ABSTRACT
All known splice isoforms of vascular endothelial growth factor A (VEGF-A) can bind to the receptor tyrosine kinases VEGFR-1 and VEGFR-2. We focus here on VEGF-A121a and VEGF-A165a, two of the most abundant VEGF-A splice isoforms in human tissueCitation1, and their ability to bind the Neuropilin co-receptors NRP1 and NRP2. The Neuropilins are key vascular, immune, and nervous system receptors on endothelial cells, neuronal axons, and regulatory T cells respectively. They serve as co-receptors for the Plexins in Semaphorin binding on neuronal and vascular endothelial cells, and for the VEGFRs in VEGF binding on vascular and lymphatic endothelial cells, and thus regulate the initiation and coordination of cell signaling by Semaphorins and VEGFs.Citation2 There is conflicting evidence in the literature as to whether only heparin-binding VEGF-A isoforms – that is, isoforms with domains encoded by exons 6 and/or 7 plus 8a – bind to Neuropilins on endothelial cells. While it is clear that VEGF-A165a binds to both NRP1 and NRP2, published studies do not all agree on the ability of VEGF-A121a to bind NRPs. Here, we review and attempt to reconcile evidence for and against VEGF-A121a binding to Neuropilins. This evidence suggests that, in vitro, VEGF-A121a can bind to both NRP1 and NRP2 via domains encoded by exons 5 and 8a; in the case of NRP1, VEGF-A121a binds with lower affinity than VEGF-A165a. In in vitro cell culture experiments, both NRP1 and NRP2 can enhance VEGF-A121a-induced phosphorylation of VEGFR2 and downstream signaling including proliferation. However, unlike VEGFA-165a, experiments have shown that VEGF-A121a does not ‘bridge’ VEGFR2 and NRP1, i.e. it does not bind both receptors simultaneously at their extracellular domain. Thus, the mechanism by which Neuropilins potentiate VEGF-A121a-mediated VEGFR2 signaling may be different from that for VEGF-A165a. We suggest such an alternate mechanism: interactions between NRP1 and VEGFR2 transmembrane (TM) and intracellular (IC) domains.
VEGF-A isoforms exhibit differential binding to VEGFRs and NRPs
VEGF-A is one of five VEGF genes in humans, the others being VEGF-B, -C, -D, and PlGF. VEGF-A encodes multiple splice isoforms, and there are additional proteolytic isoforms generated by post-translational enzymatic cleavage.Citation3,Citation4 All of the known isoforms share the same binding sites for receptor tyrosine kinases; as anti-parallel homodimers, VEGF-A ligands are bivalent and bind two monomers of their cognate receptors, VEGFR1 and/or VEGFR2. There are two additional functional binding domains, but these are present only in select isoforms. First, the longer splice isoforms can include heparin binding domains (encoded by exons 6 and/or 7) () that confer on those isoforms an ability to bind to, and be sequestered by, proteoglycans of the extracellular matrixCitation5 or cell surface (HSPGs).Citation3,Citation6,Citation7 Second, the ‘xxxa’ isoforms contain the short C-terminal exon 8a-encoded sequence, which can confer the ability to bind to the Neuropilin coreceptors (, ), while the ‘xxxb’ isoforms, containing the exon 8b-encoded sequence, cannot.Citation8-Citation10 All pro-angiogenic VEGF-A isoforms retain the exon 8a-encoded sequence. However, while the exon 8a-encoded sequence appears to be necessary for Neuropilin binding, experiments had not directly clarified whether it is sufficient; VEGF-A121a, for example, has been shown in some studies to bind Neuropilins and in other studies not to bind. Here we review and compare that literature.
Figure 1. Schematic of human VEGF-A splice isoforms. A, The VEGF gene contains multiple exons that can be alternately spliced to make over a dozen isoforms. One of exon 8a or exon 8b is present in most isoforms, and these isoforms are thus denoted xxxa or xxxb, where xxx is the number of amino acids. Exon 1 plus 4 amino acids of exon 2 encodes the 26-residue signal peptide, cleaved during secretion. B, The final form of VEGF is a dimer, containing two of the above sequences covalently linked in an antiparallel orientation by cysteine-cysteine bonds; this makes VEGF bivalent, and VEGF receptors (VEGFR1, R2) bind in the regions encoded by exons 3 and 4Citation61. Neuropilins (NRPs) and heparan sulfate (HS) chains bind VEGF in the region encoded by exons 6, 7 and 8a; thus isoforms have different receptor-binding characteristics depending on the encoding exons included. VEGF-A121a lacks a heparin binding domain (HBD: exons 6 and 7). The absence of this domain in VEGF-A121a (left) may also make the NRP-binding domain less flexible in terms of orientation and distance from the main body of the protein, compared to the presence of exon 7-encoded domain in VEGF-A165a (right).
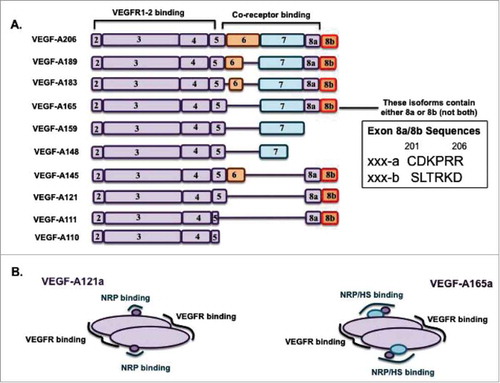
Figure 2. Schematic diagram of structure and binding characteristics of Neuropilins. NRPs are ∼ 920 residue, 130 kDa glycoproteins of the plasma membrane. The receptors consist of an extracellular domain (EC, including the ligand binding site), transmembrane domain (TM), and a short intracellular (IC) domain. The EC domain consists of two CUB calcium-binding homology domains (a1 and a2), two coagulation factor V and VII homology domains (b1 and b2) and a MAM (meprin) domain (c) critical for receptor dimerization. The b1/b2 and a1/a2 domains mediate the high-affinity binding of NRPs to their ligands – the VEGFs and the class 3 semaphorinsCitation62. The NRP cytoplasmic domain has 40 residues and allows binding of the PDZ domain-containing protein GIPC1 (or synectin)Citation49. NRP1 can form ligand-independent homodimersCitation29, and heterodimers with NRP2Citation63-Citation65 and with VEGFR2Citation48,Citation49 in the cellular plasma membrane. NRP1 (but not NRP2) has one covalent GAG (HS/CS) attachment in the EC domain at Serine 612Citation30 (conserved across speciesCitation66); The GAG modification of NRP1 enhances VEGFR2 signaling in endothelial cells by multiple mechanisms, including enhancement of VEGF-A165a binding and delayed degradation of VEGFR2 bound to VEGFCitation30.
