ABSTRACT
Introduction: Trophoblast homing to maternal spiral arteries is mandatory for successful placentation. Cell-cell adhesion molecules regulate this process and adhesion molecule expression is altered in impaired placentation. We hypothesize that, similar to immune cell recruitment, trophoblast cell adherence and rolling are primarily mediated by adhesion molecules like, cadherins, immunoglobulins, selectins and their partnering ligands. Here, the interdependence of adhesion molecule expression in trophoblastic cell lines of diverse origin was investigated in relation to their interaction with endothelial cell networks on Matrigel® co-cultures and the effect of specific adhesion molecule knockdown analyzed. Methods: Trophoblastic cells were labeled in red and co-cultured with green HUVEC networks on Matrigel®. Association was quantified after collection of fluorescence microscopy pictures using Wimasis® internet platform and software. Expression of adhesion molecules was analyzed by PCR and Western blot, immuno-fluorescence and flow cytometry. The impact of adhesion molecules on trophoblast-endothelial-cell interaction was investigated using siRNA technique. Results: N-cadherin and CD162 were specifically expressed in the trophoblast cell line HTR-8/SVneo, which closely adhere to and actively migrate toward HUVEC networks on Matrigel®. Suppression of N-cadherin led to a significant alteration in trophoblast-endothelial cell interaction. Expression of VE-cadherin in closely interacting trophoblast cells was not confirmed in vitro. Discussion: We identified N-cadherin to mediate specific interaction between HUVEC and the migrating trophoblast cells HTR-8/SVneo in a Matrigel® co-culture model. VE-cadherin contribution could not be confirmed in vitro. Our results support the hypothesis that impaired N-cadherin but not VE-cadherin expression is involved in trophoblast recruitment to the maternal endothelium.
Introduction
Adequate embryonic development and optimal fetal growth are facilitated through appropriate transportation of nutrients and gas between mother and child. This is achieved through the remodeling of maternal spiral arteries into wide, luminous conduits during placentation.Citation1,Citation2 An incomplete remodeling of maternal spiral arteries is associated with an altered utero-placental perfusion, leading to pathologies like intrauterine growth restriction (IUGR) and preeclampsia.Citation3,Citation4 The interplay and coordination of molecular and cellular mechanisms regulating this crucial interaction between invading trophoblast cells and the endothelium are still not fully understood.
Similarities in the interaction between trophoblast and endothelial cells, to the recruitment of immune cells out of the blood stream towards and through the vessel endothelium have been observed.Citation5 Here, cell adherence and rolling is primarily mediated by adhesion molecules like immunoglobulins, selectins and their partnering ligands.Citation6,Citation7 In the past years numerous adhesion molecules have been examined in the context of trophoblast invasion and many have been identified as possible candidates participating in trophoblast differentiation, migration and interaction.Citation5,Citation8–Citation12 Regulated changes in the expression of specific adhesion molecules seem to enable trophoblast migration within maternal vessels. The typical epithelial phenotype of the cytotrophoblast changes to a mesenchymal type with invasion, and to an increasingly endothelial phenotype during interaction with the maternal spiral arteries.Citation13–Citation15 Fisher et al. showed that endovascular trophoblast cells present endothelial cell type integrins, IgG-class adhesion molecules like VCAM and PECAM, as well as the endothelial specific VE-cadherin.Citation14 The authors noted this changing phenotype to be spars in placentas with IUGR and preeclampsia, leading to the interpretation, that VE-cadherin may be directly associated with trophoblast-endothelial interaction and altered vessel remodelling.Citation14,Citation16
The aim of our study was to analyse the interaction of different trophoblast cell lines with HUVEC networks on Matrigel®, in order to identify specific adhesion molecules enabling the crucial interaction of invading trophoblast cells with the endothelial cells. We hypothesize adhesion molecules known to mediate immune cell recruitment, to also regulate the homing of trophoblast cells to the vessel endothelium.
Results
Differential interaction of JEG-3 and HTR-8/SVneo trophoblast cells with endothelial cell networks on Matrigel®
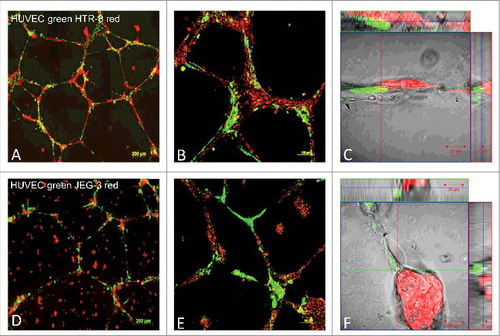
The interaction of the trophoblastic cell lines JEG-3 and HTR-8/SVneo with endothelial cells was compared by co-culture of fluorescence labelled trophoblastic cells in red and endothelial cells in green. HUVEC cell culture networks in Matrigel® were established for 24 h, before co-culture with HTR-8/SVneo or JEG-3 cells for 24 h to evaluate cell migration and co-localisation patterns. Results show significant differences of interaction between the stationary epithelial-like JEG-3 cells and the invasive HTR-8/SVneo cells (). Whereas interaction of JEG-3 with endothelial networks showed a random pattern, HTR-8/SVneo actively aligned with the pre-existing endothelial networks in the co-culture. The active movement of the HTR-8/SVneo cells towards the endothelial cell networks on Matrigel® was recorded in live cell observations (Supplemental material). Confocal images show close interaction of the two cell lines and HUVECs. During the observed time course of 24 h, cell borders where conserved ().
