ABSTRACT
Animal terminalia represent some of the most diverse and rapidly evolving structures in the animal kingdom, and for this reason have been a mainstay in the taxonomic description of species. The terminalia of Drosophila melanogaster, with its wide range of experimental tools, have recently become the focus of increased interest in the fields of development, evolution, and behavior. However, studies from different disciplines have often used discrepant terminologies for the same anatomical structures. Consequently, the terminology of genital parts has become a barrier to integrating results from different fields, rendering it difficult to determine what parts are being referenced. We formed a consortium of researchers studying the genitalia of D. melanogaster to help establish a set of naming conventions. Here, we present a detailed visual anatomy of male genital parts, including a list of synonymous terms, and suggest practices to avoid confusion when referring to anatomical parts in future studies. The goal of this effort is to facilitate interdisciplinary communication and help newcomers orient themselves within the exciting field of Drosophila genitalia.
Introduction
Insect terminalia, which usually encompass the male and female genitalia and analia, are among the most diverse and complex morphological structures [Citation1]. In Drosophila, they have been the subjects of three research disciplines that led to different terminologies. The earliest of these is ontogeny, which aimed at identifying the segmental origin of the different structures and how they sexually differentiate during development from the larval genital disc in D. melanogaster. It is thanks to this discipline that the ‘traditional terminology’ was established [Citation2–Citation4] and continues to be used by contemporary developmental biologists [Citation5]. Most of the terms currently annotated in FlyBase (www.flybase.org) are based on the traditional system.
The second discipline was phylogenetics, which aimed at describing the diversity of terminalia among drosophilids in order to group species according to their similarities in these structures. The earliest comparative studies [Citation6,Citation7] standardized the ‘traditional terminology’ in Drosophila systematics [e.g. Citation8]. However, following the publication of McAlpine’s Manual of Nearctic Diptera [Citation9], an effort to standardize morphological terms of putatively homologous structures across the Diptera emerged [Citation10,Citation11]. Subsequently, the resulting ‘revised terminology’ was widely accepted by Drosophila systematists [Citation12–Citation14], although some terms, such as parameres, paraphyses or gonopods, remained problematic because they sometimes refer to structures not related by clear homology in different species [Citation15,Citation16].
Recently, a third discipline, functional morphology, has emerged, aiming at understanding the role that each genital structure may play during copulation [Citation17–Citation23]. With advanced techniques such as laser surgery and tomography scanning, this approach has enhanced our understanding of the functional roles of genital anatomy. However, researchers in this discipline used a mixture of traditional and revised terminologies [Citation24] that can lead to confusion as community members from different disciplines assimilate the literature.
Believing that the breadth and richness of the three research disciplines offers a unique opportunity for integrative biology, the purpose of the current paper is to unify terminology of Drosophila male terminalia (). As a group of researchers working on different aspects of Drosophila terminalia, we think that a unified system would facilitate exchanges between research fields. Although some researchers highlighted the usefulness of the traditional system in providing meaningful English terms rather than obscure Latin-derived names (e.g. clasper vs. surstylus), the majority opted for the phylogenetic tradition which captures homology relationships between species. Consequently, we provide an update of the terminalia terminology found in FlyBase. For the problematic terms (parameres, paraphyses, and gonopods), we relied on Sinclair’s [Citation25] and Cumming and Wood’s [Citation26] revisions of Diptera terminalia nomenclature to propose new terms not previously used in Drosophila biology (namely, pregonites, postgonites, and gonocoxites). Although we restricted our revision to male terminalia, we do so with the intention to address female terminology later.
Figure 1. (a) Light microscope preparation of the entire male terminalia of D. melanogaster Canton S. (b) Caudolateral view of the periphallic structures. (c) Ventrolateral view of the phallic structures. Scale bars are 100 μm. Note that the exact size and shape of terminalia structures, such as the epandrial posterior lobe, vary within D. melanogaster [Citation27,Citation28].