Evidence against binding of VEGF-A121a to Neuropilins
The work of Hela Gitay-Goren, Shay Soker, Gera Neufeld, Michael Klagsbrun, and colleagues in the late 1990s elucidated the role of Neuropilins as VEGF-VEGFR2 coreceptors, and in particular showed that VEGF-A165a formed a ‘bridge’ between VEGFR2 and NRP1 by binding both receptors simultaneously.Citation11-Citation14 The authors used cross-linking to fix 125I-VEGF-A121a and 125I-VEGF-A165a to human umbilical endothelial (HUE) cells that express VEGFR1, VEGFR2, NRP1 and NRP2 at densities detectable by western blotting.Citation13 They used western blotting to assess the appearance of 125I-VEGF-A121a-bound or 125I-VEGF-A165a-bound NRP and VEGFR complexes by bands corresponding to their combined molecular weight.Citation13 In of their paperCitation13, bands corresponding to the molecular weight of NRP1+125I-VEGF-A121a did not appear with excess VEGF-A121a (10–20 ng.mL−1 ∼ 0.25–0.52 nM). Furthermore, treatment with an excess of VEGF-A121a did not inhibit formation of NRP1+125I-VEGF-A165a complexesCitation13 or does so to a small extent.Citation10 The authors concluded that either VEGF-A121a does not bind to NRPs, or that the affinity of VEGF-A121a to these receptors is lowerCitation13; competition experiments are not always sufficiently sensitive to detect low affinity ligand binding.Citation15
In another 1996 paper, the same authors used cross-linking to test whether VEGF-A165a or VEGF-A121a were capable of binding to MDA-MB-231 cells (expressing NRP1 but not VEGFR2) and HUVECs (expressing both NRP1 and VEGFR2)Citation11. As shown in figure 5 of their paper, the authors observed 5–10 ng.mL−1 of VEGF-A121a cross-linking to HUVECs but not the 231 cells, and did not observe VEGF-A121a competition with 125I-VEGF-A165a for binding to NRP1 on MDA-MB-231 cells.
In 1998, the same authors assessed whether VEGF-A121a and VEGF-A165a bound to porcine aortic endothelial (PAE) cells engineered to express VEGFR2, or NRP1, or both VEGFR2 and NRP1.Citation12 As shown in figure 5A of that paper, VEGF-A121a did not cross-link to PAE cells or PAE/NRP1 cells, but did cross-link to PAE/VEGFR2 or PAE/VEGFR2/NRP1 cells.Citation12 The difficulty in cross-linking VEGF-A121a to PAE cells in the absence of VEGFR2 could be an issue with the specific reagent used (DSS: disuccinimidyl suberate). The DSS cross-linker used here has an spacer arm length of ∼ 11.4 Å and cross-linking may depend on both the molecules (due to availability of Lysines) and the cell type (due to expression of different extracellular matrix (ECM) species).Citation16
Evidence that the exon 8a domain, which VEGF-A121a has, is responsible for Neuropilin binding
Crystal structures of NRP1 b1 domain demonstrate distinct contacts with VEGF-A exon 7 and 8a encoded domains
A VEGF-A-bound NRP1 crystal structure solved by Vander Kooi, Leahy and colleagues in 2007 demonstrated that exon 8a-encoded residues of VEGF-A165a are the direct site of interaction between NRP1 domains b1b2 and VEGF-A165a ( ofCitation17). Thus, NRP1 binding specificity towards VEGF-A isoforms is regulated by VEGF-A exon 8a-encoded sequence, not exon 7 (supplementary figure 8 ofCitation17). In 2012, Parker et al reported a crystal structure of mouse VEGF-A164a exons 7+8a-encoded sequence bound to NRP1.Citation18 The intermolecular interface, and experimental mutagenesis in the exon 8a-encoded sequence and NRP1 b1 domain residues, showed that exon 8a-encoded amino acids are critical for high affinity binding of any VEGF-A isoform to NRP1.Citation18 All NRP1-binding proteins and peptides are found to possess a C-terminal arginine.Citation17,Citation19-Citation21 The authors further showed that NRP1 has a C-terminal arginine-binding pocket in the b1 domain. Mutating VEGF's C-terminal exon 8a-encoded arginine (R164) resulted in up to 97% loss in retention of mouse VEGF-A164a by NRP1, thus this residue plays a critical role in VEGF-A/NRP1 interactions ( ofCitation18). Additional support for the key role of this arginine comes from a different VEGF ligand encoded by a different gene, VEGF-C, which can bind NRP2. Processed peptides corresponding to the VEGF-C C-terminus (219-SIIRR-223) can bind to NRP2 b1 domainCitation21, and the R223E mutation results in loss of VEGF-C binding to NRP2.Citation21
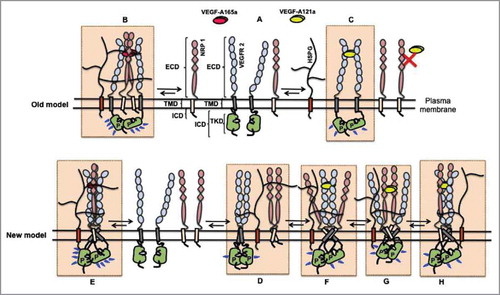
Figure 3. Models of VEGF-VEGFR2-NRP signaling. A complete picture of differential VEGFR2 phosphorylation and signaling induced by VEGF-A121a and VEGF-A165a is yet to be elucidated, and is complicated by the inclusion of HSPGs and NRPs in the signal initiation macrocomplex. The old model proposed that: (A) receptors exist only as monomers in the absence of ligands; (B) upon VEGF-A165a binding, two VEGFR2 monomers and two NRP1 monomers are bridged by the ligand, which results in formation of a macrocomplexCitation6 efficient in VEGFR2 transphosphorylation; and (C) VEGF-A121a binds only to two VEGFR2 monomers to form dimers and to activate the receptor's tyrosine kinase domain. Since VEGF-A121a does not bind to NRPs to bridge VEGFR2 and NRP1 extracellular domains in this model, it does not explain the observed modulation of VEGF-A121a signaling by NRP1. The new model explains these downstream effects by proposing two key concepts: 1) binding of VEGF-A121a to NRP1; and 2) stabilization of both VEGFR2-NRP1-VEGF-A121a (and VEGFR2-NRP1-VEGF-A165a) complexes by transmembrane and intracellular domain contacts between VEGFR2 and NRP1. In this new model: (D) VEGFR2 and NRP1 form complexes (low activity homo- and hetero-dimers) in the absence of VEGFsCitation15,Citation29,Citation48,Citation50,Citation59. These dimers are stabilized by specific ECD, TMD and ICD contacts in the absence of VEGFs. VEGFR2/NRP1 interactions are not necessary for VEGFR2 kinase activationCitation50. Furthermore, ligand-induced bridging of NRP and VEGFR2 is not necessary as contacts occur at the transmembrane and intracellular domainsCitation50,Citation67. (E) VEGF-A165a binding results in two VEGFR2 and two NRP1 monomers to form a stable, active complex. HS chains on endothelial HSPGs stabilize this complex by binding to NRP1, VEGFR2 domain 6–7 and VEGF-A165a. (F) Binding (at lower affinityCitation18,Citation27) of VEGF-A121a to NRP1 and VEGFR2 may form a weak extracellular bridge that can not be captured by immunoprecipitaion and cross-linking or (G) VEGF-A121a may alternatively only bind to VEGFR2 and activate the receptor dimer, (H) VEGF-A121a may predominantly bind to abundant NRP1 species on endothelial cell surface to activate the VEGFR2 receptor dimer. Upon binding to the receptors, VEGF-A121a and VEGF-A165a each may induce a conformationally distinct VEGFR/NRP/VEGF signaling complex (comparing E with F, G and H). The different conformations permit different signaling, but NRP can modulate bothCitation15,Citation27. Alternatively, VEGF-A121a and VEGF-A165a each may induce a VEGFR/NRP/VEGF signaling complex with similar conformation, but with different stability. The different stability would cause different levels or durations of signaling, resulting in distinct downstream cell behavior. NRP can still modulate both signaling complexes.