Figure 1. Fluorescence images of co-localisation and migration of HTR-8/SVneo cells, compared with JEG-3 cells, to pre-formed endothelial cell tubes in Matrigel®. HUVEC cell culture networks in Matrigel® (labelled with cytoplasma marker CellTracker™ Green CMFDA Dye, Invitrogen™) were established for 24h, before co-culture with HTR-8/SVneo (panels A-C) or JEG-3 cells (D-F) for 24 h to evaluate cell migration and co-localisation patterns. Both HTR-8/SVneo and JEG-3 were labelled with mitochondrial marker MitoTracker® Deep RedFM , Invitrogen™ and digital images were captured by epifluorescence microscopy (AxioObserver Z1, 10x objective, Zeiss, Jena, Germany). Images C and F represent close capture images of cell-cell interaction. Representative examples of three independent experiments, with 10 images per gel are depicted. Supplemental material available of videos capturing life cell interactions (CELL-IQ Analyser, Chip man technologies, Tampere, Finland).
Quantification of trophoblastic – endothelial cell interaction on Matrigel®
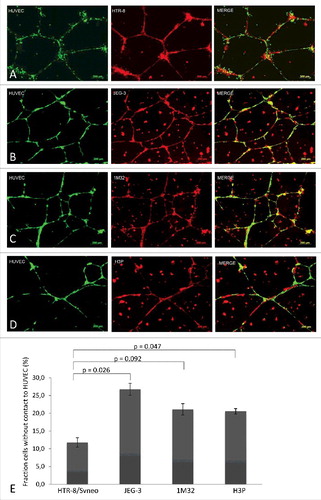
The behavior of the trophoblast cell lines JEG-3, AC-1M32, AC-H3P and HTR-8/SVneo, when added to HUVEC network formations on Matrigel®, was analyzed and compared. Visible and statistically significant differences were found between migration and lining of extravillous-like HTR-8/SVneo cells with HUVEC networks, as opposed to the cytotrophoblast-like cell lines JEG-3, AC-1M32 and AC-H3P (). The HTR-8/SVneo cells form a tight connection with the endothelial cell networks. In contrast, JEG-3, AC-1M32 and AC-H3P seem to grow mostly at random on the Matrigel®, independent of the HUVEC network formation ().
Figure 2. Fluorescence images and co-localisation analyses of different trophoblast cell lines with HUVEC networks in Matrigel®. Pre-established 24 h culture HUVEC cell networks (labelled with CellTrackerTM Green CMFDA Dye, InvitrogenTM, single label depicted on left column of picture panel) were co-incubated with four trophoblast cell lines (all labelled with MitoTracker® Deep RedFM, InvitrogenTM, single labels captured in center column): HTR-8 (panel A), JEG-3 (B), 1M32 (C), or H3P (D) for 24 h. Co-localization of HUVEC (green) and trophoblast cells (red) is illustrated in the right column of image panel. Images were captured with a 10x objective of epifluorescence microscope (AxioObserver Z1, Zeiss, Jena, Germany) and analysed using Axiovision software (AxioVs40 V 4.8.2.0, Carl Zeiss MicroImaging GmbH, Jena, Germany). Panel E illustrates quantitative differences in co-localisation of the four different trophoblast cell lines with HUVEC cells as analysed with Wimasis Analysis Software®. Percentages of trophoblast cells without contact to HUVEC cells as total of captured cells in the captured culture image are depicted in graph bars with standard deviation. Results represent the average of three independent experiments with three replicate cultures. Statistical analyses with student's t test using Excel (Microsoft). A p-value < 0.05 was considered statistically significant.
Distinct expression of adhesion molecules on differentially behaving trophoblast cell-lines
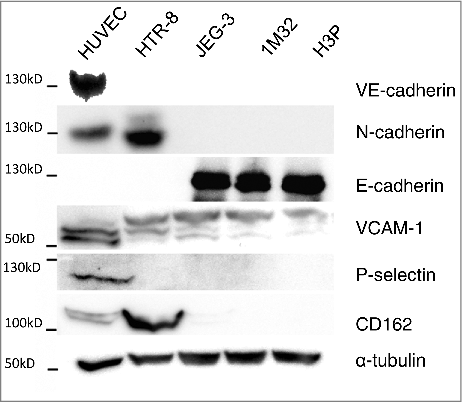
The expression of ICAM, PECAM, VCAM-1, of the cadherins VE-, N-, and E-cadherin, as well as the expression of E-, L-, and P-selectin with their corresponding ligands was analyzed in HTR-8/SVneo, JEG-3, AC-1M32, AC-H3P and HUVEC cells via PCR assays (). N-cadherin, VCAM-1, and P-selectin, with its ligand CD162, were identified as adhesion molecules potentially determining the specific migration and co-localisation of HTR-8/SVneo cells with HUVEC. Differential expression was confirmed by Western blot analysis for N-cadherin, P-selectin and CD162, whereas VCAM-1 protein was detected in all tested cell lines (). E-cadherin was tested to verify negative expression of VE-cadherin and N-cadherin in the epithelial like cell lines JEG-3, AC-1M32 and AC-H3P ().
