ABSTRACT
Previous studies indicate that TGFBR3 (transforming growth factor type III receptor, also known as betaglycan), a novel suppressor of progression in certain cancers, is down-regulated in tongue squamous cell carcinoma (TSCC). However, the role of this factor as an upstream regulator in TSCC cells remains to be elucidated. The present study was designed to elucidate whether TGFBR3 gene expression is regulated by two microRNA molecules, miR-19a and miR-424. The study also aimed to determine if these microRNAs promote migration of CAL-27 human oral squamous cells. Immunohistochemistry (IHC) and western blot analyses demonstrated that TGFBR3 protein levels were dramatically down-regulated in clinical TSCC specimens. Conversely, bioinformatics analyses and qRT-PCR results confirmed that both miR-19a and miR-424 were markedly up-regulated in clinical TSCC specimens. In this study, we observed that transfection of a TGFBR3-containing plasmid dramatically inhibited epithelial-to-mesenchymal transition (EMT) and migration in CAL-27 cells. Co-immunoprecipitation analyses also revealed that TGFBR3 forms a complex with the β-arrestin 2 scaffolding protein and IκBα. Furthermore, overexpression of TGFBR3 decreased p-p65 expression and increased IκBα expression; these effects were subsequently abolished following knockdown of β-arrestin 2. Moreover, over-expression of miR-19a and miR-424 promoted migration and EMT in CAL-27 cells. We also observed that the promotion of EMT by miR-19a and miR-424 was mediated by the inhibition of TGFBR3. Our study provides evidence that miR-19a and miR-424 play important roles in the development of TSCC. These results expand our understanding of TGFBR3 gene expression and regulatory mechanisms pertaining to miRNAs.
Introduction
Tongue squamous cell carcinoma (TSCC) is the most common type of oral cancer and is characterized by high proliferation rates and lymph nodal metastases.Citation1,Citation2 In clinical practice, neck dissections are required following observation of TSCC cervical lymph node metastases in presenting patients. Therefore, an understanding of the mechanisms that underpin TSCC cell migration is important for the future clinical treatment of TSCC disease.
TGFBR3, also known as betaglycan, is a TGF-β superfamily co-receptor, which plays a critical role in tumor cell differentiation and migration.Citation3 A study by Lambert et al reported that TGFBR3 inhibits multiple myeloma progression by suppressing cell growth, proliferation, migration, and adhesion.Citation4 Furthermore, a separate study reported that mistargeting of TGFBR3 results in enhanced proliferation, migration, and invasion in vitro breast carcinoma.Citation5 Interestingly, TGFBR3 is also down-regulated in a variety of different cancer cell types, including human TSCC specimens.Citation6,Citation7 However, the role of TGFBR3 and its upstream molecular regulators in the development of TSCC disease has yet to be elucidated.
MicroRNAs (miRNAs) are endogenously expressed small (19–24 nucleotides) noncoding RNAs that regulate gene expression by inhibiting translation or inducing degradation of target messenger RNAs (mRNAs).Citation8 Increasing numbers of abnormally expressed miRNAs have been identified in oral cancer by miRNA expression profiling methods.Citation9 Among these miRNAs, miRNA-19a and miR-424 were found to be over-expressed in human head and neck cancers specimens.Citation10,Citation11 Using bioinformatics analysis methods, it was observed that miRNA-19a and miR-424 were up-regulated in human TSCC specimens. Furthermore, two putative target sites for miR-19a or miR-424 were discovered in TGFBR3 3′UTRs, suggesting theTGFBR3 is a potential target for miR-19a and miR-424 ().
In this study, we analyzed the expression of miR-19a, miR-424, and TGFBR3 in human TSCC specimens and investigated whether TGFBR3 is a direct target of miR-19a and miR-424. We also investigated the role of these microRNAs in regulating migration of CAL-27 human oral squamous cells.
Material and methods
Tissue samples
Human TSCC cells and associated adjacent non-cancer tissues were obtained from the Department of Oral and Maxillofacial Surgery at the Second Affiliated Hospital of Harbin Medical University in China. The tissue information was shown in . In accordance with institutional guidelines, all of the patients gave informed consent prior to the collection of specimens. Tissue samples were snap-frozen in the operating room immediately after surgery and sent to pathology for diagnosis by a board-certified pathologist. For each TSCC patient, a frozen tumor sample (stored at −80oC) and a paraffin-embedded tissue specimen were obtained.
Immunohistochemistry (IHC)
Serial sections (5–6 µm thick) were prepared from paraffin-embedded tissue blocks and mounted on silane-coated glass slides (Matsunami Glass, Osaka, Japan). A single section from each tissue block was stained with hematoxylin and eosin (H&E). All other sections from the block were used for IHC. IHC staining was performed using the standard streptavidin-biotin-peroxidase complex method. Briefly, paraffin sections of TSCC tissues were deparaffinized, blocked with 10% normal goat serum for 10 min, and incubated with anti-TβRIII overnight at 4oC. The tissue section was then incubated with biotinylated goat anti-rabbit immunoglobulin at a dilution of 1:75 at 37oC for 30 min. The status of TGFBR3 expression was assessed by two independent investigators without prior knowledge of the clinicopathological data.
