ABSTRACT
Programmed cell death occurs as a normal part of oocyte development in Drosophila. For each egg that is formed, 15 germline-derived nurse cells transfer their cytoplasmic contents into the oocyte and die. Disruption of apoptosis or autophagy only partially inhibits the death of the nurse cells, indicating that other mechanisms significantly contribute to nurse cell death. Recently, we demonstrated that the surrounding stretch follicle cells non-autonomously promote nurse cell death during late oogenesis and that phagocytosis genes including draper, ced-12, and the JNK pathway are crucial for this process. When phagocytosis genes are inhibited in the follicle cells, events specifically associated with death of the nurse cells are impaired. Death of the nurse cells is not completely blocked in draper mutants, suggesting that other engulfment receptors are involved. Indeed, we found that the integrin subunit, αPS3, is enriched on stretch follicle cells during late oogenesis and is required for elimination of the nurse cells. Moreover, double mutant analysis revealed that integrins act in parallel to draper. Death of nurse cells in the Drosophila ovary is a unique example of programmed cell death that is both non-apoptotic and non-cell autonomously controlled.
Introduction
Cell death occurs throughout development, helps to maintain homeostasis, and is a major contributing factor to numerous diseases in humans. Over 40 y ago, Kerr and colleagues coined the term apoptosis, describing a common morphological form of cell death observed in development and disease.Citation1 In the 1990s, several genes controlling apoptosis in mammals and programmed cell death in C. elegans were found to be homologous, demonstrating evolutionary conservation of this fundamental cellular process.Citation2-4 Although there are differences among upstream regulators of apoptosis in different cell types and organisms, a common event in apoptosis is the activation of caspases, a family of cysteine-aspartyl proteases that cleave cellular targets to destroy the cell.Citation5
In addition to apoptosis, many other forms of cell death have been described. However, a molecular understanding of these forms of cell death has lagged significantly compared to apoptosis. Other forms of cell death, including necrosis, pyroptosis, and undoubtedly others, are likely to contribute significantly to certain human diseases, and identifying treatments hinges on a molecular understanding of these “alternative” cell death pathways.Citation6 For example, necroptosis has recently been associated with multiple sclerosis,Citation7 and pyroptosis occurs in response to bacterial or viral infection.Citation5,7 Distinct biochemical requirements for different forms of cell death have been identified (see Citation6,8,9 for reviews).
While apoptosis is typically considered a form of cell suicide, apoptosis and other forms of cell death can be controlled non-autonomously, via either “assisted suicide” or “murder.”Citation10 TNFα-induced necroptosis and apoptosis are well-characterized examples of assisted suicide, where signaling downstream of death receptors determines the form of cell death that occurs.Citation7 Natural killer T cells can also induce apoptosis non-autonomously by releasing granzyme B, which cleaves caspase substrates and triggers the caspase cascade.Citation11 Entosis is an intriguing form of cell death where one cell commits suicide by invading another cell, and requires the lysosomal machinery of the surrounding cell for its degradation.Citation12
When cells die, they are typically phagocytosed and degraded by another cell. However, in some instances phagocytic cells actively participate in the death of the target cells.Citation13 This was first demonstrated in C. elegans when engulfment mutants showed increased survival of cells that had compromised caspase activity.Citation14,15 More recently, it has been shown that a surrounding phagocytic cell affects caspase levels and activity in the precursor of a dying cell.Citation16 In another recent example in C. elegans, the engulfment machinery was found to be completely required for the death of a specific cell in the male tail.Citation17 However, these cases are examples of assisted suicide, as the cell deaths are ultimately dependent on the apoptotic machinery in the dying cell.