Table 1. RNA-Expression of different adhesion molecules in trophoblast cell lines.
Figure 3. Protein expression analysis of selected adhesion molecules in HUVEC, HTR-8/SVneo, JEG-3, 1M32 and H3P cells via western blot. Expression of VE-cadherin, N-cadherin, E-cadherin, VCAM, P-selectin and CD162 was analysed in the above mention cell lines using immunochemical detection after SDS gel electrophoresis and western blot with α-tubulin as protein concentration control.
VE-cadherin expression was not detectable in closely interacting trophoblastic HTR-8/SVneo cells
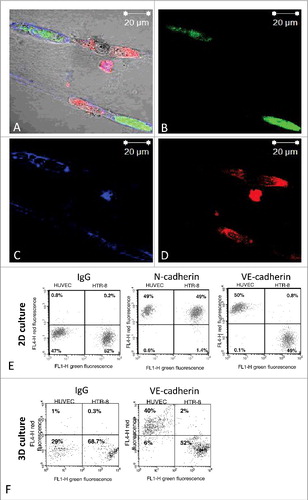
To confirm the expression of endothelial VE-cadherin in trophoblastic cells upon interaction with endothelial cells, we studied the VE-cadherin expression in closely interacting HTR-8/SVneo cells in our 3D co-culture model (). We could not detect any expression of VE-cadherin using immunostaining and confocal microscopy. These results were further confirmed by FACS-analysis of HTR-8/SVneo cells recovered from Matrigel® co-culture ().
Figure 4. Fluorescence images of immunostaining for VE-cadherin in co-cultured HTR-8/SVneo cells and endothelial cell tubes in Matrigel® and FACS-analysis for VE-cadherin expression on HTR-8/SVneo cells and endothelial tubes following co-culture in Matrigel®. HUVEC cell culture networks in Matrigel® (labelled with cytoplasma marker CellTracker™ Green CMFDA Dye, Invitrogen™) were established for 24 h, before co-culture with HTR-8/SVneo labelled with mitochondrial marker MitoTracker® Deep RedFM , Invitrogen™ for another 24 hours. Immunhistochemical staining was done using antibody against VE-cadherin (BD Transduction, 610252) and fluorescence labelled secondary antibody (Alexa Fluor 647, ThermoFischer, A32728). Fluorescence pictures were recorded using confocal microscopy (Zeiss, LSM 510). Representative pictures of at least three experiments performed are shown. (A-D) After co-culture of unlabelled HUVEC with green labelled HTR-8/SVneo cells in 2D (E) or 3D (F) cells were recovered for flow cytometry. Immunolabeling was done using VE-cadherin (BV6, Chemicon) and N-cadherin (Sigma, GC-4) antibodies followed by fluorescence labelled secondary antibody (Alexa Fluor 647). Flow cytometry was performed using the FACS Calibur™ (BD Biosciences, Heidelberg Germany). One representative experiment out of 3 performed is shown. (E and F). Flow cytometry analysis of recovered endothelial and HTR-8/SVneo cells did show N-cadherin expression in both HUVEC and HTR-8/SVneo cells (E) but not VE-cadherin upon 2D (E) and 3D-co-culture (F).
Inhibition of N-cadherin and CD162 expression in HTR-8/SVneo cells
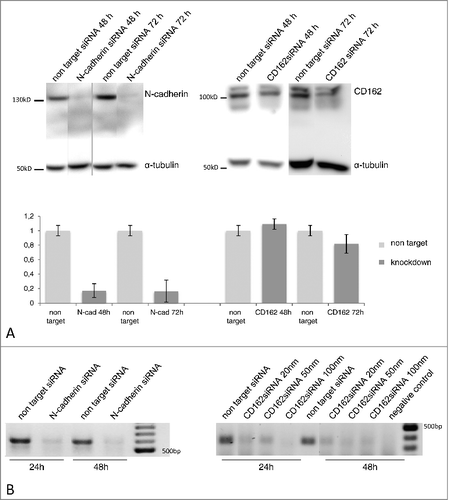
To prove the regulatory influence of N-cadherin and P-selectin/CD162 we established siRNA based expression inhibition in HTR-8/SVneo cells (). After 48 h of specific siRNA incubation a N-cadherin knockdown of 83% and after 72 h a CD162 knockdown of 18% was achieved (). Additionally, PCR-analysis confirmed inhibition of the corresponding mRNA (). Specificity of protein inhibition was tested by Western blot analysis showing unaltered expression of non-inhibited adhesion molecules (data not shown).
Figure 5. Expression of N-cadherin and CD162 after molecule specific siRNA knockdown as analysed via Western blot (A) and PCR (B). Protein specific knockdown via siRNA transfection was analysed compared to non target siRNA incubation using immunochemical detection after SDS gel electrophoresis and Western blot. After 48 and 72 h incubation N-cadherin specific knockdown was 83% and 84% (5 replicates), CD162 knockdown was only verified via western blot after 72 h siRNA incubation and showed strong interexprimental fluctuations (6 replicates). PCR confirmed RNA suppression of N-cadherin (20 nm siRNA) and CD162 (20, 50, 100 nm siRNA) after 48 and 72 h incubation.
