ABSTRACT
Receptor proteins of the Roundabout (Robo) family regulate axon guidance decisions during nervous system development. Among the three Drosophila robo family genes (robo1, robo2 and robo3), robo2 displays a dynamic expression pattern and regulates multiple axon guidance outcomes, including preventing midline crossing in some axons, promoting midline crossing in others, forming lateral longitudinal axon pathways, and regulating motor axon guidance. The identity and location of enhancer elements regulating robo2’s complex and dynamic expression pattern in different neural cell types are unknown. Here, we characterize a set of 17 transgenic lines expressing GAL4 under the control of DNA sequences derived from noncoding regions in and around robo2, to identify enhancers controlling specific aspects of robo2 expression in the embryonic ventral nerve cord. We identify individual fragments that confer expression in specific cell types where robo2 is known to function, including early pioneer neurons, midline glia and lateral longitudinal neurons. Our results indicate that robo2’s dynamic expression pattern is specified by a combination of enhancer elements that are active in different subsets of cells. We show that robo2’s expression in lateral longitudinal axons represents two genetically separable subsets of neurons, and compare their axon projections with each other and with Fasciclin II (FasII), a commonly used marker of longitudinal axon pathways. In addition, we provide a general description of each fragment’s expression in embryonic tissues outside of the nervous system, to serve as a resource for other researchers interested in robo2 expression and its functional roles outside the central nervous system.
Introduction
The Drosophila lea/robo2 gene encodes a transmembrane protein (Robo2) that is a member of the evolutionarily conserved Roundabout (Robo) family of axon guidance receptors. Robo receptors canonically regulate midline crossing of axons by signalling midline repulsion in response to Slit ligands. In addition to their evolutionarily conserved role in Slit-dependent midline repulsion, individual Robo family members also regulate other developmental processes both inside and outside of the nervous system. In Drosophila, three Robo family members are present (Robo1, Robo2 and Robo3), and each plays a distinct subset of developmental roles that depend on differences in expression pattern, differences in protein activity, or both [Citation1, Citation2, Citation3–7].
Drosophila robo2 was initially named leak (lea) and was first identified by virtue of its head development defect (“head broad”) in Nüsslein-Volhard and Wieschaus’s foundational genetic screen for mutations affecting the patterning of the larval cuticle [Citation8]. Mutations in lea were later found to fail to complement mutations in robo2 [Citation9]. robo2 is expressed in a dynamic pattern during Drosophila embryonic ventral nerve cord (VNC) development. Its expression is broad in early pioneer neurons, where it is expressed alongside robo1 during the early stages of axon guidance, and becomes more restricted as the nerve cord develops [Citation1,Citation2]. In late embryonic development, Robo2 protein in the nerve cord is largely restricted to longitudinal axons in the lateral-most region of the neuropile [Citation2,Citation4], and robo2 mRNA remains detectable in motor neurons (in particular, ventrally projecting RP motor neurons) through stage 17 [Citation10].
Outside of the developing VNC, Robo2 embryonic expression (protein and/or mRNA) has been reported in the anterior ectoderm during head involution [Citation9], thoracic visceral mesoderm and chordotonal neurons [Citation11], ectodermal stripes [Citation1,Citation12], developing heart/pericardial cells [Citation2,Citation13], tracheal branches [Citation2,Citation14,Citation15], ventral longitudinal muscles [Citation2,Citation10,Citation16] and the embryonic gonad [Citation17]. Additional aspects of robo2 expression have been reported in post-embryonic stages, including expression in the adult posterior midgut [Citation18], glial cells in the larval leg imaginal disc [Citation19], adult testis [Citation20] and motor neurons and gustatory neurons in the adult leg [Citation21,Citation22]. While some links have been established between aspects of robo2 expression and specific transcription factor proteins, for example Hb9’s role in regulating robo2 transcription in RP motor neurons [Citation10], the location or sequence of individual enhancer elements in robo2 has not been identified for any of the subsets of cells in which robo2 is expressed.
Here, we examine the embryonic expression patterns of 17 Drosophila transgenic lines expressing the GAL4 transcriptional activator under the control of genomic DNA fragments derived from intergenic and intron sequences in and around the robo2 gene [Citation23,Citation24]. We crossed each line to a UAS-TauMycGFP reporter strain and we have characterized the green fluorescent protein (GFP) expression pattern conferred by each fragment in the embryonic VNC during the major stages of axon guidance (stage 12 through 17). We identify individual fragments that confer expression in specific cell types where robo2 is known to function, including early pioneer neurons, midline glia and lateral longitudinal neurons. Our results indicate that robo2’s dynamic expression during embryonic central nervous system (CNS) development is specified by a combination of enhancer elements located both upstream of the robo2 promoter and within the large robo2 first intron that are active in different subsets of cells. We also show that robo2’s expression in lateral longitudinal axons represents at least two genetically separable subsets of commissural neurons with cell body positions and axon projection patterns that are morphologically distinct from each other. In addition, we provide a general description of expression characteristics for each fragment in embryonic tissues outside of the nervous system, to serve as a resource for other researchers who may be interested in robo2 expression and its functional roles outside of the CNS.
Materials and methods
Drosophila strains
Transgenic Drosophila strains carrying GAL4 transgenes from the FlyLight collection [Citation23,Citation24] were obtained from Bloomington Drosophila Stock Center. The following Drosophila mutant alleles and transgenes were used: w1118; snaSco/CyO,P{en1}wgen11 (Sco/CyOwg), y1 M{w[+mC] = nos-Cas9.P}ZH-2A w* (nos-Cas9.P) [Citation25], robo2myc-robo2 (this study), robo2HA-robo2 [Citation26], P{GMR27G11-GAL4}attP2, P{GMR27F07-GAL4}attP2, P{GMR28D05-GAL4}attP2, P{GMR27D10-GAL4}attP2, P{GMR27D07-GAL4}attP2, P{GMR28A12-GAL4}attP2, P{GMR28F02-GAL4}attP2, P{GMR28E10-GAL4}attP2, P{GMR28D10-GAL4}attP2, P{GMR28G05-GAL4}attP2, P{GMR28D12-GAL4}attP2, P{GMR28F12-GAL4}attP2, P{GMR27H02-GAL4}attP2, P{GMR28C04-GAL4}attP2, P{GMR28B05-GAL4}attP2, P{GMR28A10-GAL4}attP2, P{GMR28E07-GAL4}attP2, P{GawB}NP6273 (robo2GAL4) [Citation27], P{UAS-TauMycGFP}III. The genomic sequence coordinates for the DNA fragments associated with the above FlyLight GAL4 transgenes are described in .
