ABSTRACT
During development, neuroepithelial progenitors acquire apico-basal polarity and adhere to one another via apically located tight and adherens junction complexes. This polarized neuroepithelium must continue to integrate cells arising through cell divisions and intercalation, and allow for cell movements, at the same time as undergoing morphogenesis. Cell proliferation, migration and intercalation all occur in the morphing embryonic eye. To understand how eye development might depend on dynamic epithelial adhesion, we investigated the function of a known regulator of junctional plasticity, Tumour necrosis factor receptor-associated factor 4 (Traf4). traf4a mRNA is expressed in the developing eye vesicle over the period of optic cup morphogenesis, and Traf4a loss leads to disrupted evagination and elongation of the eye vesicles, and aberrant organization and apico-basal polarity of the eye epithelium. We propose a model whereby Traf4a regulates apical junction plasticity in nascent eye epithelium, allowing for its polarization and morphogenesis.
Symbols and Abbreviations: AB: apico-basal; aPKC: atypical protein kinase-C; CRISPR: clustered regularly-interspaced short palindromic repeats; GFP: green fluorescent protein; hpf: hours post-fertilization; MO: antisense morpholino oligonucleotide; pHH3: phospho histone H3; ss: somite stage; Traf4: Tumour necrosis factor receptor-associated factor 4; ZO-1: zona occludens-1
Introduction
Epithelial remodelling is a feature of tissues undergoing morphogenesis. Tissue morphogenesis is driven in part by the migration and intercalation of cells. How these dynamic behaviours occur within the context of a nascent epithelium of adherent cells is poorly understood. Presumably, molecular mechanisms exist that would allow for individual cells to change position without disrupting the integrity of the epithelium.
The eye undergoes extensive morphogenesis to achieve its final form. First, the specified eye field splits into two eye vesicles that evaginate from the anterior neural plate and elongate. Subsequently, the vesicles invaginate around the lens to form the optic cup, and lastly the two poles of the ventral optic cup meet and fuse [Citation1,Citation2]. Cell behaviours underlying these events have been studied in fish, where it is possible to track individual cells in the live embryo [Citation3–Citation7]. Formation of the eye vesicles appears to be driven both by individual cell migration and cell intercalation. In medaka, it is reported that migration of eye progenitors drives both the evagination of bilateral eye vesicles from a single eye field, and their subsequent elongation [Citation7]. Recent work in zebrafish argues that eye vesicle evagination and elongation are driven predominantly by the intercalation of mesenchymal-like progenitors from the core of the eye field into the epithelium forming at its lateral edges [Citation4]. Additional cell movements include the migration of progenitors from the telencephalon into the elongating eye vesicles [Citation5], and movement of the epithelium of the dorsal and ventral portions of the inner leaflet of the eye vesicle around the distal rim of the invaginating optic cup [Citation3,Citation6].
Cells within an epithelium exhibit apico-basal (AB) polarity – with neighbouring cells adhering to one another at their apical surface through tight junctions and cadherin-dependent adherens junctions. As cells rearrange, these junctions must maintain adhesion to not compromise epithelial integrity, but at the same time allow cells to move. One potential mechanism is the shrinkage of the junctional contacts between neighbouring cells [Citation8]. Thus, apical adhesive junctions that are plastic with regard their extent and stability may be an important feature of an embryonic epithelium. The molecules that control such epithelial plasticity, however, are poorly understood.
Tumour necrosis factor receptor-associated factor 4 (TRAF4) belongs to the TRAF family of proteins, but unlike other TRAFs, TRAF4 has no defined role in the immune system. TRAF4 encodes a well-conserved adaptor protein with cytoplasmic, nuclear and membrane localization [Citation9–Citation11]. Moreover, TRAF4 is associated with both tight and adherens junctions [Citation12,Citation13], and can control the subcellular location of proteins [Citation13,Citation14]. In Drosophila, Traf4 regulates the association of Armadillo (β-Catenin) in the adherens junctions of constricting mesoderm cells [Citation13], and is required for proper formation of the imaginal eye discs and a correct photosensory neuronal array in the brain [Citation15]. While TRAF4 is required in mouse for neural tube closure and neural crest cell development [Citation16,Citation17], a role for Traf4 in the control of cell-cell adhesion in vertebrate systems has not been explored.
Here we employ loss of Traf4a in zebrafish to understand the need for dynamic regulation of adhesive interactions between progenitors of the eye vesicle for morphogenesis. We find that traf4a mRNA is expressed in the developing eye field and vesicles. Loss or knockdown of Traf4a protein in the early embryo results in disrupted symmetric emergence and elongation of the bilateral eye vesicles, as well as failure of some eye progenitors within the core of the eye field to integrate into the eye epithelium. Additionally, the organization of the eye epithelium is disrupted, with cells of the evaginating vesicles exhibiting aberrant AB polarity, morphology, and radial orientation of their nuclei. We propose a model whereby Traf4a controls apical junction distribution and stability to allow for plasticity of adhesion between epithelial cells that accommodates eye morphogenesis, while maintaining epithelial integrity.
Results
Traf4a mRNA is expressed by eye progenitors
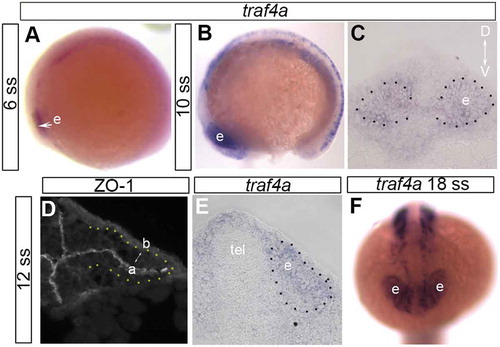
Two traf4 orthologs of human TRAF4 are present in zebrafish, traf4a and traf4b [Citation10]. In the embryo, traf4b mRNA is weakly and ubiquitously expressed, whereas traf4a mRNA is expressed in a highly specific manner. While traf4a expression in the eye of the 3–10 somite stage (ss) zebrafish embryo was reported previously [Citation10], spatial and temporal aspects of the expression in the eye vesicle between the 10 ss and 24 hours post fertilization (hpf) were not described. Thus, we investigated the expression of traf4a by wholemount in situ hybridization from the 6 ss to 18 hpf. The zebrafish eye field is specified during gastrulation and separates into bilateral eye vesicles that start to evaginate at the 5 ss, and elongate over the next 3 hours [Citation1,Citation5]. traf4a mRNA was expressed throughout the 6–12 ss eye vesicle (), and eye expression continued through to at least 18 hpf (). Of note, Traf4 protein is reported to localize to the apical surface of epithelia [Citation12,Citation13], labeled at the 12 ss by the tight junction associated protein ZO-1 [Citation18] (), and the adherens junction protein E-cadherin [Citation19] (data not shown).
Figure 1. traf4a is expressed in the developing eye vesicle. A-C,E: Wholemount in situ hybridization viewed laterally (A,B) and in anterior (C) and posterior (E) transverse sections with antisense riboprobe for traf4a mRNA. D: Immunolabeling of transverse sections through an eye vesicle and forebrain of a 12ss embryo with an antibody against ZO-1 to identify apical surface of eye vesicle. F: traf4a mRNA is still expressed in the 18 ss eye vesicle. Orientation bar in C applies to panels C-E. a, apical; b, basal; D, dorsal; e, eye vesicle; tel, telencephalon; V, ventral.