Disruption of HTR-8/SVneo-HUVEC interaction upon expression inhibition of N-cadherin and CD162
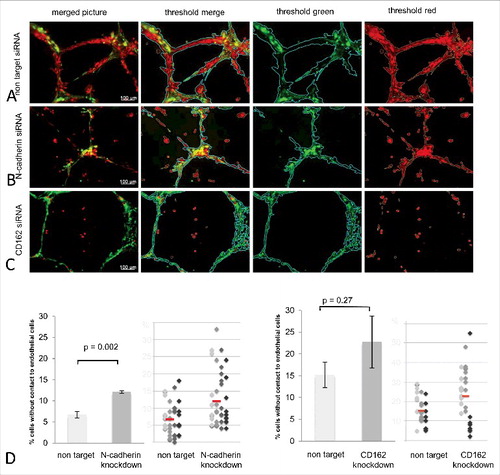
Upon expression inhibition of N-cadherin, HTR-8 interaction with endothelial cell networks in Matrigel® co-culture was significantly reduced by 44,5% (p = 0.002) ( and ). Additionally, sole inhibition of CD162 also revealed a decrease in HTR-8/SVneo and endothelial cell interaction by 33,1%, not reaching significant values (p = 0.27) ( and ). Pictures show green fluorescent and red fluorescent thresholds as well as the merged threshold representing the algorithm used to calculate the percentage of red fluorescent HTR-8/SVneo cells not connected to the green matrix of endothelial cell networks ().
Figure 6. Quality and quantity analysis of HTR-8/SVneo and HUVEC tubuli interaction after N-cadherin and CD162 manipulation. Matrigel® co-culture (images top to bottom) after non target (A), N-cadherin specific (B) and CD162 specific (C) siRNA incubation (left to right) as original microscopic image, with Wimasis® analysis matrix, filtered for HUVEC (green) and HTR-8/SVneo cells (red). D shows the percentage of trophoblast cells without contact to endothelial cells as means, with standard deviation in bar graphs, as well as via dot blots where each colour represents one experiment performed and the red line depicts the median of all experiments done. (4 replicates N-cadherin, 3 replicates CD162).
Discussion
Our data demonstrates the dependency of cell-cell interaction on specific adhesion molecule expression. It reveals an elementary mechanism possibly involved in the interaction of trophoblastic and endothelial cells. The expression of N-cadherin and CD162/P-selectin was associated with the closely interacting phenotype of the extravillous trophoblastic cell line HTR-8/SVneo. In our experimental system we measured, that a diminished expression of N-cadherin or CD162 molecules in HTR-8/SVneo cells changes their migratory behaviour and association with the endothelial cell line HUVEC. This reinforces the hypothesis that adhesion molecules play an essential role in trophoblast invasion and therefore, possibly in the transformation of spiral arteries during pregnancy. Considering the undisputed requirement of an undisturbed interaction of invading trophoblast cells with the maternal vessel endothelium in the process of spiral artery remodelling, our results offer a relevant input on further understanding the pathophysiology of altered trophoblast cell recruitment to maternal vessels during the process of placentation. Thus, defective expression of N-cadherin or CD162 in trophoblast cells, as well as defective expression of N-cadherin or P-selectin in endothelial cells could be part of the pathogenesis of placenta associated pregnancy complications.
Cadherins are calcium-dependent integral membrane glycoproteins that primarily mediate cell-cell adhesion and interaction.Citation17 They not only serve a mechanical function, by stabilizing and regulating cell shape and polarity, but through association with the intracellular actin cytoskeleton additionally contribute to the balance of cell proliferation, migration and tissue homeostasis.Citation18 N-cadherin has been shown to participate in the epithelial-mesenchymal transition that is necessary for well organized, coherently layered villous cytotrophoblasts to change into extravillous, migratory and invasive trophoblasts.Citation13 A defective transformation to an N-cadherin dominant expression within the extravillous trophoblast could therefore inhibit the cells ability to invade and interact with maternal endothelial cells.Citation13,Citation19 In 1992, Salomon et al. discovered the expression of N-cadherin in HUVEC-cells.Citation20 Even though the role of N-cadherin in endothelial cells is only partially understood, it likely mediates contacts and communication of endothelial cells with other cell types.Citation20 A participation in cell interaction during trophoblast invasion is therefore plausible, yet was not further investigated. New research supports this hypothesis showing that through a suppression of the transcription factor Twist, N-cadherin expression in the trophoblast cell line HTR-8/SVneo declines and cell invasion is significantly decreased.Citation21 The correlation of N-cadherin expression with HTR-8/SVneo invasiveness has additionally been confirmed by others.Citation22,Citation23 In this context our data emphasizes the impact of N-cadherin during trophoblast invasion and placental maturation.
