ABSTRACT
Sugen kinases (SgK)269 (also known as PEAK1), and SgK223, an orthologue of rat pragmin and mouse NACK, are human pseudokinases that are implicated in the progression of several cancers. Both are scaffolding proteins that recruit distinct repertoires of signalling proteins and regulate a variety of biological endpoints including cell migration and invasion. To date, SgK269 and SgK223 have been largely studied as separate signalling entities. However, recent work has demonstrated that SgK269 and SgK223 undergo homo- and heterotypic association that determines signal output and biological response. Further characterization of the mechanism of action of these two pseudokinases will provide novel insights into how they promote cancer progression and may reveal novel therapeutic strategies. Here we review their structure, mechanism and function and roles they play in cancer pathogenesis.
Introduction
Protein kinases regulate diverse cellular processes, including proliferation, survival, metabolism and motility, and aberrant signalling by these enzymes is implicated in a variety of human diseases and pathologies, including cancer.Citation1,Citation2 Approximately 10% of annotated protein kinases are classified as pseudokinases, as they lack at least one of the conserved amino acid motifs predicted to be essential for catalytic activity.Citation2 Emerging evidence indicates that pseudokinases function as allosteric regulators and/or as scaffolds rather than a protein kinases capable of catalysing phosphoryl transfer reactions.Citation3,Citation4 Despite impaired or absent catalytic activity, the signalling potential of pseudokinases is reflected in the growing number of these proteins implicated in human disease, including cancer.Citation3
SgK269 and SgK223: Structure
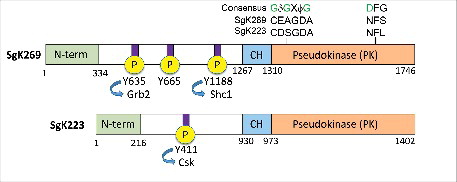
Sugen kinases (SgK)269, also known as PEAK1Citation5, and SgK223, the human orthologue of rat pragminCitation6 and mouse NACKCitation7 are related proteins of 193 and 149 kDa, respectively (). They do not exhibit close structural, sequence or functional similarity with the bona fide protein kinases SGK1-3, where SGK is an abbreviation for serum and glucocorticoid kinase. They exhibit a similar overall molecular architecture as well as significant sequence homology over the most N-terminal 100 amino acids, the pseudokinase (PK) domains and a preceding short, 43 amino acid α-helical segment defined by our group as the C-terminal α-helical (CH) region ().Citation8 Amongst the key conserved motifs that characterise bona fide kinases, the aspartate of the DFG motif that is required for Mg2+-ATP binding is substituted by an asparagine residue in SgK269 and SgK223. Additionally, the conserved glycine-rich loop that also contributes to ATP binding is absent (). These features indicate that both proteins may be pseudokinases. Consistent with this prediction, and despite an early characterization of SgK269 as an active tyrosine kinaseCitation5, both SgK269 and SgK223 are devoid of nucleotide binding activity, as detected by a thermal-shift assay.Citation9 A further feature of both proteins is the presence of tyrosine phosphorylation sites that recruit specific src homology (SH)2 and phosphotyrosine binding (PTB) domain-containing effectors, indicating that these pseudokinases are likely to function as signalling scaffolds.Citation8 The role of these recruitment sites is discussed in later sections of this review. The following sections first address the expression, function and mechanism for each of SgK269 and SgK223 separately, before addressing their interaction and likely integrated roles.
Figure 1. Schematic representation of SgK269 and SgK223 structure. Amino acid substitutions in the pseudokinase domain relative to consensus sequence motifs in bona fide kinases (glycine-rich loop and DFG motif) are highlighted. Functionally-annotated tyrosine phosphorylation sites are also shown. SgK269 Y665 is known to regulate focal adhesion turnover. CH: predicted C-terminal α-helical region. For full details please refer to text.