Table 1. DNA sequence fragments from the Drosophila GAL4 lines used in this study.
Generation of myc-tagged robo2myc-robo2 allele
CRISPR modification of robo2 was carried out as described by Howard et al. [Citation26], with an N-terminal 4xMyc tag replacing the 4xHA tag in the full-length robo2robo2 donor plasmid. robo2 gRNA sequences (GTATCTTTTATACCATGCCA and GGAGCAAAGGTCAGACATTA) were cloned into the tandem expression vector pCFD4 [Citation25] via PCR followed by Gibson assembly using the PCR product and BbsI-digested pCFD4 backbone. The robo2 gRNA plasmid and homologous donor plasmid were co-injected into nos-Cas9.P embryos (Bloomington Drosophila Stock Center stock #54591) [Citation25] by BestGene Inc (Chino Hills, CA). Injected individuals (G0) were crossed as adults to Sco/CyOwg. Founders (G0 flies producing F1 progeny carrying modified robo2 alleles) were identified by testing two pools of three F1 females per G0 cross by genomic PCR. From each identified founder, 5–10 F1 males were then crossed individually to Sco/CyOwg virgin females. After 3 d, the F1 males were removed from the crosses and tested by PCR to determine if they carried the modified allele. F2 flies from positive F1 crosses were used to generate balanced stocks, and the modified alleles were fully sequenced by amplifying the entire modified locus from genomic DNA, then sequencing the PCR product after cloning via CloneJET PCR cloning kit (Thermo Scientific).
Embryo collection, antibody staining/immunofluorescence and imaging
Drosophila embryo collection, fixation and antibody staining were carried out as described by Patel [Citation28]. Flies carrying each GAL4 transgene were mated with flies carrying a UAS-TauMycGFP transgene, and offspring from the crosses were collected as embryos every 24 h for 4–5 d. 0–24-h-old embryos were collected on apple juice agar plates containing a dollop of yeast paste (50/50 mix by volume of water and dried active baker’s yeast). A 50% bleach solution was applied directly to the embryos on the agar plate for 2–5 min to dissolve the embryonic chorion, then embryos were collected in mesh baskets and rinsed well with water. Dechorionated embryos were transferred to glass scintillation vials containing 5 ml heptane and 5 ml 3.7% formaldehyde and fixed for 12–15 min at room temperature while rocking on a nutator. After fixation, the formaldehyde solution was removed and 10 ml methanol was added, followed by vigorous shaking for 30 s to remove embryonic vitelline membranes. Devitellinized embryos were rinsed well with methanol to remove residual heptane and formaldehyde, transferred to 1.5 ml microcentrifuge tubes and stored in methanol at −20°C. For antibody staining, embryos were rehydrated in phosphate-buffered saline (PBS) + 0.1% Triton X-100 (PBT), blocked with PBT + 5% normal goat serum (NGS), incubated with primary antibodies diluted in PBT+NGS at 4°C overnight, washed in PBT, incubated with secondary antibodies diluted in PBT+NGS for 1–2 h at room temperature, then washed again in PBT. Embryos stained with horseradish peroxidase (HRP)-conjugated antibodies were developed by incubation with stable diaminobenzidine (DAB) solution (Invitrogen #750118) according to the manufacturer’s instructions. Stained embryos were stored in 70% glycerol/PBS at room temperature. VNCs from fluorescently stained embryos of the desired genotype and developmental stage were dissected and mounted in 70% glycerol/PBS.
The expression of each GAL4 line was examined in whole-mount embryos stained with an anti-GFP primary antibody followed by a HRP-conjugated secondary antibody, and developed with 3,3-diaminobenzidine (DAB), which produces a brown precipitate in the presence of HRP. Whole embryo images were acquired using a Leica M165FC stereo microscope with attached MC170 digital camera and processed by Adobe Photoshop software. The general aspects of GFP expression throughout the embryo (e.g. brain, VNC, gut, epidermis, muscle, salivary glands) from the initial stages of axon outgrowth through the end of embryogenesis were noted (embryonic stages 12–17).
Expression of each GAL4 line in the VNC during embryonic development was examined via immunofluorescence staining with an anti-GFP primary antibody in combination with antibodies against HRP (anti-HRP), which cross-reacts with a pan-neural epitope in Drosophila and labels all of the axons in the embryonic VNC [Citation29,Citation30], and Fasciclin II (anti-FasII), which labels pioneer axons during the early stages of axon guidance and a subset of mature longitudinal axon pathways in older embryos [Citation31]. Nerve cords were dissected from these fluorescently stained embryos and confocal z-stack images of nerve cords at each developmental stage from 12 to 17 were collected using a Leica SP5 confocal microscope. Confocal z-stacks were processed by Fiji/ImageJ [Citation32] and Adobe Photoshop software.
The following antibodies were used: rabbit anti-GFP (Invitrogen #A11122, 1:1000), rabbit anti-c-Myc (Sigma #C3956), mouse anti-Fasciclin II (Developmental Studies Hybridoma Bank [DSHB] #1D4, 1:100), mouse anti-HA (BioLegend #901503, 1:1000), mouse anti-wrapper (DSHB #10D3, 1:100), HRP-conjugated goat anti-mouse (Jackson ImmunoResearch #115-035-003, 1:500), HRP-conjugated goat anti-rabbit (Jackson #111-035-003, 1:500), Alexa 488-conjugated goat anti-HRP (Jackson #123-545-021, 1:200), Alexa 647-conjugated goat anti-HRP (Jackson #123-605-021, 1:100), Cy3-conjugated goat anti-mouse (Jackson #115-165-003, 1:1000), Cy3-conjugated goat anti-rabbit (Jackson #111-165-003, 1:500), Alexa 647-conjugated goat anti-mouse (Jackson #115-605-003, 1:500), Alexa 488-conjugated goat anti-rabbit (Jackson #111-545-003, 1:500), Alexa 647-conjugated goat anti-rabbit (Jackson #111-605-144, 1:500).
Results
To identify potential enhancer sequences regulating robo2 transcription during embryonic VNC development, we took advantage of a series of transgenic strains generated as part of a large-scale effort to identify Drosophila enhancers expressed in neuronal subsets in the adult brain (FlyLight collection, https://flweb.janelia.org/cgi-bin/flew.cgi) [Citation23]. These transgenic lines each carry a small segment of DNA (average size approx. 3,000 bp) derived from introns or upstream intergenic regions of genes known to be expressed in the brain, cloned upstream of the GAL4 transcriptional activator coding sequence [Citation24]. We obtained 17 of these GAL4 lines carrying sequences derived from in or around the robo2 gene (; ). We crossed each of these lines to a reporter line carrying a GAL4-responsive UAS-TauMycGFP (UAS-TMG) transgene, and used an anti-GFP antibody to examine the expression of each GAL4 line throughout embryogenesis in the progeny of each cross. The TauMycGFP (TMG) reporter protein binds to microtubules and labels the cell bodies and axons of neurons in which GAL4 is expressed.