Fisher et al. has shown that endovascular trophoblast cells present endothelial cell type integrins, IgG-class adhesion molecules like VCAM and PECAM, as well as the endothelial specific VE-cadherin while integrating in the maturating maternal vessels.Citation14 In addition, the authors noted this changing phenotype imitation to be inadequate in placentas with IUGR and preeclampsia, interpreting VE-cadherin to be directly associated with trophoblast-endothelial interaction and altered vessel remodelling.Citation14,Citation16 This data, however, was mainly aquired from histopathological studies of fixed samples taken from placentas of affected individuals after delivery. Thus, results are concluded from end-point evaluation of a dynamic process mainly taking place during placental develpment in the first trimester. In our experimental in vitro setting we could neither confirm the dependence of trophoblast-endothelial interaction on the expression of VE-cadherin, nor detect VE-cadherin expression in HTR-8/SVneo cells upon close interaction.
CD162 is predominantly expressed in cells of the hematopoietic linages. P-selectin is primarily found on activated endothelial cells and thrombocytes and mediates the rolling of leucocytes on the endothelium.Citation24 Together they create adhesions that are initiated under strong physical force, as would be the case during trophoblast migration against the blood flow.Citation25 After gene silencing of CD162 in mice, a significant reduction of leucocyte recruitment, migration and adhesion to the endothelium has been described.Citation26 A contribution to trophoblast-endothelium interaction seemed therefore be conceivable. Our results showing a markedly decreased interaction of HTR-8/SVneo cells with endothelial cells on Matrigel® upon inhibition of CD162 support the possible participation in trophoblast vessel invasion. However, the effect was much less profound than that for N-cadherin inhibition. These results could be due to an insufficient protein knockdown (18%) using siRNA transfection even though mRNA knockdown as analysed via PCR was adequate. Insufficient protein knockdown could be explained by prolonged turnover of CD162. Taylor et al. have described CD162 to be stored within the cell and integrated into the cell wall upon stimulation.Citation25 CD162 expression could therefore be relatively independent of acute translation inhibition via siRNA.
Considering that N-cadherin promotes not only interaction with endothelial cells, but also migration towards the endothelial cell networks on Matrigel® a less pronounced effect of CD162 inhibition is also in agreement with the distinctive function of the two molecules.
Using a Matrigel® system for co-culture of endothelial cells and trophoblasts enables these cells to display the phenotypic and morphological characteristics they are likely to assume in vivo.Citation27,Citation28 Nevertheless, the use of immortalized trophoblast cell lines for experiments will inevitably change cellular properties and behaviour in comparison to primary cells in vivo. Although differences have been found, many studies have shown a close approximation of HTR-8/SVneo cells to primary extravillous trophoblasts, allowing possible conclusions to in vivo activity and pathogenesis.Citation29-Citation31 The employed HUVEC cells are pooled primary human umbilical vein endothelial cells and represent the maternal spiral artery endothelium. These are the predominantly used endothelial cells in placentation experiments and analyses. To achieve experimental conditions simulating the in vivo situation, several models on Matrigel® have been established to permit more accurate examinations of cell function, invasion, migration and communication.Citation27,Citation28,Citation32 We refined the methods by cultivating endothelial cells on Matrigel® and controlling for network formation first, before adding trophoblastic cells. Additionally, the development of a computerized analysing tool using the Wimasis Image Analysis© platform offered a valid method to quantify cell interaction in our model. The development of a fitting algorithm was achieved though visual controls. Once optimized, we combined observer-blinded microscopy, where pictures were taken in a standardized grid-guided manner, with the computerized quantification of fluorescence ratios. This new combination strengthens the data presented here.
We focused our investigations on the possible expression of adhesion molecules, known to be involved in immune cell homing, in the extravillous trophoblast cell line HTR-8/SVneo and the endothelial cell line HUVEC. Our aim was to determine if specific adhesion molecules may be involved in the interaction of invasive extravillous trophoblasts with maternal endothelial cells, thus participating in the remodelling of maternal spiral arteries during placentation, or the lack thereof. We have demonstrated that a disruption of N-Cadherin expression on the extravillous trophoblast cell line HTR-8/SVneo significantly interferes with the ability to interact with endothelial HUVEC tubes. Conversely, it is possible that the defect lies within the endothelial cells. A lack of specific adhesion molecules on the endothelial cells of the maternal spiral arteries could well explain the limited in-depth trophoblast invasion that has been found in placentas of preeclampsia and IUGR. The familial clustering of placenta associated pregnancy complications, like preeclampsia and intrauterine growth restriction, indicates a maternal factor and genetic variations are believed to be part of the genesis of disturbed placentation.Citation33,Citation34 Further analysis of N-cadherin and CD162 expression in vivo should be performed to validate the necessity in trophoblast invasion of maternal spiral arteries. Biopsies from first and third trimester placentas, as well as of placenta bed from term deliveries could be examined by immunohistochemistry to verify reduced N-Cadherin expression associated with impaired placentation. Localisation of N-Cadherin impairment on trophoblast and, or endothelial cells could than lead to further understand the origin and time course of the underlying pathology.