![Figure 1. (a) Light microscope preparation of the entire male terminalia of D. melanogaster Canton S. (b) Caudolateral view of the periphallic structures. (c) Ventrolateral view of the phallic structures. Scale bars are 100 μm. Note that the exact size and shape of terminalia structures, such as the epandrial posterior lobe, vary within D. melanogaster [Citation27,Citation28].](/cms/asset/b2327cd5-5121-4d28-b28f-544bfb7d7619/kfly_a_1653733_f0001_oc.jpg)
Results and discussion
A visual atlas of adult D. melanogaster male terminalia
In much of the past literature, genital morphology was rendered by hand-drawings, and the names of different parts were indicated by lines pointing to each structure. As new researchers join this growing field, it can be quite difficult to grasp the exact extent of a structure based on these drawings. In order to make the revised nomenclature as useful as possible, we provide here a visual guide to this terminology which shows both drawings and cuticle images that outline the full extent of each named part (, ). It is important to note that the exact size and shape of these structures, such as the epandrial posterior lobe, can vary within D. melanogaster [Citation27,Citation28]. In , we propose a unified nomenclature of the various anatomical elements containing definitions and references to previously used terms. Conversely, provides correspondence from previously used terms to the unified nomenclature. Although the current set of nomenclature is centered around D. melanogaster, we have adopted general terms such that most should also apply to other Drosophilidae species.
Table 1. Definition of the terms in the standardized nomenclature.
Table 2. Table of correspondence between terms previously used in publications and term of the standardized nomenclature.
Figure 2. Visual atlas of periphallic structures. Light microscopy images (Canton S strain) and diagrams representing the broad divisions and substructures of epandrium and cercus. The images are oriented dorsal (top) to ventral (bottom). Previous FlyBase terms are on the left and 2019 revised terms are on the right.
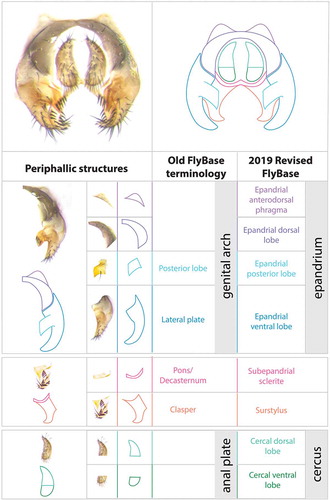
Figure 3. Visual atlas of phallic structures. Light microscopy images (Canton S strain) and diagrams representing the broad divisions and substructures of phallus and hypandrium. The images are oriented posterior (top) to anterior (bottom). Previous FlyBase terms are on the left and the 2019 revised terms are on the right.
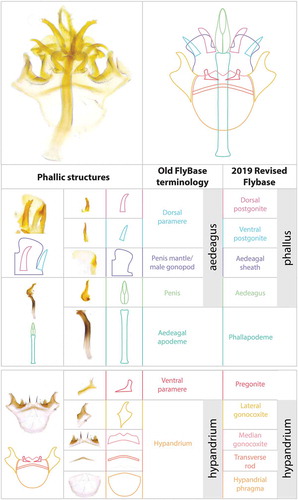
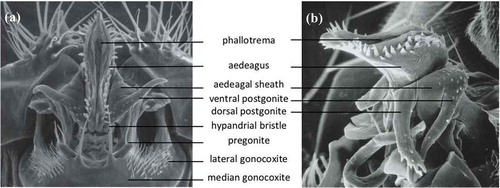
Figure 4. Scanning electron micrographs of the phallic structures in (a) ventral and (b) lateral views, from L. Tsacas’ collection at the National Museum of Natural History, Paris (Courtesy of the Museum).
The male terminalia of D. melanogaster corresponds to the entire set of external structures in the distal half of the male abdomen (–), i.e. segments 8–10. It derives from the genital disc, which comprises three primordia: a reduced Abdominal segment 8 primordium, which in females gives rise to most genital structures but in males gives rise only to a miniature eighth tergite (here termed the epandrial anterodorsal phragma, see below); an Abdominal segment 9 primordium, forming the male genitalia, and the Abdominal segment 10 primordium, making the analia [Citation29]. During development, the D. melanogaster male genitalia rotate 360 degrees clockwise, causing the internal organs to loop around the gut; this rotation and thus the dorsal/ventral designation of the genitalia varies within Diptera [Citation30]. We dissected and imaged adult cuticle preparations of a D. melanogaster wild type strain (Canton S), and provide cuticle images as well as drawings of the distinct parts in –. To maximize clarity, we present each part both in isolation and in the context of intact tissue, and we indicate the outlines of each anatomical component (, ).