Figure 1. Robo2 enhancer fragments and summary of GAL4 transgene expression patterns.
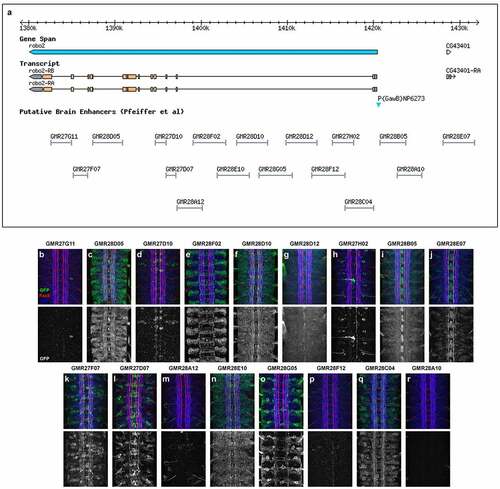
In the following sections, we first provide an overview of the embryonic expression patterns conferred by each of the 17 putative enhancer fragments, compared with endogenous Robo2 expression and a robo2GAL4 enhancer trap line, followed by a more in-depth description of fragments of particular interest which direct expression in neuronal and glial subsets in which Robo2 is known to be functionally important, including lateral longitudinal neurons and midline glia.
Endogenous Robo2 protein expression in the embryo
Robo2 protein is broadly expressed in CNS neurons beginning at stage 12, when early extending axons pioneer the initial longitudinal and commissural axon pathways [Citation1,Citation2]. robo2 mRNA is detectable slightly earlier, at stage 11 [Citation4]. Robo2 protein becomes more restricted as the VNC develops, and at later stages is detectable only on longitudinal axons in the most lateral region of the neuropile [Citation2,Citation4]. We and others have shown that anti-HA staining of modified robo2 loci including an N-terminal 4xHA tag reproduces the pattern seen with antibodies against Robo2 protein in the late-stage embryonic CNS [Citation5,Citation26]. In whole robo2HA-robo2 embryos stained with anti-HA, Robo2 protein is also clearly detectable in the gut, head and epidermis in addition to its CNS expression (). We used a similar engineered robo2 allele with an N-terminal 4xMyc tag to detect Robo2 protein expression in embryos co-labelled with anti-HRP (which labels all axons in the embryonic CNS) and anti-FasII (which labels motor axons and a subset of longitudinal axon pathways in the VNC) from stages 12 to 17, which illustrates Robo2’s early broad expression and later restriction to lateral axons (). We used different tags for immunohistochemistry (HA) and immunofluorescence (Myc) because in our hands mouse anti-HA antibodies produce the cleanest signal, but rabbit anti-Myc antibodies are cleaner than rabbit anti-HA for co-staining with the mouse monoclonal anti-FasII. We have not observed any differences in function or protein localization between our HA-tagged and Myc-tagged robo2robo2 alleles. Like Robo1, Robo2 protein is cleared from commissural segments of midline-crossing contralateral axons [Citation1], so it is not clear from examining endogenous Robo2 protein which Robo2-expressing axons are ipsilateral and which (if any) are contralateral.
Figure 2. Endogenous expression of Robo2 protein.
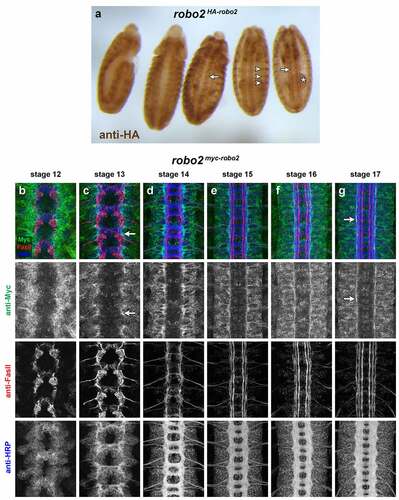
Embryonic expression pattern of a robo2GAL4 enhancer trap insertion
P{GawB}NP6273 is a GAL4 enhancer trap transgene insertion located 217 bp upstream of the predicted robo2 transcriptional start site, recovered in a large-scale screen for novel insertions of the GAL4 enhancer trap element P{GawB} [Citation27]. GAL4 expression in this line recapitulates aspects of the robo2 expression pattern including midline glia, early pioneer neurons and lateral longitudinal neurons [Citation7]. In whole embryos carrying robo2GAL4 and UAS-TMG, GFP is strongly and broadly expressed throughout the embryonic epidermis. Although this makes it difficult to detect internal aspects of GFP expression in these intact embryos, some internal GFP can clearly be seen, including strong midline expression in the VNC ().
Figure 3. Expression of a robo2GAL4 enhancer trap allele.
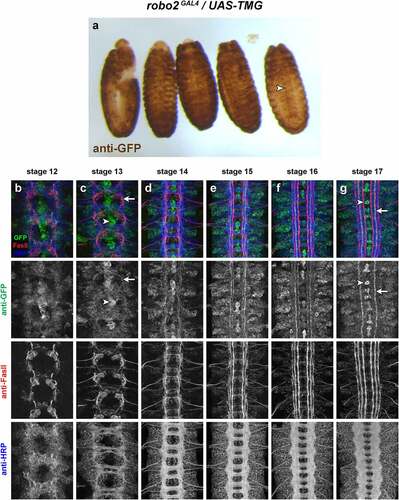
Similarly, fluorescent staining with anti-GFP antibodies in combination with anti-HRP and anti-FasII in robo2GAL4/UAS-TMG dissected embryonic VNCs reveals broad GFP expression at all stages of VNC development (). The strongest expression is in midline glia and midline-adjacent pioneer neurons; by early-stage 13, the axons of these neurons are clearly distinguishable and strong GFP expression remains in these cells through stage 17, where their axons projecting in intermediate longitudinal pathways remain strongly GFP-positive (). The lower-level broad GFP expression throughout the neuropile makes it difficult to clearly identify other specific subsets of GFP-positive axons in these embryos, or to connect GFP-positive axons with the corresponding neuronal cell bodies in order to identify specific subset(s) of robo2-expressing neurons.
