ABSTRACT
The Semaphorin/Neuropilin/Plexin (SNP) complexes control a wide range of biological processes. Consistently, activity deregulation of these complexes is associated with many diseases. The increasing knowledge on SNP had in turn validated these molecular complexes as novel therapeutic targets. Targeting SNP activities by small molecules, antibodies and peptides or by soluble semaphorins have been proposed as new therapeutic approach. This review is focusing on the latest demonstration of this potential and discusses some of the key questions that need to be addressed before translating SNP targeting into clinically relevant approaches.
Introduction
The first semaphorin (at that time called collapsin) has been discovered in 1993 by the group of JA Raper.Citation1 This secreted protein turned out to be a strong inhibitor of axon growth by inducing rapid depolymerization of the actin cytoskeleton. This property consequently was shown to transiently destabilize the axonal growth cone thereby impeding proper axonal guidance. From this discovery, a huge amount of data has been generated over the last 2 decades that describe the now called semaphorin family and its pleiotropic functions. Almost all organs and tissues express several semaphorins from early development up to the adult which occurs in both healthy and damaged tissues. The many properties of semaphorinsCitation2 can explain the diversity and multitude of functions they have on cell death, proliferation, migration and differentiation. Consequently, dysregulated semaphorin expression or signaling is associated with a variety of diseases in the central nervous system (CNS), the cardio-vascular system (CV), the immune system (IS) and cancer.
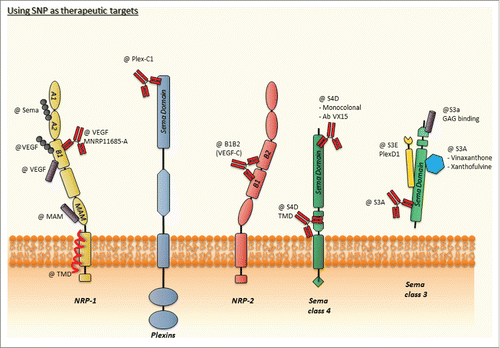
However, the main challenge when studying the semaphorin functions is their extraordinary versatility inherent to their many signaling pathways. Semaphorins can exhibit promoting or inhibiting effects depending on the receptor complex.Citation3-6 Indeed, the class 3 semaphorins (secreted molecules) bind to Neuropilins that in turn associate with co-receptors to trigger cellular signaling. Neuropilin-1 (NRP1) which was initially identified as a cell surface receptor in the nervous system,7 is the primary class 3 semaphorin binding partnerCitation8 with the exception of Sema3E which directly binds Plexin D1.Citation9 NRP1 lacks direct signaling activities and therefore needs to associate with co-receptors to transduce the semaphorin signal. It has been largely described that Plexins are the main co-receptors of NRP1 to transduce class 3 semaphorin signaling while transmembrane semaphorins can directly interact with Plexins .10,Citation11 Different combinations of Plexins and NRP1 can generate a variety of complexes that modulate semaphorin signaling.Citation12 Moreover, additional coreceptors such as the cell adhesion molecule L1 for Sema3ACitation13 or CD72 for Sema4DCitation14 have also been described. In addition, NRP1 and its homolog Neuropilin-2 (NRP2) do not only bind semaphorins but also bind VEGF and several other molecules.Citation15 These interactions are evidently important to control normal and tumor associated angiogenesis.Citation16 Moreover, NRPs interact with many other growth factors such as FGF, PDGF or TGF-β1 and their cognate tyrosine kinase receptors (RTKs).Citation17 NRPs are therefore entering the complex hub of RTKs and their intricate network of signaling pathways. This already large signaling versatility was even further increased when NRPs were identified as partners of integrin signalingCitation18 that could regulate fibronectin fibril assembly.Citation19 Hence, besides transmembrane semaphorins which do not seem to require NRPs for signalingCitation10 most of the secreted semaphorins compete with several other ligands (directly or indirectly) involving NRPS to establish their bona fide signaling pathways.
The obvious diversity of semaphorin/Neuropilin/Plexin (SNP) complexes and their involvement in diseases raises the frequently asked question whether they are good therapeutic targets.Citation20 Indeed, several approaches have been tested to regulate semaphorin signaling either by inhibitors or by using the soluble molecule itself. An excellent review by Mishra and colleaguesCitation21 is providing detailed information about semaphorins and NRPs. Here, this review will summarize in vivo studies that were undertaken to evaluate SNP complexes as potential targets in curing various pathologies.