SgK269: Expression and function
SgK269 was first identified by a proteomic strategy aimed at identifying tyrosine-phosphorylated proteins enriched in cell pseudopodia.Citation5 It is widely-expressed in normal tissuesCitation5, and overexpressed in multiple human malignancies, including colon cancer.Citation5, Citation10 Furthermore, increased SgK269 protein expression is observed in pancreatic intraepithelial lesions as well as pancreatic ductal adenocarcinoma (PDAC), identifying SgK269 as a potential biomarker for pancreatic cancer development.Citation10 In breast cancer, SgK269 is overexpressed in a subset of luminal, HER2 and basal subtypesCitation11 and SgK269 gene expression positively correlates with several markers of poor prognosis including metastatic lesions and disease relapse.Citation12 Interestingly, SgK269 protein expression across a breast cancer cell line panel is associated with the basal subtype, but this relationship is not observed at the mRNA level, indicating a major contribution from post-transcriptional or -translational regulatory mechanisms.Citation11 In this regard, it is interesting to note that in PDAC, SgK269 is an effector of eukaryotic translation initiation factor 5a (eIF5a) in driving cancer cell proliferation, with the latter upregulated in response to expression of active KRas.Citation13
SgK269 localises to the actin cytoskeleton and focal adhesions of NIH-3T3 fibroblasts, and regulates both cell spreading and migration in Cos-7 cells.Citation5 It undergoes tyrosine phosphorylation in HEK 293T cells and murine embryonic fibroblasts in response to EGF stimulation, and this modification is dependent on the activity of Src family kinases (SFKs).Citation5 A key mechanism underpinning the effect of SgK269 on cell migration is regulation of focal adhesion turnover, which requires dynamic phosphorylation of Y665 by SFKs, as demonstrated in HT1080 fibrosarcoma cells.Citation14 Consistent with a regulatory role at focal adhesions, SgK269 modulates tyrosine phosphorylation of p130Cas and paxillin in HEK 293T cells, and co-immunoprecipitates with p130Cas and its downstream effector Crk.Citation5 SgK269 also represents an important regulator of epithelial-mesenchymal transition (EMT). Overexpression of SgK269 in MCF-10A mammary epithelial cells results in an elongated, highly motile and invasive cell phenotype with gene expression changes characteristic of EMT, while stable knockdown of this protein in MDA-MB-231 breast cancer cells results in acquisition of a more epithelial phenotype.Citation11 Furthermore, SgK269 enhances TGFβ-induced migration and EMT of MCF-7 breast cancer cells grown on fibronectin, and promotes a switch from Smad2/3 to Src/Erk signalling under these conditions.Citation12 Consistent with its effects on cell migration and invasion, SgK269 is required for TGFβ/fibronectin-induced metastasis of transformed MCF-10A cells in a xenograft modelCitation12, as well as systemic spread of PDAC cancer cells following establishment as orthotopic xenografts.Citation10
SgK269 also plays a pro-proliferative role, albeit in a context-dependent manner. For example, while SgK269 overexpression does not affect monolayer proliferation of MCF-10A cells, it results in formation of large, aberrant acini in 3D Matrigel culture.Citation11 In addition, SgK269 modulates the proliferative response of breast cancer cells to TGFβ12 and promotes anchorage-independent proliferation of MDA-MB-435 breast cancer cells, as well as their growth as mouse xenografts.Citation5 It also promotes cellular resistance to specific therapies, including trastuzumab and gemcitabine in PDAC cellsCitation10, and the small molecule Src inhibitor AZM 475271 in a breast cancer model.Citation12
SgK269: Signalling mechanism
As described in the previous section, the localization of SgK269 to focal adhesions and the actin cytoskeleton, together with its association with p130Cas and Crk and ability to regulate focal adhesion dynamics, likely contribute to its demonstrated effects on cell migration and invasion.Citation5,Citation14 Further mechanistic details regarding the impact of SgK269 on these endpoints were provided by a landmark study led by the Pawson laboratory.Citation15 Here, characterization of the interactome of the Shc1 scaffold in Rat-2 fibroblasts at different timepoints following EGF stimulation revealed that at early time points, Shc1 recruits a suite of proteins that includes Grb2 in order to promote proliferative and survival signalling, while at later time points the interactome switches to one centred on SgK269 in order to regulate endpoints related to cell morphology and invasion. Specifically, the Shc1 PTB domain binds to tyrosine-phosphorylated SgK269 Y1188, and knockdown studies demonstrated that SgK269 ‘bridges’ Shc1 to several proteins including the serine/threonine protein phosphatases Ppp1ca and Ppp1cc, the Ras GTPase activating protein (GAP) Dab2ip and the Arf GAP, Asap215. This study greatly extended our understanding of the scaffolding ability of SgK269 and highlighted additional effectors that may contribute to its impact on focal adhesion dynamics and cytoskeletal organization. For example, Asap2 is known to regulate paxillin recruitment to focal adhesions and cell migration.Citation16
In MCF-10A cells, the proliferative effect of SgK269 is dependent upon Lyn-mediated tyrosine phosphorylation of SgK269 Y635, which creates a binding site for the Grb2 SH2 domain and enhances activation of the Ras/Erk pathway. Indeed, normalization of Erk activation in SgK269-overexpressing cells restored acinar size to control levels.Citation11 Interestingly, SgK269 also enhanced Stat3 activation in this system, and this was also dependent upon Y635 phosphorylation. However, the mechanistic basis for this observation is currently unclear.