Though VEGF-A121a (human) and VEGF-A120a (mouse) both contain the exon 8a-encoded domain, a crystal structure for either isoform binding to NRPs is yet to be elucidated. Binding affinities of mouse VEGF-A120a and 164a to NRP1 ( ofCitation18) were measured to be 3 nM and 22 nM respectively. VEGF-A120a, which lacks exons 6 and 7 (the ‘heparin-binding domain’Citation22), binds with higher affinity to NRP2 than to NRP1; mouse VEGF-A164a, which has exon 7, binds to NRP1 with higher affinity than to NRP2 ( and 5 ofCitation18). Thus, the exon 8a-encoded arginine is essential for the VEGF-A-NRP interaction, and the exon 7-encoded domain provides additional contacts (a larger intermolecular interface) that may provide specificity towards NRP1 but not NRP2.Citation18
The crystal structure of VEGF-A165a has been determined, but as two separate fragments: a 110-amino acid fragment that includes the VEGFR-binding domains (, exons 2–5), and a 55-amino acid fragment (corresponding to exons 6–8a) that includes the NRP-binding domain. The inability to crystallize both together suggests that the two domains are not rigid with respect to each other. Thus, the NRP1-binding site likely has some range of motion (distance and orientation) from the main VEGFR-binding domain in VEGF-A165a (), due to the additional amino acids encoded by exon 7; this may make NRP binding less restricted in longer VEGF isoforms.
Mutations, peptidomimetics, and antagonists: The exon 8a-encoded tertiary fold is key to NRP1 binding
In a 2006 paper, Cébe Suarez et al showed that VEGF-A splice variants which either lack the exon 8-encoded domain entirely (i.e. VEGF-A159), or have their exon 8a replaced by exon 8b (8a: CDKPRR; 8b: SLTRKD; i.e. VEGF-A121b and VEGF-A165b), do not bind NRP1 at all (figure 4 ofCitation23). They conclude that exon 8a-encoded sequence is required for NRP1 binding, that the heparin-binding domains (exons 6 and/or 7) are not sufficient for NRP1 binding, and that exon 8a is necessary for proper folding of the heparin-binding domains, as VEGF-A165b has reduced HS binding, no NRP1 binding and altered (delayed and attenuated) signaling characteristics via VEGFR2 and ERK kinases.Citation9,Citation10,Citation23 Only VEGF-A isoforms containing the exon 8a-encoded sequence (VEGF-Axxxa; ) compete with VEGF-A165a for binding to NRP1.Citation23
In 2006, von Wronski et al demonstrated that Tuftsin, a naturally occurring short peptide antagonist mimicking the C-terminal sequence of VEGF-A (Tuftsin: TKPR; exon 8a: CDKPRR), blocks VEGF-A binding to NRP1 without blocking VEGF-A binding to VEGFR2.Citation24 The peptide competed with VEGF-A165a on NRP1 and NRP2, and displaced VEGF-A165a from endothelial cells. The authors further showed that the peptide inhibits VEGF-A165a-induced phosphorylation of VEGFR2 without directly inhibiting VEGF-A binding to VEGFR2. These results and homology between Tufstin and the exon 8a-encoded sequence of VEGF-A suggests a specific role for exon 8a's C-terminal residues in NRP1 binding.
In another 2006 article, Jia et al identified the NRP1 binding region of VEGF-A165a to be the C-terminal region of the peptide encoded by exons 7+8a, and showed that EG3287a, a VEGF-A165a mimetic bicyclic peptide with the three-dimensional conformation of VEGF-A exon 8a-encoded fold, binds to NRP1. This peptide significantly inhibited VEGF-A165a binding to NRP1 while lacking most of the exon 7-encoded domain and comprising largely of the exon 8a-encoded sequence ( ofCitation25). The peptide inhibited VEGF-A165a-induced VEGFR2, PLC-γ and ERK activation in HUVECs. The authors further found that peptides lacking VEGF-A exon 7-encoded residues and comprised largely of the exon 8a-encoded residues retained most of their inhibitory activity with reduced potency compared to EG3287a. Mutagenesis on exon 8a residues, Lysine (K), Proline (P) and Arginine (R), demonstrated that all these residues are essential for VEGF-A binding to NRP1. These results demonstrate the significance of exon 8a-encoded sequence in NRP1 binding to VEGF-A isoforms; the presence of these exon 8a-encoded residues in VEGF-A121a, therefore, suggests that that isoform can also bind NRP1.