Traf4a is required for eye development
Given the embryonic expression of traf4a, we tested its involvement in eye development by reducing traf4a mRNA using an antisense morpholino oligonucleotide (MO) that successfully targeted the splice junction between exon 1 and intron 1 (e1i1) (). The MO was injected into one cell Tg(rx3:GFP) embryos, in which the rx3 promoter drives GFP expression in eye and hypothalamic progenitors [Citation7]. The bilateral eye vesicles of controls had elongated at the 12 ss and were of comparable size (). While the eye fields of traf4a morphants also separated into bilateral eye vesicles that evaginated, one or both vesicles were significantly smaller than control (), with vesicles asymmetric in size in over 40% of embryos (3/30 control vs. 18/41 morphants present with ≥ 10% size difference). Three hours later at the 18 ss, when the eye vesicles invaginate around the lens to form the optic cups, the size disparity had not resolved (; 2/25 control and 11/33 morphant embryos).
Figure 2. Traf4a knockdown results in small and asymmetric eye vesicles. A-G: Dorsal views of 12 ss (A-D) and 18 ss (E-G) Tg(rx3:GFP) control embryos (A,E), and embryos injected with either e1i1 MO (B,F), e3i3 MO (G), wild-type zebrafish traf4a mRNA (C), or e1i1 MO along with wild-type zebrafish traf4a mRNA (D). In G, the left eye vesicle outline is superimposed on the right eye. Morphant eye vesicles (outlined) are misshaped, and can be smaller in antero-posterior length. e, eye vesicle; hy, hypothalamus. H: RT-PCR showing knockdown or missplicing of the traf4a transcript with the e1i1 and the e3i3 antisense MOs, respectively, with ef1α as a loading control. I,K,M: Eye size (µm) as measured by the antero-posterior length of the shorter eye vesicle for 18 ss control, and e1i1 (I) and e3i3 (K) traf4a morphant embryos; 12 ss control embryos, and embryos injected with e1i1 with or without wild-type zebrafish traf4a mRNA (M). J,L: Mean percentage difference between the length of e1i1 (J) or e3i3 (L) embryos’ two eye vesicles. Error bars are standard deviation, and numbers above bars indicate number of embryos. Statistics (* p < 0.05, **, p < 0.01) represent: two-tailed, unpaired Student’s t-tests for I,K (N = 3) and J,L (N = 4); One Way ANOVA, Dunnett’s post-hoc test (M, N = 4).
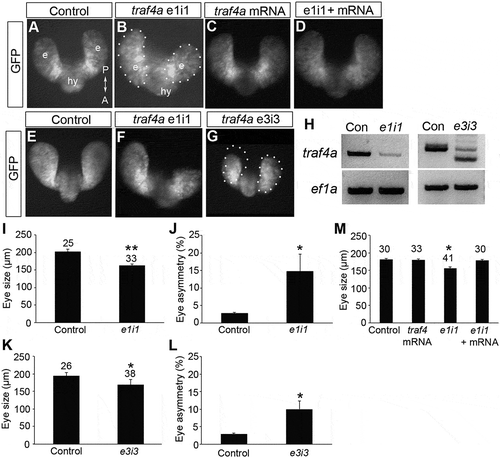
Several lines of evidence support the specificity of the eye phenotype for traf4a knockdown. First, a similar phenotype was observed with a second MO targeting the splice junction between exon 3 and intron 3 (e3i3) (,K,L), which caused mis-splicing of the traf4a mRNA (): morphants exhibited small eyes, sometimes with the two eyes of an embryo being of different sizes (1/27 control and 8/38 MO embryos). The e1i1 MO was used for all subsequent experiments. Second, the phenotype did not arise from inappropriate p53 upregulation, a documented off-target response of antisense MOs in fish [Citation20], in that simultaneous knockdown of both traf4a and p53 failed to rescue the eye phenotype seen with Traf4a knockdown (Supp. Figure 1). Finally, the traf4a morphant eye phenotype was rescued by co-injection of the traf4a MO along with full-length zebrafish traf4a mRNA (not targeted by the e1i1 splice MO) (). Interestingly, Traf4a overexpression had no significant effect on eye vesicle size or morphogenesis (). Finally, because TRAF4 is upregulated in several cancers [Citation21], and controls the asymmetric cell division of Drosophila neuroblasts [Citation22,Citation23], we also investigated cell proliferation. An antibody against phospho-Histone H3 (pHH3), which labels cells actively undergoing mitosis, reveals similar numbers of pHH3 positive cells in both traf4a morphant and control 12 ss embryos (Supp Figure 2).
Anterior neural tube patterned normally with Traf4a knockdown
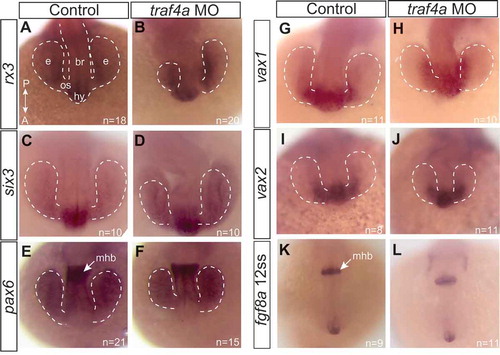
Aberrant development of the eye vesicles could result from defects in the patterning of the early anterior neural tube from which it derives, and where traf4a is expressed (). To address this possibility, we examined by in situ hybridization the mRNA expression of several transcription factors key in the development of anterior neural structures, including: rx3, critical for eye morphogenesis and proliferation [Citation24,Citation25](); six3, key for retinal specification [Citation26](); pax6, known to function in forebrain and eye development [Citation27,Citation28] (); vax1, an optic stalk marker [Citation29] (-H), and; vax2, a marker of the early anterior eye vesicle [Citation30] (-J). In addition, we visualized fibroblast growth factor 8a (fgf8a) mRNA (-L), a morphogen that patterns the forebrain and midbrain [Citation31]. Despite obvious defects in eye vesicle elongation, the general patterns of expression of all of these genes were similar in controls and traf4a morphants.
Figure 3. Gross patterning of the anterior neural keel is not disrupted with Traf4a knockdown. Dorsal views of 12 ss control (A,C,E,G,I,K) and traf4a morphant (B,D,F,H,J,L) embryos processed with antisense riboprobes for rx3 (A-B; N = 2), six3 (C-D; N = 2), pax6 (E-F; N = 2), vax1 (G-H; N = 1), vax2 (I-J; N = 1), and fgf8a (K-L; N = 1). Eye vesicles outlined in white dots. br, brain; e, eye vesicle; hy, hypothalamus; mhb, midbrain/hindbrain border; os, optic stalk.