The expression profile of TGFBR3-targeting miRNAs following bioinformatics analysis
The prediction of miRNA targets using multiple algorithms is likely to be more reliable than the deployment of singular algorithms; thus, miRNA-target interactions appearing in at least two of nine databases (TargetScan, miRanda, PicTar, miRBase, DIANA-microT, PITA, miRNAMap, miRTarBase, and miRecords) were included in our analysis. Oral tongue miRNA expression data (level 3) for 133 oral tongue squamous cell carcinoma samples and 44 normal controls were downloaded from The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/). A two-sample t-test was performed to select differentially expressed miRNAs. The P values were adjusted using the Benjamini and Hochberg correction procedure. This procedure accounts for multiple tests with a false discovery rate (FDR) of < 0.05. All of the bioinformatics analyses were performed using R software (http://www.r-project.org/). Network visualization was performed using Cytoscape software (http://cytoscape.org/).
Quantification of miRNA-19a and miR-424 expression levels
Quantitative real-time PCR (qRT-PCR) kits obtained from Applied Biosystems (Foster City, CA, USA) were used to assess the expression of miR-19a and miR-424. For each sample, the ΔCt (target–reference) value was calculated. The fold-change between the TSCC samples and normal controls for miR-19a and miR-424 were calculated using the 2-ΔΔCt method, where ΔΔCT = ΔCT (target–reference) – ΔCT (target–reference) (in untreated samples). qRT-PCR was performed in triplicate for each sample, and an average 2-ΔΔCt value (along with the associated standard error (SE) value was calculated for expression of miR-19a and miR-424 (relative to the normal controls)). U6 and GeNorm were used as internal reference genes to facilitate miR-19a and miR-424 normalization.
Cell culture and transfection
CAL-27, a human tongue squamous cell line, was provided by Harbin Medical University. The cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) purchased from Gibco. All cells were supplemented with 10% FBS and maintained at 37oC in an incubator containing humidified air with 5% (v/v) CO2. Cells were transfected with 1 µg/ml of the pc-DNA3.1-hTGFBR3 plasmid (GeneChem Co., Ltd., Shanghai, China). The pc-DNA3.1-plasmid was used as an empty vector control. Transient transfections were carried out using Fugene 6 (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. GenePharma (Shanghai, China) synthesized the oligonucleotides including 2′ OMe-miR-19a (5′-ugugcaaaucuaugcaaaacuga-3′), miR-19a negative control oligonucleotide (5′-caguacuuuuguguaguacaa-3′), antimiRNA oligonucleotides specific to miR-19a (AMO-19a) (5′-ucaguuuugcauagauuugcaca-3′), 2′OMe-miR-424 (5′- cagcagcaauucauguuuugaa-3′), miR-424 negative control oligonucleotide (5′-uucuccgaacgugucacgutt-3′), and anti-miRNA oligonucleotides specific to miR-424 (AMO-424) (5′-uucaaaacaugaauugcugcug-3′). The afore-mentioned oligonucleotides were transfected into CAL-27 cells (200 nmol per well) using Lipofectamine 2000 reagent (Invitrogen, USA).
Luciferase reporter assay
The TGFBR3 3′-UTR containing the conserved miR-19a or miR-424 binding site was synthesized by Invitrogen and amplified by PCR. The PCR fragment was cloned downstream of the luciferase gene between the SacI and HindIII sites in pMIR-Report (Promega). Next, 0.1 μg of the luciferase reporter constructs containing the 3′-UTR were co-transfected with miR-19a or miR-424 mimics into CAL-27 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). A Renilla luciferase reporter (10 ng) was used as an internal control. Finally, the cells were collected 48 h after transfection, and dual luciferase activities were measured using a luminometer according to the manufacturer's instructions.
RNA interference
RNA interference was performed according to the manufacturer's protocol using Lipofactamine 2000 (Invitrogen). siRNA duplexes (GenePharma, Shanghai) harboring sequences that specifically targeted β-arrestin 2 (5′-aaggaccgcaaaguguuugug-3′) or control non-specific siRNA were utilized to facilitate the interference reactions. Knockdown of expression of the target was confirmed by western blot analysis.
Transwell migration assay
Transwell migration was performed using 24-well cell culture inserts without Matrigel chambers (8-µm pore; BD Biosciences). Briefly, 5 × 104 cells were resuspended in 250 µl of serum-free RPMI-1640 and added into the inserts. A total of 500 µl DMEM with 10% FBS was added to the lower chamber. After allowing cells to migrate for 24 h, cells on the upper surface of the membrane were removed using a cotton swab, and the membranes were fixed with methanol and stained with crystal violet. The number of migrating or invading cells was determined by averaging cell counts from nine randomly selected 100x fields.
Co-immunoprecipitation (Co-IP) and western blot analysis
Total protein samples were extracted from the cultured cells. Approximately 5 µg of antibody specific to TGFBR3 or β-arrestin 2 was added to cell lysates and incubated for 12 h at 4°C. The antibody-protein immune complexes were precipitated together with protein A/G PLUS-Agarose (rabbit polyclonal; Santa Cruz Biotechnology). This agarose facilitates the binding of most antibodies. The precipitation mixture was subsequently incubated overnight at 4°C. The control (n-IgG) was also included for each sample. Briefly, proteins were resolved by SDS-polyacrylamide gel electrophoresis, and transferred to PVDF membranes (Amersham Biosciences). The blots were subsequently blocked with 5% non-fat dry milk powder in Tris-buffered saline with Tween-20 for 2 h and then incubated with primary rabbit anti-TGFBR3, anti-p-p65, anti-β-arrestin 2, anti-IκBα, anti-Vimentin or anti-E-cadherin (1:1,000; Cell Signaling Technology, Beverly, MA, USA). Western blot bands were quantified using Odyssey v1.2 software by measuring the band intensity (area × OD) for each group and normalizing the associated intensity using GAPDH or β-actin (Zhongshan, Beijing, China) as an internal control.