In mammals, cell death dependent on the phagocytic machinery has been coined “phagoptosis.”Citation13 For example, LPS-stimulated microglia can kill otherwise healthy neurons and the large scale turnover of erythrocytes in mammals has been attributed to phagoptosis. Recently it has been shown that activated microglia produce nitric oxide, which promotes transient caspase activity and phosphatidylserine exposure in neurons, prior to phagocytosis.Citation18 However, it is not clear how widely this mechanism is used, or whether other mechanisms are used by phagocytes to promote the death of cells.
Developmental nurse cell death
One mysterious type of cell death is the developmental cell death of nurse cells in the Drosophila melanogaster ovary.Citation19-21 Each egg is derived from a germline cyst of 16 cells, where one cell differentiates as the oocyte and the other 15 cells become polyploid nurse cells. At the end of oogenesis during stages 10-11, the 15 nurse cells transfer their cytoplasm to the oocyte and then during stages 12-14, the nurse cell nuclei are degraded. Genetic studies have ruled out a major role for apoptosis in developmental nurse cell death.Citation20-23 Mutants of caspase genes or the apoptosis initiators, reaper, hid and grim, fail to show any strong disruption to nurse cell death.Citation21,23 Similar mild phenotypes are observed with over-expression of caspase inhibitors.Citation20-22 There is some evidence that autophagy is induced during nurse cell death, but mutants of autophagy genes also fail to significantly disrupt nurse cell death suggesting that nurse cells do not die by autophagic cell death.Citation24,25 Even combined inhibition of apoptosis and autophagy does not cause a disruption to nurse cell death.Citation26 So how do nurse cells die?
In our recent paper,Citation27 we investigated whether nurse cell death was controlled non-autonomously. In late oogenesis, the cluster of 15 nurse cells is surrounded by a thin layer of approximately 50 “stretch” follicle cells (). As nurse cell death proceeds, the stretch follicle cells extend processes between the nurse cell nuclei, gradually surrounding each nurse cell nucleus individually. This intimate association with the nurse cells implicated the stretch follicle cells as candidates for controlling nurse cell death. Indeed, we found that the stretch follicle cells are required for nurse cell death, and that many of the nurse cell death events are mediated by the phagocytic machinery of the stretch follicle cells.
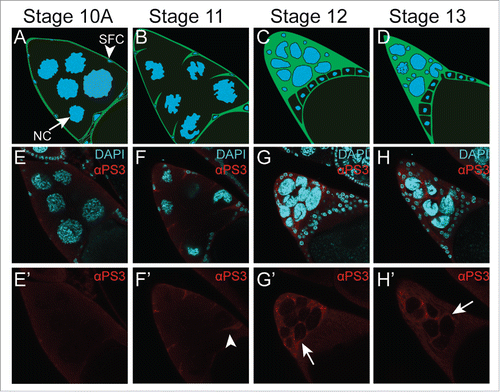
Figure 1. Integrin αPS3 (Scab, FBgn0003328) is expressed in stretch follicle cells in late oogenesis. (A-D) Illustration of stretch follicle cell interactions with nurse cells at the end of oogenesis during stages 10-13. Stretch follicle cells (green with small blue nuclei, arrowhead) extend processes that surround each nurse cell (large blue nuclei, arrow). SFC = stretch follicle cell, NC = nurse cell. (E-H’) Egg chambers stained with antibodies against αPS3Citation37,42 and DAPI to visualize nuclei. αPS3 is first detectable during stage 11 (arrows, F’), and increases during stages 12-13 (G’-H’).
Role of engulfment genes in nurse cell death
The Drosophila engulfment receptor Draper has been shown to play an important role in the clearance of apoptotic cells by hemocytes, glia, and epidermal cells.Citation28-31 Previously, we found that Draper was required earlier in oogenesis for the clearance of nurse cells undergoing apoptosis in response to starvation.Citation32 Interestingly, we also found that overexpression of Draper in follicle cells was sufficient to kill the underlying germline cells. Thus, our previous work suggested that follicle cells were capable of phagoptosis.