In VEGF-A121a, domains encoded by both exons 5 and 8a are necessary for NRP binding
Delcombel et al demonstrated that VEGF-A111a (a biologically active and proteolysis-resistant isoformCitation26), which contains the exon 8a-encoded sequence, does not bind to NRP1 ( ofCitation9). Thus, the exon 8a-encoded sequence is not sufficient by itself to confer NRP1 binding. Unlike VEGF-A121a, VEGF-A111a has part of exon 5 missing, and thus Delcombel et al synthesized exon 5+8a encoded peptides. The results showed that exon 5 or 8a alone are not sufficient for NRP1 binding, and both domains must be present and intact (as is the case for VEGF-A121a), for binding to occur.Citation9
Evidence supporting direct VEGF-A121a binding to Neuropilins
Cell-free SPR demonstrates that VEGF-A121a can bind NRP1
In 2007, Pan et al examined binding of VEGF-A165a, VEGF-A121a and VEGF-A109 to NRP1 using surface plasmon resonance (SPR) methodology.Citation27 VEGF-A109 is a proteolytically-processed VEGF-A isoform which only contains exons 1–4 and most of exon 5; since it lacks the exon 8a-encoded sequence it should not and does not bind to Neuropilins. Figures 4 and 6 of that paper show that VEGF-A121a produced by a number of different sources binds to NRP1.Citation27 This shows that well-characterized VEGF-A121a, possessing the correct sequence (including exon 8a), has a three dimensional structure that enables its binding to NRP1 with binding affinity comparable to VEGF-A165a (Kd of 220 nM compared to Kd of 110 nM). Note that SPR measurements represent the affinities for the interaction of a ligand with monomeric truncated NRP1, comprising only the a1/12-b1/b2 domains and lacking the MAM domain known to induce NRP oligomerization,Citation28 the transmembrane domain known to homodimerizeCitation29 and the NRP1 GAG modificationCitation30, which may explain why these SPR-measured affinities are lower than those measured for on-cell VEGF-NRP interactions.Citation31
Pan et al and Kawamura et al emphasized the importance of expression, purification or purchase of sequenced VEGF isoforms that are not degraded and have the correct exon 8a-encoded sequence and tertiary structure for proper receptor binding, and suggest that lack of VEGF-A121a/NRP1 binding in previous experiments could be due to inadvertently cleaved VEGF-A121a lacking the exon 8a-encoded sequence, as demonstrated in and 4C of.Citation27,Citation32 Other technical considerations for any experiment probing ligand-receptor interactions in general include: 1) the presence of tags (e.g. myc, Flag) on purified or purchased VEGF-A121a that may sterically interfere with ligand-receptor binding; and 2) expression and purification in E. coli instead of mammalian cells, which may cause incorrect posttranslational modifications or VEGF-A disulfide bond formation, or low affinity of VEGF-A121a due to improper folding/structure.
In 2012, Parker et al also used SPR to demonstrate that VEGF-A121a binds to NRP1.Citation18 In 2013 Delcombel et al also confirmed via SPR that VEGF-A121a bound to NRP1 with lower affinity compared to VEGF-A165a, and with distinct kinetics ( ofCitation9). Going further, using multiple VEGF-Axxxa and VEGF-Axxxb isoforms, Delcombel et al demonstrated that: all VEGF-Axxxa isoforms containing exon 8a encoded sequence except VEGF-A111a bind to NRP1. As noted above, this one exception (VEGF-A111a) is likely due to the lack of part of exon 5 encoded sequence on the VEGF-A111a isoform. The authors suggest that the lack of observed NRP1-binding by VEGF-A121a in earlier publicationsCitation11-Citation13 is due to degradation of exon 8a encoded sequence during expression and preparation of the ligand or during the experiments. They also showed that VEGF-Axxxb isoform failing to bind NRP1 is due to lack of exon 8a encoded sequence/tertiary fold and is not due to the presence of exon 8b encoded sequence/tertiary fold.Citation9
Parker et al also showed that VEGF-A120a (mouse) binds to both NRP1 and NRP2. However, VEGF-A120a binds to NRP2 with higher affinity than VEGF-A164a (mouse)Citation33 (as summarized in Figure 6 ofCitation18). VEGF-A164a (mouse) binds to NRP2 with close to 50-fold lower affinity as compared to its binding to NRP1.Citation18
VEGF-A121a and VEGF-A165a binding to NRP1 and NRP2 on the cell surface
VEGF-A165a/NRP binding can be experimentally measured in endothelial cells expressing only NRP1 or NRP2.Citation11,Citation14,Citation31 VEGF-A121a, however, does not appear to bind to porcine aortic endothelial cells expressing only NRP1Citation11. Shraga-Heled et al showed that VEGF-A121a binding affinity to VEGFR2 is ∼10 fold higher in the presence of NRPsCitation15, and coexpression of NRPs and VEGFRs on the same endothelial cell may affect VEGF-A121a association with NRPs.Citation14,Citation15 As discussed later, HSPG binding to VEGFR2 and NRP1 may also affect VEGF-A121a association.
VEGF-A165a binds NRP1 with higher affinity than to NRP2 (kd ∼100–300 pM for NRP1Citation14,Citation31, ∼700 pM for NRP2Citation14; as noted in the previous section, the measured cell surface affinities are stronger than in cell-free systems). VEGFR2 phosphorylation on Y951 and Y1175 is lower for VEGF-A121a-ligated receptor complexes than for VEGF-A165a-ligated complexes in the presence of NRP1, but higher for VEGF-A121a-ligated complexes in the presence of NRP2 (Figure 5 ofCitation15). This suggests that there are distinct mechanisms by which NRP1 and 2 modulate VEGF-A121a-mediated VEGFR2 activation.
Evidence supporting Neuropilins modulating VEGF-A121a-induced signaling
Anti-NRP1 antibodies inhibit VEGF-A121a-induced EC migration and sprouting
In a cell-free system, Pan et al showed that an anti-NRP1B antibody blocks NRP1 binding to VEGF-A165a and VEGF-A121a immobilized on a surface (Figure 4 E-F ofCitation27). The same anti-NRPB antibody also inhibits VEGF-A165a-induced and VEGF-A121a-induced HUVEC migration and sprouting, and it did not affect VEGF-A109-induced migration ( ofCitation27).
Extracellular ‘Bridging’ of VEGFR2 and NRP1 by VEGF is not necessary for NRP1 modulation of VEGFR2 activation
In a 2007 paper, Shraga-Heled, Neufeld, and colleagues characterized VEGF-A165aKF, a VEGF-A165a mutant that does not bind to VEGFR1 or VEGFR2 but does bind to NRP1, NRP2 and HSPGs. This mutant induced significant phosphorylation of VEGFR2 on Y951 and Y1175, and ERK1/2 phosphorylation, in PAE/VEGFR2/NRP1 cells even at low VEGF-A165KF concentrations; but did not activate VEGFR2 in cells that lacked NRP1 ( ofCitation15). Thus, VEGF-A165a activation of VEGFR2 appears to be possible without the simultaneous binding (so called ‘extracellular bridging’) of VEGF-A165a to NRP1 and VEGFR2. This is important, as it appears that VEGF-A121a cannot bridge these two receptors extracellularly.