Statistical analysis
Data are presented as the mean ± SE. Statistical comparison was performed using the Student's t test and analysis of variance (ANOVA), with a value of p < 0.05 considered statistically significant.
Results
Down-regulation of TGFBR3 protein in TSCC Clinical Specimens
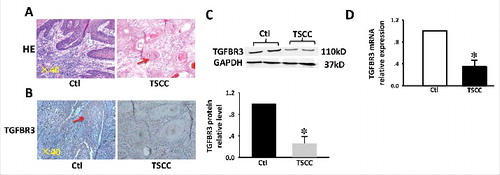
A reduction in the expression of TGFBR3 protein has previously been reported in TSCC disease.Citation7 Representative samples are shown in . H&E staining revealed papillary morphology in normal tongue tissue and cancer pearl patterns in TSCC tissue. Consistent with previous studies, immunohistochemical staining (IHC) showed strong TGFBR3 expression in normal tongue tissues.Citation7 Conversely, TSCC cells showed weak reactivity to TGFBR3, and the number of TGFBR3-positive cells was markedly reduced (). Western blot and RT-PCR analyses also revealed significantly lower levels of TGFBR3 protein and mRNA in TSCC specimens compared with non-tumor tissues (, ).
Figure 1. Expression of TGFBR3 in TSCC tissues. (A) H&E stain and IHC analysis. (B) TGFBR3 expression in matched normal human tongue tissue and TSCC specimens ( × 400 magnification). (C) Expression of TGFBR3 protein in specimens was evaluated by western blot analysis. (D) Expression of TGFBR3 mRNA in specimens was evaluated by RT-PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. These changes were quantitated using densitometry in (C). Data are expressed as the mean ± SEM from three independent experiments (n = 6). *p < 0.05 vs. control.
miR-19a and miR-424 are up-regulated in TSCC Clinical Specimens
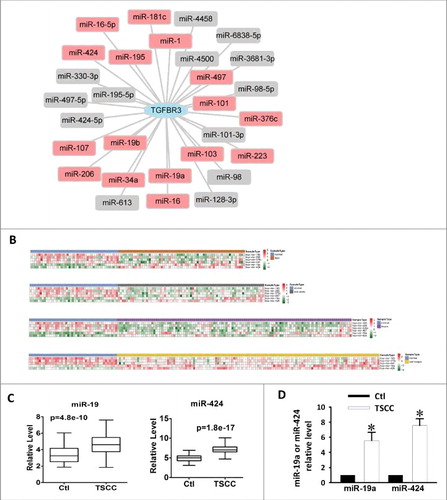
According to the miRNA-target interaction information in at least two of the nine databases analyzed (See Methods), 29 miRNAs are predicted to target TGFBR3 (). Sixteen of the 29 miRNAs were detected with the expression level in the level 3 miRNA expression profile of TSCC from The Cancer Genome Atlas (TCGA). We observed five differentially expressed miRNAs in TSCC samples compared with normal controls (FDR < 0.05, t test) (). We also found that these five miRNAs are differentially expressed in cancers of the floor of the mouth, the oral cavity and the larynx in the TCGA datasets (FDR < 0.05, t test) (). Among the five miRNAs, miR-19a was significantly up-regulated in TSCC (, p = 4.8e-10). MiR-424 was also significantly up-regulated in TSCC (, p = 1.8e-17). Finally, qRT-PCR was utilized to detect the expression of miR-19a and miR-424 in the normal tongue and TSCC specimens. Following analysis of miR-19a and miR-424 expression levels, it was found that both of these microRNAs were markedly increased in TSCC specimens (). Thus, we hypothesize that the upregulation of miR-19a and miR-424 could cause concomitant reductions in TGFBR3 expression. This suggests that TGFBR3 is a potential target of miR-19a and miR-424, and these microRNAs might participate in the progression of TSCC.
Figure 2. Upregulation of miR-19a and miR-424 in clinical TSCC specimens. (A) miRNA-TGFBR3 interaction network. The hexagon represents the TGFBR3 gene and rounded rectangles represent miRNAs. The red rectangles are the miRNAs that were detected in the expression profile of TSCC in the TCGA; additional miRNAs are colored gray. (B) Heatmap of differentially expressed miRNAs in TSCC and controls. Rows represent miRNAs and columns represent samples. Red represents high expression levels and green represents low expression values. (C) Box-plots of miR-19a and miR-424 expression in TSCC and control samples from the TCGA datasets. (D) Up-regulation of miR-19a and miR-424 in clinical TSCC specimens according to qRT-PCR. Values are shown as the mean ± SEM. n = 6 for each group, *p < 0.05 vs. control.