Because the P{GawB}NP6273 transgene is located near the robo2 promoter, it is likely to be influenced by all of the enhancer elements that normally regulate robo2 expression. Given that robo2 is broadly and dynamically transcribed throughout the VNC and other tissues at various developmental stages, and that the GAL4 and GFP proteins likely perdure in cells long after transcription ceases from the transgene’s promoter, it is not surprising that most cells that express robo2 during embryonic development may remain GFP-positive through later stages. Examining the expression patterns conferred by isolated putative enhancer regions may allow a more precise characterization of robo2 expression in specific subsets of neurons in addition to other non-neural embryonic tissues. We therefore now turn to a characterization of expression patterns for each of the 17 robo2 cloned enhancer fragment transgenes.
Summary of whole-embryo expression in each robo2 GAL4 line
In the following sections, we summarize the GFP expression patterns in embryos carrying each GAL4 transgene in combination with UAS-TMG and describe specific aspects of VNC expression where applicable. Whole-mount embryos and confocal z-stack projection images of dissected VNCs from embryonic developmental stages 12 to 17 are shown in a corresponding figure for each line ().
Figure 4. GMR27G11 lacks expression in the embryonic ventral nerve cord.
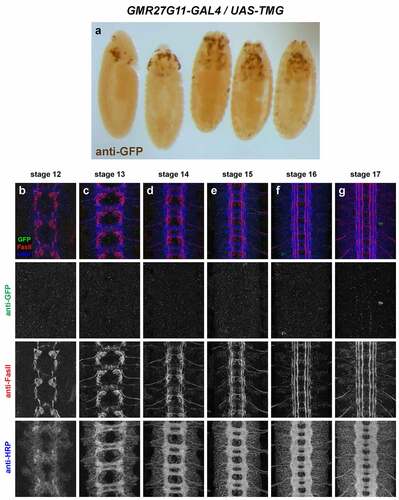
GMR27G11 (). No VNC expression. Sparse expression in anterior/head begins around stage 11/12 and persists through stage 17.
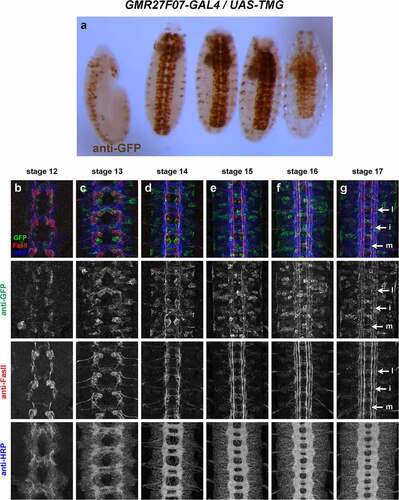
GMR27F07 (). Broadly expressed throughout brain and VNC, beginning around stage 11/12 and persisting through stage 17. Expression in a few peripheral cells (possibly peripheral nervous system [PNS] neurons) begins around stage 13, with afferent processes visible reaching the nerve cord by stage 15. Expression in pioneer VNC neurons at stage 12–13, some FasII-positive and some FasII-negative. GFP-positive axons are present in medial and intermediate longitudinal pathways by stage 14, and in lateral longitudinal pathways by stage 15. GFP persists in all three longitudinal pathways through stage 17. Very little expression outside the nervous system.
Figure 5. GMR27F07 is expressed broadly in the embryonic ventral nerve cord.
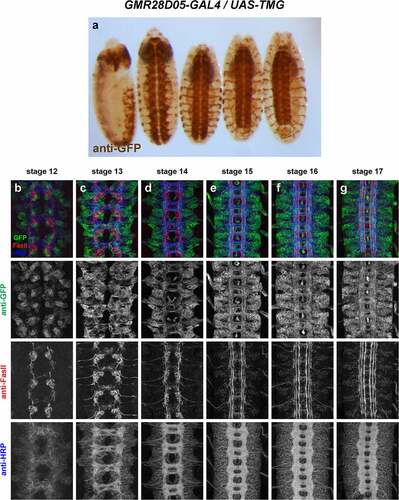
GMR28D05 (). Strongly expressed throughout brain and VNC, beginning around stage 11/12 and persisting through stage 17. Many commissural and longitudinal axons are labelled but broad expression makes it difficult to distinguish individual pathways or fasicles. Expression in a few peripheral cells (possibly PNS neurons) begins around stage 13. Likely peripheral glia exiting VNC are labelled at stage 13 and expression extends along perpipheral nerve persisting through stage 17. Moderate expression throughout the gut/visceral mesoderm beginning around stage 13 and persisting through stage 17.
Figure 6. GMR28D05 is expressed broadly in the embryonic ventral nerve cord.
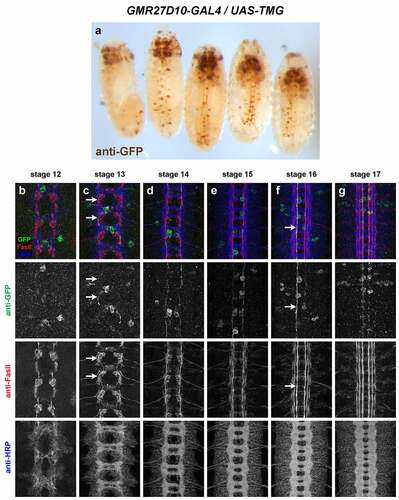
GMR27D10 (). Broadly expressed in anterior/head and brain by stage 12, persisting through stage 17. Stochastic labelling of one or two ipsilateral pioneer neurons in some segments at stage 12/13 (likely vMP2/dMP2) whose axons remain in the medial FasII pathway through stage 17. Some expression in anterior head lobes at late stages (16/17). Little or no other expression outside of the CNS.
Figure 7. GMR27D10 is stochastically expressed in a small number of longitudinal pioneer neurons in the ventral nerve cord.
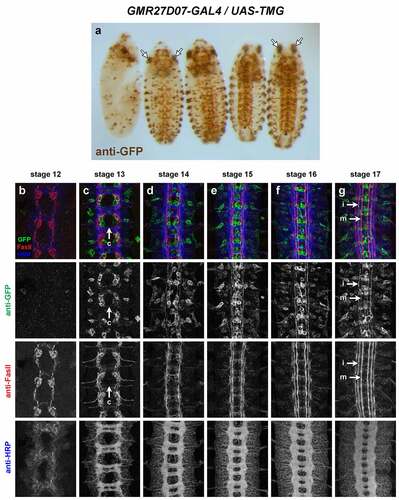
GMR27D07 (). Broadly expressed in anterior/head and brain by stage 12, persisting through stage 17. Expressed in clusters of neurons near the midline beginning around stage 13, including some commissural pioneer neurons. GFP persists in these neurons and their axons in or near medial and intermediate FasII pathways through stage 17. Expression in peripheral cells (possibly PNS neurons) begins around stage 13. Sensory and/or motor axons labelled beginning stage 14 through stage 17.