Using soluble semaphorins as therapeutic agents
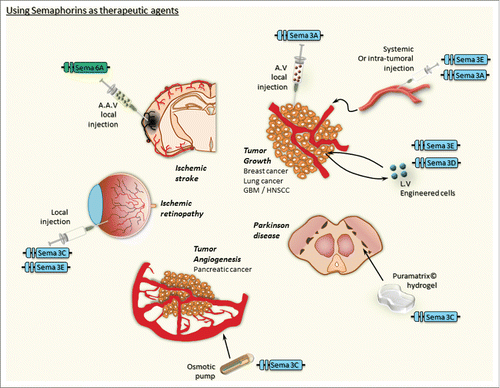
Numerous studies have shown that semaphorins can act as inhibitors of cell migration, cell proliferation or even inducers of apoptosis. Their pro-apoptotic role is particularly interesting in the cancer context. Another good reason to consider regulation of semaphorins in tumor development is the clear anti-angiogenic activity of class 3 semaphorins that has been proven in several models.Citation22-26 Thus, it is tempting to use the natural properties of semaphorins to trigger inhibition of tumor cell growth and/or tumor angiogenesis by synthetic versions of semaphorins. Indeed, intraocular injection of Sema3E displays an anti-angiogenic activity on developing normal vessels through binding to Plexin D1.Citation27 While affecting also tumor associated vessels, a negative impact on normal vessels may represent a major risk of bleeding as side effect in some tissues. However, intravitreal administration of the Sema3E protein selectively suppressed extraretinal vascular outgrowth without affecting the regeneration of the retinal vasculature in a model of ischemic retinopathy ().Citation28 The use of semaphorins as therapeutic agents to treat abnormal vessel development is again strengthened by the demonstration that Sema3C inhibits pathological angiogenesis in a murine oxygen-induced retinopathy model.Citation29 In these studies, recombinant proteins were locally administrated. It would be interesting to know whether systemic administrations could provide similar beneficial effects. Consistently, a stabilized form of Sema3C (a furin cleavage-resistant Sema3C) was able to inhibit tumor angiogenesis as well as tumor lymphangiogenesis and tumor.Citation30 The continuing effort devoted to analyze the therapeutic potential of additional semaphorins is also fruitful in other diseases, as the transmembrane Sema4B was shown to inhibit non-small cell lung cancer growth in vivo when overexpressed in the cancer cells.Citation31 Because gene delivery-based therapies are still under debate and somehow still difficult to achieve, it remains to be shown whether administration of a soluble form of the protein would be sufficient to recapitulate the effects of ectopically expressed Sema4B. The same comment applies to glioblastoma, where Sema3D or Sema3E when ectopically expressed by the tumor cells (using lentivirus-based strategies) reduced tumor growth.32 It would be interesting to see whether local or systemic administration of recombinant Sema3D and Sema3E molecules are able to reach the tumor and block glioblastoma growth, where, despite a high tumor blood vessels leakiness, crossing the blood brain barrier may represent an issue (). Alternatively, lentivirus delivery in vivo (by direct intracerebral injection) into established tumors should be tested because a similar strategy applying AAV-mediated delivery and expression of Sema6A in the cortex enhanced post-ischemic recovery of animals ().33 This study showed that at least membrane bound semaphorins can be produced at the right place, in the right cells and at the right concentration to exert therapeutic effects. These expression properties will certainly be more difficult to achieve with secreted semaphorins because autocrine effects or gradient mediated-effects may generate cell type specific and opposing results. Sema3A is an example to illustrate this complexity as it can stimulate glioma cell dispersion when being overexpressed by the tumor cellsCitation34 while it inhibits breast tumor growth when delivered systemically.Citation35 A similar tumor growth inhibitory effect has also been reported for head and neck squamous cell carcinoma upon intratumoral injections of Sema3A-encoding adenoviruses ().Citation36 Another interesting option comes from the description of the Sema3C-dependent promotion of dopaminergic axons in view of cell therapy for Parkinson disease.Citation37 In this in vitro study, Sema3C was incorporated in a hydrogel (PuraMatrix) to ensure stable long-term release and trigger enhanced axon outgrowth, thus arguing for the use of use such biocompatible hydrogels to deliver semaphorins in vivo as e.g. as inhibitors to block tumor growth ().
Thus, semaphorins can be considered as therapeutic agents, yet the delivery mode needs to be solved. Moreover, the biodistribution profiles should be determined as a function of the administration mode. A special attention should also be given to the semaphorins that are not involved in the maintenance and integrity of adult tissues as a recent study showed that an excess of Sema3A causes severe diabetic nephropathyCitation38 and neuronal toxicity.Citation39 Future development is also needed to increase stability, target binding, and preferential delivery to only abnormal tissues. In this context it is interesting to note the study from the Tamagnone laboratory who investigated local delivery of an uncleavable Sema3E (by using an Alzet osmotic mini-pump) and observed inhibition of tumor angiogenesis in the pancreatic Rip1Tag2 model ().Citation40
Using small molecules as inhibitors of semaphorin signaling
Inhibitory small molecules represent a very classical approach. In case of SNP complexes, very few studies have however tried or succeeded by using small interfering molecules. Xanthofulvin and Vinaxanthone are specific natural inhibitors of Sema3A (refs.Citation41,42,43) and showed strong regenerative properties in a model of spinal cord lesion.Citation44 Recent work identified a synthesis procedure helping to characterize the structures of the compounds.Citation45,46 Such a better characterization of the compounds should now lead to a better understanding of the mode of action, a prerequisite to further develop these compounds as therapeutics. Another mode of semaphorin inhibition is provided by the so called ligand-caging system that predominantly uses soluble forms of SNPs such as NRP1 or NRP2 (refs. Citation47, 48). As an example, expression of the Sema3E ligand trap derived from Plexin D1 (SD1, containing the Sema domain of Plexin D1) by the tumor cells reduced tumor growth.Citation49 A multistep in silico / in vitro screening procedure recently led to the identification of a non-peptidic VEGF-A165/NRP protein-protein interaction antagonist.Citation50 Based on this molecule, several other antagonists were recently designed.Citation51 While originally designed to selectively block VEGF binding, it would be interesting to determine whether these compounds potentially also affect semaphorin binding and signaling. Any compound antagonizing both semaphorins and VEGF ligand binding would certainly be interesting in the context of cancer since both pathways are strong promoters of cancer.