TGFBR3 is a direct target of miR-19a and miR-424
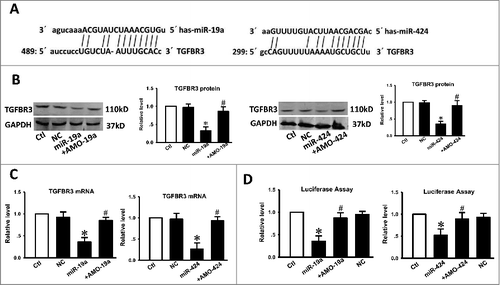
Following analysis of miR-19a, miR-424, and TGFBR3 3′-UTR sequences, we observed that the seed sites of miR-19a and miR-424 matched the TGFBR3 3′UTR (), suggesting that TGFBR3 may be a target of miR-19a and miR-424. Following transfection of miR-19a or miR-424 into CAL-27 cells, western blot analysis confirmed this hypothesis. As depicted in , TGFBR3 expression was significantly downregulated in CAL-27 cells that were transfected with miR-19a or miR-424. Conversely, TGFBR3 expression levels were restored in cells co-transfected with miR-19a and antisense-miR19a (AMO-19a) or miR-424 and antisense-miR-424 (AMO-424). To evaluate whether miR-19a or miR-424 could interfere with the TGFBR3 3′-UTR, pMIR-report constructs containing TGFBR3 binding sites for miR-19a or miR-424 were utilized. Notably, qRT-PCR demonstrated that miR-19a or miR-424 also inhibited the expression of TGFBR3 mRNA (), indicating that both miR-19a and miR-424 regulate TGFBR3 at the transcriptional level. As illustrated in , overexpression of miR-19a or miR-424 (20 nmol/L) with luciferase expression constructs carrying target fragments caused a significant reduction in luciferase activity compared with that of the negative control (NC), whereas the reduction in luciferase activity was efficiently reversed by AMO-19a or AMO-424 (10 nmol/L), suggesting that TGFBR3 is a direct target of miR-19a and miR-424.
Figure 3. The TGFBR3 gene is a direct target for miR-19a and miR-424. (A) Sequence analysis revealed the two binding sites of miRNA: mRNA complementary between miR-19a, miR-424, and human TGFBR3 gene. (B) Compared with the control, transfection of miR-19a or miR-424 resulted in a significant reduction in TGFBR3 protein expression. Co-transfection of miR-19a with AMO-19a or miR-424 with AMO-424 alleviated the reduction in TGFBR3 protein expression. (C) qRT-PCR analysis revealed that miR-19a and miR-424 markedly inhibited TGFBR3 mRNA expression. (D) Compared with the control, transfection of miR-19a or miR-424 with the luciferase reporter gene linked to the wild-type 3′-UTR of TGFBR3 resulted in a significant reduction in luciferase activity. Co-transfection of miR-19a with AMO-19a or miR-424 with AMO-424 alleviated the reduction in luciferase activity. Three independent experiments (n = 3) were performed for each condition. Data are shown as the mean ± SEM. *p < 0.05 vs. control,# p < 0.05 vs. miR-19a or miR-424 correspondingly. Ctl control, NC transfection of negative control, miR-19a transfection of miR-19a, +AMO-19a co-transfection of miR-19a and its inhibitor (AMO-19a), miR-424 transfection of miR-424, +AMO-424 co-transfection of miR-424 and its inhibitor (AMO-424).
Over-expression of TGFBR3 decreases migration and inhibits EMT of CAL-27 cells while decreasing p-p65 in vitro
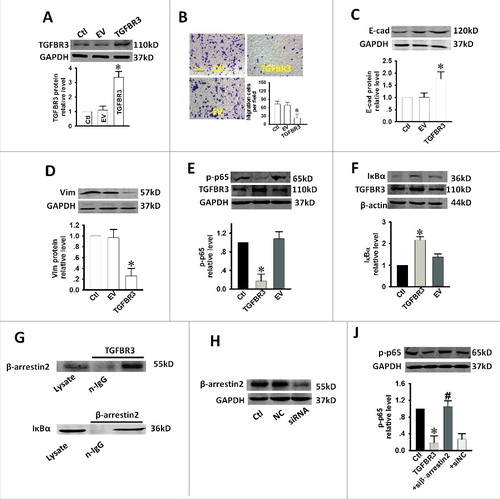
We further examined whether TGFBR3 overexpression affected cell migration in CAL-27 cells. Western blot analysis revealed that TGFBR3 expression was increased by 3.27-fold in cells treated with 1 μg/mL of TGFBR3 plasmid DNA (). As shown in , transwell assays revealed that TGFBR3 overexpression significantly inhibited cellular transmigration compared with the controls. These results strongly suggest that TGFBR3 also regulates the cell migration ability of CAL-27 cells. EMT is a central mechanism that contributes to the migration of various cancer cell types. To better quantify the EMT process, we measured the expression levels of several EMT markers including E-cadherin (E-cad) and Vimentin (Vim). We observed that over-expression of TGFBR3 enhanced E-cad expression while inhibiting Vim expression (, ). Moreover, increased NF-κB activity has been reported in diverse human malignancies, including TSCC disease. This increased activity is believed to enhance tumor cell migration by accelerating EMT.Citation12,Citation13 You et al reported that TGFBR3 is involved in NF-κB regulation via its interaction with β-arrestin 2 in MCF10A breast epithelial and MDA-MB-231 breast cancer cells. This interaction results in an increase in TGFBR3 expression and a concomitant reduction in NF-κB-mediated transcriptional activation and IκBα degradation.Citation14 It is known that β-arrestin 2 can function as an effective suppressor of UV-induced NF-κB activation through its direct interaction with IκBα.Citation15 We next investigated the effect of TGFBR3 overexpression on NF-κB activity in CAL-27 cells. Our results indicate that transient increases in TGFBR3 expression resulted in concomitant decreases and increases in p-p65 expression () and IκBα expression (), respectively. These results suggest that inhibition of NF-κB signaling represents a potential mechanism for TGFBR3-mediated inhibition of cell migration and EMT in CAL-27 cells. Our results also confirm that TGFBR3 interacts with β-arrestin 2, and β-arrestin 2 interacts with IκBα (). TGFBR3 overexpression decreased p-p65 expression, and co-transfection of TGFBR3 and β-arrestin 2 siRNA resulted in an increase in p65 phosphorylation (, ). These results suggest that TGFBR3, through its interaction with β-arrestin 2, negatively regulates NF-κB signaling in CAL-27 cells.