Materials and methods
Cell culture
JEG-3, AC-H3P and AC-1M59 cells (DSMZ company, ACC 463, AC-H3P kindly provided by Hans-Georg Frank, Department of anatomy, Ludwig-Maximilian-University Munich, Germany,Citation35 ACC 442) were cultured in Ham's F12 with L-Glutamin (PAA, E15-813) and 10% fetal calf serum (FCS, Sigma, Cat. F7524). The HTR-8/SVneo trophoblast cell line was kindly provided by C.H. Graham, Queen's University, CA,Citation29 and cultured in Roswell Park Memorial Institute (RPMI) 1640 with L-Glutamin (PAA, E15-840) supplemented with 10% FCS. HUVEC cells (PromoCell, C-12253) were cultured in Endothelial Cell Basal Medium with Supplement Mix (ECGM, PromoCell, C-22010) and 10% FCS. All cells were incubated under standard culturing conditions (37°C, 5% CO2, humidified atmosphere). Cell pellets of confluent cultures were processed for protein and RNA expression analysis.
Western blot
For Western blot analysis cell pellets were lysed in RIPA buffer (NaCl 0,2 M, sodium desoxycholate 1%, Triton X-100 1%, SDS 0,1%, Tris-HCl 0,05 M pH 7,5, EDTA 4 mM) on ice for 30 min and then centrifuged at 4°C and 16,000 × g. The supernatant was used for analysis and western blot was performed as previously described.Citation36 The antibodies used were directed against N-cadherin (Sigma, C2542) at 1:200, VCAM-1 (Abcam, ab98954) at 1:500, CD162 (Abcam, ab66882) at 1:250, VE-Cadherin (Santa Cruz Biotech, sc-28644) at 1:100, E-Cadherin (BD Biosciences, 610181) at 1:1000, P-selectin (Santa Cruz, sc-6943) at 1:50, alpha-tubulin (Abcam, ab7291-100) at 1:104. Label application was conducted with horseradish peroxidase (HRP) conjugated anti-mouse (Cell Signalling, 7076), anti-rabbit (Cell Signalling, 7074S) or anti-goat (Jackson ImmunoResearch Laboratories Inc., 705-495-147) IgG in 5% (w/v) milk buffer at 1:103. Detection was performed using the MF-ChemiBIS 3.2 detection system (biostep, DNR Bio-Imaging-Systems, Jerusalem, Israel) and visualized via GelCapture Acquisition Software (DNR Bio-Imaging-Systems Version 5.1).
PCR
Total RNA was isolated from cell pellets with RNeasy Mini Kit (Qiagen, 74104) according to the manufacturer's protocol. Quality and quantity of the extracted RNA was checked with the ND-1000 spectrophotometer at 260 nm and analysed with ND-1000 Software Version 3.2.1 (Thermo Scientific). One microgram of RNA was used for cDNA synthesis via SuperScriptTM III First-Strand Synthesis System (Invitrogen, 18080-051) according to manufacturer's protocol. The primer sets employed are listed in . The PCR products were separated on 2% agarose gels by electrophoresis, photographed with the MF-ChemiBIS Version 3.2 (biostep, DNR Bio-Imaging-Systems, Jerusalem, Israel) and analyzed via GelCapture Acquisition Software (DNR Bio-Imaging-Systems Version 5.1).
Table 2. Primer used during PCR experiments.
siRNA transfection
For protein knockdown experiments HTR-8/SVneo cells were transfected with ON-TARGETplus SMARTpool siRNA for N-cadherin, CD162 or VCAM-1 (Thermo Scientific Dharmacon, L-003641-00-005, L-011774-00-0005, L-013351-00-0005) and the transfection reagent DharmaFECT® (Thermo Fisher Scientific, T-2001-01) according to the manufacture's protocol. Controls consisted of non-target siRNA (Thermo Fisher Scientific Dharmacon, D-001810-10-05). Cells were cultured in 6-well plates over night (105/well) up to an estimated confluence of 70–80%, washed with PBS (Thermo Fisher Scientific, 14190-094) and transfected at a final concentration of 20 nM siRNA with DharmaFECT®. Transfections were performed for 24, 48 or 72 h after which the cells were washed and harvested for, either, staining and Matrigel® co-culture with HUVEC cells, to analyse changes in cell-cell interaction, for PCR to assess mRNA expression, or for Western blot or FACS analysis to assess protein expression levels, and to confirm successful knockdown.
Matrigel®
Culture slides (BD Falcon™ Culture Slides, BD Biosciences, 354104) were coated with 250 µl growth factor reduced Matrigel® (BD Matrigel™ Basement Membrane Matrix, BD Biosciences, 354234) per chamber and incubated in Endothelial Cell Basal Medium with Supplement Mix (ECGM, PromoCell, C-22010) and 10% FCS over night. 105 HUVEC cells, labelled with Cell Tracker Green CMFDA Dye® (Thermo Fisher Scientific, C2925) were added to each chamber and incubated in fresh cell specific medium for 24 h, in which time they formed a network of capillary-like structures. The following day, medium was removed, cells carefully washed with PBS and 5 × 104 MitoTracker Deep Red FM® (Thermo Fisher Scientific, M22426) stained HTR-8/SVneo cells were added and after 24 h of co-culture the medium was removed, chambers carefully washed with PBS and cells fixed with ice-cold methanol-DMSO (4:1) at 4°C over night. The cellular interactions were recorded using epifluorescence microscopy (AxioObserver Z1, Zeiss, Jena, Germany). Per chamber 10 images were photographed by a qualified independent investigator using the grid-function of Axiovision software (AxioVs40 V 4.8.2.0, Carl Zeiss MicroImaging GmbH, Jena, Germany). Live cell imaging of co-culture was performed using the CELL-IQ Analyser (Chip man technologies, Tampere, Finland).