We examined homozygous draper mutant ovaries and found that there was a profound effect on developmental nurse cell death.Citation27 Whereas wild-type egg chambers showed an average of 0.2 nurse cell nuclei remaining by stage 14, draper mutants had an average of 8 persisting nurse cell nuclei. A similar phenotype was also observed when draper was knocked down by RNAi specifically in stretch follicle cells, demonstrating that draper was non-autonomously required for nurse cell removal. Knockdown of another engulfment gene, Ced-12, showed a comparable phenotype. In C. elegans, Ced-12 and the draper ortholog Ced-1 have been shown to function in parallel.Citation33 To determine whether this held true in the stretch follicle cells, we generated double Ced-12 draper knockdowns. Indeed, double mutants showed a stronger phenotype with over 10 persisting nuclei, suggesting that Ced-12 and draper function in parallel during nurse cell death. We screened through a number of other engulfment genes using RNAi and identified several other genes important for nurse cell removal (e.g. shark, Gprk2, Src42A, mbc, Rac1 and the JNK pathway).
The persistence of nurse cell nuclei could indicate a defect in clearance, or a defect in cell death.Citation27 To distinguish between these possibilities, we stained ovaries for several cell death markers. In wild-type ovaries, signs of nurse cell death first become apparent in stage 10B, when nurse cell nuclei become permeable, releasing nuclear proteins such as the reporter nuclear ß-galactosidase.Citation34 Cytoplasmic actin bundles also form during stage 10B, prior to the rapid transfer of nurse cell cytoplasm to the oocyte.Citation34 After the transfer of cytoplasm in stage 11, nurse cell nuclei become more compact, and eventually become acidified and stain positively for TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) Citation23,24,35,36 which labels the 3′OH groups of fragmented DNA. We found that TUNEL and acidification were almost completely abolished in draper mutants.Citation27 While nurse cell dumping was not affected, the leakage of nuclear proteins was impaired. Together these findings indicated that the nuclei remained intact in draper mutants, suggesting that nurse cell death as well as clearance was inhibited.
Stretch follicle cells control nurse cell death
To directly address the requirement of stretch follicle cells in nurse cell death, we eliminated them by expressing RNAi against the caspase inhibitor Diap1 specifically in the stretch follicle cells.Citation27 We found that these egg chambers not only had persisting nuclei, but they were also “dumpless,” indicating that there was a failure in the transport of nurse cell cytoplasm to the oocyte. Actin bundles failed to form in the cytoplasm, and the nurse cell nuclei failed to become acidified or TUNEL-positive. These findings demonstrate that the stretch follicle cells control all aspects of nurse cell death. Moreover, it shows that the stretch follicle cells activate other pathways in addition to the phagocytic machinery, as actin bundles and nurse cell cytoplasm dumping occurred normally in phagocytosis mutants.
Engulfment receptors integrins and Draper function in parallel
One puzzling aspect of our analysis was that nurse cell removal was not completely blocked in draper mutants. One possibility is that another receptor acts in parallel to draper in late oogenesis. A strong candidate is integrins, which have been shown to function during engulfment in C. elegans, mammals, and recently flies.Citation37 In flies, there are 7 different integrin subunits that can form different combinations of α/ ß heterodimers. Knockdown of most of the integrin subunits did not affect programmed cell death in late oogenesis.Citation38 However, knockdown of either the αPS3 or ßPS subunit in follicle cells led to moderate to strong persisting nurse cell nuclei phenotypes in late oogenesis.Citation27 The knockdown of ßPS had additional defects in egg chamber development,Citation38 so we focused on αPS3, which seemed to have a specific defect in nurse cell removal.