VEGF-A165aKF also induced substantial angiogenesis in vitro in PAE/VEGFR2/NRP1 cells and in HUVECs ( ofCitation15,Citation34,Citation35 ). Similarly, using PAE/VEGFR2, PAE/VEGFR2/NRP1 and PAE/VEGFR2/NRP2 cell lines expressing similar VEGFR2 levels, the authors showed that both VEGF-A165a and VEGF-A121a substantially enhanced VEGFR2 activation (Y951 and Y1175 phosphorylation) when NRP1 is present (Figure 5 ofCitation15). Furthermore, siRNA knockdown of NRP1 inhibited VEGF-A121a-induced, VEGF-A165aKF-induced, and VEGF-A165a-induced HUVEC proliferation (Figure 6 ofCitation15). A combination of siRNA against both NRP1 and NRP2 inhibited EC proliferation induced by either isoform more potently than NRP1-only siRNA, demonstrating that NRP2 also contributes to VEGF-A121a- and VEGF-A165a-induced VEGFR2 signaling in ECs (Figure 6 ofCitation15). VEGF-A165a promoted formation of stable VEGFR2-VEGF-A-NRP1 complexes (Figure 7 ofCitation15). According to the authors, while the VEGFR2-VEGF-A165a-NRP1 complexes survive immunoprecipitation, the VEGFR2-NRP1-VEGF-A165aKF and VEGFR2-NRP1-VEGF-A121a only do so at higher concentrations of ligand (1µg/mL).Citation15,Citation32
Thus, it appears that NRP1 can enhance VEGF-A signaling – including VEGF-A121a-induced VEGFR2 signaling – through mechanisms other than VEGFR2-NRP extracellular bridging. We note that in that paperCitation15, the authors state that “VEGF-A121a does not bind to Neuropilins,” however, based on their own results and those of other publications described above, it is more likely that it can (certainly it does in cell-free systems), and that the mechanisms may be different than for VEGF-A165a. Whether or not VEGF-A121 binds neuropilins in appreciable quantities on cells in vitro, or on cells in vivo where VEGF concentrations are lower, is less certain. Regardless, the impact of NRP on VEGF-A121a signaling suggests that, in the absence of VEGF-A121a mediating a weak extracellular bridging of VEGFR2 and NRP1 not captured by immunoprecipitation or cross-linking, then either: 1) VEGF-A121a binds to NRP1 and affects VEGFR2 signaling without bridging; and/or 2) VEGF-A121a binds only VEGFR2 and its signaling is modulated by NRP1 without bridging. In either case, VEGFR2-NRP1 interactions at the transmembrane and intracellular level could explain this (see next section).
NRP1 and NRP2 both enhance and modulate VEGF-A121a- and VEGF-A165a-induced VEGFR2 activation and signaling in HUVECs.Citation15 Interestingly, Shraga-Heled et al further demonstrated that VEGF-A165aKF (a VEGF-A165a mutant with impaired VEGFR binding but intact NRP and HSPG binding) did not modulate VEGFR2 or ERK phosphorylation in the presence of NRP2, suggesting that extracellular bridging by VEGF-A165a might be required for NRP2 to enhance VEGFR2 signaling. NRP2 appears to present VEGF-A165a to VEGFR2 (i.e. to act as a concentrator/reservoir of VEGF-A), since VEGF-A165aKF does not induce VEGFR2 or ERK1/2 phosphorylation in the presence of NRP2.Citation15
Evidence for modulation of VEGF and NRPs by HSPGs
Heparan sulfate proteoglycans (HSPGs) consist of a core protein and two or three attached heparan sulfate (HS) or chondroitin sulfate (CS) chains.Citation36 The abundant negatively-charged HS/CS chains of endothelial-cell-surface proteoglycans bind VEGF-A165a and, as with HS/CS chains in the extracellular matrix, can differentially regulate accessibility/storage of this isoform (and other isoforms with a heparin-binding domain, with affinities depending on the exon 6–8 combinationsCitation37 and tertiary fold). These HS/CS chains can also modify the ability of VEGF isoforms to bind to VEGFR2 and NRPs, which themselves interact with and colocalize with cell surface HSPGs (VEGFR2 was shown to directly interact with HS chains on HSPGs expressed in endothelial cells via a stretch of residues between D6-D7 of VEGFR2Citation38-Citation41; heparin also binds to VEGFR1Citation42-Citation44, NRP1Citation17 and NRP2Citation45 independent of VEGFs).
Heparin and HS are known to increase the affinity of VEGF-A165a for VEGFRs and NRPsCitation46. In contrast, VEGF-A121a lacks both exons 6 and 7 and does not bind to HS, as demonstrated by multiple assays.Citation10,Citation32 However, HS appears to be required for binding of VEGF-A121a to VEGFR1.Citation43 There are effects downstream of receptor binding too: in vivo alteration of HS biosynthesis, or inhibition of the HS-VEGFR interaction, inhibit VEGF-A121a-induced hyperpermeability; and removal of the HS chains decreases VEGF-A121a/VEGFR2/NRP1 and VEGF-A165a/NRP1/VEGFR2 assembly level and attenuates effective VEGFR2 phosphorylation in endothelial cells.Citation39
NRP1 was shown to interact with HS via a streak of positive residues stretching over both extracellular b1-b2 domainsCitation45; a 3-O sulfated modification specifically facilitates this binding.Citation38 Additionally NRP1-specific GAG modification of HS/CS chain on Serine 612Citation30 can enhance VEGF-A165a binding to the NRP core protein, which may play a role in the VEGF-A165a-responsiveness of endothelial cells.
A new model: VEGFR-NRP interactions outside of the extracellular domain
The extracellular domains of VEGFR2 and NRP1 interact very weakly with one another or not at allCitation27,Citation47. This supported the concept that VEGF needed to ‘extracellularly bridge’ VEGFR2 and NRP1 in order for NRP1 to enhance VEGFR2 activation. However, while NRP1 substantially increases VEGF-A121a affinity to VEGFR2 and VEGF-A121a activation of VEGFR2Citation15,Citation27, as described earlier, VEGF-A121a cannot bridge the extracellular domains of VEGFR2 and NRP1 as VEGF-A165a can. Therefore, ‘extracellular bridging’ appears not to be required for NRP1 enhancement of VEGFR2 activation, suggesting that NRP1 and VEGFR2 also associate in a different, ligand-independent, non-extracellular domain way. Thus, we need a new model. Interactions of NRP1 with VEGFR2 via transmembrane (TM) and intracellular (IC) domain contacts are a strong possibility.Citation9,Citation15,Citation48 Evidence of functional cytoplasmic interactions between VEGFR2 and NRP1 was presented by Prahst et al in 2008. Blockade of VEGFR2 phosphorylation disrupts formation of VEGFR2-NRP1 complexes, and removal of the IC domain of NRP1 reduces the number of VEGFR/NRP/VEGF-A165a complexes.Citation49 If intracellular domain contacts can supplement or replace extracellular ligand bridging, then NRP1 can impact VEGF-A121a activation of VEGFR2. This mechanism may even occur in the absence of VEGF-A121a binding to NRP1, as it would be the interaction between VEGFR2 and NRP1 that would be key. Sequence-specific contacts at the transmembrane and intracellular domains in VEGFR2-NRP interactionsCitation29,Citation48-Citation50 can provide a mechanistic interpretation for the Shraga-Heled et al results demonstrating enhancement of VEGFR2 activation by VEGF-A165a, VEGF-A121a, and VEGF-A165aKF (a VEGF-A165a mutant with impaired VEGFR binding but intact NRP and HSPG binding).