We have subdivided the terminalia into two parts, periphallic structures, which are secondarily connected to the intromittent organ () and the phallic structures, which comprise the intromittent organ and structures directly connected to it (). These two classes are easily separable via dissection in the adult.
Periphallic portions of the terminalia
Periphallic structures comprise the cercus (former anal plate), the epandrium (former genital arch), the pair of surstyli (former claspers) and the subepandrial sclerite (former pons) that connects the surstyli to the other periphallic structures (). Although periphallic structures are not directly involved in transferring sperm, several of them (cercus, surstylus, and epandrial posterior lobe) have been implicated in grasping onto the female during copulation [Citation17–Citation19,Citation22,Citation31–Citation34]. Additionally, although many of these structures do not show obvious boundaries in D. melanogaster, they are far more complex in its close relatives, suggesting that there are natural subdivisions of these structures in some species. For example, while the ventral margin of the cercus forms a relatively flat cuticle in D. melanogaster, it bears a lobe-like extension in D. bipectinata that affects copulatory success [Citation23,Citation24]. Furthermore, the dorsal and ventral parts of the cercus accumulate distinct levels of engrailed in D. melanogaster (Figure 3(f) in [Citation35]).
Subdivision and nomenclature of phallic parts
During copulation, several parts of the male genitalia enter the female vagina: the aedeagus, part of the phallapodeme, the ventral and dorsal postgonites, and the aedaegal sheath [Citation18,Citation36]. All of these structures except the phallapodeme form the intromittent organ or phallus. The aedeagus is perhaps the most complex structure of the male genitalia of D. melanogaster (): it is covered with cuticular projections and its shape varies broadly between closely related species (see Figures 13, 14, 16, 17 of [Citation37, Citation38]). The postgonites are flexible relative to the aedeagus; they move progressively during mating and have been implicated in producing copulatory wounds in females [Citation18]. The aedeagal sheath surrounds the aedeagus and the postgonites dorso-laterally. It also moves outwards during mating. The movement of the postgonites and the aedeagal sheath may be induced through the complex musculature found in the phallus (; [Citation18]).
Figure 5. Musculature of the phallic structures. Same diagram of cuticular parts as in (ventral view). Muscles are indicated in distinct colors and numbered I to VI [Citation18]. These muscles are bilateral. For sake of clarity, muscles are shown either on the left or on the right side of the diagram. See for muscles description.
![Figure 5. Musculature of the phallic structures. Same diagram of cuticular parts as in Figure 3 (ventral view). Muscles are indicated in distinct colors and numbered I to VI [Citation18]. These muscles are bilateral. For sake of clarity, muscles are shown either on the left or on the right side of the diagram. See Table 1 for muscles description.](/cms/asset/866c45b4-bbbd-4cfa-8b1c-54d145ee15e5/kfly_a_1653733_f0005_oc.jpg)
The hypandrium is a large structure that surrounds the phallus ventrally. It can be broken down into several identifiable substructures. We consider the posterior part to be fused gonocoxites (see below) and divide each gonocoxite into two parts, lateral and median. The fused median gonocoxites host a pair of large bristles [Citation39,Citation40]. They connect to the phallus via the two pregonites. Each pregonite displays two to three smaller bristles.
Justifying the separation/individuality of parts
It is important to note that the boundary of each anatomical element is based largely on defined cuticular ridges observed in the adult. However, some key parts lack clear boundaries with other adjacent tissues. Examples include the epandrial posterior lobe, cercal ventral and dorsal lobes, and sub-parts of the hypandrium. We envision that in these cases, a careful analysis of cellular formation during development will be necessary to precisely define the boundaries of separate parts. Experiments that map the spatial expression patterns of regulatory genes such as transcription factors further support the boundaries of each anatomical element, and could motivate further refinements into smaller sub-parts [Citation35,Citation41].
