-8; http://dx.doi.org/10.1038/mt.2012.161
- Pace, A. et al. Hsp60, a novel target for antitumor therapy: structure-function features and prospective drugs design. Curr Pharm Des 2013; 19:2757–2764.
- Akerfelt M, Morimoto RI, Sistonen L Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 2010; 11:545-55; PMID:20628411; http://dx.doi.org/10.1038/nrm2938
- Beckmann RP, Mizzen LE, Welch WJ Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 1990; 248:850-4; PMID:2188360; http://dx.doi.org/10.1126/science.2188360
- Gething MJ, Sambrook J Protein folding in the cell. Nature 1992; 355:33-45; PMID:1731198; http://dx.doi.org/10.1038/355033a0
- Lanneau D, Wettstein G, Bonniaud P, Garrido C Heat shock proteins: cell protection through protein triage. ScientificWorldJournal 2010; 10:1543-52; PMID:20694452; http://dx.doi.org/10.1100/tsw.2010.152
- Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion 2007; 1:53-60; PMID:19164900; http://dx.doi.org/10.4161/pri.1.1.4059
- De Maio A Extracellular Hsp70: export and function. Curr Protein Pept Sci 2014; 15:225-31; PMID:24694368; http://dx.doi.org/10.2174/1389203715666140331113057
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 1995; 61:272-9; PMID:7705958; http://dx.doi.org/10.1002/ijc.2910610222
- Liao W-C, Wu M-S, Wang H-P, Tien Y-W, Lin J-T Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 2009; 38:422-6; PMID:19214136; http://dx.doi.org/10.1097/MPA.0b013e318198281d
- De Maio A, Vazquez D Extracellular heat shock proteins: a new location, a new function. Shock Augusta Ga 2013b; 40:239-46; http://dx.doi.org/10.1097/SHK.0b013e3182a185ab
- Sidera K, Patsavoudi E Extracellular HSP90: conquering the cell surface. Cell Cycle Georget Tex 2008; 7:1564-8; http://dx.doi.org/10.4161/cc.7.11.6054
- Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DFV, Barbieri G, David S, Farina F, Zummo G, et al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PloS One 2012; 7:e42008; PMID:22848686; http://dx.doi.org/10.1371/journal.pone.0042008
- Schorey JS, Bhatnagar S Exosome function: from tumor immunology to pathogen biology. Traffic Cph Den 2008; 9:871-81; http://dx.doi.org/10.1111/j.1600-0854.2008.00734.x
- Théry C, Ostrowski M, Segura E Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009b; 9:581-93; http://dx.doi.org/10.1038/nri2567
- Blagosklonny MV Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia 2002; 16:455-62; PMID:11960322; http://dx.doi.org/10.1038/sj.leu.2402415
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle Georget Tex 2006; 5:2592-601.
- Neckers L, Ivy SP Heat shock protein 90. Curr Opin Oncol 2003; 15:419-24; PMID:14624223; http://dx.doi.org/10.1097/00001622-200311000-00003
- Sun J, Liao JK Induction of Angiogenesis by Heat Shock Protein 90 Mediated by Protein Kinase Akt and Endothelial Nitric Oxide Synthase. Arterioscler Thromb Vasc Biol 2004; 24:2238-44; PMID:15486309; http://dx.doi.org/10.1161/01.ATV.0000147894.22300.4c
- Calderwood SK, Gong J Heat Shock Proteins Promote Cancer: It's a Protection Racket. Trends Biochem Sci 2016; 41:311-23; PMID:26874923; http://dx.doi.org/10.1016/j.tibs.2016.01.003
- Gibert B, Eckel B, Gonin V, Goldschneider D, Fombonne J, Deux B, Mehlen P, Arrigo A-P, Clézardin P, Diaz-Latoud C Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. Br J Cancer 2012; 107:63-70; PMID:22627320; http://dx.doi.org/10.1038/bjc.2012.188
- Gong J, Weng D, Eguchi T, Murshid A, Sherman MY, Song B, Calderwood SK Targeting the hsp70 gene delays mammary tumor initiation and inhibits tumor cell metastasis. Oncogene 2015; 34:5460-71; PMID:25659585; http://dx.doi.org/10.1038/onc.2015.1
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin J-P, Boireau W, Rouleau A, Simon B, Lanneau D, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120:457-71; PMID:20093776
- Tamura Y, Torigoe T, Kutomi G, Hirata K, Sato N New paradigm for intrinsic function of heat shock proteins as endogenous ligands in inflammation and innate immunity. Curr Mol Med 2012; 12:1198-206; PMID:22804242; http://dx.doi.org/10.2174/156652412803306710.
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005; 65:5238-47; PMID:15958569; http://dx.doi.org/10.1158/0008-5472.CAN-04-3804
- Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol Baltim Md 1950 2008; 180:4299-307
- De Maio A Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 2011; 16:235-49; PMID:20963644; http://dx.doi.org/10.1007/s12192-010-0236-4
- Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 2006; 36:1598-607; PMID:16708399; http://dx.doi.org/10.1002/eji.200535501
- Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med 2010; 14:2655-66; PMID:19627400; http://dx.doi.org/10.1111/j.1582-4934.2009.00851.x
- Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, Lin H-M, Shang C-Z, Chen Y-J, Min J Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012; 287:15874-85; PMID:22396543; http://dx.doi.org/10.1074/jbc.M112.340588
- Stangl S, Varga J, Freysoldt B, Trajkovic-Arsic M, Siveke JT, Greten FR, Ntziachristos V, Multhoff G Selective in vivo imaging of syngeneic, spontaneous, and xenograft tumors using a novel tumor cell-specific hsp70 peptide-based probe. Cancer Res 2014; 74:6903-12; PMID:25300920; http://dx.doi.org/10.1158/0008-5472.CAN-14-0413
- McCready J, Sims JD, Chan D, Jay DG Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010; 10:294; PMID:20553606; http://dx.doi.org/10.1186/1471-2407-10-294
- WO2015/189395. “HSP70 peptide aptamers: Methods and compositions for diagnosis, monitoring and treating cancer.”
- Campanella C, Rappa F, Sciumè C, Marino Gammazza A, Barone R, Bucchieri F, David S, Curcurù G, Caruso Bavisotto C, et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015; 121:3230-9; PMID:26060090; http://dx.doi.org/10.1002/cncr.29499