Assembly via homo- and heterodimerization is common among integral membrane proteins as demonstrated for multiple receptor tyrosine kinase families.Citation51 All 58 RTK transmembrane domains show a self-association propensity as measured by the TOXCAT assay.Citation52 VEGFR2 homodimerizes in the absence and presence of VEGFs.Citation50 VEGFRs form heterodimers as well: intact VEGFR2/VEGFR3 and VEGFR2/VEGFR1 dimers form as demonstrated by a number of biochemical techniques including PLA and co-immunoprecipitation.Citation53-Citation56 NRP1, though not an RTK, homodimerizes in the absence of VEGFsCitation29, and the transmembrane domain plays a role in NRP-NRP interactions. The NRP1 transmembrane domain includes two GxxxG dimerization motifs in the form of GxxxGxxxG, (where G is an amino acid with a small side chain such as Glycine, Serine or Alanine); this motif is long recognized to promote higher order structures of alpha-helical transmembrane domainsCitation57,Citation58. There is also evidence for the formation of VEGFR2-NRP1 dimers on endothelial cells, in the absence and presence of VEGFs. Prahst et al and others have shown by co-immunoprecipitation that NRP1 and VEGFR2 heterodimerize in the absence and presence of VEGFs.Citation48,Citation49 A number of studies have used other techniques to report evidence for spontaneously formed (pre-formed) VEGFR2-NRP1 complexes.Citation15,Citation48,Citation59 In addition, NRP1 D320K (NRP1 with impaired VEGF binding) is found to be capable of regulating VEGFR2 activationCitation48, and NRP1 modulates VEGFR2 levels independent of VEGF binding to NRP1.Citation15,Citation49,Citation59 Dimerization propensity of NRP1 TM domains has been measured using reporter genes in ToxLuc and FRET assays in detergent micelles. Roth et al demonstrated that the TM domain drives NRP1 homodimerization as mutation of the GxxxG motifs in the TM domains diminished NRP1-induced Sema3A activation.Citation29 Thus, this motif is important for NRP1-involved oligomerization with VEGFR and Semaphorin family receptor complexes in the plasma membrane and biological function of NRP1 requires the GxxxGxxxG motif integrity. NRP1-NRP1 and NRP1-VEGFR2 complexes potentially modulate signaling upon differential binding to ligands and/or formation of distinct VEGFR2-NRP1 dimer conformations (dimer structures) upon binding to VEGF-A121a and VEGF-A165a ().
Conclusions
Binding of the VEGF-A165a C-terminal exon 8a-encoded domain to NRP1 has been captured via crystallography (Figure 6 ofCitation18, ofCitation17). NRP1 binding to VEGF-A is isolated to C-terminal portion of VEGF-A165a and VEGF-A165a exon 8a encoded residues are indispensible for NRP1 binding.Citation9,Citation18,Citation23,Citation24,Citation60 Crystallographic studies are not yet available for intact VEGF-A121a, but this ligand has the entire exon 5 and exon 8a encoded sequence intact and is shown by the SPR technique (cell-free) to bind NRP1, with lower affinity compared to VEGF-A165aCitation18,Citation27 and to NRP2 with similar affinity as NRP1.Citation18 Modulation of VEGF-A121a-VEGFR2 signaling by the expression of (or interference with) NRP1 on cells in vitro suggests that these components are interacting (or at least interdependent), although it is also established that VEGF-A121a cannot form the extracellular VEGFR2-VEGF-NRP1 bridge that VEGF-A165a is capable of forming (or if a weak extracellular VEGFR2-VEGF-A121a-NRP1 bridge exists, it can not be measured experimentally).
A VEGF-A165a mutant (VEGF-A165aKF) that binds to HSPGs and NRPs but not VEGFRs, demonstrates that ‘extracellular bridging’ of VEGFR2 and NRP1 by VEGF-A, previously thought central to the modulation of VEGFR2 signaling by NRP1, is not required for that modulation.Citation15 Here, we proposed an alternate model for NRP modulation of VEGFR2 signaling, through transmembrane and intracellular association (specific contacts) rather than extracellular bridging (). Interestingly, although the evidence points to the ability of VEGF-A121a to bind NRP1 and NRP2 in a cell-free system, this model does not require that this binding interaction occur on the endothelial cells in vitro; rather than a VEGFR2-VEGF-NRP1 extracellular bridge, this would be a VEGF-VEGFR2-NRP1 complex, with the VEGF-A-VEGFR2 and/or VEGF-A-NRP binding extracellularly and the VEGFR2-NRP1 binding via transmembrane and intracellular domains.
Based on this proposed model, the interactions between VEGFR2 and NRP1 at the transmembrane and intracellular domains joins ligand binding specificity, HSPG interactions and priming of VEGFRs and NRPs towards VEGF-A121a binding, receptor dimerization propensity, and conformational changes in the VEGFR kinase domainCitation50 as key regulators of active VEGFR2-NRP1-VEGF-A signaling complexes on the cell surface (). If this interaction mechanism mediated by transmembrane domains and intracellular domains exists, it may be important for VEGF-A165a signaling as well.
Abbreviations
| RTK | = | Receptor Tyrosine Kinase |
| VEGF | = | Vascular endothelial growth factor |
| ECD | = | Extracellular domain |
| TMD | = | Transmembrane domain |
| ICD | = | Intracellular domain |
| TKD | = | Tyrosine kinase domain |
| HBD | = | heparin binding domain |
| GAGs | = | Glycosaminoglycans |
| HSPG | = | heparin sulfate proteoglycan |
| VEGFR | = | Vascular endothelial growth factor receptor |
| NRP | = | Neuropilin |
| PlGF | = | Placenta growth factor |
| SEMA3A | = | Semaphorin-3A |
| ECM | = | Extracellular matrix |
| DSS | = | Disuccinimidyl suberate |
| SPR | = | Surface Plasmon Resonance |
| PLA | = | proximity ligation assay |
| CO-IP | = | co-immunoprecipitation |
| HUEs or HUVECs | = | Human umbilical vascular endothelial cells |
| ECs | = | Endothelial cells |
| PAECs or PAOECs | = | Porcine aortic endothelial cells |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant R01-HL101200.
References
- Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97:978-85. doi:10.1038/sj.bjc.6603923. PMID:17912242.