Figure 4. Effect of over-expression of TGFBR3 on migration, EMT and NF-κB activity in CAL-27 cells. CAL-27 cells were transfected for 24 h with 1 µg/ml of plasmid encoding TGFBR3. EV represents empty vector (1 µg/ml of pc-DNA3.1 plasmid)-transfected CAL-27 cells. (A) TGFBR3 expression was determined by western blot analysis and the average band densities from three independent experiments are shown. (B) Scale bars, 10 µm. TGFBR3 overexpression inhibits migration in CAL-27 cells. Following plasmid transfection, cells were subjected to Transwell migration. (C, D) The EMT-related markers, E-cadherin (E-cad) and Vimentin (Vim) were up-regulated and down-regulated after TGFBR3 over-expression for 24 h, respectively. (E) Transient increases in TGFBR3 expression decreased p-p65 expression. (F) Transient increases in TGFBR3 expression resulted in concomitant increases in IκBα expression. (G) TGFBR3 interacts with β-arrestin 2; β-arrestin 2 interacts with IκBα. (J) CAL-27 cells transiently transfected with β-arrestin 2 siRNA. (H) TGFBR3 overexpression decreased p-p65 expression, and co-transfection of TGFBR3 and β-arrestin 2 siRNA resulted in an increase in phosphorylation of p65. Data are presented as mean ± standard deviation following three independent experiments. *p < 0.05 vs. control; # p < 0.05 vs. TGFBR3.
miR-19a and miR-424 induce EMT and promote migration of CAL-27 cells by downregulating TGFBR3
Given the established effects known to be mediated by TGFBR3 with respect to migration and EMT in CAL-27 cells, we proposed that miR-19a or miR-424 might promote migration and EMT by targeting TGFBR3. As expected, over-expression of miR-19a and miR-424 promoted cell migration and EMT processes, while AMO-19a and AMO-424 abolished these changes (). These data suggest that miR-19a and miR-424 are stimulatory factors in relation to migration and EMT processes. In order to determine if TGFBR3 plays a critical role in EMT activities induced by miR-19a or miR-424, we transfected the TGFBR3 plasmid into CAL-27 cells. Mir-19a or miR-424-induced migration was subsequently inhibited following TGFBR3 overexpression (, ). Similarly, TGFBR3 overexpression also reduced the ability of miR-19a/miR-424 to promote EMT processes (). Accordingly, the stimulatory effects of miR-19a/miR-424 on p-p65 expression were also reversed by TGFBR3 overexpression (, ). These results suggest that TGFBR3 is a key target for miR-19a/miR-424-mediated pro-EMT effects.
Figure 5. miR-19a and miR-424 promote migration and EMT in CAL-27 cells. (A, B) Scale bars, 10 µm. Enhanced migration in CAL-27 cells by miR-19a or miR-424. N = 5. Down-regulation of E-cad (C, D) and up-regulaiton of Vim (E, F) by miR-19a and miR-424. n = 5. *p < 0.05 vs. control; # p < 0.05 vs. miR-19a or miR-424 alone.
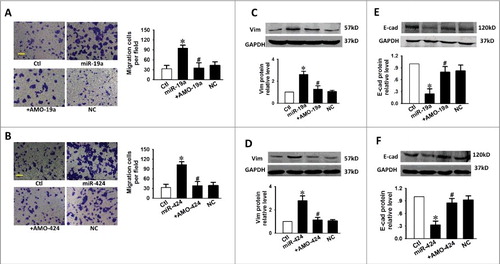
Figure 6. TGFBR3 attenuates the pro-EMT effects caused by miR-19a and miR-424 in cardiac fibroblasts. (A, B) Scale bars, 10 µm. Transwell assay indicates that TGFBR3 abolished miR-19a/miR-24-mediated migration. +TGFBR3: co-transfection of miR-19a or miR-424 and TGFBR3, +EV: co-transfection of miR-19a or miR-424 and empty vector (EV), n = 4. Effects of TGFBR3 on the EMT marker proteins, E-Cad (C, D) and Vim (E, F), following transfection with miR-19a or miR-424. (G, H) TGFBR3 reduces p-p65 expression induced by miR-19a or miR-424. n = 4, *p < 0.05 vs. control; # p < 0.05 vs. miR-19a or miR-424 alone.