To determine where αPS3 was expressed in late oogenesis, we stained ovaries with antibodies against αPS3. In early stages of oogenesis, αPS3 was not detected () but by stage 11 (), αPS3 was specifically detected on stretch follicle cell membranes. Staining became more intense during stage 12 and 13 (), resembling the expression and staining pattern of Draper.Citation27 ßPS, while expressed at low levels throughout oogenesis, also became enriched on stretch follicle cells during stages 10-13.Citation38
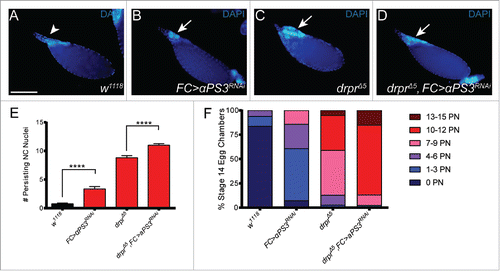
To determine if integrins and Draper functioned in parallel in late oogenesis, we generated double mutants and counted persisting nuclei in stage 14 egg chambers (). As we reported previously,Citation27 the control w1118 had on average less than one nurse cell nucleus remaining at stage 14 (). Knockdown of αPS3 in follicle cells led to an average of 3 persisting nurse cell nuclei (). The double mutants, draperΔ5 (null) combined with the αPS3 RNAi knockdown in follicle cells, showed a stronger phenotype than draper alone, with an average of 11 persisting nuclei (). In over 15% of egg chambers, the disruption to nurse cell removal was nearly complete with 13-15 nuclei remaining. These data indicate that αPS3 acts in parallel to draper for the removal of nurse cell nuclei in late oogenesis. The strength of the αPS3 draper double mutant phenotype was comparable to the phenotype of the Ced-12 draper double mutant,Citation27 suggesting that integrins could act upstream of Ced-12, as has been shown in C. elegans.Citation39
Figure 2. αPS3 and draper act in parallel to remove nurse cells in late oogenesis. (A-D) Stage 14 egg chambers of the indicated genotypes stained with DAPI to label nuclei. (A) The control w1118 does not have persisting nurse cell nuclei. (B-D) Loss of engulfment receptors leads to persisting nurse cell nuclei (arrows). The follicle cell (FC) αPS3 RNAi knockdown was generated using GR1-GAL4 Citation43 and TRiP strain JF02696. draperΔ5 (FBgn0027594) was generated by Marc Freeman.Citation29 The genotype of the double mutant in (D) is draperΔ5 UAS-αPS3 (dsRNA JF02696)/ draperΔ5, GR1-GAL4. (E) Persisting nuclei were quantified in stage 14 egg chambers. (F) Data from (E) sorted into bins. PN = Persisting Nuclei. Number of egg chambers was >22 for all genotypes.
It was intriguing, however, that the double mutants still did not show a complete block to nurse cell removal. We also examined a triple mutant including the CD36 family receptor Croquemort, and found that nurse cell death was still not completely inhibited.Citation38 These findings suggest that there may be redundant or compensatory mechanisms, perhaps involving another engulfment receptor, or some of the nurse cells may be removed by other mechanisms that do not involve phagocytosis genes.
Future perspectives
One major open question is: how does the phagocytosis machinery mediate nurse cell death and removal? When nurse cells die in mid-oogenesis in response to starvation, they are engulfed by the surrounding follicle cells in a typical fashion, with the formation of phagosomes containing apoptotic debris.Citation32,37 The phagosomes are then processed through the canonical phagosome maturation pathway.Citation40 Phagosome formation and processing during starvation-induced cell death in mid-oogenesis are dependent on the phagocytosis genes draper, Ced-12, integrins, and the JNK pathway. However, during developmental nurse cell death in late oogenesis, we have not seen any evidence of vesicle uptake into the stretch follicle cells. We do see LysoTracker-positive puncta in follicle cells,Citation27 but these may be trafficking toward the nurse cell nuclei, rather than away from them, which would be expected if the phagocytic material was being processed.