Apical junction complexes disrupted in Traf4a morphants
In Drosophila, Traf4 is associated with adherens junctions and is required for apical constriction during ventral furrow formation [Citation13]. Epithelial adherens junctions are comprised of the cell adhesion molecule E-cadherin, which associates intracellularly with ß–catenin and the actin cytoskeleton [Citation19]. We investigated the localization of all three proteins. In control, a ß-catenin antibody (; N = 2, 11/11) and rhodamine-phalloidin that marks F-actin (-L; N = 3, 5/5) labeled the membranes of the cells that spanned the width of the brain and eye epithelium, with particularly bright apical and basal signal (). In contrast, the epithelium of morphant eyes was disorganized, with disrupted ß-Catenin labeling of the basal epithelium (-H; N = 2, 16/16; compare white arrowheads in ), and bright accumulations of ß-catenin at the apical surface (,H; white arrows). F-actin label was also dis-continuous basally (-P; N = 3, 26/26; white arrowheads N,P), and disorganized within the epithelium (). Finally, E-cadherin immunoreactivity in control accumulated at the apical surface of the eye vesicle epithelium (-S arrowheads; N = 4, 15/17), whereas in morphants E-Cadherin failed to collect at the apical surface (-W, arrows; N = 4, 17/18). Disrupted localization of apical adherens junction proteins, along with physical gaps between the two normally adherent leaflets of the eye vesicle (), as well as progenitors with no obvious apical attachment (), suggest that adhesive junctions are disrupted in the traf4a morphants. Interestingly, bulges off the eye vesicle (, white arrow) were sometimes observed, as well as clusters of GFP+ cells within the ventricles of the eye vesicles and forebrain (,N,T,X). These cells expressed the polarity protein atypical Protein Kinase C (aPKC) ().
Figure 4. traf4a knockdown disrupts apical adherens junction protein localization. Cryostat sections of 12 ss Tg(rx3:GFP) control (A-D,I-L,Q-T) and traf4a morphant (E-H,M-P,U-X) embryos labeled with antibodies for β-Catenin (B,D,F,H), E-Cadherin (Q,U,S,W), and aPKC (T,X), and rhodamine-phalloidin to reveal F-actin (J-L,N-P). Shown is the merge with the corresponding GFP+ eye progenitors (C,G,K,O,S,W). High magnification views in D,H,L,P,S,T,W,X. Red arrows (F,G,U) point to open ventricle, and white arrow in G to a bulge off the eye vesicle. In morphants, β-Catenin shows aberrant apical accumulations (F,H, white arrows), patches where the radial orientation of F-actin label evident in control (L) is lacking (P), and breaks in β-Catenin and F-actin label basally (B,D,F,H,J,N,L,P arrowheads). E-Cadherin accumulation at the apical surface in control (Q,S arrowheads) is lost in morphant (U,W arrows). Of note, E-Cadherin is expressed at high levels in the skin epithelium (S). T,X: aPKC+ ectopic cells within the forebrain/eye vesicle ventricle (ve) in morphant (E,F,N,X asterisks) but not control (A,B,T). Orientation in A applies to all panels. Scale bar in A: 15 µm (L,P), 25 µm for D,H,S,T,W,X and 50 µm for remaining panels.
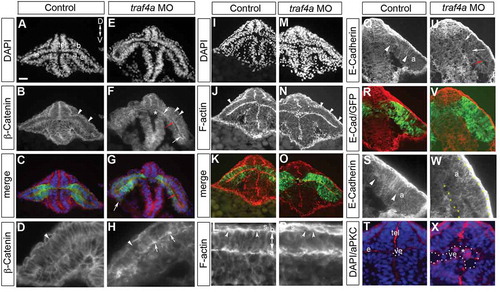
Figure 5. The traf4a morphant eye phenotype is phenocopied in a traf4a genetic mutant. A. The nucleotide sequence of exon1 of the traf4a gene in wild-type and traf4a-/- fish indicating the 10 bp deletion caused by CRISPR mutagenesis, and wild-type and mutant sequence chromatograms showing the premature stop codon introduced in the mutant by the resulting frameshift. B-G: Dorsal views (B,D-G) of traf4a+/+, traf4a+/- and traf4a-/- 14 ss embryos in a Tg(rx3:GFP) background, and a traf4a-/- embryo in brightfield (C). H-I: Lateral view of 48 hpf eyes. Scale bar in B is 100 µm for panels B, D-I. J-K: Quantitation of mean eye size (J) and % difference between the sizes of the two eyes (K). Number of embryos analyzed is shown. *** p < 0.001, non-parametric Mann-Whitney Rank Sums Test.
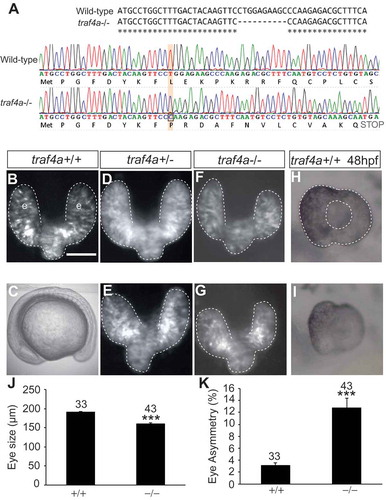
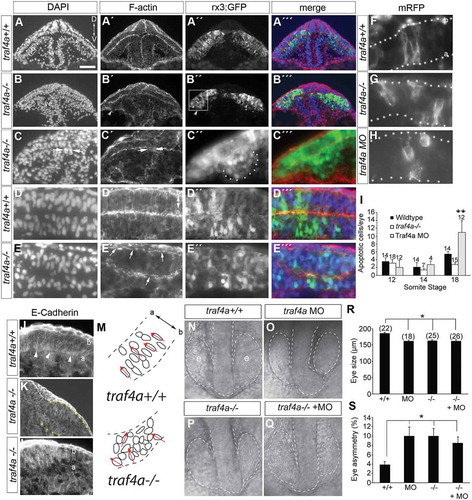
Figure 6. Eye epithelium disrupted in traf4a CRISPR mutant. A-E: Transverse sections through the eye vesicles (e) of wild-type (A,D) and traf4a-/- 12 ss Tg(rx3:GFP) (B,C,E) embryos revealing DAPI-stained nuclei (A-E) and rhodamine-phalloidin stained F-actin (A’-E’), with merge in A’’’-E’’’. In B’ arrowhead points to a bulge off the eye vesicle (shown at higher magnification in C’’), and in C,C’ arrows point to cell cluster in ventricle (ve). Scale bar in A is 100 µm for A-B, 75 µm for C,C’ and 25 µm for C’’,C’’’,D-E,J-L. Higher magnification view (D,E) reveals disruption of organization of DAPI nuclei (E) and non continuous F-actin (E’, arrows) in mutant. F-H: mRFP expressing progenitors in the eyes of wildtype (F), traf4a-/- (G) and traf4a morphant (H) 14 ss embryos. I: Graph of the mean number of activated Caspase-3 positive cells in the whole eye of embryos at the indicated somite stages. Error bars are standard error of the mean (s.e.m.) and numbers above the bars indicate the numbers of embryos assessed (data pooled from two independent experiments). **, p < 0.01 One Way ANOVA, Bonferroni correction. J-L: E-Cadherin labeling of 14 ss wildtype (J) and traf4a-/- (K) rx3:GFP embryo (high power in L). Arrowheads show accumulation of E-Cadherin label at the apical surface in wildtype. M: Schematic of DAPI-labeled nuclei in boxed areas in A and B, with the orientation of selected nuclei indicated by red arrows. N-Q: Dorsal brightfield images of traf4a+/+ (N), e1i1 MO+ traf4a+/+ (O), traf4a-/- (P), and e1i1 MO+ traf4a-/- (Q) embryos with the eye vesicle and brain outlined. R-S: Quantitation of size of smallest 12 ss eye (R) and the % difference between the sizes of the two eyes (S). Number of embryos analyzed is shown. Error bars are standard deviation. *p < 0.05, Kruskal Wallis One Way ANOVA, followed by Dunn’s method for multiple comparisons. a, apical; b, basal.