Implications of our system to the terminological debate within Diptera
The term ‘surstylus’ has been proposed by Crampton [Citation42] to refer to the clasping organs that are associated with the dorsal compartment of the genitalia (i.e. epandrium) in Eremoneuran (Higher) Diptera to which Drosophila belongs. In non-Eremoneuran (Lower) Diptera and in other related insect orders, the clasping organs consist of appendices, the gonopods, comprised of two substructures, the gonocoxites and the gonostyli, that are associated with the ventral compartment of the genitalia (i.e. the hypandrium). Crampton’s view, which would later be called the ‘surstylar concept’ [Citation43], postulates that the gonostyli have been lost whereas the gonocoxites remain associated with the hypandrium in Eremoneurans. This view has a wide acceptance among Dipterologists [Citation25,Citation26,Citation44], as well as between Drosophila systematists who have opted for a revised terminology [Citation10,Citation15,Citation16]. For example, the term gonopod, whereas used for different structures in D. melanogaster (see and ), has always been applied to ventral structures associated with the phallic portions.
However, alternative hypotheses for the origin of the Eremoneuran dorsal claspers exist, i.e. the ‘gonostylar concept’, postulating that Eremoneuran dorsal claspers are homologous to the ventral gonopods (mostly to the gonostyli) of non-Eremoneuran Diptera [reviewed in Citation43]. Zatwarnicki [Citation43] further considered the subepandrial sclerite [medandrium in Citation43] to be homologous to the gonocoxites. Although evaluating these concepts goes far beyond the scope of our paper, we believe that further research in Drosophila could ultimately help elucidating the origin of the Eremoneuran dorsal claspers. For example, Abd-B mutants in D. melanogaster genital disc transform the phallic structures, as well as a part of the surstylus (clasper), into a leg [Citation45]. This supports the idea that the ventral parts of the Eremoneuran genitalia are of appendicular origin (as the name gonopod, i.e. genital leg, would suggest), but it also suggests that a part (not the whole) of the surstylus might be of appendicular origin. Further mapping of transcription factors expression in the different compartments of the male terminalia between D. melanogaster [e.g. Citation41] and other non-Eremoneuran Diptera could shed light on the deep homology between these structures. At the time being, and because our major aim is to unify terms used by Drosophila biologists, we have opted here for the terminology based on the ‘surstylar concept’, and we hope that this would prompt further research on these questions.
Incorporating the new standardized terminology into diverse ongoing studies
The revised terminology described here should facilitate cross-disciplinary synthesis of our knowledge of genital function, development, and evolution. We have worked with the FlyBase team to integrate these terms into their anatomy ontology [Citation46,Citation47]. Although we focused on the D. melanogaster terminalia, a standardized terminology is vital/crucial for the ease of comparing various species. Thus, it is our hope that these terms will facilitate descriptions of homologous and novel structures in other insect species. It was important for us to include as much of the community of researchers working on Drosophila genital morphology as possible to reach consensus in the definition and deployment of this terminology. We suggest that when publishing studies that name these structures, authors use the terms of the revised terminology, while parenthetically citing alternate synonyms such as familiar terms, e.g. surstylus (clasper). For those who would like to use familiar terms (perhaps for the purpose of continuity with previous publications), we would strongly recommend that the revised terminology is presented parenthetically, e.g. clasper (surstylus). This way, the broader scientific community can understand and integrate results with as few barriers to comprehension as possible.
Studies of Drosophila genitalia have provided examples of large-scale differences between males and females, vital taxonomic traits to distinguish species from one another, and important factors in the reproductive incompatibility between species. Yet, the complexity of the genitalia itself presents barriers to the study of these fascinating anatomical parts. This problem has been aggravated by variability in nomenclature, which has further impeded entry into this field. The revision and visual atlas of male genital structures provided here will hopefully allow for increased communication across a range of disciplines and welcome new scientists to this growing field.