Using function blocking antibodies to inhibit semaphorin signaling
Currently, except small molecules monoclonal antibodies represent the most important targeted therapeutics. Several antibodies have been generated to block semaphorins or their receptors. Using a neutralizing monoclonal antibody targeting Sema3A, the group of Y. Goshima was able to ameliorate lipopolysaccharide-induced sepsis in mice.Citation52 Considering the numerous conditions involving abnormal Sema3A signaling, this antibody may represent a very interesting therapeutic tool with a large spectrum of indications. A neutralizing antibody had also been generated against the transmembrane Sema4D and was shown to markedly prevent bone loss in a model of postmenopausal osteoporosis when administrated every 3 days for 3 weeks.Citation53 Additional Sema4D blocking antibodies have been developed and characterized in various disease conditions including cancer,Citation54,55 Huntington disease,Citation56 experimental autoimmune encephalomyelitisCitation57 and rheumatoid arthritis.Citation58 The Sema4D blocking VX15/2503 antibody is currently in clinical studies (conducted by Vaccinex Inc. in Huntington disease and non-small cell lung cancer) after successful preclinical characterization and thanks to a good tolerance profile in human patients ().Citation59 Few years ago, the structural analysis of the extracellular domain of NRPs described 2 independent binding sites for semaphorins and VEGF,Citation60 thereby providing an interesting rational for the design of function blocking antibodies. Until today, NRP1 is the most advanced target for antibody development. The MNRP1685-A antibody that targets the VEGF-binding domain has been evaluated in a promising phase 1 studyCitation61 followed by a phase 1B study showing unexpected side effect (high proteinuria) in combination with Bevacizumab (anti-VEGF) with or without paclitaxel ().Citation62 An interesting study recently showed that the Fab’ fragment of a mouse NRP1 antibody can be used to functionalize cytotoxic drug-containing liposomes. This turned out to be an effective method to concentrate the drug at the tumor site. The confirmation of such results with a humanized antibody could represent an interesting therapeutic potential. In addition, the description of an anti-NRP2 antibodyCitation63 with strong anti-metastatic properties is highly interesting (), yet this approach has not been further pursued for clinical translation. Moreover, despite development of a new anti-NRP2 antibody that targets the b1b2 domainCitation64 functional in vivo assays are missing. Function blocking antibodies were also raised against Plexins. Based on its promoting role in acute inflammation,Citation65 Plexin C1 may be a good therapeutic target. Indeed, a function blocking antibody against Plexin-C1 reduced hepatic ischemia-reperfusion injury by a mechanism that blocked transmigration of neutrophils.Citation66 One major difficulty when targeting the SNP complexes remains the redundancy of semaphorins and the multiplicity of the receptors which are moreover shared with several other ligands. This implies to develop smart compounds overcoming this bottleneck to produce selective but very potent molecules that bypass the competition of ligands and block the multiple receptors involved in the signaling.
Using antagonist peptides to target or inhibit SNP complexes
The development of therapeutic peptides is growing incredibly fast in the pharmaceutical race.Citation67 If therapeutic peptides such as insulin have undoubtedly shown their efficacy and safety,Citation68 the classical challenges of peptide and protein immunogenicityCitation69 or deliveryCitation70 slows down the emergence of new drugs in comparison to the development of small molecule inhibitors (). Peptides mimicking ligands can be used to antagonize receptor binding, or can be used to mirror the biological activity of the ligand. Consistently a wide range of agonistic or antagonistic peptides can be developed by partially or totally using the original sequences of the respective molecules. Chemical modifications can also be applied to stabilize the structure and/or the stability of therapeutic peptides. In case of SNP complexes, one of the first peptide-based strategy has been proposed by Doherty and colleagues who characterized a peptide targeting the extracellular MAM domain of NRP1 ().Citation71 The recent publication of the crystal structure of the MAM domain may provide interesting information to optimize docking of peptides in this crucial domain.Citation72 Another interesting approach designed a peptide with a motif recognizing the VEGF binding domain of Neuropilin-1.Citation73 The latest development of this approach led to the design of multifunctional nanoplatforms exhibiting strong potential for photodynamic therapy of brain tumors.Citation74 Cationic peptides and peptidomimetics have also been developed to antagonize Sema3A functions presumably by interfering with Sema binding to glycoaminoglycans.Citation75 A peptide recognizing the VEGF binding domain of NRP1 showed interesting inhibitory properties in experimental rheumatoid arthritis ().Citation76