Eye phenotype present in Traf4a-/- CRISPR genetic mutant
Because of potential concerns about the specificity of phenotypes arising through the use of antisense MOs [Citation32], we generated a traf4a genetic mutant by using CRISPR gene silencing technology [Citation33]. A guide RNA complementary to exon1 of the traf4a gene was injected along with Cas-9 nuclease into the one cell embryo. A single F0 female founder was bred to homozygosity in the F2 generation: sequencing identified a 10 bp deletion that produced a frameshift introducing a premature stop codon (), as seen by a smaller RT-PCR product amplified with traf4a specific primers from mRNA of mutants than wildtype (data not shown). traf4a homozygous mutants were viable and fertile, and embryogenesis was grossly normal (). The eyes of 12 ss F3 embryos showed a comparable phenotype to the traf4a morphants. In the majority of embryos (), one or both eyes were smaller than observed in wild-type or traf4a± embryos (). In many cases an asymmetry in the size of the two eyes was present (,K; 0/33 wildtype and 24/43 traf4a-/- embryos), though in some embryos both eyes were equally small in size (). The generally smaller size of the mutant eyes remained at 48 hpf (). Nonetheless, optic cup morphogenesis and retinal pigment epithelium expansion over the optic cup appeared to occur normally.
Compared to wild-type eye vesicles (; N = 2, 10/10), the epithelium of the mutant (; N = 3, 16/16) eye vesicles were disorganized: nuclei often failed to orient perpendicular to the apical surface of the eye vesicle epithelium (), and F-actin on both basal and apical surfaces of the epithelium was disrupted and unevenly distributed (compare Fig. 6A’,D’ with B’,C’,E’). Similar to the traf4a morphants, mutants sometimes exhibited ectopic GFP positive cell clusters in the ventricles of the eye epithelium (,C’ arrows), and aberrant bulges from the eye vesicle epithelium (Fig. 6B’,B’’,C’’,C’’’). Moreover, with Traf4a loss, membrane associated red fluorescent protein (mRFP)-expressing eye cells (from a cDNA plasmid injected at the one-cell stage) were present that did not span the epithelium, and had no obvious apically directed process with an attachment to the apical surface, similar to what is seen in traf4a morphants (). Finally, comparable to what was observed in traf4a morphants (), E-Cadherin failed to accumulate at the apical surface of the eye epithelium in 14 ss traf4a-/- mutants (compare ).
TRAF4 can act as either an anti- or pro- apoptotic factor (Rousseau et al., 2011). As such, we examined cell death in our traf4a mutant and morphant eye vesicles by immunohistochemistry for activated Caspase 3, at different time points between the 12 ss and 18 ss. traf4a mutant 12–18 ss eye vesicles exhibited similar numbers of apoptotic cells as compared to control (). More apoptotic cells were present in the eye vesicles of traf4a morphants at the 18ss, though numbers were relatively small, and importantly eye vesicle defects were seen in morphant embryos at embryonic stages (12 and 14 ss) when apoptosis was minimal. Thus, apoptosis does not appear to be a significant contributor towards the eye vesicle phenotypes observed at the 12–14 ss with the loss of Traf4a. Importantly, for the validity of the morphant phenotypes we described, we saw no exacerbation of the mutant phenotype () when we injected traf4a MO into mutant embryos, either in terms of the length of the smallest eye vesicle () or the size asymmetry of the two eyes ().
Epithelial polarity disrupted by Traf4a loss
Given that Traf4 regulates adhesion junctions [Citation12,Citation13], and apical E-Cadherin is disrupted with Traf4a loss, we next asked if the polarity of the eye epithelium was also affected. We first performed immunolabeling for aPKC, which localizes to the apical surface of epithelia [Citation34]. In control, aPKC () was found at the apical midline of the forebrain and eye vesicle epithelia (N = 4, 18/18 embryos). In traf4a morphants (; N = 4, 15/17 embryos) and mutants (; 10/10 embryos), while this polarized localization was somewhat preserved, aPKC was unevenly distributed, with mosaic patches of little or intense apical immunoreactivity, and ectopic non-apical expression. Further, a marker of the basal surface of the epithelium, Laminin, appeared downregulated and discontinuous on the dorsal surface of the eye epithelium in the traf4a morphants (; N = 2, 11/13 embryos) and mutants (; 10/10 embryos) as compared to controls (; N = 2, 0/20 embryos).
Figure 7. Traf4a loss disrupts apical-basal polarity of the eye epithelium. Transverse sections through the anterior forebrain (br) and eye vesicles (e) of control (A-C,J,M-O,V), traf4a morphant (D-F,K,P-R,W), and traf4a-/- (G-I,L,S-U,X) 12-14ss Tg(rx3:GFP) embryos. A-L: aPKC (A,D,G,J-L) immunolabeling, and merge (C,F,I) with GFP (B,E,H). Unevenly distributed apical (D,G arrows), and patches of non-apically localized (D,G,K,L; arrowheads), aPKC immunolabel are evident with Traf4a loss. M-U: Laminin immunolabeling (M,P,S) is basally localized in control, but patchy (arrows) and occasionally ectopically expressed (arrowheads) in morphants and mutants. V-X: ZO-1 immunolabeling is predominantly at the apical surface of the eye epithelium, but is also ectopic to the apical surface (W,X; arrowheads) with Traf4a loss. Orientation in A applies to all panels. a, apical; b, basal; s, skin. Scale bar in J is 50 µm (A-I), 75 µm (M-U), and 20 µm (J-L, V-X).
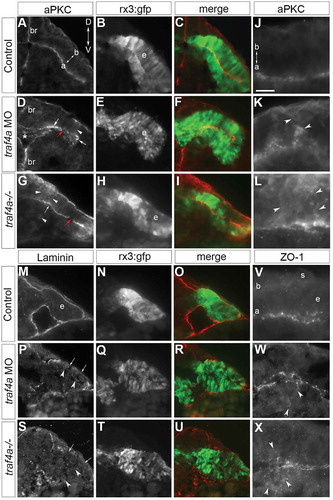
TRAF4 is reported to associate with apically localized tight junctions [Citation35]. As such, we also investigated the expression of the tight junction protein ZO-1 [Citation18]. In control, ZO-1 immunoreactivity was associated with the apical surface of the neuroepithelium of the eye vesicle (; N = 3, 20/20). With Traf4a knockdown (; N = 3, 19/19) and loss (; 14/14 embryos), ZO-1 immunoreactivity was still associated with the apical surface but in a disorganized fashion, and like aPKC could be mis-localized away from the apical surface of the eye vesicle.