Materials and methods
A Canton S line of Drosophila melanogaster (Bloomington # 64349) was used for all imaging. Adult males were dissected in 100% EtOH with micro-forceps and mounted in PVA Mounting Medium (BioQuip). For ), the sample was imaged at 500× magnification with a digital microscope VHX 2000 (Keyence) using lens VH-Z20R/W. For ,) digital images were taken at different depths of focus using a Dino-Lite® Microscope Eyepiece Camera (AM7025X, AnMo Electronics Corporation) on an Olympus BX50 microscope and stacked with CombineZP 1.0 (https://combinezp.software.informer.com/). For and , samples were imaged at 16× magnification on a Leica M205C microscope with a Leica DFC425 camera or at 20× magnification on a Leica DM 2000 with a Leica DFC540 C camera. Images from the former microscope were Z stack-compiled with the Leica Application Suite to allow for optimal focus. Images of the epandrial anterodorsal phragma, epandrial dorsal lobe, epandrial posterior lobe, epandrial ventral lobe, subepandrial sclerite, cercal dorsal lobe, cercal ventral lobe, lateral gonocoxite, median gonocoxite, transverse rod, and hypandrial phragma were modified in Adobe Photoshop via the eraser tool to isolate full parts along sutures to provide the clearest view of each part in its entirety. Photoshop was used because dissection of the various parts would be difficult.
Acknowlegements
We thank Clare Pilgrim and Steve Mangold for working with us to integrate our terminology into the FlyBase anatomy ontology, as well as the reviewers and Tadeusz Zatwarnicki for their useful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Eberhard WG. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press; 1985.
- Bryant P. Pattern formation in imaginal discs. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. Vol. 2c. London, England: Academic Press; 1978. p. 229–335. chapter 22. 1978.
- Ferris GF. External morphology of the adult. In: Demerec M, editor. Biology of Drosophila. New York, NY: Cold Spring Harbor Laboratory Press; 1950. p. 368–419. chapter 5. facsimile edition, 1994.
- Dobzhansky T. Studies on the intersexes and supersexes in Drosophila melanogaster. Izvestia Biuro Po Evgenike. 1930;8:91–158. in Russian, with English summary.
- Chatterjee RN, Chatterjee P, Kuthe S, et al. Intersex (ix) mutations of Drosophila melanogaster cause nonrandom cell death in genital disc and can induce tumours in genitals in response to decapentaplegic (dppdisk) mutations. J Genet. 2015;94(2):207–220.
- Okada T. Comparative morphology of the drosophilid flies. I. Phallic organs of the melanogaster group. Vol. 22. Kontyu; 1954.p. 36–46.
- Hsu TC. The external genital apparatus of male Drosophilidae in relation to systematics. Vol. 4920. The University of Texas Publication; 1949. p. 80–142.
- Bock IR, Wheeler MR. The Drosophila melanogaster species group. Vol. 7213. The University of Texas Publication; 1972. p. 1–102.
- McAlpine JF. Morphology and terminology-adults. In: McAlpine JF, editor. Manual of nearctic diptera. Vol. 1. Ottawa: Minister of Supply and Services; 1981. chapter 2. Agriculture Canada Monograph No. 27.p. 45–63
- Grimaldi DA. A phylogenetic, revised classification of genera in the Drosophilidae (Diptera). Bull Am Mus Nat Hist. 1990;197:1–139.
- Grimaldi DA. Phylogenetics and taxonomy of Zygothrica (Diptera: Drosophilidae). Bull Am Mus Nat Hist. 1987;186:103–268.
- Zhang W, Toda MJ. A new species-subgroup of the Drosophila immigrans Species-group (Diptera, Drosophilidae), with description of two new species from China and revision of taxonomic terminology. Jpn J Entomol. 1992;60:839–850.
- Vilela CR, Bachli G. Taxonomic studies on neotropical species of seven genera of drosophilidae (Diptera). Mitteilungen der Schweizerische Entomologischen Gesellschaft. 1990;63:1–332.