An unexpected alternative approach emerged from studies of the so far largely ignored transmembrane domain (TMD) of Neuropilin-1.Citation77 Indeed, the transmembrane domain of bitopic receptors is usually considered as a membrane anchoring element without further functions. However, the TMDs play a crucial role in receptor dimerization and together with juxta membrane domains they are able to control and modulate extracellular domain ligand-binding capacity or structure.Citation78,79 Several mutations in TMD are associated with severe human diseases in cancer.Citation80 The existence of dimerizing motives such as the GAS motivesCitation81 is known to be the source of selective TMD interactions either for homo- or hetero-dimerization. The TMDs of Neuropilins and Plexins are indeed now well characterized and several studies have shown the specificity of interactions.Citation82 The systematic analysis of TMD / TMD interactions is progressively contributing to the demonstration of a TMD interaction code defining very precise and specific rules of receptor dimerization and heterodimerization. Consistently, peptides targeting the TMD of NRP1 act as receptor dimerization inhibitor leading to the blockade of related biological functions.Citation77 This property turned out to be an efficient strategy to fight against glioblastoma growth in vivo with the corresponding Membrane Targeting Peptide MTP-NRP1 ().Citation83 The strength of such therapeutic peptides is their relative independence toward ligand binding and their intrinsic capability to selectively and specifically interfere with several receptors. Strikingly, such TMD targeting peptides can exhibit biological effects in preclinical in vivo models with a very low dose of 1 µg/kg (or 10 µg/kg for peptides targeting Neu/ ErbB2 receptor),Citation84 doses delivered every 3 days. Such treatments are well tolerated even when administrated for several weeks.Citation85 Another exciting feature is the recent demonstration of the anti-metastatic effect of MTP-NRP1 in a triple negative preclinical breast cancer model.Citation85 In this study, it was shown that MTP-NRP1 could exert a protective effect by reducing metastases growth if administrated before grafting of metastasizing cells. This result suggests the possibility to design preventive treatments attacking the metastasis process upon detection of a primary tumor before metastasis occurs.
Conclusion
The SNP complexes clearly define an exquisite molecular target for drug design. The way to produce drugs tackling these molecules however is not solved yet because of the functional versatility of the SNP complexes. Promoting the natural inhibitory effects of semaphorins in one condition may have deleterious effects in another organ while blocking the semaphorin receptors may counteract positive effects of semaphorins in certain disease contexts. The existence of gradients of soluble semaphorins and the highly diverse and dynamic composition of the receptor complexes are creating additional complexity. A detailed analysis of the biodistribution and safety profiles for SNP targeting drugs is urgently required. The next step will be to study the combination of SNP targeting drugs together with other anti-cancer drugs to address the question of additive, compensatory or resistance mechanisms. Hence, the dual targeting and inhibitory properties of SNP drugs could be better explored by designing smart compounds such as nanoparticles to ensure carrier-mediated specific delivery to damaged tissues while normal tissues are spared. If the last decade was asking the question whether SNP complexes were potential therapeutic targets, the next decade will be the one to clarify what are the best drug design strategies and application modes.
Figure 1. Using semaphorins as therapeutic agents. This cartoon is illustrating the major pathological conditions in which semaphorins have been shown to produce a potential therapeutic effect. The delivery mode is mentioned for each experimental in vivo models. AV, Adenovirus; AAV, Adeno-associated virus; LV, Lentivirus.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been published within the LABEX ANR-10-LABX-0034_Medalis and received a financial support from French government managed by “Agence National de la Recherche” under “Program d'investissement d'avenir.” The authors thank Dr G. Orend for critical reading of the manuscript.
References
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993; 75:217-27; PMID:8402908; http://dx.doi.org/10.1016/0092-8674(93)80064-L
- Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci CMLS 2009; 66:649-66; PMID:18953684; http://dx.doi.org/10.1007/s00018-008-8518-z
- Bagnard D, Lohrum M, Uziel D, Püschel AW, Bolz J. semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Dev Camb Engl 1998; 125:5043-53.