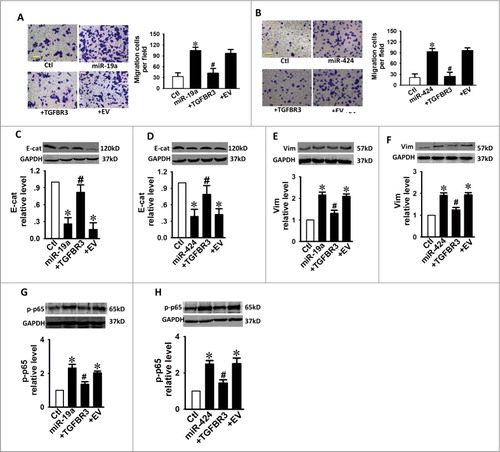
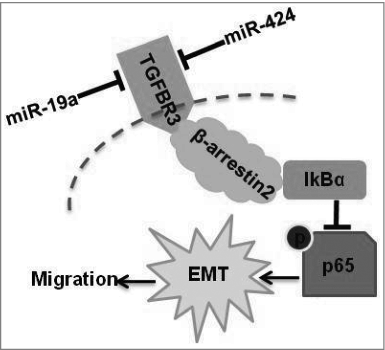
Figure 7. A diagram of miR-19a- and miR-424-mediated down-regulation of TGFBR3 expression. Down-regulation of TGFBR3 resulted in the promotion of migration, and EMT in tongue squamous cell carcinoma cells. TGFBR3 is a direct target for miR-19a and miR-424. β-arrestin 2 facilitates the interaction between TGFBR3 and IκBα and is required for TGFBR3-mediated inhibition of p-p65, EMT and migration in CAL-27 cells.
Discussion
TGFBR3 is specifically down-regulated in TSCC patients, and is implicated in the accelerated migration of various cancer cell types.Citation6,Citation7,Citation16 At the transcriptional level, TGFBR3 expression is positively regulated by dexamethasone, aldosterone, and hydrocortisone.Citation6 However, previous studies have not elucidated how TGFBR3 expression is down-regulated in various human cancer specimens. In this study, we reveal significant decreases in TGFBR3 protein and mRNA expression following over-expression of miR-19a and miR-424 in TSCC specimens (). miRNAs facilitate the control of expression levels of target genes. Thus, down-regulation of TGFBR3 expression in human TSCC disease might be due to dysregulation of miRNAs. A recent study reported that TGFBR3 is a direct target for miR-328 while also exerting inhibitory effects in relation to collagen production in cardiac fibroblastss.Citation17 However, due to the fact that a given target can be targeted by multiple miRNAs, it is essential that further studies are conducted to identify key miRNAs in the regulation of TGFBR3 expression in TSCC disease. Our results show that increased expression of miR-19a and miR-424 in tongue squamous cell carcinoma cells induces EMT by targeting TGFBR3; this causes a concomitant increase in the migration of CAL-27 cells.
Identification of key factors involved in EMT and investigations into the molecular mechanisms that underpin EMT are of critical importance in aiding our understanding of tumor metastasis. This information is crucial for the development of novel interventions for the treatment of TSCC cancer.Citation18 We also observed that over-expression of TGFBR3 independently inhibits migration and EMT progression in CAL-27 cells. However, this over-expression had no significant effect on cell proliferation (data not shown). Accordingly, Turley et al reported that the restoration of TGFBR3 expression in prostate cancer cells inhibits migration and invasion.Citation19 A study by Lambert KE et al showed that loss of TGFBR3 expression during multiple myeloma progression contributes to disease progression through its inhibitory effects on proliferation, migration, and adhesion in human myeloma cells.Citation4 A recent study revealed that transient overexpression of TGFBR3 promoted TGF-β1-induced cyclin-dependent kinase inhibitor 2b (CDKN2b) and p38 protein activity, and restores TGF-β1 sensitivity in CAL-27 cells.Citation20 Moreover, ALK-3, ALK-6, bone morphogenetic protein 4 (BMP4), MMPs, p21, p27 and additional TGFBR3 targets were identified in various tumor cells including breast cancer, renal cancer, prostate cancer and colon cancer cells.Citation21 Citation22-Citation24
Recently, new insights have been gained into the structure and function of the cytoplasmic TGFBR3 domain. The associated reports suggest that this domain plays an essential role in ligand-dependent and ligand-independent functions through interactions with β-arrestin 2 and Gα-interacting protein–interacting protein, C terminus (GIPC).Citation6,Citation25,Citation26 Lee JD et al demonstrated that TGFBR3-mediated inhibition of migration occurs following interaction of the cytoplasmic domain of TGFBR3 with GIPC.Citation27 Sun F et al observed that simvastatin alleviates cardiac fibrosis induced by infarction via up-regulation of TGFBR3. This up-regulation was found to be associated with GIPC-mediated inhibition of the ERK1/2/JNK pathway.Citation25 Most notably, a separate study reported that TGFBR3 had direct effects in relation to the regulation of cardiac myocyte hypertrophy. These effects were mediated without any cytokines or ligands and were predicated by β-arrestin 2-mediated CaMKII activation.Citation28 Moreover, NF-κB has an essential role in the initiation and progression of TSCC cancer and specifically mediates the induction of EMT.Citation12 A study has shown that β-arrestin 2 directly interacts with IκBα and prevents phosphorylation and degradation of IκBα.Citation29 Similar results were also reported by Witherow et al.Citation30 Furthermore, You et al demonstrated that TGFBR3, through its interaction with β-arrestin 2, negatively regulates NF-κB signaling in breast cancer.Citation14 These studies reveal that the nature of output signaling caused by the interaction of TGFBR3 with β-arrestin 2 or GIPC may depend upon cell type. In the present study, we confirmed that TGFBR3 is a negative regulator of NF-κB. Our results reveal a novel interaction between TGFBR3 and the scaffolding protein, β-arrestin 2, which results in TGFBR3 internalization and down-regulation of TGF-β signaling. β-arrestin 2 also scaffolds interacting receptors with IκBα (). These results are consistent with those generated by other groups. TGFBR3 has been shown to interact with the cytoplasmic domain of β-arrestin 2.