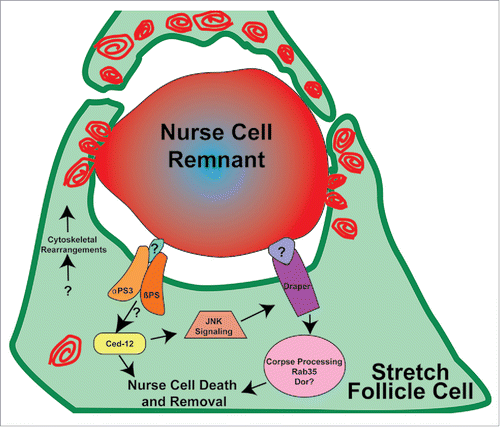
Another possibility is that the stretch follicle cells form a very large phagocytic cup to surround each nurse cell nucleus, which then fuses with multiple lysosomes until each nurse cell remnant is acidified (). Our observations suggest that multiple stretch follicle cells may collaborate to remove each nucleus,Citation27 so a simple phagocytic cup model is not likely. It is clear however, that nurse cell removal involves lysosomal activity because we identified many lysosomal trafficking genes required for nurse cell removal.Citation27 It is possible that the stretch follicle cells utilize this lysosomal machinery to acidify and degrade the nurse cells in a mechanism distinct from phagosome formation. For example, osteoclasts utilize lysosomal machinery to secrete hydrogen ions to acidify and resorb bone, specifically by targeting V-ATPases to the plasma membrane.Citation41
Figure 3. Model of nurse cell death and removal in late oogenesis. In the drawing, one nurse cell nucleus is surrounded by stretch follicle cells (green). Red swirls represent lysosomes and/or other acidified organelles that surround the nurse cell remnant, eventually leading to its acidification, indicated by the gradient of red staining.
In addition to the acidification step, it is possible that the phagocytic machinery signals to the nurse cells to promote their death. We showed previously that over-expression of draper in follicle cells can induce the death of the germline, prior to any phagocytic activity.Citation32 This implicates pro-death signaling between follicle cells and nurse cells. The molecules that mediate nurse cell death in response to draper overexpression remain to be determined. One clue is that draper and Ced-12 can both activate the JNK pathway, and the transcription factor Kayak is required for nurse cell removal.Citation27 Thus there may be a transcriptional response activated by the phagocytic machinery that mediates nurse cell death independent of phagocytic activity per se. Further investigation into exactly how phagocytic cells can contribute to the death of cells by phagoptosis remains to be determined.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of our lab for helpful discussions, Eric Shepherd for illustrations, and Trudi Schüpbach, Estee Kurant, the Bloomington Drosophila stock center and the Harvard Transgenic RNAi Project (TRiP) for fly strains.
Funding
This work was supported by NIH R01 GM060574 (to KM) and NIH F31GM115177 (to AAM).
References
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis, a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26:239-57; PMID:4561027; http://dx.doi.org/10.1038/bjc.1972.33
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1ß-converting enzyme. Cell 1993; 75:641-52; PMID:8242740; http://dx.doi.org/10.1016/0092-8674(93)90485-9
- Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 1994; 76:665-76; PMID:7907274; http://dx.doi.org/10.1016/0092-8674(94)90506-1
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997; 90:405-13; PMID:9267021; http://dx.doi.org/10.1016/S0092-8674(00)80501-2
- Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 2016; 44:221-31; PMID:26885855; http://dx.doi.org/10.1016/j.immuni.2016.01.020
- Tait SW, Ichim G, Green DR. Die another way–non-apoptotic mechanisms of cell death. J Cell Sci 2014; 127:2135-44; PMID:24833670; http://dx.doi.org/10.1242/jcs.093575
- Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, et al. Activation of necroptosis in multiple sclerosis. Cell Rep 2015; 10:1836-49; PMID:25801023; http://dx.doi.org/10.1016/j.celrep.2015.02.051
- Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 2015; 22:58-73; PMID:25236395; http://dx.doi.org/10.1038/cdd.2014.137
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19:107-20; PMID:21760595; http://dx.doi.org/10.1038/cdd.2011.96
- Eroglu M, Derry WB. Your neighbours matter - non-autonomous control of apoptosis in development and disease. Cell Death Differ 2016; 23:1110-8; PMID:27177021; http://dx.doi.org/10.1038/cdd.2016.41
- Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med 1991; 173:1099-109; PMID:2022921; http://dx.doi.org/10.1084/jem.173.5.1099
- Krishna S, Overholtzer M. Mechanisms and consequences of entosis. Cell Mol Life Sci 2016; 73(11-12):2379-86; PMID:27048820
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 2012; 37:325-32; PMID:22682109; http://dx.doi.org/10.1016/j.tibs.2012.05.002
- Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 2001; 412:202-6; PMID:11449279; http://dx.doi.org/10.1038/35084103
- Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature 2001; 412:198-202; PMID:11449278; http://dx.doi.org/10.1038/35084096
- Chakraborty S, Lambie EJ, Bindu S, Mikeladze-Dvali T, Conradt B. Engulfment pathways promote programmed cell death by enhancing the unequal segregation of apoptotic potential. Nat Commun 2015; 6:10126; PMID:26657541; http://dx.doi.org/10.1038/ncomms10126
- Johnsen HL, Horvitz HR. Both the apoptotic suicide pathway and phagocytosis are required for a programmed cell death in Caenorhabditis elegans. BMC Biol 2016; 14:39; PMID:27185172; http://dx.doi.org/10.1186/s12915-016-0262-5
- Hornik TC, Vilalta A, Brown GC. Activated microglia cause reversible apoptosis of pheochromocytoma cells, inducing their cell death by phagocytosis. J Cell Sci 2016; 129:65-79; PMID:26567213; http://dx.doi.org/10.1242/jcs.174631
- Peterson JS, Timmons AK, Mondragon AA, McCall K. The end of the beginning: cell death in the germline. Curr Top Dev Biol 2015; 114:93-119; PMID:26431565; http://dx.doi.org/10.1016/bs.ctdb.2015.07.025
- Mazzalupo S, Cooley L. Illuminating the role of caspases during Drosophila oogenesis. Cell Death Differ 2006; 13:1950-9; PMID:16528381; http://dx.doi.org/10.1038/sj.cdd.4401892
- Baum JS, Arama E, Steller H, McCall K. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ 2007; 14:1508-17; PMID:17464325; http://dx.doi.org/10.1038/sj.cdd.4402155
- Peterson JS, Barkett M, McCall K. Stage-specific regulation of caspase activity in Drosophila oogenesis. Dev Biol 2003; 260:113-23; PMID:12885559; http://dx.doi.org/10.1016/S0012-1606(03)00240-9
- Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development 1998; 125:1075-82; PMID:9463354
- Bass BP, Tanner EA, Mateos San Martin D, Blute T, Kinser RD, Dolph PJ, McCall K. Cell-autonomous requirement for DNaseII in nonapoptotic cell death. Cell Death Differ 2009; 16:1362-71; PMID:19557011; http://dx.doi.org/10.1038/cdd.2009.79
- Nezis IP, Shravage BV, Sagona AP, Lamark T, Bjorkoy G, Johansen T, Rusten TE, Brech A, Baehrecke EH, Stenmark H. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J Cell Biol 2010; 190:523-31; http://dx.doi.org/10.1083/jcb.201002035
- Peterson JS, McCall K. Combined inhibition of autophagy and caspases fails to prevent developmental nurse cell death in the Drosophila melanogaster ovary. PLoS One 2013; 8:e76046; PMID:24098761; http://dx.doi.org/10.1371/journal.pone.0076046