Figure 8. GMR27D07 is expressed in commissural pioneer and longitudinal neurons in the ventral nerve cord.
GMR28A12 (). Little or no GFP expression is detectable in the VNC, apart from a single bilateral pair of cells in each segment beginning around stage 13, located dorsally to the neuropile with processes extending laterally out of the CNS, likely dorsal channel glia [Citation33] or transverse nerve (TN) exit glia [Citation34]. Expressed in visceral mesoderm beginning stage 12 (two broad longitudinal stripes at stage 13/14, expanding to cover entire gut by stage 15). Strongly expressed in salivary glands beginning around stage 14/15. Expression in anterior/head beginning stage 12 or earlier, persisting through stage 17 including anterior head lobes.
Figure 9. GMR28A12 exhibits little or no expression in the embryonic ventral nerve cord.
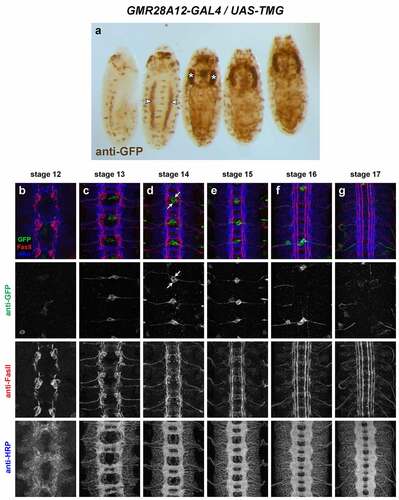
GMR28F02 (). Expressed mainly in brain/VNC and visceral mesoderm. Visceral mesoderm expression begins by stage 12–13 (two broad longitudinal stripes at stage 13/14, expanding to cover entire gut by stage 15). In VNC, expressed consistently in a large cluster of neurons with lateral cell bodies plus some midline-adjacent neurons in each hemisegment beginning stage 12. Commissural axons from this cluster are detectable crossing the midline at stage 13: one bundle near posterior edge of PC and 2–3 bundles in the anterior half of AC. Lateral longitudinal axons are detectable at stage 13, forming an intact lateral pathway connecting adjacent segments by stage 14 (at this stage the lateral FasII pathways have not yet formed). Axon and cell body GFP persists through stage 17, where GFP+ longitudinal axons are detectable in or near the intermediate and lateral FasII pathways. Some motor nerve and/or peripheral glia expression visible by stage 15, persisting through stage 17. Expressed in dorsal channel glia/TN exit glia beginning around stage 13.
Figure 10. GMR28F02 is expressed in a subset of commissural longitudinal neurons.
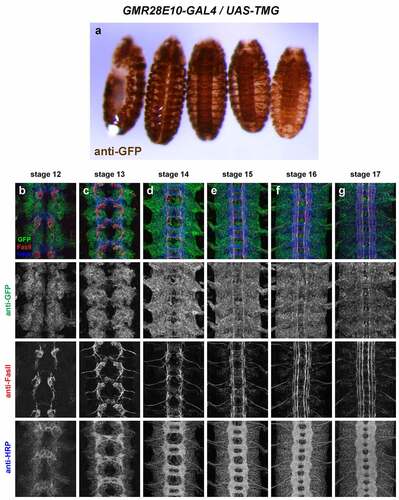
GMR28E10 (). Very broadly expressed including nerve cord and epidermis beginning at stage 11 or earlier. Broad expression in neuronal cell bodies from stage 12 through stage 17; few axons individually discernable due to uniform expression in nerve cord. The expression pattern of GMR28E10 looks very similar to that of GMR28D10, with which it overlaps.
Figure 11. GMR28E10 is expressed broadly in the embryonic ventral nerve cord.
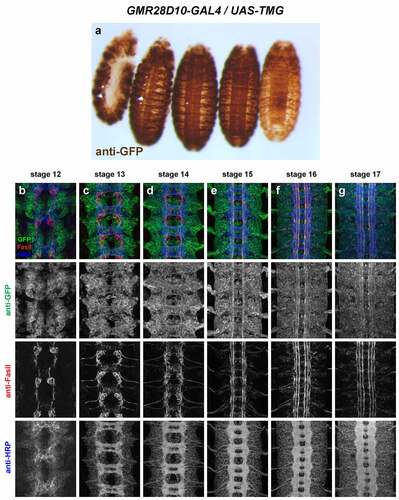
GMR28D10 (). Very broadly expressed including nerve cord and epidermis beginning at stage 11 or earlier. Broad expression in neuronal cell bodies from stage 12 through 17; few axons individually discernable due to uniform expression in nerve cord. The expression pattern of GMR28D10 looks very similar to that of GMR28E10, with which it overlaps.
Figure 12. GMR28D10 is expressed broadly in the embryonic ventral nerve cord.
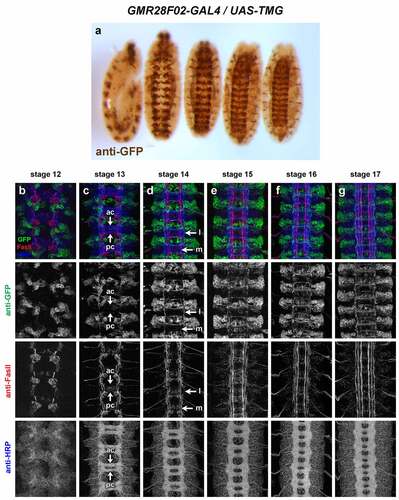
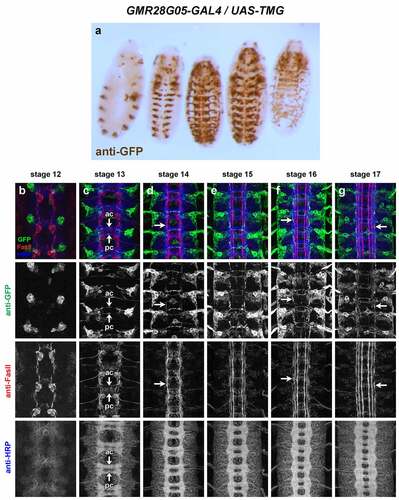
GMR28G05 (). Expression mainly restricted to brain/head and VNC. In VNC, expressed in a consistent cluster of neurons in each hemisegment with lateral cell bodies beginning stage 12. Commissural axons from this cluster are detectable crossing the midline at stage 13: one bundle near posterior edge of AC and 1–2 bundles at anterior edge of PC. Axons that cross in AC begin turning anteriorly by stage 14 and overlap with lateral FasII pathways by stage 15. Axons that cross in PC also appear to turn anteriorly by stage 14 but grow along medial or intermediate FasII pathways. Axon and cell body GFP persists through stage 17, where GFP+ longitudinal axons are detectable mainly in or near lateral FasII pathways, with a few in or near medial or intermediate FasII pathways. Peripheral glia expression is visible by stage 13/14 and persists through stage 17.