- Gurrapu S, Tamagnone L. Transmembrane semaphorins: Multimodal signaling cues in development and cancer. Cell Adhes Migr 2016; 10(6):1-17; http://dx.doi.org/10.1080/19336918.2016.1197479
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004; 44:961-75; PMID:15603739; http://dx.doi.org/10.1016/j.neuron.2004.12.002
- Kolk SM, Gunput RA, Tran TS, van den Heuvel DMA, Prasad AA, Hellemons AJ, Adolfs Y, Ginty DD, Kolodkin AL, Burbach JPH, et al. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci Off J Soc Neurosci 2009; 29:12542-57; http://dx.doi.org/10.1523/JNEUROSCI.2521-09.2009
- Takagi S, Hirata T, Agata K, Mochii M, Eguchi G, Fujisawa H. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron 1991; 7:295-307; PMID:1908252; http://dx.doi.org/10.1016/0896-6273(91)90268-5
- He Z, Tessier-Lavigne M. Neuropilin Is a Receptor for the Axonal Chemorepellent semaphorin III. Cell 1997; 90:739-51; PMID:9288753; http://dx.doi.org/10.1016/S0092-8674(00)80534-6
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 2005; 307:265-8; PMID:15550623; http://dx.doi.org/10.1126/science.1105416
- Battistini C, Tamagnone L. Transmembrane semaphorins, forward and reverse signaling: have a look both ways. Cell Mol Life Sci CMLS 2016; 73:1609-22; PMID:26794845; http://dx.doi.org/10.1007/s00018-016-2137-x
- Cagnoni G, Tamagnone L. Semaphorin receptors meet receptor tyrosine kinases on the way of tumor progression. Oncogene 2014; 33:4795-802; PMID:24213581; http://dx.doi.org/10.1038/onc.2013.474
- Sabag AD, Smolkin T, Mumblat Y, Ueffing M, Kessler O, Gloeckner CJ, Neufeld G. The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction. J Cell Sci 2014; 127:5240-52; PMID:25335892; http://dx.doi.org/10.1242/jcs.155960
- Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 2002; 21:6348-57; PMID:12456642; http://dx.doi.org/10.1093/emboj/cdf645
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 2000; 13:621-31; PMID:11114375; http://dx.doi.org/10.1016/S1074-7613(00)00062-5
- Prud'homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012; 3:921-39; http://dx.doi.org/10.18632/oncotarget.626
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013; 13:871-82; PMID:24263190; http://dx.doi.org/10.1038/nrc3627
- Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans 2011; 39:1583-91; PMID:22103491; http://dx.doi.org/10.1042/BST20110697
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol 2009; 7:e25; PMID:19175293; http://dx.doi.org/10.1371/journal.pbio.1000025
- Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, de Assuncao TM, Cao Y, Szabolcs A, Thorgeirsson S, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012; 72:4047-59; PMID:22738912; http://dx.doi.org/10.1158/0008-5472.CAN-11-3907
- Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov 2014; 13:603-21; PMID:25082288; http://dx.doi.org/10.1038/nrd4337
- Mishra R, Kumar D, Tomar D, Chakraborty G, Kumar S, Kundu GC. The potential of class 3 semaphorins as both targets and therapeutics in cancer. Expert Opin Ther Targets 2015; 19:427-42; PMID:25434284; http://dx.doi.org/10.1517/14728222.2014.986095
- Guo H-F, Li X, Parker MW, Waltenberger J, Becker PM, Vander Kooi CW. Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry (Mosc) 2013; 52:7551-8; http://dx.doi.org/10.1021/bi401034q
- Nakayama H, Huang L, Kelly RP, Oudenaarden CRL, Dagher A, Hofmann NA, Moses MA, Bischoff J, Klagsbrun M. Infantile hemangioma-derived stem cells and endothelial cells are inhibited by class 3 semaphorins. Biochem Biophys Res Commun 2015; 464:126-32; PMID:26086095; http://dx.doi.org/10.1016/j.bbrc.2015.06.087
- Serini G, Bussolino F, Maione F, Giraudo E. Class 3 semaphorins: physiological vascular normalizing agents for anti-cancer therapy. J Intern Med 2013; 273:138-55; PMID:23198760; http://dx.doi.org/10.1111/joim.12017
- Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res 2004; 64:1008-15; PMID:14871832; http://dx.doi.org/10.1158/0008-5472.CAN-03-3090
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest 2004; 114:1260-71; PMID:15520858; http://dx.doi.org/10.1172/JCI21378
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol 2010; 30:3086-98; PMID:20385769; http://dx.doi.org/10.1128/MCB.01652-09
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S-I, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 2011; 121:1974-85; PMID:21505259; http://dx.doi.org/10.1172/JCI44900
- Yang W-J, Hu J, Uemura A, Tetzlaff F, Augustin HG, Fischer A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med 2015; 7:1267-84; PMID:26194913; http://dx.doi.org/10.15252/emmm.201404922
- Mumblat Y, Kessler O, Ilan N, Neufeld G. Full-Length semaphorin-3C Is an Inhibitor of Tumor Lymphangiogenesis and Metastasis. Cancer Res 2015; 75:2177-86; PMID:25808871; http://dx.doi.org/10.1158/0008-5472.CAN-14-2464
- Jian H, Zhao Y, Liu B, Lu S. SEMA4B inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signal 2015; 27:1208-13; PMID:25746385; http://dx.doi.org/10.1016/j.cellsig.2015.02.027
- Sabag AD, Bode J, Fink D, Kigel B, Kugler W, Neufeld G. Semaphorin-3D and semaphorin-3E Inhibit the Development of Tumors from Glioblastoma Cells Implanted in the Cortex of the Brain. PLoS One 2012; 7:e42912; PMID:22936999; http://dx.doi.org/10.1371/journal.pone.0042912
- Rogalewski A, Dittgen T, Klugmann M, Kirsch F, Krüger C, Pitzer C, Minnerup J, Schäbitz W-R, Schneider A. Semaphorin 6A Improves Functional Recovery in Conjunction with Motor Training after Cerebral Ischemia. PLoS One 2010; 5:e10737; PMID:20505770; http://dx.doi.org/10.1371/journal.pone.0010737
- Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene 2009; 28:3537-50; PMID:19684614; http://dx.doi.org/10.1038/onc.2009.204
- Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, Squadrito ML, Venneri MA, Mazzone M, Larsson E, et al. Systemic and Targeted Delivery of semaphorin 3A Inhibits Tumor Angiogenesis and Progression in Mouse Tumor Models. Arterioscler Thromb Vasc Biol 2011; 31:741-9; PMID:21205984; http://dx.doi.org/10.1161/ATVBAHA.110.211920
- Wang Z, Chen J, Zhang W, Zheng Y, Wang Z, Liu L, Wu H, Ye J, Zhang W, Qi B, et al. Axon guidance molecule semaphorin3A is a novel tumor suppressor in head and neck squamous cell carcinoma. Oncotarget 2016; 7:6048-62; PMID:26755661.