- Timmons AK, Mondragon AA, Schenkel CE, Yalonetskaya A, Taylor JD, Moynihan KE, Etchegaray JI, Meehan TL, McCall K. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc Natl Acad Sci U S A 2016; 113:E1246-55; PMID:26884181; http://dx.doi.org/10.1073/pnas.1522830113
- Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van Goethem E, Eid JP, Quirin M, Franc NC. Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell 2008; 135:524-34; PMID:18984163; http://dx.doi.org/10.1016/j.cell.2008.08.033
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 2003; 38:567-80; PMID:12765609; http://dx.doi.org/10.1016/S0896-6273(03)00289-7
- Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, Henson P, Nakanishi Y. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J Biol Chem 2004; 279:48466-76; PMID:15342648; http://dx.doi.org/10.1074/jbc.M408597200
- Han C, Song Y, Xiao H, Wang D, Franc NC, Jan LY, Jan YN. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron 2014; 81:544-60; PMID:24412417; http://dx.doi.org/10.1016/j.neuron.2013.11.021
- Etchegaray JI, Timmons AK, Klein AP, Pritchett TL, Welch E, Meehan TL, Li C, McCall K. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 2012; 139:4029-39; PMID:22992958; http://dx.doi.org/10.1242/dev.082776
- Reddien PW, Horvitz HR. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu Rev Cell Dev Biol 2004; 20:193-221; PMID:15473839; http://dx.doi.org/10.1146/annurev.cellbio.20.022003.114619
- Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell 1992; 69:173-84; PMID:1339308; http://dx.doi.org/10.1016/0092-8674(92)90128-Y
- McCall K, Steller H. Requirement for DCP-1 caspase during Drosophila oogenesis. Science 1998; 279:230-4; PMID:9422696; http://dx.doi.org/10.1126/science.279.5348.230
- Cavaliere V, Taddei C, Gargiulo G. Apoptosis of nurse cells at the late stages of oogenesis. Dev Genes Evol 1998; 208:106-12; PMID:9569352; http://dx.doi.org/10.1007/s004270050160
- Meehan TL, Kleinsorge SE, Timmons AK, Taylor JD, McCall K. Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells in the Drosophila ovary. Dis Model Mech 2015; 8:1603-14; PMID:26398951; http://dx.doi.org/10.1242/dmm.021998
- Meehan TL. Analysis of engulfment and cell corpse processing by epithelial cells in the Drosophila ovary. Ph.D. Dissertation. Boston University, 2016
- Hsieh HH, Hsu TY, Jiang HS, Wu YC. Integrin α PAT-2/CDC-42 signaling is required for muscle-mediated clearance of apoptotic cells in Caenorhabditis elegans. PLoS Genet 2012; 8:e1002663; PMID:22615577; http://dx.doi.org/10.1371/journal.pgen.1002663
- Meehan TL, Joudi TF, Timmons AK, Taylor JD, Habib C, Peterson JS, Emmanuel S, Franc NC, McCall K. Components of the engulfment machinery have distinct roles in corpse processing. PLoS One 2016; 11:e0158217; PMID:27347682; http://dx.doi.org/10.1371/journal.pone.0158217
- Vaananen HK, Karhukorpi EK, Sundquist K, Wallmark B, Roininen I, Hentunen T, Tuukkanen J, Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol 1990; 111:1305-11; PMID:2144003; http://dx.doi.org/10.1083/jcb.111.3.1305
- Wada A, Kato K, Uwo MF, Yonemura S, Hayashi S. Specialized extraembryonic cells connect embryonic and extraembryonic epidermis in response to Dpp during dorsal closure in Drosophila. Dev Biol 2007; 301:340-9; PMID:17034783; http://dx.doi.org/10.1016/j.ydbio.2006.09.020
- Goentoro LA, Yakoby N, Goodhouse J, Schupbach T, Shvartsman SY. Quantitative analysis of the GAL4/UAS system in Drosophila oogenesis. Genesis 2006; 44:66-74; PMID:16425298; http://dx.doi.org/10.1002/gene.20184