SgK223: Expression and function
Pragmin, the rat orthologue of human SgK223, was originally identified as a downstream effector of Rnd2, a member of the Rho family of small GTPases.Citation6 In the rat, pragmin is highly-expressed in brain, including cortical and hippocampal pyramidal neurons, as well as in kidney, spleen, colon and small intestine.Citation6 Unlike SgK269, the phenotype of a gene knock-out mouse has been reported, with global knockout of the mouse ortholog being embryonic lethal.Citation7 Similar to SgK269, SgK223 is also implicated in human cancer. SgK223 is overexpressed in many PDAC cell lines compared to human pancreatic ductal epithelial (HPDE) cells, as well as in primary PDAC, esophageal adenocarcinoma and non-small cell lung cancer (NSCLC) versus normal tissue.Citation7,Citation17,Citation18 Importantly, in NSCLC, high SgK223 expression is associated with poor patient prognosis.Citation17
In AGS gastric epithelial cells, SgK223 and ectopically-expressed pragmin localize to the plasma membrane, cytosol and focal adhesionsCitation19, suggesting a potential role in regulation of cell adhesion and related biological endpoints. In this regard, the effects of pragmin on cell morphology appear to be context-dependent. Pragmin co-operates with active Rnd2 to promote cell contraction of HeLa cells in a Rho kinase-dependent manner, and to inhibit NGF-induced neurite outgrowth.Citation6 However, overexpression of pragmin in HPDE or MKN7 gastric epithelial cells results in a more elongated phenotype.Citation18,Citation19 In the former model, pragminpromotes both cell migration and invasionCitation18, and consistent with these data, knockdown of SgK223 in SW620 colorectal cancer cells overexpressing Src leads to decreased invasive potential.Citation20 SgK223 knockdown also reduces migration and invasion of NSCLC cells.Citation17
Interestingly, recent studies have demonstrated that this pseudokinase also has an important nuclear function. The Capobianco group identified the mouse ortholog, Notch activation complex kinase (NACK), as a binding partner for the Notch1 intracellular domain and an important component of the Notch transcriptional activation complex.Citation7 Interestingly, NACK also represents a Notch target gene, indicating the presence of a feed-forward loop for regulating NACK expression. In addition, NACK/SgK223 is required for Notch-mediated transformation of HC11 mammary epithelial cells, and NACK/SgK223 knockdown reduces growth of esophageal adenocarcinoma cells in a xenograft model, demonstrating an important role for this pseudokinase in Notch-driven tumourigenesis.Citation7 A transforming role for SgK223 has also been demonstrated in NSCLC, where SgK223 knockdown reduces cell proliferation in vitro and xenograft growth in vivo.Citation17
SgK223: Signalling mechanism
Pragmin binds to Rnd2 via the C-terminal region of pragmin, which harbours the pseudokinase domain, and stimulates RhoA activity in cells.Citation6 To date, Rnd2 represents the only downstream effector documented to bind this region of pragmin or SgK269. However, as with SgK269, SgK223 also functions as a scaffold through a tyrosine phosphorylation-dependent mechanism. Here, phosphorylation of Y391 in pragmin by SFKs creates a binding site for the SH2 domain of Csk, a negative regulator of SFKs. Furthermore, overexpression of pragmin leads to SFK activation, presumably due to cytoplasmic sequestration of Csk away from plasma membrane-anchored SFKs, indicating the presence of a positive feedback loop.Citation21 Interestingly, Y391 resides within a conserved Glu-Pro-Ile-Tyr-Ala (EPIYA) motif that is also present in effector proteins of specific pathogenic bacteria used to alter host cell signalling.Citation22 For example, Helicobacter pylori CagA localizes to the plasma membrane and binds to Csk via CagA EPIYA motifs, in this case leading to Src inhibition. Consequently, bacterial EPIYA effectors appear to subvert the normal cellular function of pragmin.
More recently, Csk itself has been identified as an additional kinase for pragmin Y39119. This study demonstrated a feed-forward mechanism whereby tyrosine-phosphorylated pragmin activated Csk, which then phosphorylated pragmin further. In addition, Csk and pragmin co-localized at focal adhesions, and co-operated to promote cell elongation and scattering in a Y391-dependent manner.Citation19 An additional signalling pathway regulated by pragmin is Stat3 activation, which is required for pragmin to enhance cell migration and invasion in HPDE cells.Citation18 While the detailed mechanism is unclear, pragmin associates with Stat3 in co-immunoprecipitation studies, and enhances activation of the Stat3 kinase, JAK118.