Figure 13. GMR28G05 is expressed in a subset of commissural longitudinal neurons.
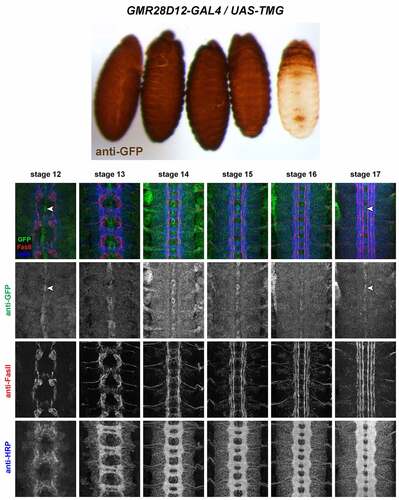
GMR28D12 (). Uniformly expressed throughout ectoderm beginning at stage 11 or earlier. Also uniformly expressed throughout VNC from stage 12 through 17. Higher expression in midline glia beginning at stage 12, persisting through stage 17. Similar expression to GMR28B05 except lower in midline glia.
Figure 14. GMR28D12 is expressed broadly in the embryonic ventral nerve cord.
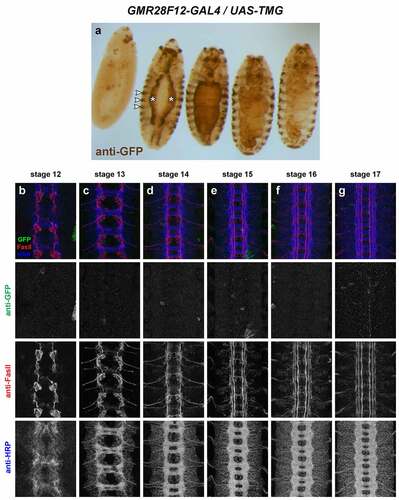
GMR28F12 (). No detectable VNC expression. Strong staining in some cells in brain/head region beginning around stage 13 and persisting through stage 17 in anterior head lobes.High expression in visceral mesoderm beginning around stage 13 (two broad longitudinal stripes at stage 13/14, expanding to cover entire gut by stage 15). Striped expression in ectoderm also beginning around stage 13. The visceral mesoderm and striped ectodermal expression in this line resembles the Robo2 expression pattern described by Kraut and Zinn [Citation11].
Figure 15. GMR28F12 lacks expression in the embryonic ventral nerve cord.
GMR27H02 (). Sparse but regular expression in a few neurons per hemisegment in the VNC beginning around stage 14, with lateral cell bodies and medial/intermediate ipsilateral axons. Expressed in dorsal channel glia/TN exit glia beginning around stage 13. Restricted expression in brain/head from stage 12 through stage 17, including anterior head lobes.
Figure 16. GMR27H02 is expressed in a small number of longitudinal neurons in the embryonic ventral nerve cord.
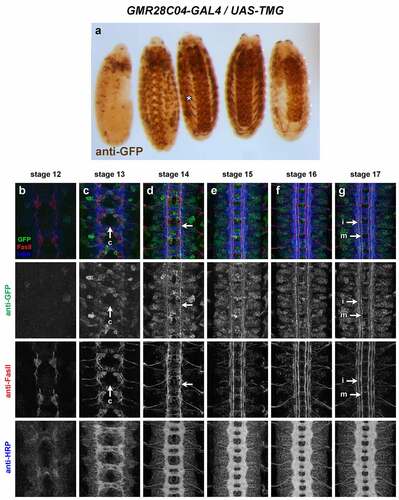
GMR28C04 (). Expression mainly restricted to brain/VNC and body wall muscles, in particular ventral muscles. Broad expression throughout VNC neurons starting at stage 13. Some commissural axons visible early (stage 13) and medial/intermediate longitudinal axons prominently labelled by stage 16/17. Two strong foci of expression in lateral head at stage 12, persist through stage 17 at tips of anterior head lobes.
Figure 17. GMR28C04 is expressed broadly in the embryonic ventral nerve cord.
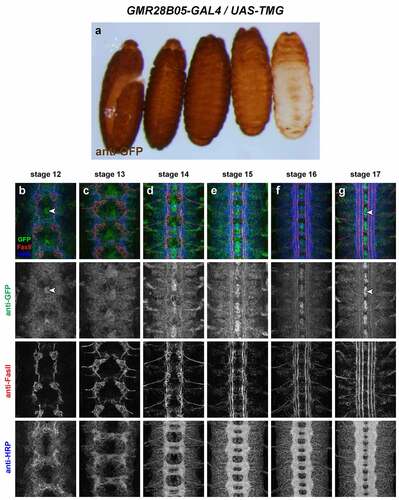
GMR28B05 (). Uniformly expressed throughout ectoderm beginning at stage 11 or earlier. Also uniformly expressed throughout VNC from stage 12 through 17. Higher expression in midline glia beginning at stage 12, persisting through stage 17. Similar expression to GMR28D12 except higher in midline glia.
Figure 18. GMR28B05 is expressed broadly in the embryonic ventral nerve cord.
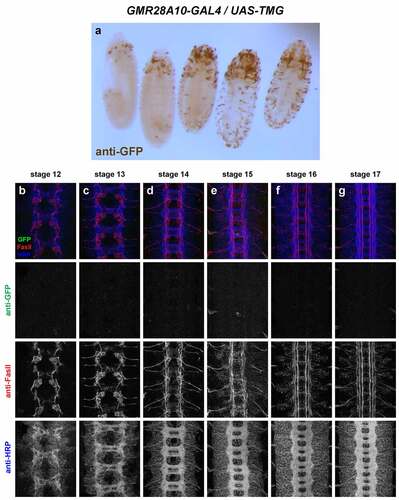
GMR28A10 (). Little or no VNC expression. Head/anterior expression from stage 12 through stage 17, and scattered peripheral cells, possibly PNS neurons or peripheral glia.
Figure 19. GMR28A10 exhibits little or no expression in the embryonic ventral nerve cord.