Improper eye vesicle evagination and elongation with Traf4a loss
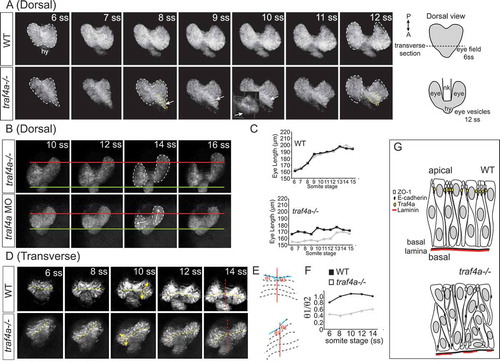
To better understand the defects of early eye development observed with loss of Traf4a, we followed the morphogenesis of control and traf4a loss Tg(rx3:GFP) eye vesicles by confocal time-lapse microscopy, over the period that eye vesicles evaginate and elongate (6–18 ss) (). In a dorsal view, in the control embryo the eye field separated and the eye vesicles evaginated in a symmetrical manner from the diencephalon (, Supp. Movie 1). The control eye vesicles then elongated gradually over time and separated fully at the midline (). In the traf4a-/- embryo, however, the eye field at the 6 ss was not symmetric in shape, exhibiting an aberrant bulge (, arrow; Supp. Movie 2), and elongation of the two eye vesicles was impaired (). Nonetheless, the eye vesicles did separate completely. In other traf4a-/- mutant embryos, one eye vesicle appeared to evaginate and elongate normally, while the other failed to elongate (, Supp. Movie 3). A similar failure of one or both eye vesicles to elongate was observed in the traf4a morphant embryos (). To assess whether prior to defects in eye vesicle elongation, evagination of the eye vesicles was affected, we followed early eye morphogenesis in transverse optical sections starting at the 6 ss (). In the traf4a-/- mutant eye vesicle, evagination appeared defective at the 6ss, both in relation to the initial size of the two eye vesicles (), and the direction of evagination (). As a result, spatial symmetry of the eye vesicles centred at the midline of the neural keel was lost (), as revealed by the ratio of the angles the ventricle of the two eye vesicles make with the midline of the neural keel ().
Figure 8. Eye vesicle evagination and elongation abnormal with Traf4a loss. Sequential confocal maximal projection of optical sections from Tg(rx3:GFP) control, e1i1 MO injected and traf4a-/- eye vesicles over the period of eye evagination and elongation (6–18 ss). Time-lapse sequences were obtained for the dorsal view from n = 6 control, n = 3 morphant and n = 5 traf4a-/- embryos, and for the transverse view from n = 8 control, n = 9 morphant and n = 3 traf4a-/- embryos. A-B: Dorsal view, from the 6–12 ss (A) and from the 10–16 ss (B). Eye vesicles outlined in white, and a bulge in the eye field (ef) outlined in yellow, and shown as a 3-D reconstruction (inset). Asymmetric evagination of the eye vesicles indicated by arrows. In B, the green and red lines mark the anterior and posterior extent of the left eye vesicles at the 10 ss. C: Length of the two eye vesicles over time for the two embryos shown in A. D-F: Transverse view (D), from the 6–14 ss. Dotted yellow lines mark the ventricles, and the red line the midline of the neural keel. These lines are represented anew in E, and the ratios of the angles of the ventricle to the midline for the two eye vesicles over time are shown in F. G: Model for Traf4a function in the eye epithelium. Traf4a is an adaptor protein found at the plasma membrane that associates with both adherens (E-cadherin) and tight (ZO-1) junction proteins in other systems. Based on the literature, we propose that Traf4a is co-localized with these proteins at the apical surface of the nascent zebrafish eye epithelium. In the absence of Traf4a, ZO-1 is present in a patchy fashion on the apical surface and ectopic to the apical surface, while E-Cadherin does not accumulate apically. Further, expression of the basally located protein Laminin is disrupted. Consequently a disorganized epithelium is formed, whose cells can lose their apical attachment and radially-oriented nuclei, which is associated with a failure of eye vesicles to evaginate and elongate.
Discussion
Our data support an important role for the apical junction-associated adaptor protein Traf4a in the embryonic zebrafish eye in allowing proper formation of a polarized pseudostratified eye epithelium – a feature that appears critical for morphogenesis. traf4a is expressed by early eye progenitors. Both knockdown and loss of Traf4a protein result in a disorganized embryonic eye epithelium with disrupted AB polarity and apical progenitor attachments, and improper evagination and elongation of the eye vesicles. Our data support the idea [Citation4] that development of an eye epithelium with appropriately localized apical adhesive junctions and basal proteins is required to allow for the cell movements that drive early eye vesicle morphogenesis.
Traf4a loss or knockdown causes several distinct defects in eye epithelial organization. Importantly, comparable defects are present in both the traf4a morphants and CRISPR genetic mutant. As such, the disruptions in epithelial organization we described are highly likely to be specific to low or absent Traf4a. These defects include many eye progenitors showing disrupted AB polarity, a loss of the elongated, radial orientation of nuclei within the pseudo-stratified eye epithelium, and an abnormally distributed actin cytoskeleton. Also present are clusters of adherent GFP+ eye progenitors in the ventricle of the eye vesicle, which could reflect core eye field progenitors that failed to intercalate into the eye epithelium [Citation4], or the delamination and extrusion of progenitors [Citation6]. Loss of Traf4a presents with extra evaginations at random locations off the eye vesicle. Interestingly, time-lapse imaging suggests that these protrusions are transient and resorb into the eye vesicle proper (data not shown). Finally, the antero-posterior extent of one or both of the eye vesicles is often smaller than in control. The time lapse microscopy suggests a few possible explanations. First, with Traf4a loss the eye field can split in a non-symmetric fashion, resulting in eye vesicles of different sizes. Second, eye vesicles do not always elongate appropriately. Elongation of the eye vesicles in zebrafish is thought to be driven by the intercalation of mesenchymal-like eye progenitors from the core of the eye field into the lateral eye vesicle epithelium [Citation4], and so potentially the loss of Traf4a alters the adhesiveness of the epithelium and blocks progenitor intercalation. Indeed, we do observe GFP+ eye progenitors within the ventricle of the forebrain and eye vesicles with Traf4a loss. Survival of progenitors within the eye vesicles did not appear to be impacted by Traf4a loss, and so likely did not contribute directly to a smaller eye vesicle size.
The disrupted AB polarity of eye progenitors is reminiscent of that reported for zebrafish missing Laminin function [Citation4,Citation36]. Laminin-1 is a component of the basal lamina and is required for many eye progenitors to maintain proper AB polarity and an elongated radial morphology. In the absence of Laminin-1, apical proteins are present both apically and ectopically on the basal surfaces of the eye epithelium, progenitors lose their apical attachments, and eye evagination is consequently disrupted [Citation4,Citation36]. We find the basal expression of Laminin is patchy with Traf4a loss. Thus, the defects in eye evagination and elongation that occur when Traf4a is absent might result similarly from disruptions of normal AB cellular polarity and epithelial organization. Interestingly, despite the disorganized epithelium, eye vesicles usually do evaginate and elongate to some extent, arguing that early eye morphogenesis is a robust phenomenon. Subsequent events of eye morphogenesis, including eye vesicle invagination around the lens and choroid fissure fusion, appear to not require Traf4a, while Laminin-alpha1 is necessary for both early eye vesicle elongation and for subsequent optic cup morphogenesis [Citation36].