Fluorescence staining of co-cultured cells on Matrigel®
To perform immunostaining of co-cultured cells on Matrigel® in order to analyse VE-cadherin expression in interacting cells, fixed samples were washed and cells were permeabilized by incubation with 0.5% TritonX/PBS for 5 min. Antibody against VE-cadherin (BD Transduction, 610252) was used at 1:20 and unspecific IgG (Chemicon, AQ127) at 1:100 as well as fluorescence labelled secondary antibody (Alexa Fluor 647 goat anti-mouse, 1:500, ThermoFischer, A32728) at 1:1000. Fluorescence pictures were recorded using confocal microscopy (Zeiss, LSM 510).
Flow cytometry
To recover cells from Matrigel® after co-culture for flow cytometry Cell Recovery Solution (BD Biosciences, 354253) was used as described in the manufacturers' protocol. Recovered cell pellets were resuspended in 1mM EDTA/PBS and incubated with primary antibody detecting VE-cadherin (BV6, Chemicon) at 1:50; N-cadherin (Sigma, GC-4) at 1:100 or unspecific IgG (Sigma) at 1:50 followed by incubation with fluorescence labelled secondary antibody (Alexa Fluor 647 goat anti-mouse, 1:500, ThermoFischer, A32728) at 1:1000. Flow cytometry was performed using the FACS Calibur™ (BD Biosciences, Heidelberg Germany).
Image analysis and statistical calculation
The captured Matrigel® images were sent to Wimasis Image Analysis® for the investigation of cell-cell interaction. In collaboration, an algorithm was developed that identifies and constructs a matrix-image of the green fluorescent HUVEC networks. Based on this matrix the percentage of red fluorescence not connected to the green was determined. Thus, we quantified the amount of red fluorescent HTR-8/SVneo cells that did not interact with the HUVEC network. Statistical analyses of all experiments were performed with the paired student's t test (typ 1, tail 2) using Excel (Microsoft). A p-value < 0.05 was considered statistically significant.
Contributors
AM is a MD student, who performed the majority of presented experiments, contributed to analyzing and interpretation of the results and provided the graphical illustrations. As a native speaker, she was responsible for manuscript revision and preparation. MM and CG are master students who performed parts of the experimental results presented. MB contributed to microscopic imaging and the performance of image analysis. BH contributed to study design and conception. Furthermore he provided equipment and conceptual support during the performance of the live cell experiments. UM and ES contributed to study design and writing. TG is the principal investigator of the study and wrote the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Files
Download Zip (10.3 MB)Acknowledgments
We would like to thank C.H. Graham from the Queen's University for providing the HTR-8/SVneo cells. We thank Nadin Gebhard for her technical assistant establishing Matrigel® co-culture and staining.
Funding
The present study was supported by grant GR 1955/2-1 received from the Deutsche Forschungsgemeinschaft (DFG) to TG and by a Promotionsstipendium (MD/PhD scholarship) from the Center for interdisciplinary Research (IZKF) at the University Hospital Jena to AM.
References
- Kaufmann P, Black S and Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biology of reproduction. 2003;69(1):1–7. doi:10.1095/biolreprod.102.014977.
- Harris LK. Review: Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31 Suppl:S93–8.
- Madazli R, Somunkiran A, Calay Z, Ilvan S and Aksu MF. Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta. 2003;24(5):510–6. doi:10.1053/plac.2002.0945.
- Pijnenborg R, Vercruysse L and Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9-10):939–58. doi:10.1016/j.placenta.2005.12.006.
- Burrows TD, King A and Loke YW. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta. 1994;15(1):21–33. doi:10.1016/S0143-4004(05)80233-4.
- Fernandez-Borja M, van Buul JD and Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res. 2010;86(2):202–10. doi:10.1093/cvr/cvq003.
- Vestweber D, Wessel F and Nottebaum AF. Similarities and differences in the regulation of leukocyte extravasation and vascular permeability. Seminars in immunopathology. 2014;36(2):177–92. doi:10.1007/s00281-014-0419-7.
- Kaspi E, Guillet B, Piercecchi-Marti MD, Alfaidy N, Bretelle F, Bertaud-Foucault A, Stalin J, Rambeloson L, Lacroix O, Blot-Chabaud M, Dignat-George F and Bardin N. Identification of soluble CD146 as a regulator of trophoblast migration: potential role in placental vascular development. Angiogenesis. 2013;16(2):329–42. doi:10.1007/s10456-012-9317-6.
- Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S and Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Experimental cell research. 2005;303(1):101–13.
- Zhang Q, Tan D, Luo W, Lu J and Tan Y. Expression of CD82 in human trophoblast and its role in trophoblast invasion. PloS one. 2012;7(6):e38487. doi:10.1371/journal.pone.0038487.