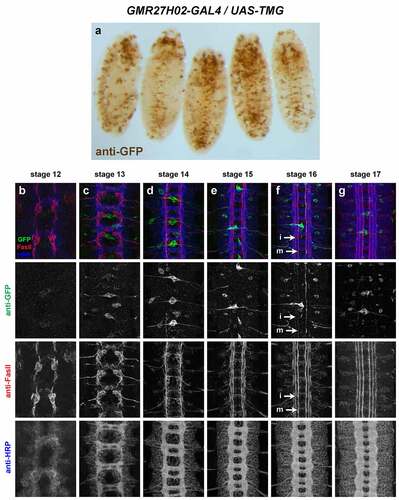
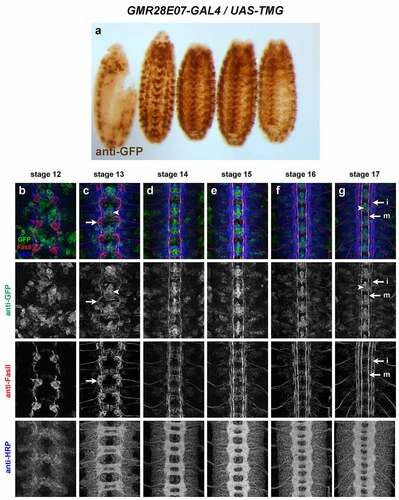
GMR28E07 (). Broadly expressed in nerve cord, body wall muscles/peripheral ectoderm and head, beginning around stage 12/13 and persisting through stage 17. Strong expression in pioneer neurons near the midline and in midline glia at stage 12/13. Broad low-level expression throughout VNC persists through stage 17, along with higher expression in midline glia and midline-adjacent neurons with axons in medial and intermediate longitudinal pathways. Expressed in dorsal channel glia/TN exit glia beginning around stage 13.
Figure 20. GMR28E07 is expressed strongly in midline glia and pioneer longitudinal neurons.
Enhancer fragments promoting robo2 expression in midline glia
We have previously shown that robo2 mRNA is transiently expressed in midline glia during the early stages of axon pathfinding (stages 12–13) and that the robo2-GAL4 enhancer-trap insertion P{GawB}NP6273 and HA-tagged Robo2 protein expressed from the robo2robo2 knock-in allele are also expressed in midline glia at these stages [Citation7]. Robo2 expression in midline cells is important for its pro-midline crossing activity, as it antagonizes Slit-Robo1 repulsion in trans in pre-crossing commissural axons to allow Robo1-expressing growth cones to cross the Slit-expressing midline [Citation7].
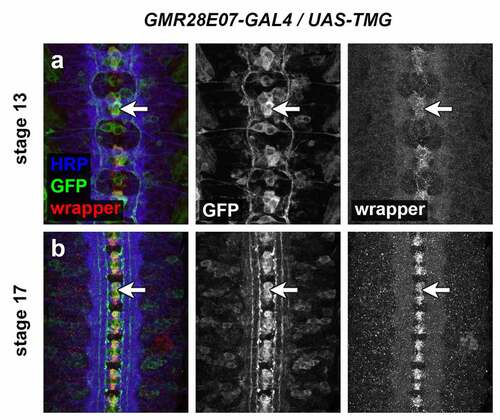
Three of the lines described here are strongly expressed in midline cells, including cells with positions and morphologies consistent with midline glia (GMR28D12, GMR28B05 and GMR28E07) (). The GMR28E07 and GMR28B05 fragments are both located upstream of the robo2 promoter but do not overlap with each other, while GMR28D12 is located within the first intron of robo2. Overall, GMR28E07 appears to most closely reproduce the midline expression seen in the robo2-GAL4 line, with strong GFP expression in midline cells (both midline glia and midline-adjacent neurons) beginning early (stage 12–13) and persisting through stage 17 (compare with ). Both robo2-GAL4/UAS-TMG and GMR28E07/UAS-TMG embryos also include GFP-labelled neuronal axons visible in the medial (pCC/dMP2) pathway and intermediate (MP1) pathway. To confirm midline glial expression of GMR28E07, we stained GMR28E07/UAS-TMG embryos with anti-wrapper, a marker for midline glia [Citation35], along with anti-GFP. We found that GFP clearly labelled wrapper-positive midline glia at stage 13, and GFP/wrapper co-localization persisted through stage 17 ().
Figure 21. GMR28E07 is expressed in wrapper-positive midline glia.
Enhancer fragments promoting robo2 expression in morphologically distinct and genetically separable subsets of lateral longitudinal axons
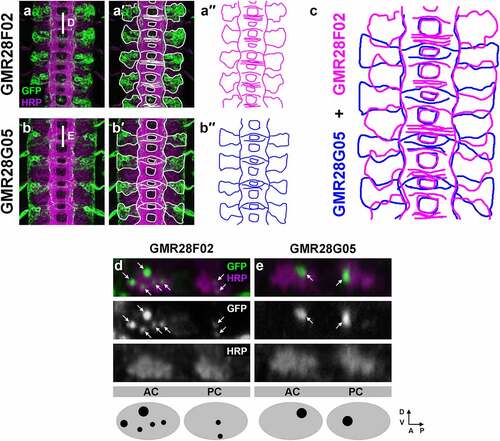
A number of the putative enhancer fragments presented here exhibit broad expression in the embryonic VNC which may overlap with Robo2’s endogenous expression pattern in lateral longitudinal axons. Two fragments in particular exhibit more restricted expression where GFP is specifically detectable in longitudinal axons in the lateral zone: GMR28F02 and GMR28G05 (). These two fragments are non-overlapping (), and their GFP expression patterns do not appear to be identical, suggesting that they may represent (at least) two molecularly distinct enhancer regions promoting expression in two distinct subsets of longitudinal neurons. Both subsets include early crossing commissural axons, with GFP-positive axons visible crossing midline as early as stage 13 in both lines, and both include some GFP-positive longitudinal axons in pathways outside of the lateral zone. As Robo2 protein is not normally detectable on medial/intermediate longitudinal axons at late stages, it is possible that GFP-positive axons visible in the medial/intermediate zones at stage 17 may be due to perdurance of early GFP expression in medial/intermediate pathway pioneer neurons.
To more closely compare the expression patterns of GMR28F02 and GMR28G05, and to determine whether the neurons expressing GAL4 in these two lines represent distinct neuronal subsets, we first compared the cell body positions of the neuron clusters expressing GMR28F02 and GMR28G05 (). We traced the cell body clusters and the midline-crossing portions of GFP-positive axons in stage 16 GMR28F02/UAS-TMG and GMR28G05/UAS-TMG embryos (′, a′′, b′, b′′) and overlaid the traces, using the outline of the HRP-positive axon scaffold to register the two traces with each other (). We found that the GFP-positive cell body clusters in GMR28F02 and GMR28G05 exhibit little or no overlap in these registered traces. GFP-positive cell bodies in GMR28F02 are found mainly in the anterior portion of each segment, located between the AC of one segment and the PC of the next anterior segment (′, a′′). In contrast, GFP-positive cell bodies in GMR28G05 are clustered near the PC in each segment, with the anterior boundary of each cluster located near the posterior edge of the AC in the same segment (′, b′′).