Interestingly, the defects in AB polarity of the eye progenitors with Traf4a loss are mosaic in nature. Disruptions in polarity protein, and actin and β-Catenin localization are patchy in nature and vary in location and severity from eye vesicle to eye vesicle. The mosaicism could be explained by redundant mechanisms permitting proper AB polarity to be achieved in some regions of the epithelium. In support, a Traf4-independent mechanism must carry out similar functions to Traf4a in organizing the location of proteins of the Xenopus eye epithelium, where traf4 is not obviously expressed [Citation16]. The fact that only 30% of Traf4-/- mice exhibit embryonic lethality, with the remainder showing a number of less penetrant defects [Citation17], and that we find that some zebrafish embryos exhibit apparently normal eye vesicle development with Traf4a loss, also argues for redundant mechanisms. Mosaic penetrance of the defects within the eye epithelium could explain the differences in phenotype observed between the two eye vesicles of a single embryo. Presumably, morphogenetic defects arise only if large enough patches of the epithelium are disorganized and contain cells with defective AB polarity and morphology. One possibility is that if the associations between eye progenitors are sufficiently disrupted at the eye field stage to impact the extent and/or direction of evagination of the tissue from the diencephalon, eye elongation is impacted. If not, eye elongation occurs normally.
How might Traf4a control the organization and polarity of a nascent epithelium? Traf4 is known to regulate the subcellular localization of proteins, to control cell apoptosis and proliferation, and to associate with both tight and adherens junctions. Traf4a is unlikely to be controlling epithelial homeostasis as it does for cultured mammary epithelial cells [Citation12], in that Traf4a is absent from the nuclei of eye progenitors and Traf4a loss causes no dramatic proliferation or apoptosis defects over the early stages of eye development. Instead, based on the known roles for Traf4 in the literature, our data suggest a model () whereby Traf4a controls the localization and function of apical adhesive junctions to control associations between eye progenitors and maintain the organization of the pseudostratified eye epithelium, and allow for proper eye evagination and elongation. In support, Traf4a protein is enriched at the apical surface of the eye epithelium, along with tight junction and adherens junction associated proteins. Moreover, we find the apical localization of both the E-Cadherin and β-Catenin adherens junction proteins, and the ZO-1 tight junction protein, along with the apical attachments of progenitors, are disrupted with Traf4a loss.
In some systems, Traf4 promotes the plasticity of junctional complexes by negatively regulating junction stability. For instance, in breast cancer cells Traf4 is upregulated and destabilizes tight junctions [Citation35], and indirectly regulates adherens junctions by promoting TGFβR1 signaling-dependent down regulation of E-cadherin [Citation37]. Such plasticity is important to allow epithelial cells to retain contacts while undergoing morphogenesis [Citation38]. While Traf4 can regulate tight junctions [Citation35], a critical role for tight junctions in epithelial remodelling is unclear [Citation39], and we find ZO-1 is only mildly impacted by Traf4a loss. In contrast, the regulation of adherens junctions is key for epithelial remodelling [Citation40], and Traf4 associates with and fine tunes the assembly of adherens junction complexes in invaginating Drosophila mesodermal cells [Citation13]. Thus, our data revealing a loss of the accumulation of E-Cadherin at the apical surface of the eye epithelium, and non-uniform apical localization of β-Catenin and aPKC, support the idea that the eye phenotype observed with Traf4a loss is best explained by Traf4a regulation of adherens junctions. Our data, however, argue against Traf4a de-stabilizing apical junctions between eye epithelial cells, as with Traf4a loss E-Cadherin fails to accumulate at the apical surface. Whether normally Traf4a controls localization of E-Cadherin or β-Catenin to the apical surface of eye progenitors is unclear, though data suggests Traf4a can interact directly with β-Catenin [Citation13]. Nonetheless, the two proteins critical for adherens junction function become mislocalized with Traf4a loss, with apical β-Catenin released from E-Cadherin interactions clumping, and E-Cadherin failing to collect at the apical surface, respectively. The apparent result is that adhesive interactions in the eye vesicle are impacted, with progenitors losing their apical attachments and AB polarity, and the two leaflets of the eye vesicles failing to adhere to one another.
The idea that disrupted adherens junction function could explain some of the epithelial phenotypes we observe is supported by the literature. For instance, increases in the levels of proteins that promote apical localization of adherens junctions cause ectopic folds of the dorsal epithelium during Drosophila gastrulation [Citation41]. Thus, one possibility is that in the absence of Traf4a, mis-localized adherens junction proteins cause ectopic folds in the eye epithelium, and account for the ectopic evaginations we observe. Traf4a may also, via the regulation of adherens junctions, control the intercalation of progenitors of the eye field core [Citation4], in that a role for adherens junctions in cell intercalation is well established [Citation42–Citation44].
We also find Traf4a concentrated at the basal surface of the eye vesicle epithelium, where we observe disruptions in the localization of Laminin, F-actin and β-Catenin expression. The function of Traf4 that is best supported by the literature is as a regulator of the localization of apically targeted proteins [Citation12,Citation13], and certainly we see disruptions in the localization of apical proteins with Traf4a loss. Thus, the defects in basally located proteins may arise secondary to apical defects. Nonetheless, we cannot rule out a direct role of Traf4a at the basal surface of eye epithelial cells. Certainly, Laminin is known for its role in polarizing epithelia [Citation45]. Moreover, in platelets, Traf4 is in a complex with a glycoprotein collagen receptor, upstream of focal adhesion kinase [Citation46], suggesting it may play roles in extracellular matrix protein signaling. Future experiments will need to resolve the exact role Traf4 plays in controlling adhesion in the eye epithelium.
traf4a expression exhibits dynamic spatial changes over embryonic development, and Traf4a in zebrafish is expressed in tissues other than the eye, such as the somites, which also undergo morphogenesis. In cancers where Traf4 is overexpressed [Citation47,Citation48], cells fail to adhere to their neighbours, epithelial integrity is compromised and migration is promoted. Thus, Traf4a may be a general regulator of the plasticity of adhesion between cells within epithelia that are experiencing cell movement and morphogenesis.
Materials and methods
Zebrafish husbandry
Zebrafish embryos were developmentally staged as per [Citation49]. Dr. Wittbrodt (U. Heidelberg) provided Tg(rx3:GFP) fish. The University of Calgary Animal Care Committee approved all procedures.
In situ hybridization
RNA in situ hybridization was performed as described previously [Citation50]. Antisense riboprobe was made for traf4a by using linearized Image Clone 6970326 with the UTR removed (MGC:77418 Accession BC0659691, Open Biosystems). rx3, vax1, and six3 riboprobes were provided by Dr. D. Kurrasch (U. Calgary), and the vax2 riboprobe from an Image clone (#9038293). fgf8a and pax6 riboprobes were synthesized from PCR fragments amplified from embryonic zebrafish cDNA using the primers: fgf8a: forward primer; GATGAGACTCATACCTTCAC, and reverse primer+ SP6: ATTTAGGTGACACTATAGATCAACGCTCTCCTGAGTAG. pax6: forward primer: CTGACGTTTTTGCACGAGAA, reverse+ T7: GAAATTAATACGACTCACTATAGGGTTTGCAGTGCAGGATGAGTC. Probes were synthesized by using SP6 or T7 RNA polymerase (Roche).