- Carballo-Molina OA, Sánchez-Navarro A, López-Ornelas A, Lara-Rodarte EJR, Salazar P, Campos-Romo A, Ramos-Mejia V, Velasco I. Semaphorin 3C released from a biocompatible hydrogel guides and promotes axonal growth of rodent and human dopaminergic neurons. Tissue Eng Part A 2016; 22(11-12):850-61; PMID:27174503
- Aggarwal PK, Veron D, Thomas DB, Siegel D, Moeckel G, Kashgarian M, Tufro A. Semaphorin3a promotes advanced diabetic nephropathy. Diabetes 2015; 64:1743-59; PMID:25475434; http://dx.doi.org/10.2337/db14-0719
- Shirvan A, Shina R, Ziv I, Melamed E, Barzilai A. Induction of neuronal apoptosis by semaphorin3A-derived peptide. Mol Brain Res 2000; 83:81-93; PMID:11072098; http://dx.doi.org/10.1016/S0169-328X(00)00198-4
- Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant semaphorin 3E isoform. EMBO Mol Med 2012; 4:234-50; PMID:22247010; http://dx.doi.org/10.1002/emmm.201100205
- Kikuchi K, Kishino A, Konishi O, Kumagai K, Hosotani N, Saji I, Nakayama C, Kimura T. In Vitro and in Vivo Characterization of a Novel semaphorin 3A Inhibitor, SM-216289 or Xanthofulvin. J Biol Chem 2003; 278:42985-91; PMID:12933805; http://dx.doi.org/10.1074/jbc.M302395200
- Zheng CJ, Sohn M-J, Kim W-G. Vinaxanthone, a new FabI inhibitor from Penicillium sp. J Antimicrob Chemother 2009; 63:949-53; PMID:19282328; http://dx.doi.org/10.1093/jac/dkp058
- Zhang L, Kaneko S, Kikuchi K, Sano A, Maeda M, Kishino A, Shibata S, Mukaino M, Toyama Y, Liu M, et al. Rewiring of regenerated axons by combining treadmill training with semaphorin3A inhibition. Mol Brain 2014; 7:14; PMID:24618249; http://dx.doi.org/10.1186/1756-6606-7-14
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 2006; 12:1380-9; PMID:17099709; http://dx.doi.org/10.1038/nm1505
- Axelrod A, Eliasen AM, Chin MR, Zlotkowski K, Siegel D. Syntheses of xanthofulvin and vinaxanthone, natural products enabling spinal cord regeneration. Angew Chem Int Ed Engl 2013; 52:3421-4; PMID:23086682; http://dx.doi.org/10.1002/anie.201205837
- Chin MR, Zlotkowski K, Han M, Patel S, Eliasen AM, Axelrod A, Siegel D. Expedited access to vinaxanthone and chemically edited derivatives possessing neuronal regenerative effects through ynone coupling reactions. ACS Chem Neurosci 2015; 6:542-50; PMID:25615693; http://dx.doi.org/10.1021/cn500237z
- Schuch G, Machluf M, Bartsch G, Nomi M, Richard H, Atala A, Soker S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood 2002; 100:4622-8; PMID:12453880; http://dx.doi.org/10.1182/blood.V100.13.4622
- Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res MCR 2010; 8:1063-73; PMID:20651020; http://dx.doi.org/10.1158/1541-7786.MCR-10-0157
- Luchino J, Hocine M, Amoureux M-C, Gibert B, Bernet A, Royet A, Treilleux I, Lécine P, Borg J-P, Mehlen P, et al. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 2013; 24:673-85; PMID:24139859; http://dx.doi.org/10.1016/j.ccr.2013.09.010
- Borriello L, Montès M, Lepelletier Y, Leforban B, Liu W-Q, Demange L, Delhomme B, Pavoni S, Jarray R, Boucher JL, et al. Structure-based discovery of a small non-peptidic Neuropilins antagonist exerting in vitro and in vivo anti-tumor activity on breast cancer model. Cancer Lett 2014; 349:120-7; PMID:24752068; http://dx.doi.org/10.1016/j.canlet.2014.04.004
- Liu W-Q, Megale V, Borriello L, Leforban B, Montès M, Goldwaser E, Gresh N, Piquemal J-P, Hadj-Slimane R, Hermine O, et al. Synthesis and structure-activity relationship of non-peptidic antagonists of neuropilin-1 receptor. Bioorg Med Chem Lett 2014; 24:4254-9; PMID:25091928; http://dx.doi.org/10.1016/j.bmcl.2014.07.028
- Yamashita N, Jitsuki-Takahashi A, Ogawara M, Ohkubo W, Araki T, Hotta C, Tamura T, Hashimoto S, Yabuki T, Tsuji T, et al. Anti-semaphorin 3A neutralization monoclonal antibody prevents sepsis development in lipopolysaccharide-treated mice. Int Immunol 2015; 27:459-66; PMID:25855660; http://dx.doi.org/10.1093/intimm/dxv014
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011; 17:1473-80; PMID:22019888; http://dx.doi.org/10.1038/nm.2489