- Guo HF, Vander Kooi CW. Neuropilin Functions as an Essential Cell Surface Receptor. J Biol Chem. 2015;290:29120-6. doi:10.1074/jbc.R115.687327. PMID:26451046.
- Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1-19. doi:10.1016/j.cytogfr.2013.11.002. PMID:24332926.
- Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2010;1804:567-80. doi:10.1016/j.bbapap.2009.09.002.
- Gengrinovitch S, Berman B, David G, Witte L, Neufeld G, Ron D. Glypican-1 is a VEGF(165) binding proteoglycan that acts as an extracellular chaperone for VEGF(165). J Biol Chem. 1999;274:10816-22. doi:10.1074/jbc.274.16.10816. PMID:10196157.
- Teran M, Nugent MA. Synergistic Binding of Vascular Endothelial Growth Factor-A and Its Receptors to Heparin Selectively Modulates Complex Affinity. J Biol Chem. 2015;290:16451-62. doi:10.1074/jbc.M114.627372. PMID:25979342.
- Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellen L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell. 2006;10:625-34. doi:10.1016/j.devcel.2006.03.009. PMID:16678777.
- Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487-95. doi:10.1242/jcs.016410. PMID:18843117.
- Delcombel R, Janssen L, Vassy R, Gammons M, Haddad O, Richard B, Letourneur D, Bates D, Hendricks C, Waltenberger J, et al. New prospects in the roles of the C-terminal domains of VEGF-A and their cooperation for ligand binding, cellular signaling and vessels formation. Angiogenesis. 2013;16:353-71. doi:10.1007/s10456-012-9320-y. PMID:23254820.
- Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683-92. doi:10.1158/0008-5472.CAN-07-6577. PMID:18559514.
- Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF(165) via its exon 7-encoded domain. J Biol Chem. 1996;271:5761-7. doi:10.1074/jbc.271.10.5761. PMID:8621443.
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735-45. doi:10.1016/S0092-8674(00)81402-6. PMID:9529250.
- GitayGoren H, Cohen T, Tessler S, Soker S, Gengrinovitch S, Rockwell P, Klagsbrun M, Levi BZ, Neufeld G. Selective binding of VEGF(121) to one of the three vascular endothelial growth factor receptors of vascular endothelial cells. J Biol Chem. 1996;271:5519-23. doi:10.1074/jbc.271.10.5519. PMID:8621410.
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected]. J Biol Chem. 2000;275:18040-5. doi:10.1074/jbc.M909259199. PMID:10748121.
- Shraga-Heled N, Kessler O, Prahst C, Kroll J, Augustin H, Neufeld G. Neuropilin-1 and neuropilin-2 enhance VEGF121 stimulated signal transduction by the VEGFR-2 receptor. FASEB J. 2007;21:915-26. doi:10.1096/fj.06-6277com. PMID:17185751.
- Peters K, Richards FM. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem 1977;46:523-51. doi:10.1146/annurev.bi.46.070177.002515. PMID:409338.
- Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci U S A. 2007;104:6152-7. doi:10.1073/pnas.0700043104. PMID:17405859.
- Parker MW, Xu P, Li X, Vander Kooi CW. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem. 2012;287:11082-9. doi:10.1074/jbc.M111.331140. PMID:22318724.
- Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A. 2009;106:16157-62. doi:10.1073/pnas.0908201106. PMID:19805273.
- Starzec A, Ladam P, Vassy R, Badache S, Bouchemal N, Navaza A, du Penhoat CH, Perret GY. Structure-function analysis of the antiangiogenic ATWLPPR peptide inhibiting VEGF(165) binding to neuropilin-1 and molecular dynamics simulations of the ATWLPPR/neuropilin-1 complex. Peptides. 2007;28:2397-402. doi:10.1016/j.peptides.2007.09.013. PMID:17983687.
- Parker MW, Linkugel AD, Goel HL, Wu T, Mercurio AM, Vander Kooi CW. Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure. 2015;23:677-87. doi:10.1016/j.str.2015.01.018. PMID:25752543.
- Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure with Folding & Design 1998;6:637-48. doi:10.1016/S0969-2126(98)00065-3..
- Suarez SC, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067-77. doi:10.1007/s00018-006-6254-9. PMID:16909199.
- von Wronski MA Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, Ramalingam K, Arunachalam T, Eaton S, Linder KE, et al. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702-10. doi:10.1074/jbc.M511941200. PMID:16371354.
- Jia H, Bagherzadeh A, Hartzoulakis B, Jarvis A, Lohr M, Shaikh S, Aqil R, Cheng L, Tickner M, Esposito D, et al. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J Biol Chem. 2006;281:13493-502. doi:10.1074/jbc.M512121200. PMID:16513643.
- Mineur P, Colige AC, Deroanne CF, Dubail J, Kesteloot F, Habraken Y, Noel A, Voo S, Waltenberger J, Lapiere CM, et al. Newly identified biologically active and proteolysis-resistant VEGF-A isoform VEGF111 is induced by genotoxic agents. J Cell Biol. 2007;179:1261-73. doi:10.1083/jcb.200703052. PMID:18086921.
- Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049-56. doi:10.1074/jbc.M703554200. PMID:17575273.
- Barton R, Driscoll A, Flores S, Mudbhari D, Collins T, Iovine MK, Berger BW. Cysteines in the Neuropilin-2 MAM Domain Modulate Receptor Homooligomerization and Signal Transduction. Biopolymers. 2015;104:371-8. doi:10.1002/bip.22619. PMID:25656526.
- Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Cremel G, Hubert P, Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646-54. doi:10.1091/mbc.E07-06-0625. PMID:18045991.
- Shintani Y, Takashima S, Asano Y, Kato H, Liao YL, Yamazaki S, Tsukamoto O, Seguchi O, Yamamoto H, Fukushima T, et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. Embo J. 2006;25:3045-55. doi:10.1038/sj.emboj.7601188. PMID:16763549.
- Vintonenko N, Pelaez-Garavito I, Buteau-Lozano H, Toullec A, Lidereau R, Perret GY, Bieche I, Perrot-Applanat M. Overexpression of VEGF189 in breast cancer cells induces apoptosis via NRP1 under stress conditions. Cell Adh Migr. 2011;5:332-43. doi:10.4161/cam.5.4.17287. PMID:21897119.
- Kawamura H, Li XJ, Goishi K, van Meeteren LA, Jakobsson L, Cebe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer K, Kjellen L, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112:3638-49. doi:10.1182/blood-2007-12-125856. PMID:18664627.
- Parker MW, Xu P, Guo HF, Vander Kooi CW. Mechanism of selective VEGF-A binding by neuropilin-1 reveals a basis for specific ligand inhibition. PLoS One. 2012;7:e49177. doi:10.1371/journal.pone.0049177. PMID:23145112.