RNA isolation and RT-PCR
Total RNA from 50–75 embryos at the 21 ss was prepared by using Trizol/chloroform (Invitrogen). First strand cDNA was made using the Superscript II RT-PCR protocol (Invitrogen), and amplified by PCR using HiFi polymerase (Thermo Scientific) and the following primers: traf4a e1i1 MO; forward: TTCTCGTCCTCTCGGTTCAT, reverse: TAATCCAATGGGAGCTGGTC. traf4a e3i3 MO; forward: GACCAGCTCCCATTGGATTA, reverse: GACAGTCGTGTTGCAGGTGT. ef1α; forward: CGGTGACAACATGCTGGAGG, reverse: ACCAGTCTCCACACGACCCA.
Embryo injections
Antisense morpholino oligonucleotides (Gene Tools LLC), mRNA (450 pg mRNA), or morpholino/mRNA combinations were injected at the 1-cell stage. mRNA for embryo injection was made by using cDNA plasmids encoding either a zebrafish traf4a, or membrane-associated red fluorescent protein (mRFP; provided by Dr. P. Huang) and the mMessage Machine T7 Kit (Ambion) as per kit instructions. Morpholino concentrations and sequences are as follows; traf4a e1i1, 4–8 ng (GGCCAAACGTTGCTCTTACCTGAGA), and traf4a e3i3 12 ng (GAGATGAAAAGCGTGATTACCTGTA). The p53 MO is Danio rerio p53 from Gene Tools, LLC. For rescue experiments, traf4a e1i1 MO was injected on its own, or with mRNA encoding full-length zebrafish traf4a. Injected embryos that were delayed in development (> 1 hr behind control) and those with severe convergent extension defects where embryo staging was not possible were not included in our analysis.
CRISPR/Cas9 targeted mutation of traf4a
The single guide RNA (sgRNA) target sequence for traf4a (AGCGTCTCTTGGGCTTCTCC) was identified by using MIT Optimized CRISPR Design software (Zhang lab), and made using the Maxiscript T7 In Vitro Transcription Kit (Ambion) [Citation51]. 1nL of a mixture of traf4a sgRNA (82ng/uL) and Cas9 Nuclease (80ng/uL, New England Biolabs) were injected into single cell Tupfel Long fin embryos. RNA was quantified by using a Nanodrop spectrophotometer. Injected embryos were pooled at 24 hpf, genomic DNA was extracted and the targeted region was amplified by PCR to detect mutations via a T7 Endonuclease assay (F: CGAGCTCTAGCCTGCATTGA, R: TTCCTGCAGGCAGGTATCAC, product 350bp). Founders were outcrossed onto the Tg(rx3:gfp) background and germline transmission was determined in progeny by T7 Endonuclease and High Resolution Melt (HRM) analysis (F: TGCCTGGCTTTGACTACAAGT, R: CACATGTGGAAACTTGGACCG). An indel mutation was determined by subcloning into TOPO (Invitrogen) for sequencing. Heterozygous F1 adults carrying the same frameshift mutant alleles were crossed to one another to yield F2 homozygous mutant fish.
Confocal time-lapse imaging
Tg(rx3:GFP) transgenic embryos were live imaged from the 6–12 ss or the 10–18 ss with a 10X or 20X objective. Embryos were embedded in 0.8% low melt agarose on glass bottom dishes (Mat-Tek). For transverse optical sections, embryos were positioned at a slight angle with their yolk sac touching the bottom of the dish and caudal end closer to the bottom than the head. For both transverse and dorsal orientations, optical sections of 3 µm step size through the eye vesicle were taken on a Zeiss LSM 700 inverted microscope every 5 minutes over a 3.5-hour window. Stacks were processed in Zen Lite and presented as maximal projections of 4–5 sections, or for the movies as weighted averages of 30 sections.
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde, infiltrated in 25% followed by 35% sucrose (EM Science), and embedded in Optimal Cutting Temperature (OCT; Tissue Tek). Twelve µm sections were cut on a Microm HM 500 OM cryostat, and immunostaining performed as described previously [Citation52]. The following antibodies were used; activated Caspase-3 1:500 (Promega, G748A lot#47473), Laminin 1:200 (Sigma, L9393, Batch 103M4779), pHH3 1:500 (Millipore, 06–570, lot#2202541), ZO-1 1:150 (Invitrogen, 339100, lot#QA210455), E-Cadherin 1:250 (GeneTex, GTX125890, lot#41388), β-Catenin 1:500 (Santa Cruz), aPKC 1:500 (Santa Cruz, SC-216, lot#J2604), anti-mouse or anti-rabbit Alexa Fluor 546 secondary 1:1000 (Invitrogen). Hoechst dye (1:1000, Invitrogen) and AlexFluor 546 phalloidin (Invitrogen) were used to label nuclei and F-actin, respectively. Images were taken on a Zeiss compound microscope using an Axiovision MRc camera (Zeiss). Images were processed in Adobe Photoshop for brightness and contrast. pHH3 and activated Caspase-3 immunostaining were performed in 12 ss wholemount embryos. pHH3 labeled embryos were embedded in JB4 media, 7 µm transverse sections cut on a Leica microtome, and pHH3+ cells labeled cells counted within the GFP+ eye where it evaginates from the brain. For activated Caspase-3+, cells with the GFP+ eye vesicle were counted in wholemount. In both experiments, the experimenter was blinded to the treatment.
Eye size measurements
Photomicrographs of the dorsal view of the bilateral eye vesicles were taken of embryos with a dissecting stereocompound epifluorescent microscope with an Axiovision HRc camera (Zeiss). The antero-posterior lengths of the right and left eye vesicles (µm) were measured.
Statistics
Statistical analyzes were performed by using SigmaStat 3.0. Error bars represent the standard deviation of the average of data from independent replicates (N). Statistical tests, numbers of independent replicates, and the numbers of embryos analyzed, are presented in the figures and their corresponding legends.
Author Contributions
CH did all the experiments except for the time-lapse imaging done by RH, and both CH and RH provided experimental guidance and edited the manuscript. SM designed the experiments, collected data, and wrote the manuscript.
Supplemental Material
Download Zip (10 MB)Acknowledgments
The authors thank Dr. G.E. Bertolesi for technical assistance, Ms. Paula Cechmanek and Dr. G.E. Bertolesi for comments on the manuscript, and Dr. S. Childs for the use of her fish facility.
Disclosure statement
No potential conflict of interest was reported by the authors.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Graw J. Eye development. Curr Top Dev Biol. 2010;90:343–386.
- Sinn R, Wittbrodt J. An eye on eye development. Mech Dev. 2013;130(6–8):347–358.
- Heermann S, Schütz L, Lemke S, et al. Eye morphogenesis driven by epithelial flow into the optic cup facilitated by modulation of bone morphogenetic protein. eLife. 2015;4:e05216.