Further mechanistic details have recently been provided for the role of NACK in Notch signalling. Here, NACK is recruited to the Notch1 ternary complex via Mastermind-like 1, following acetylation of the latter by p300 and CBP. NACK then recruits RNA polymerase II to the promoter of Notch target genes to drive their transcription.Citation23
SgK269/SgK223: A novel oncogenic alliance
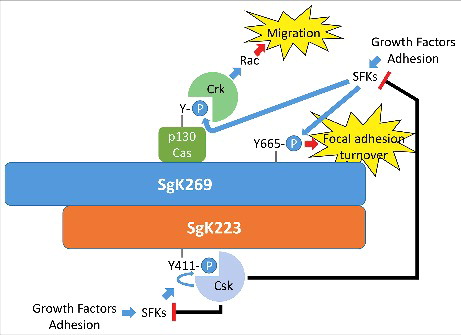
Until recently it was assumed that SgK269 and SgK223 signal and function as separate entities. However, it is now evident that this is not the case, and they in fact exhibit a close mechanistic relationship. The starting point for this finding was a structure/function analysis demonstrating that the ability of SgK269 to promote cell elongation and migration in MCF-10A cells was dependent upon the CH region and PK domain.Citation8 In an effort to identify binding partners for these regions, a proteomic analysis was undertaken, identifying SgK223 as one of the most prominent interactors. Subsequent work demonstrated that the two proteins associate at physiological expression levels, the interaction is direct, and requires the CH and PK regions. Furthermore, homotypic association of these two pseudokinases could also be detected, and exhibited the same structural requirements. Importantly, functional dependency between SgK269 and SgK223 could also be demonstrated. SgK223 required SgK269 expression in order to co-immunoprecipitate with Grb2, indicating that SgK269 ‘bridges’ SgK223 to Grb2, and SgK269 required SgK223 to efficiently promote cell migration and activate Stat3 in MCF-10A cells. This led to a model whereby homotypic and heterotypic association of the two pseudokinases acts a mechanism to diversity and integrate signal output, in a manner akin to that utilized by receptor tyrosine kinases, such as those of the erbB family.Citation8 provides a schematic representation of how this signalling mechanism might be utilized by SgK269 and SgK223 to regulate focal adhesion dynamics.
Figure 2. A potential role for SgK269/SgK223 heterotypic association in regulation of focal adhesion dynamics. Both SgK269 and SgK223 localize to focal adhesions, and SgK269 is known to associate with specific focal adhesion components. SgK269 also regulates focal adhesion turnover in a manner dependent on dynamic phosphorylation of Y665. Heterotypic association of SgK269 with SgK223, which recruits Csk, provides a potential mechanism for dynamic regulation of Src family kinases and hence focal adhesion turnover.
Summary and perspectives
It is now evident that the scaffolding functions of the SgK269 and SgK223 pseudokinases regulate a variety of important signalling pathways and thereby impact diverse biological processes, including regulation of cell morphology, migration, invasion and proliferation. In doing so, they play important functional roles in both normal and transformed cells. However, critical questions remain to be answered. For example, the exact mechanisms whereby these pseudokinases regulate specific processes, such as focal adhesion turnover, or pathways, such as Stat3 activation, remain to be resolved. In addition, key spatiotemporal aspects of their regulation and coupling to specific pathways need to be defined. This is particularly important in the context of homotypic and heterotypic association of the two pseudokinases, where the heterotypic complex presents the opportunity for signal integration. For example, the presence of SgK223-bound Csk is likely to impact the ability of SgK269 to regulate focal adhesion proteins such as p130Cas, which are known SFK substrates (). Furthermore, it is unclear whether the nuclear role recently identified for NACK involves interaction with previously-identified effectors, such as Csk, or indeed SgK269. From a cancer perspective, it will be interesting to determine how co-overexpression of the two pseudokinases impacts disease progression and patient prognosis, compared to either one alone. Finally, structural characterization of these pseudokinases and their mode of association will provide further insights into their signalling mechanisms and may also identify key interfaces amenable to therapeutic intervention.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Additional information
Funding
References
- Fleuren ED, Zhang L, Wu J, Daly RJ. The kinome ‘at large’ in cancer. Nat Rev Cancer. 2016;16:83–98. doi:10.1038/nrc.2015.18.
- Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi:10.1126/science.1075762.
- Reiterer V, Eyers PA, Farhan H. Day of the dead: pseudokinases and pseudophosphatases in physiology and disease. Trends Cell Biol. 2014;24:489–505. doi:10.1016/j.tcb.2014.03.008.