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249-58. PMID:10504330.
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. Faseb J. 2001;15:447-57. doi:10.1096/fj.00-0139com. PMID:11156960.
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030-7. doi:10.1038/nature05817. PMID:17460664.
- El-Sheikh A, Liu C, Huang HN, Edgington TS. A novel vascular endothelial growth factor heparin-binding domain substructure binds to glycosaminoglycans in vivo and localizes to tumor microvascular endothelium. Cancer Res. 2002;62:7118-23. PMID:12460934.
- Thacker BE, Seamen E, Lawrence R, Parker MW, Xu Y, Liu J, Vander Kooi CW, Esko JD. Expanding the 3-O-Sulfate Proteome–Enhanced Binding of Neuropilin-1 to 3-O-Sulfated Heparan Sulfate Modulates Its Activity. ACS Chem Biol. 2016;11:971-80. doi:10.1021/acschembio.5b00897. PMID:26731579.
- Xu D, Fuster MM, Lawrence R, Esko JD. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J Biol Chem. 2011;286:737-45. doi:10.1074/jbc.M110.177006. PMID:20974861.
- Chiang MK, Flanagan JG. INTERACTIONS BETWEEN THE FLK-1 RECEPTOR, VASCULAR ENDOTHELIAL GROWTH-FACTOR, AND CELL-SURFACE PROTEOGLYCAN IDENTIFIED WITH A SOLUBLE RECEPTOR REAGENT. Growth Factors 1995;12:1-10. doi:10.3109/08977199509003208. PMID:8527158.
- Dougher AM, Wasserstrom H, Torley L, Shridaran L, Westdock P, Hileman RE, Fromm JR, Anderberg R, Lyman S, Linhardt RJ, et al. Identification of a heparin binding peptide on the extracellular domain of the KDR VEGF receptor. Growth Factors 1997;14:257-68. doi:10.3109/08977199709021524. PMID:9386990.
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 1993;90:10705-9. doi:10.1073/pnas.90.22.10705. PMID:8248162.
- Cohen T, Gitaygoren H, Sharon R, Shibuya M, Halaban R, Levi BZ, Neufeld G. Vegf(121), a Vascular Endothelial Growth-Factor (Vegf) Isoform Lacking Heparin-Binding Ability, Requires Cell-Surface Heparan Sulfates for Efficient Binding to the Vegf Receptors of Human-Melanoma Cells. J Biol Chem. 1995;270:11322-6. doi:10.1074/jbc.270.19.11322. PMID:7744769.
- Park M, Lee ST. The fourth immunoglobulin-like loop in the extracellular domain of FLT-1, a VEGF receptor, includes a major heparin-binding site. Biochem Biophys Res Commun. 1999;264:730-4. doi:10.1006/bbrc.1999.1580. PMID:10544000.
- Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818-25. doi:10.1074/jbc.M200730200. PMID:11986311.
- Tessler S, Rockwell P, Hicklin D, Cohen T, Levi BZ, Witte L, Lemischka IR, Neufeld G. Heparin Modulates the Interaction of Vegf(165) with Soluble and Cell-Associated Flk-1 Receptors. J Biol Chem. 1994;269:12456-61. PMID:8175651.
- Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690-5. PMID:10842181.
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, Gu C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. Elife. 2014;3:e03720. doi:10.7554/eLife.03720. PMID:25244320.
- Prahst C, Heroult M, Lanahan AA, Uziel N, Kessler O, Shraga-Heled N, Simons M, Neufeld G, Augustin HG. Neuropilin-1-VEGFR-2 complexing requires the PDZ-binding domain of neuropilin-1. J Biol Chem. 2008;283:25110-4. doi:10.1074/jbc.C800137200. PMID:18628209.
- Sarabipour S, Ballmer-Hofer K, Hristova K. VEGFR-2 conformational switch in response to ligand binding. Elife. 2016;5:e13876. doi:10.7554/eLife.13876. PMID:27052508.
- Sarabipour S. Parallels and Distinctions in FGFR, VEGFR, and EGFR Mechanisms of Transmembrane Signaling. Biochemistry. 2017;56:3159-73. doi:10.1021/acs.biochem.7b00399. PMID:28621531.
- Finger C, Escher C, Schneider D. The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Sci Signal. 2009;2:ra56. doi:10.1126/scisignal.2000547. PMID:19797273.
- Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:972. doi:10.1038/ncomms1977. PMID:22828632.
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243-50. doi:10.1182/blood-2005-11-4447. PMID:16621967.
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973-9. doi:10.1074/jbc.M304499200. PMID:12881528.
- Huang K, Andersson C, Roomans GM, Ito N, Claesson-Welsh L. Signaling properties of VEGF receptor-1 and -2 homo- and heterodimers. Int J Biochem Cell Biol. 2001;33:315-24. doi:10.1016/S1357-2725(01)00019-X. PMID:11312102.
- Senes A, Gerstein M, Engelman DM. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J Mol Biol. 2000;296:921-36. doi:10.1006/jmbi.1999.3488. PMID:10677292.
- Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296:911-9. doi:10.1006/jmbi.1999.3489. PMID:10677291.
- Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem. 2001;276:25520-31. doi:10.1074/jbc.M102315200. PMID:11333271.
- von Wronski MA Tweedle MF, Nunn AD. Binding of the C-terminal amino acids of VEGF121 directly with neuropilin-1 should be considered. FASEB J. 2007;21:1292; author reply 3. doi:10.1096/fj.07-0504ufm. PMID:17470574.
- Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271:5638-46.
- Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069-76. doi:10.1074/jbc.M201681200. PMID:11886873.
- Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902-12. doi:10.1038/sj.emboj.7601906. PMID:17989695.
- Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766-76. doi:10.1091/mbc.E09-12-1061. PMID:21653826.
- Sawma P, Roth L, Blanchard C, Bagnard D, Cremel G, Bouveret E, Duneau JP, Sturgis JN, Hubert P. Evidence for New Homotypic and Heterotypic Interactions between Transmembrane Helices of Proteins Involved in Receptor Tyrosine Kinase and Neuropilin Signaling. J Mol Biol. 2014;426:4099-111. doi:10.1016/j.jmb.2014.10.007. PMID:25315821.
- Esko JD, Zhang LJ. Influence of core protein sequence on glycosaminoglycan assembly. Curr Opin Struct Biol. 1996;6:663-70. doi:10.1016/S0959-440X(96)80034-0. PMID:8913690.
- Antoniu SA, Kolb MR. Intedanib, a triple kinase inhibitor of VEGFR, FGFR and PDGFR for the treatment of cancer and idiopathic pulmonary fibrosis. IDrugs. 2010;13:332-45. PMID:20432191.