MiR-19a and miR-424 are capable of mediating EMT progression in various types of cancer cells.Citation31-Citation35 For instance, Huang L et al. revealed that miR-19a is associated with lymph metastasis and mediates TNF-α induced EMT in colorectal cancer.Citation31 Moreover, a report by Lu W et al. demonstrated that MiR-19a promotes EMT via the PI3K/AKT pathway in gastric cancer.Citation32 Recently, Li J et al found that miR-19 triggers EMT and this reaction is believed to play an important role in accelerating the migration of lung cancer cells.Citation36 Furthermore, ectopic transient and stable miR-424 expression induced EMT, with a concomitant reduction in epithelial marker expression and increased cell scattering.Citation35 Wang F et al also found that miR-424 participates in esophageal squamous cell carcinoma invasion and metastasis via smad7 pathway-mediated EMT.Citation33 Importantly, we observed that transfection of a plasmid carrying the TGFBR3 gene into CAL-27 cells not only inhibited miR-19a- or miR-424-induced migration and EMT progress, but also resulted in decreased expression of p-p65 compared with the control group. These results indicate that over-expression of miR-19a or miR-424 stimulates EMT and promotes cell migration following targeting of TGFBR3.
Table 1. Clinicopathologic Features of TSCC Samples.
In summary, we observed that over-expression of miR-19a or miR-424 resulted in down-regulation of the TGFBR3 gene in human TSCC specimens. We further demonstrated that the TGFBR3 gene is a direct target of both miR-19a and miR-424. These results further our understanding of the mechanisms that underlie EMT and migration during TSCC disease progression. The modulation of miR-19a-/miR-424-mediated targeting of the TGFBR3 pathway may potentially be exploited in the clinical treatment of TSCC in the future.
Abbreviations
| AMO-19a | = | antisense-miR19a |
| AMO-424 | = | antisense-miR424 |
| β-arrestin 2 | = | Scaffolding protein-arrestin 2 |
| co-ip | = | Co-immunoprecipitation |
| DMEM | = | Dulbecco's modified Eagle's medium |
| EMT | = | Epithelial-to-mesenchymal transition |
| GIPC | = | Gα-interacting protein–interacting protein, C-terminus |
| GAPDH | = | Glyceraldehyde 3-phosphate dehydrogenase |
| HE | = | Hematoxylin and eosin |
| IHC | = | Immunohistochemistry |
| TGFBR3 | = | Transforming growth factor type III receptor |
| TSCC | = | Tongue squamous cell carcinoma |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Letpub for providing language editing and proofreading services.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC; 81672827).
References
- Tanaka T, Tanaka M, Tanaka T. Oral carcinogenesis and oral cancer chemoprevention: a review. Patholog Res Int. 2011;2011:431246. PMID:21660266.
- Zhang T, Lubek JE, Salama A, Dyalram D, Liu X, Ord RA. Treatment of cT1N0M0 tongue cancer: outcome and prognostic parameters. J Oral Maxillofac Surg. 2014;72:406-14. doi:10.1016/j.joms.2013.05.028. PMID:24045188.
- Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339:180-9. doi:10.1016/j.mce.2011.04.014. PMID:21550381.
- Lambert KE, Huang H, Mythreye K, Blobe GC. The type III transforming growth factor-beta receptor inhibits proliferation, migration, and adhesion in human myeloma cells. Mol Biol Cell. 2011;22:1463-72. doi:10.1091/mbc.E10-11-0877. PMID:21411633.
- Meyer AE, Gatza CE, How T, Starr M, Nixon AB, Blobe GC. Role of TGF-beta receptor III localization in polarity and breast cancer progression. Mol Biol Cell. 2014;25:2291-304. doi:10.1091/mbc.E14-03-0825. PMID:24870032.
- Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163-74. doi:10.1016/j.cellsig.2010.01.016. PMID:20153821.
- Meng W, Xia Q, Wu L, Chen S, He X, Zhang L, Gao Q, Zhou H. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi:10.1186/1471-2407-11-88. PMID:21352603.
- Krutzfeldt J. Strategies to use microRNAs as therapeutic targets. Best practice & research Clinical endocrinology & metabolism. 2016;30:551-61. doi:10.1016/j.beem.2016.07.004.
- Jamali Z, Asl Aminabadi N, Attaran R, Pournagiazar F, Ghertasi Oskouei S, Ahmadpour F. MicroRNAs as prognostic molecular signatures in human head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2015;51:321-31. doi:10.1016/j.oraloncology.2015.01.008. PMID:25677760.
- Christopher AF, Gupta M, Bansal P. Micronome revealed miR-19a/b as key regulator of SOCS3 during cancer related inflammation of oral squamous cell carcinoma. Gene. 2016;594:30-40. doi:10.1016/j.gene.2016.08.044. PMID:27581787.
- Nair J, Jain P, Chandola U, Palve V, Vardhan NR, Reddy RB, Kekatpure VD, Suresh A, Kuriakose MA, Panda B. Gene and miRNA expression changes in squamous cell carcinoma of larynx and hypopharynx. Genes Cancer. 2015;6:328-40.