- Evans EE, Jonason AS, Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, et al. Antibody Blockade of semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies. Cancer Immunol Res 2015; 3:689-701; PMID:25614511; http://dx.doi.org/10.1158/2326-6066.CIR-14-0171
- Evans EE, Hu-Lieskovan S, Bussler H, Torno S, Mallow C, Reilly C, Scrivens M, Klimatcheva E, Winter LA, Kirk R, et al. Antibody blockade of semaphorin 4D breaks down barriers to enhance tumoricidal immune infiltration and supports rational immunotherapy combinations. J Immunother Cancer 2015; 3:P220; http://dx.doi.org/10.1186/2051-1426-3-S2-P220
- Southwell AL, Franciosi S, Villanueva EB, Xie Y, Winter LA, Veeraraghavan J, Jonason A, Felczak B, Zhang W, Kovalik V, et al. Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease. Neurobiol Dis 2015; 76:46-56; PMID:25662335; http://dx.doi.org/10.1016/j.nbd.2015.01.002
- Smith ES, Jonason A, Reilly C, Veeraraghavan J, Fisher T, Doherty M, Klimatcheva E, Mallow C, Cornelius C, Leonard JE, et al. SEMA4D compromises blood–brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol Dis 2015; 73:254-68; PMID:25461192; http://dx.doi.org/10.1016/j.nbd.2014.10.008
- Fisher TL, Seils J, Reilly C, Litwin V, Green L, Salkowitz-Bokal J, Walsh R, Harville S, Leonard JE, Smith E, et al. Saturation monitoring of VX15/2503, a novel semaphorin 4D-specific antibody, in clinical trials. Cytometry B Clin Cytom 2016; 90:199-208; PMID:26566052; http://dx.doi.org/10.1002/cyto.b.21338
- Patnaik A, Weiss GJ, Leonard JE, Rasco DW, Sachdev JC, Fisher TL, Winter LA, Reilly C, Parker RB, Mutz D, et al. Safety, Pharmacokinetics, and Pharmacodynamics of a Humanized Anti-semaphorin 4D Antibody, in a First-In-Human Study of Patients with Advanced Solid Tumors. Clin Cancer Res Off J Am Assoc Cancer Res 2016; 22:827-36; http://dx.doi.org/10.1158/1078-0432.CCR-15-0431
- Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J 2007; 26:4902-12; PMID:17989695; http://dx.doi.org/10.1038/sj.emboj.7601906
- Weekes CD, Beeram M, Tolcher AW, Papadopoulos KP, Gore L, Hegde P, Xin Y, Yu R, Shih LM, Xiang H, et al. A phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Invest New Drugs 2014; 32:653-60; PMID:24604265; http://dx.doi.org/10.1007/s10637-014-0071-z
- Patnaik A, LoRusso PM, Messersmith WA, Papadopoulos KP, Gore L, Beeram M, Ramakrishnan V, Kim AH, Beyer JC, Mason Shih L, et al. A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 2014; 73:951-60; PMID:24633809; http://dx.doi.org/10.1007/s00280-014-2426-8
- Caunt M, Mak J, Liang W-C, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 2008; 13:331-42; PMID:18394556; http://dx.doi.org/10.1016/j.ccr.2008.01.029
- Yang Y, Chen N, Li Z, Wang X-J, Wang S-Y, Tingwu null, Luo F-H, Yan J-H. Preparation, Purification, and Identification of a Monoclonal Antibody Against NRP2 b1b2 Domain. Monoclon Antibodies Immunodiagn Immunother 2015; 34:354-9; http://dx.doi.org/10.1089/mab.2015.0025
- König K, Marth L, Roissant J, Granja T, Jennewein C, Devanathan V, Schneider M, Köhler D, Zarbock A, Rosenberger P. The plexin C1 receptor promotes acute inflammation. Eur J Immunol 2014; 44:2648-58; PMID:24890788; http://dx.doi.org/10.1002/eji.201343968
- König K, Granja T, Eckle V-S, Mirakaj V, Köhler D, Schlegel M, Rosenberger P. Inhibition of Plexin C1 Protects Against Hepatic Ischemia-Reperfusion Injury. Crit Care Med 2016; 44(8):e625-32
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des 2013; 81:136-47; PMID:23253135; http://dx.doi.org/10.1111/cbdd.12055
- Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev 2016; 32:21-39; PMID:25865292; http://dx.doi.org/10.1002/dmrr.2653
- Baldo BA. Chimeric fusion proteins used for therapy: indications, mechanisms, and safety. Drug Saf 2015; 38:455-79; PMID:25832756; http://dx.doi.org/10.1007/s40264-015-0285-9
- Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013; 4:1443-67; PMID:24228993; http://dx.doi.org/10.4155/tde.13.104
- Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel semaphorin/neuropilin antagonists: Complementary semaphorin/neuropilin antagonists. J Neurochem 2005; 92:1180-90; PMID:15715668; http://dx.doi.org/10.1111/j.1471-4159.2004.02950.x
- Yelland T, Djordjevic S. Crystal Structure of the Neuropilin-1 MAM Domain: Completing the Neuropilin-1 Ectodomain Picture. Structure 2016; 24:2008-15; PMID:27720589; http://dx.doi.org/10.1016/j.str.2016.08.017
- Tirand L, Frochot C, Vanderesse R, Thomas N, Trinquet E, Pinel S, Viriot M-L, Guillemin F, Barberi-Heyob M. A peptide competing with VEGF165 binding on neuropilin-1 mediates targeting of a chlorin-type photosensitizer and potentiates its photodynamic activity in human endothelial cells. J Control Release Off J Control Release Soc 2006; 111:153-64; http://dx.doi.org/10.1016/j.jconrel.2005.11.017
- Bechet D, Auger F, Couleaud P, Marty E, Ravasi L, Durieux N, Bonnet C, Plénat F, Frochot C, Mordon S, et al. Multifunctional ultrasmall nanoplatforms for vascular-targeted interstitial photodynamic therapy of brain tumors guided by real-time MRI. Nanomedicine Nanotechnol Biol Med 2015; 11:657-70; http://dx.doi.org/10.1016/j.nano.2014.12.007
- Corredor M, Bonet R, Moure A, Domingo C, Bujons J, Alfonso I, Pérez Y, Messeguer À. Cationic Peptides and Peptidomimetics Bind Glycosaminoglycans as Potential Sema3A Pathway Inhibitors. Biophys J 2016; 110:1291-303; PMID:27028639; http://dx.doi.org/10.1016/j.bpj.2016.01.033
- Kong J-S, Yoo S-A, Kim J-W, Yang S-P, Chae C-B, Tarallo V, De Falco S, Ryu S-H, Cho C-S, Kim W-U. Anti-neuropilin-1 peptide inhibition of synoviocyte survival, angiogenesis, and experimental arthritis. Arthritis Rheum 2010; 62:179-90; PMID:20039409; http://dx.doi.org/10.1002/art.27243
- Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Crémel G, Hubert P, Bagnard D. Transmembrane Domain Interactions Control Biological Functions of Neuropilin-1. Mol Biol Cell 2008; 19:646-54; PMID:18045991; http://dx.doi.org/10.1091/mbc.E07-06-0625
- Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE, Kuriyan J, Shaw DE. Architecture and membrane interactions of the EGF receptor. Cell 2013; 152:557-69; PMID:23374350; http://dx.doi.org/10.1016/j.cell.2012.12.030
- Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y, Pelton JG, Shan Y, Shaw DE, Wemmer DE, et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell 2013; 152:543-56; PMID:23374349; http://dx.doi.org/10.1016/j.cell.2012.12.032
- Hubert P, Sawma P, Duneau J-P, Khao J, Hénin J, Bagnard D, Sturgis J. Single-spanning transmembrane domains in cell growth and cell-cell interactions: More than meets the eye? Cell Adhes Migr 2010; 4:313-24; http://dx.doi.org/10.4161/cam.4.2.12430
- Teese MG, Langosch D. Role of GxxxG Motifs in Transmembrane Domain Interactions. Biochemistry (Mosc) 2015; 54:5125-35; http://dx.doi.org/10.1021/acs.biochem.5b00495
- Aci-Sèche S, Sawma P, Hubert P, Sturgis JN, Bagnard D, Jacob L, Genest M, Garnier N. Transmembrane Recognition of the semaphorin Co-Receptors Neuropilin 1 and Plexin A1: Coarse-Grained Simulations. PLoS One 2014; 9:e97779; http://dx.doi.org/10.1371/journal.pone.0097779
- Nasarre C, Roth M, Jacob L, Roth L, Koncina E, Thien A, Labourdette G, Poulet P, Hubert P, Crémel G, et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene 2010; 29:2381-92; PMID:20140015; http://dx.doi.org/10.1038/onc.2010.9
- Arpel A, Sawma P, Spenlé C, Fritz J, Meyer L, Garnier N, Velázquez-Quesada I, Hussenet T, Aci-Sèche S, Baumlin N, et al. Transmembrane Domain Targeting Peptide Antagonizing ErbB2/Neu Inhibits Breast Tumor Growth and Metastasis. Cell Rep 2014; 8:1714-21; PMID:25220456; http://dx.doi.org/10.1016/j.celrep.2014.07.044
- Arpel A, Gamper C, Spenlé C, Fernandez A, Jacob L, Baumlin N, Laquerriere P, Orend G, Crémel G, Bagnard D, et al. Inhibition of primary breast tumor growth and metastasis using a neuropilin-1 transmembrane domain interfering peptide. Oncotarget [ Internet] 2016 [ cited 2016 Jun 23]; 5. Available from: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=10101.