- McEvey SF. New species of scaptomyza from Madagascar and Mauritius with a note on terminology (Diptera: Drosophilidae). Annales de la Société entomologique de France. 1990;26(N.Ser.):51–64.
- Bächli G, Vilela CR, Andersson Escher S, et al. The Drosophilidae (Diptera) of Fennoscandia and Denmark. In: Fauna Entomologica Scandinavica. Vol. 39. Leiden: Brill; 2004. p. 362.
- Hu Y-G, Toda MJ. Polyphyly of Lordiphosa and its relationships in Drosophilinae (Diptera: Drosophilidae). Syst Entomol. 2001;26:15–31.
- LeVasseur-Viens H, Polak M, Moehring AJ. No evidence for external genital morphology affecting cryptic female choice and reproductive isolation in Drosophila. Evolution. 2015;69(7):1797.
- Kamimura Y. Copulation anatomy of Drosophila melanogaster (Diptera: Drosophilidae): wound-making organs and their possible roles. Zoomorphology. 2010;129(3):163–174.
- Mattei AL, Riccio ML, Avila FW, et al. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc Nat Acad Sci. 2015;112(27):8475–8480.
- Tanaka KM, Kamimura Y, Takahashi A. Mechanical incompatibility caused by modifications of multiple male genital structures using genomic introgression in Drosophila. Evolution. 2018;72:2406–2418.
- Acebes A, Cobb M, Ferveur JF. Species-specific effects of single sensillum ablation on mating position in Drosophila. J Exp Biol. 2003;206:3095–3100.
- Frazee SR, Masly JP. Multiple sexual selection pressures drive the rapid evolution of complex morphology in a male secondary genital structure. Ecol Evol. 2015;5(19):4437–4450.
- Polak M, Rashed A. Microscale laser surgery reveals adaptive function of male intromittent genitalia. Proc R Soc B. 2010;277:1371–1376.
- Kamimura Y, Polak M. Does surgical manipulation of Drosophila intromittent organs affect insemination success? Proc R Soc B. 2011;278:815–816.
- Sinclair BJ. Morphology and terminology of Diptera male terminalia. In: Papp L, Darvas B, editors. Contributions to a manual of palaearctic diptera. Vol. 1. Budapest: Science Herald; 2000. p. 53–74.
- Cumming JM, Wood DM. Adult morphology and terminology. In: Kirk-Spriggs AH, Sinclair BJ, editors. Manual of afrotropical diptera. v. 1. Introductory chapters and keys to diptera families. Suricata 4. Pretoria: South African National Biodiversity Institute; 2017. p. 89–133.
- Liu J, Mercer JM, Stam LF, et al. Genetic analysis of a morphological shape difference in the male genitalia of Drosophila simulans and D mauritiana. Genetics. 1996;142:1129–1145.
- McNeil C, Bain C, Macdonald S. Multiple quantitative trait loci influence the shape of a male-specific genital structure in Drosophila melanogaster. G3: Genes | Genomes | Genetics. 2011;1(5):343–351.
- Keisman EL, Christiansen AE, Baker BS. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Dev Cell. 2001;1(2):215–225.
- Suzanne M, Petzoldt A, Spéder P, et al. Coupling of apoptosis and L/R patterning controls stepwise organ looping. Curr Biol. 2010;20(19):1773–1778.
- Kamimura Y, Mitsumoto H. Comparative copulation anatomy of the Drosophila melanogaster species complex (Diptera: Drosophilidae). Entomol Sci. 2011;14:399–410.
- Eberhard W, Ramirez N. Functional morphology of the male genitalia of four species of drosophila: failure to confirm both lock and key and male-female conflict predictions. Ann Entomol Soc Am. 2004;97(5):1007–1017.
- Robertson HM. Mating asymmetries and phylogeny in the Drosophila melanogaster species complex. Pac Sci. 1988;42:72–80.
- Jagadeeshan S, Singh RS. A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: role of cryptic female choice and male sex-drive in the evolution of male genitalia. J Evol Biol. 2006;19:1058–1070.