- Wang LJ, Zhou X, Wang W, Tang F, Qi CL, Yang X, Wu S, Lin YQ, Wang JT, Geng JG. Andrographolide inhibits oral squamous cell carcinogenesis through NF-kappaB inactivation. J Dent Res. 2011;90:1246-52. doi:10.1177/0022034511418341. PMID:21841043.
- Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu Q, Duan W, Yu S, Wang F, et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappaB pathway. Curr Med Chem. 2013;20:4185-94. doi:10.2174/09298673113209990251. PMID:23992306.
- You HJ, How T, Blobe GC. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30:1281-7. doi:10.1093/carcin/bgp071. PMID:19325136.
- Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. EMBO J. 2005;24:4237-46. doi:10.1038/sj.emboj.7600882. PMID:16308565.
- Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117:206-17. doi:10.1172/JCI29293. PMID:17160136.
- Du W, Liang H, Gao X, Li X, Zhang Y, Pan Z, Li C, Wang Y, Liu Y, Yuan W, et al. MicroRNA-328, a Potential Anti-Fibrotic Target in Cardiac Interstitial Fibrosis. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;39:827-36. doi:10.1159/000447793. PMID:27497782.
- Jiao J, Zhao X, Liang Y, Tang D, Pan C. FGF1-FGFR1 axis promotes tongue squamous cell carcinoma (TSCC) metastasis through epithelial-mesenchymal transition (EMT). Biochem Biophys Res Commun. 2015;466:327-32. doi:10.1016/j.bbrc.2015.09.021. PMID:26362179.
- Turley RS, Finger EC, Hempel N, How T, Fields TA, Blobe GC. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090-8. PMID:17283142.
- Li D, Xu D, Lu Z, Dong X, Wang X. Overexpression of transforming growth factor type III receptor restores TGF-beta1 sensitivity in human tongue squamous cell carcinoma cells. Bioscience reports. 2015;35(4):e00243. doi:10.1042/BSR20150141. PMID: 26205654.
- Lee NY, Kirkbride KC, Sheu RD, Blobe GC. The transforming growth factor-beta type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Mol Biol Cell. 2009;20:4362-70. PMID:19726563.
- Sharifi N, Hurt EM, Kawasaki BT, Farrar WL. TGFBR3 loss and consequences in prostate cancer. The Prostate. 2007;67:301-11. PMID:17192875.
- Gatza CE, Holtzhausen A, Kirkbride KC, Morton A, Gatza ML, Datto MB, Blobe GC. Type III TGF-beta receptor enhances colon cancer cell migration and anchorage-independent growth. Neoplasia. 2011;13:758-70. PMID:21847367.
- Bandyopadhyay A, Wang L, Lopez-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. The Prostate. 2005;63:81-90. PMID:15468171.
- Sun F, Duan W, Zhang Y, Zhang L, Qile M, Liu Z, Qiu F, Zhao D, Lu Y, Chu W. Simvastatin alleviates cardiac fibrosis induced by infarction via up-regulation of transforming growth factor, beta receptor III expression. Br J Pharmacol. 2015;172:3779-92. PMID:25884615.
- You HJ, Bruinsma MW, How T, Ostrander JH, Blobe GC. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007;28:2491-500. PMID:17768179.
- Lee JD, Hempel N, Lee NY, Blobe GC. The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis. 2010;31:175-83. PMID:19955393.
- Lou J, Zhao D, Zhang LL, Song SY, Li YC, Sun F, Ding XQ, Yu CJ, Li YY, Liu MT, et al. Type III Transforming Growth Factor-beta Receptor Drives Cardiac Hypertrophy Through beta-Arrestin2-Dependent Activation of Calmodulin-Dependent Protein Kinase II. Hypertension. 2016;68:654-66. PMID:27432858.
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303-17. PMID:15125834.
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603-7. PMID:15173580.
- Huang L, Wang X, Wen C, Yang X, Song M, Chen J, Wang C, Zhang B, Wang L, Iwamoto A, et al. Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-alpha induced epithelial-to-mesenchymal transition in colorectal cancer. Sci Rep. 2015;5:13350. PMID:26302825.
- Lu W, Xu Z, Zhang M, Zuo Y. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. Int J Clin Exp Pathol. 2014;7:7286-96. PMID:25400827.
- Wang F, Wang J, Yang X, Chen D, Wang L. MiR-424-5p participates in esophageal squamous cell carcinoma invasion and metastasis via SMAD7 pathway mediated EMT. Diagn Pathol. 2016;11:88. PMID:27628042.
- Xiao X, Huang C, Zhao C, Gou X, Senavirathna LK, Hinsdale M, Lloyd P, Liu L. Regulation of myofibroblast differentiation by miR-424 during epithelial-to-mesenchymal transition. Arch Biochem Biophys. 2015;566:49-57. PMID:25524739.
- Banyard J, Chung I, Wilson AM, Vetter G, Le Bechec A, Bielenberg DR, Zetter BR. Regulation of epithelial plasticity by miR-424 and miR-200 in a new prostate cancer metastasis model. Sci Rep. 2013;3:3151. PMID:24193225.
- Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, Xie RY, Wang SC, Jin W, Gao F, et al. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Laboratory investigation; a journal of technical methods and pathology. 2015;95:1056-70. PMID:26098000.