- Harris LK, Jones CJ and Aplin JD. Adhesion molecules in human trophoblast – a review. II. extravillous trophoblast. Placenta. 2009;30(4):299–304. doi:10.1016/j.placenta.2008.12.003.
- Shih Ie M, Hsu MY, Oldt RJ, 3rd, Herlyn M, Gearhart JD and Kurman RJ. The Role of E-cadherin in the Motility and Invasion of Implantation Site Intermediate Trophoblast. Placenta. 2002;23(10):706–15. doi:10.1053/plac.2002.0864.
- Kokkinos MI, Murthi P, Wafai R, Thompson EW and Newgreen DF. Cadherins in the human placenta–epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31(9):747–55. doi:10.1016/j.placenta.2010.06.017.
- Damsky CH and Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Current opinion in cell biology. 1998;10(5):660–6. doi:10.1016/S0955-0674(98)80043-4.
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M and Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? The Journal of clinical investigation. 1997;99(9):2139–51.
- Zhou Y, Damsky CH and Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? The Journal of clinical investigation. 1997;99(9):2152–64.
- Maitre JL and Heisenberg CP. Three functions of cadherins in cell adhesion. Current biology: CB. 2013;23(14):R626–33. doi:10.1016/j.cub.2013.06.019.
- Yap AS, Crampton MS and Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Current opinion in cell biology. 2007;19(5):508–14. doi:10.1016/j.ceb.2007.09.008.
- Gheldof A and Berx G. Cadherins and epithelial-to-mesenchymal transition. Progress in molecular biology and translational science. 2013;116:317–36. doi:10.1016/B978-0-12-394311-8.00014-5.
- Salomon D, Ayalon O, Patel-King R, Hynes RO and Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. Journal of cell science. 1992;102 (Pt 1):7–17.
- Ng YH, Zhu H and Leung PC. Twist modulates human trophoblastic cell invasion via regulation of N-cadherin. Endocrinology. 2012;153(2):925–36. doi:10.1210/en.2011-1488.
- Peng B, Zhu H and Leung PC. Gonadotropin-releasing hormone regulates human trophoblastic cell invasion via TWIST-induced N-cadherin expression. The Journal of clinical endocrinology and metabolism. 2015;100(1):E19–29. doi:10.1210/jc.2014-1897.
- Li Y, Klausen C, Cheng JC, Zhu H and Leung PC. Activin A, B, and AB increase human trophoblast cell invasion by up-regulating N-cadherin. The Journal of clinical endocrinology and metabolism. 2014;99(11):E2216–25. doi:10.1210/jc.2014-2118.
- Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leukemia & lymphoma. 1998;29(1-2):1–15. doi:10.3109/10428199809058377.
- Taylor ML, Brummet ME, Hudson SA, Miura K and Bochner BS. Expression and function of P-selectin glycoprotein ligand 1 (CD162) on human basophils. The Journal of allergy and clinical immunology. 2000;106(5):918–24. doi:10.1067/mai.2000.110230.
- Slotta JE, Braun OO, Menger MD and Thorlacius H. Capture of platelets to the endothelium of the femoral vein is mediated by CD62P and CD162. Platelets. 2009;20(7):505–12. doi:10.3109/09537100903215417.
- Highet AR, Zhang VJ, Heinemann GK and Roberts CT. Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta. 2012;33(7):586–8. doi:10.1016/j.placenta.2012.04.003.
- Pampaloni F, Reynaud EG and Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nature reviews Molecular cell biology. 2007;8(10):839–45. doi:10.1038/nrm2236.
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N and Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Experimental cell research. 1993;206(2):204–11. doi:10.1006/excr.1993.1139.
- Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O and Knofler M. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010;31(11):989–96. doi:10.1016/j.placenta.2010.08.011.
- Suman P and Gupta SK. Comparative analysis of the invasion-associated genes expression pattern in first trimester trophoblastic (HTR-8/SVneo) and JEG-3 choriocarcinoma cells. Placenta. 2012;33(10):874–7. doi:10.1016/j.placenta.2012.06.017.
- Scherberich A and Beretz A. Culture of vascular cells in tridimensional (3-D) collagen: a methodological review. Therapie. 2000;55(1):35–41.
- Melchiorre K, Sutherland GR, Liberati M and Thilaganathan B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension. 2012;60(2):437–43. doi:10.1161/HYPERTENSIONAHA.112.194159.
- Roberts JM and Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30 Suppl A:S32–7. doi:10.1016/j.placenta.2008.11.009.
- Frank HG, Gunawan B, Ebeling-Stark I, Schulten HJ, Funayama H, Cremer U, Huppertz B, Gaus G, Kaufmann P and Fuzesi L. Cytogenetic and DNA-fingerprint characterization of choriocarcinoma cell lines and a trophoblast/choriocarcinoma cell hybrid. Cancer genetics and cytogenetics. 2000;116(1):16–22. doi:10.1016/S0165-4608(99)00107-7.
- Groten T, Gebhard N, Kreienberg R, Schleussner E, Reister F and Huppertz B. Differential expression of VE-cadherin and VEGFR2 in placental syncytiotrophoblast during preeclampsia – New perspectives to explain the pathophysiology. Placenta. 2010;31(4):339–43. doi:10.1016/j.placenta.2010.01.014.