- Ivanovitch K, Cavodeassi F, Wilson SW. Precocious acquisition of neuroepithelial character in the eye field underlies the onset of eye morphogenesis. Dev Cell. 2013;27(3):293–305.
- Kwan KM, Otsuna H, Kidokoro H, et al. A complex choreography of cell movements shapes the vertebrate eye. Devt. 2012;139(2):359–372.
- Picker A, Cavodeassi F, Machate A, et al. Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina. PLoS Biol. 2009;7(10):e1000214.
- Rembold M, Loosli F, Adams RJ, et al. Individual cell migration serves as the driving force for optic vesicle evagination. Science. 2006;313(5790):1130–1134.
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192(6):907–917.
- Glauner H, Siegmund D, Motejadded H, et al. Intracellular localization and transcriptional regulation of tumor necrosis factor (TNF) receptor-associated factor 4 (TRAF4). Eur J Biochem FEBS. 2002;269(19):4819–4829.
- Kedinger V, Alpy F, Tomasetto C, et al. Spatial and temporal distribution of the traf4 genes during zebrafish development. Gene Expr Patterns. 2005;5(4):545–552.
- Régnier CH, Tomasetto C, Moog-Lutz C, et al. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem. 1995;270(43):25715–25721.
- Kédinger V, Alpy F, Baguet A, et al. Tumor necrosis factor receptor-associated factor 4 is a dynamic tight junction-related shuttle protein involved in epithelium homeostasis. PloS One. 2008;3(10):e3518.
- Mathew SJ, Rembold M, Leptin M. Role for Traf4 in polarizing adherens junctions as a prerequisite for efficient cell shape changes. Mol Cell Biol. 2011;31(24):4978–4993.
- Wu RF, Xu YC, Ma Z, et al. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171(5):893–904.
- Cha G-H, Cho KS, Lee JH, et al. Discrete functions of TRAF1 and TRAF2 in Drosophila melanogaster mediated by c-Jun N-terminal kinase and NF-kappaB-dependent signaling pathways. Mol Cell Biol. 2003;23(22):7982–7991.
- Kalkan T, Iwasaki Y, Park CY, et al. Tumor necrosis factor-receptor-associated factor-4 is a positive regulator of transforming growth factor-beta signaling that affects neural crest formation. Mol Biol Cell. 2009;20(14):3436–3450.
- Régnier CH, Masson R, Kedinger V, et al. Impaired neural tube closure, axial skeleton malformations, and tracheal ring disruption in TRAF4-deficient mice. Proc Natl Acad Sci U S A. 2002;99(8):5585–5590.
- Kiener TK, Sleptsova-Friedrich I, Hunziker W. Identification, tissue distribution and developmental expression of tjp1/zo-1, tjp2/zo-2 and tjp3/zo-3 in the zebrafish, Danio rerio. Gene Expr Patterns. 2007;7(7):767–776.
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15(4):225–242.
- Robu ME, Larson JD, Nasevicius A, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3(5):e78.
- Rousseau A, M-C R, Alpy F. TRAF4, at the Crossroad between Morphogenesis and Cancer. Cancers. 2011;3(2):2734–2749.
- Peng CY, Manning L, Albertson R, et al. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408(6812):596–600.
- Wang H, Cai Y, Chia W, et al. Drosophila homologs of mammalian TNF/TNFR-related molecules regulate segregation of Miranda/Prospero in neuroblasts. EMBO J. 2006;25(24):5783–5793.
- Loosli F, Staub W, Finger-Baier KC, et al. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4(9):894–899.
- Stigloher C, Ninkovic J, Laplante M, et al. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Devt. 2006;133(15):2925–2935.
- Liu W, Lagutin O, Swindell E, et al. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J Clin Invest. 2010;120(10):3568–3577.
- Gehring WJ. The evolution of vision. Wiley Interdiscip Rev Dev Biol. 2014;3(1):1–40.
- Georgala PA, Carr CB, Price DJ. The role of Pax6 in forebrain development. Dev Neurobiol. 2011;71(8):690–709.
- Take-Uchi M, Clarke JDW, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Devt. 2003;130(5):955–968.
- Erickson T, French CR, Waskiewicz AJ. Meis1 specifies positional information in the retina and tectum to organize the zebrafish visual system. Neural Dev. 2010;5:22.
- Suzuki-Hirano A, Shimogori T. The role of Fgf8 in telencephalic and diencephalic patterning. Semin Cell Dev Biol. 2009;20(6):719–725.
- Blum M, De Robertis EM, Wallingford JB, et al. Morpholinos: antisense and sensibility. Dev Cell. 2015;35(2):145–149.
- Chang N, Sun C, Gao L, et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 2013;23(4):465–472.
- Tawk M, Araya C, Lyons DA, et al. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446(7137):797–800.
- Rousseau A, McEwen AG, Poussin-Courmontagne P, et al. TRAF4 is a novel phosphoinositide-binding protein modulating tight junctions and favoring cell migration. PLoS Biol. 2013;11(12):e1001726.
- Bryan CD, Chien C-B, Kwan KM. Loss of laminin alpha 1 results in multiple structural defects and divergent effects on adhesion during vertebrate optic cup morphogenesis. Dev Biol. 2016;416(2):324–337.
- Zhang L, Zhou F, García De Vinuesa A, et al. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell. 2013;51(5):559–572.
- Collinet C, Lecuit T. Stability and dynamics of cell-cell junctions. Prog Mol Biol Transl Sci. 2013;116:25–47.
- Balda MS, Matter K. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol. 2016;42:94–101.
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397–410.
- Wang Y-C, Khan Z, Kaschube M, et al. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature. 2012;484(7394):390–393.
- Morita H, Heisenberg C-P. Holding on and letting go: cadherin turnover in cell intercalation. Dev Cell. 2013;24(6):567–569.
- Song S, Eckerle S, Onichtchouk D, et al. Pou5f1-dependent EGF expression controls E-cadherin endocytosis, cell adhesion, and zebrafish epiboly movements. Dev Cell. 2013;24(5):486–501.
- Winklbauer R. Cell adhesion in amphibian gastrulation. Int Rev Cell Mol Biol. 2009;278:215–275.
- Lee JL, Streuli CH. Integrins and epithelial cell polarity. J Cell Sci. 2014;127(Pt 15):3217–3225.
- Carrim N, Walsh TG, Consonni A, et al. Role of focal adhesion tyrosine kinases in GPVI-dependent platelet activation and reactive oxygen species formation. PloS One. 2014;9(11):e113679.
- Camilleri-Broët S, Cremer I, Marmey B, et al. TRAF4 overexpression is a common characteristic of human carcinomas. Oncogene. 2007;26(1):142–147.
- Rhodes DR, Yu J, Shanker K, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci U S A. 2004;101(25):9309–9314.
- Kimmel CB, Ballard WW, Kimmel SR, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310.
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3(1):59–69.
- Gagnon JA, Valen E, Thyme SB, et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PloS One. 2014;9(5):e98186.
- Ebert AM, Childs SJ, Hehr CL, et al. Sema6a and Plxna2 mediate spatially regulated repulsion within the developing eye to promote eye vesicle cohesion. Devt. 2014;141(12):2473–2482.