- Sánchez L, Casares F, Gorfinkiel N, et al. The genital disc of Drosophila melanogaster. II. Role of the genes hedgehog, decapentaplegic and wingless. Dev Genes Evol. 1997;207:229–241.
- Mattei AL, Kamimura Y, Wolfner MF. Intimate intimas: positioning of copulatory organs in mating Drosophila. Mol Repro Devt. 2017;84:1117.
- Yassin A, Orgogozo V. Coevolution between male and female genitalia in the drosophila melanogaster species subgroup. PLoS ONE. 2013;8:e57158.
- Tsacas L, Bocquet C, Daguzan M, et al. Comparaison des genitalia males de Drosophila melanogaster, de Drosophila simulanset de leurs hybrides (Dipt. Drosophilidae). Annales de la Société entomologique de France. 1971;7:75–93.
- Nagy O, Nuez I, Savisaar R, et al. Correlated evolution of two copulatory organs via a single cis-regulatory nucleotide change. Curr Biol. 2018;28(21):3450–3457.
- Taylor BJ. Sexually dimorphic neurons in the terminalia of drosophila melanogaster: I. Development of sensory neurons in the genital disc during metamorphosis. J Neurogenet. 1989;5(3):173–192.
- Vincent BJ, Rice GR, Wong GM, et al. An atlas of transcription factors expressed in the Drosophila melanogaster pupal terminalia. Biorxiv. 2019. DOI:10.1101/677260
- Crampton GC. The genitalia of male diptera and mecoptera compared with those of related insects, from the standpoint of phylogeny. Trans Am Entomol Soc. 1923;48(3):207–225.
- Zatwarnicki T. A new reconstruction of the origin of eremoneuran hypopygium and its implications for classification (Insecta: Diptera). Genus. 1996;7:103–175.
- Yeates DK, Wiegmann BM. Congruence and controversy: toward a higher-level phylogeny of Diptera. Annu Rev Entomol. 1999;44:397–428.
- Estrada B, Sanchez-Herrero E. The hox gene abdominal-B antagonizes appendage development in the genital disc of Drosophila. Development. 2001;128:331–339.
- the FlyBase Consortium. Thurmond J, Goodman JL, Strelets VB, et al. FlyBase 2.0: the next generation. Nucleic Acids Res. 2019;47(D1):D759–DD765.
- Costa M, Reeve S, Grumbling G, et al. The Drosophila anatomy ontology. J Biomed Semantics. 2013;4:32.
- Salles H. Sobre a Genitalia dos Drosofilidios (Diptera): I. Drosophila melanogaster E. D. simulans. Summa Brasiliensis Biologiae Vol 1. 1947;15:1–73.
- Bryant P, Hsei B. Pattern formation in asymmetrical and symmetrical imaginal discs of Drosophila melanogaster. Am Zool. 1977;17:595–611.
- Sánchez L, Guerrero I. The development of the Drosophila genital disc. BioEssays. 2001;23:698–707.
- Wheeler MR. Sternite modification in males of the Drosophilidae (Diptera). Ann Entomol Soc Am. 1960;53:133–137.
- Okada T. Cladogenetic differentiation of Drosophilidae in relation to material compensation. Mushi. 1963;37:79–100.
- Wheeler MR. Drosophilidae (Chap. 95). In: editor, McAlpine JF. Manual of Nearctic Diptera Vol 2 Research Branch Agriculture Canada monogr. Vol. 28. Ottawa: Minister Supply and Services Canada; 1987. p. 1011–1018.
- Chassagnard MT. Esquisse phylogénétique du genre Zaprionus Coq. (Diptera: Drosophilidae) et description de trois nouvelles espèces afrotropicales. Naturaliste Can. 1988;115:305–322.
- Okada T. Systematic study of Drosophilidae and allied families of Japan. Tokoyo: Gihodo Co., Ltd.; 1956.
- Kopp AV, True JR. Evolution of male sexual characters in the oriental drosophila melanogaster species group. Evol Dev. 2002;4(4):278–291.