- Zeqiraj E, van Aalten DM. Pseudokinases-remnants of evolution or key allosteric regulators? Curr Opin Structural Biol. 2010;20:772–81. doi:10.1016/j.sbi.2010.10.001.
- Wang Y, Kelber JA, Tran Cao HS, et al. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression [corrected]. Proc Natl Acad Sci U S A. 2010;107:10920–5. doi:10.1073/pnas.0914776107.
- Tanaka H, Katoh H, Negishi M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol Chem. 2006;281:10355–64. doi:10.1074/jbc.M511314200.
- Weaver KL, Alves-Guerra MC, Jin K, et al. NACK is an integral component of the Notch transcriptional activation complex and is critical for development and tumorigenesis. Cancer Res. 2014; 74:4741–51. doi:10.1158/0008-5472.CAN-14-1547.
- Liu L, Phua YW, Lee RS, et al. Homo- and Heterotypic Association Regulates Signaling by the SgK269/PEAK1 and SgK223 Pseudokinases. J Biol Chem. 2016;291:21571–83. doi:10.1074/jbc.M116.748897.
- Murphy JM, Zhang Q, Young SN, et al. A robust methodology to subclassify pseudokinases based on their nucleotide-binding properties. Biochem J. 2014;457:323–34. doi:10.1042/BJ20131174.
- Kelber JA, Reno T, Kaushal S, et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 2012;72:2554–64. doi:10.1158/0008-5472.CAN-11-3552.
- Croucher DR, Hochgrafe F, Zhang L, et al. Involvement of Lyn and the atypical kinase SgK269/PEAK1 in a basal breast cancer signaling pathway. Cancer Res. 2013;73:1969–80. doi:10.1158/0008-5472.CAN-12-1472.
- Agajanian M, Campeau A, Hoover M, et al. PEAK1 acts as a molecular switch to regulate context-dependent TGFbeta responses in breast cancer. PLoS One. 2015;10:e0135748. doi:10.1371/journal.pone.0135748.
- Fujimura K, Wright T, Strnadel J, et al. A hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014; 74:6671–81. doi:10.1158/0008-5472.CAN-14-1031.
- Bristow JM, Reno TA, Jo M, et al. Dynamic phosphorylation of tyrosine 665 in pseudopodium-enriched atypical kinase 1 (PEAK1) is essential for the regulation of cell migration and focal adhesion turnover. J Biol Chem. 2013;288:123–31. doi:10.1074/jbc.M112.410910.
- Zheng Y, Zhang C, Croucher DR, et al. Temporal regulation of EGF signalling networks by the scaffold protein Shc1. Nature. 2013;499:166–71. doi:10.1038/nature12308.
- Kondo A, Hashimoto S, Yano H, et al. A new paxillin-binding protein, PAG3/Papalpha/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol Biol Cell. 2000;11:1315–27. doi:10.1091/mbc.11.4.1315.
- Kong R, Feng J, Ma Y, et al. Silencing NACK by siRNA inhibits tumorigenesis in non-small cell lung cancer via targeting Notch1 signaling pathway. Oncol Rep. 2016;35:2306–14. doi:10.3892/or.2016.4552.
- Tactacan CM, Phua YW, Liu L, et al. The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol Cancer. 2015;14:139. doi:10.1186/s12943-015-0412-3.
- Senda Y, Murata-Kamiya N, Hatakeyama M. C-terminal Src kinase-mediated EPIYA phosphorylation of Pragmin creates a feed-forward C-terminal Src kinase activation loop that promotes cell motility. Cancer Sci. 2016;107:972–80. doi:10.1111/cas.12962.
- Leroy C, Fialin C, Sirvent A, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–86. doi:10.1158/0008-5472.CAN-08-2354.
- Safari F, Murata-Kamiya N, Saito Y, et al. Mammalian Pragmin regulates Src family kinases via the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif that is exploited by bacterial effectors. Proc Natl Acad Sci U S A. 2011;108:14938–43. doi:10.1073/pnas.1107740108.
- Hayashi T, Morohashi H, Hatakeyama M. Bacterial EPIYA effectors–where do they come from? What are they? Where are they going? Cell Microbiol. 2013;15:377–85. doi:10.1111/cmi.12040.
- Jin K, Zhou W, Han X, et al. Acetylation of mastermind-like 1 by p300 drives the recruitment of NACK to initiate notch-dependent transcription. Cancer Res. 2017;77:4228–37. doi:10.1158/0008-5472.CAN-16-3156.