Figure 22. GMR28F02 and GMR28G05 label two distinct subsets of commissural longitudinal neurons.
We next compared y,z confocal cross-sections through the anterior (AC) and posterior (PC) commissures in these same stage 16 GMR28F02/UAS-TMG and GMR28G05/UAS-TMG embryos and found that GFP-positive commissural axons in the two subsets cross the midline in distinct locations. GMR28F02 axons cross in one bundle near the posterior edge of the PC and two to three bundles in the anterior half of the AC, while GMR28G05 axons cross in one bundle near posterior edge of the AC and one to two bundles at anterior edge of the PC (), again suggesting that the neurons labelled by these two GAL4 lines represent distinct subsets of cells.
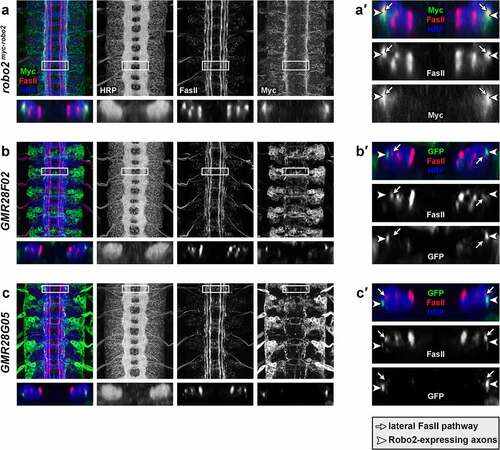
Finally, we used x,z confocal cross-sections to examine the positions of GFP-positive lateral longitudinal axons and FasII-positive axon pathways in stage 16 GMR28F02/UAS-TMG and GMR28G05/UAS-TMG embryos, compared with equivalent x,z cross-sections in robo2myc-robo2 embryos (). We confirmed that myc-tagged endogenous Robo2 protein overlaps with lateral FasII-positive longitudinal pathways in stage 16 robo2myc-robo2 embryos, as predicted by previous studies which examined Robo2 and FasII expression in different embryos (′) [Citation2,Citation4]. However, we found that GFP-positive longitudinal axons in GMR28F02/UAS-TMG and GMR28G05/UAS-TMG embryos did not overlap with lateral FasII pathways (′, c, c′), suggesting that these two subsets of Robo2-expressing axons are not among the subset of lateral longitudinal axons that are predicted to be both Robo2-positive and FasII-positive.
Figure 23. Lateral longitudinal axons labelled by GMR28F02 and GMR28G05 are distinct from FasII-positive lateral axon pathways.
Discussion
Among the three Drosophila robos, robo2 exhibits the broadest and most dynamic expression pattern and the most diverse set of molecular and developmental roles during embryonic development. Accordingly, the transcriptional regulation of robo2 expression appears to be more complex than for robo1. We and others have constructed robo1 rescue transgenes containing less than 5 kb of flanking genomic sequence that recapitulate robo1’s full expression pattern in the embryonic CNS [Citation5,Citation36,Citation37]. However, our attempts to create an equivalent rescue transgene for robo2 with similarly sized flanking sequences have not been successful, suggesting that the regulatory sequences necessary for proper robo2 expression may be more numerous and/or located farther from the robo2 transcription start site than for robo1 (T.A.E., unpublished data). Notably, the robo2 transcription unit is approximately 40 kb in size, nearly five times the size of robo1 (~8,400 bp) and unlike robo1 includes a very large first intron (22 kb), which could potentially contain regulatory sequences in addition to any located upstream of the robo2 promoter.
Here, we identify enhancer regions located upstream of the robo2 promoter and in the robo2 first intron which can drive GAL4 expression in specific cell types where robo2 is known to function, including early pioneer neurons, midline glia and lateral longitudinal neurons. The identification of fragments containing regulatory sequences described here should enable similar rescue constructs for robo2, and may allow rescue of specific phenotypes/subsets of neurons (e.g. restoring robo2 expression in lateral longitudinal neurons with the GMR28G05 or GMR28F02 fragments, alone or in combination, may allow specific rescue of robo2’s role in lateral pathway formation). Our results here also provide insight into the regulatory logic of robo2 expression, by identifying genetically separable enhancer regions that individually specify expression in distinct subsets of robo2-expressing cells (e.g. midline glia vs lateral longitudinal neurons).
Differences between Robo2 protein expression and GAL4-induced GFP expression
Importantly, the initation and termination of GAL4 transcription in the transgenic lines described here should closely reproduce the temporal dynamics of transcription specified by each enhancer fragment, but the GAL4 and/or TauMycGFP proteins are likely to perdure well after transcription ceases. Thus, cells that express a given enhancer early during development are likely to remain GFP-positive through later stages, even if the enhancer is no longer active. For this reason, the GFP expression patterns we describe at later stages of development are likely to reflect a summation of each enhancer’s expression throughout all stages of embryogenesis.
The subcellular localization of TauMycGFP will likely also be distinct from Robo2 protein. Cell bodies and the entire length of axons are labelled by TauMycGFP, while Robo2 is primarily localized to axons and is likely not uniformly expressed along the entire length of all axons. For example, the lateral longitudinal neurons labelled by GMR28G05 and GMR28F02 are clearly commissural neurons and the midline-crossing portions of their axons are labelled by TauMycGFP (), but there are few or no commissural axon segments with detectable Robo2 protein at any stage in robo2myc-robo2 or robo2HA-robo2 embryos (). The endosomal sorting receptor Commissureless (Comm) prevents Robo1 protein from reaching the growth cone surface as commissural axons grow towards and across the midline, which accounts for the exclusion of Robo1 protein from commissural axon segments [Citation38–40]. In comm gain of function embryos, Robo2 protein is also downregulated, but it is not clear whether Comm normally regulates Robo2 protein the same way it does Robo1 [Citation1,Citation2].
Dispensability of robo2 enhancer elements in introns 2–13
We note that several of the tested fragments located within robo2 introns 2–13 (GMR27G11, GMR27F07, GMR28D05, GMR27D10 and GMR27D07) were deleted in a series of knock-in and CRISPR-modified alleles created by us and others in which exons 2–14 of robo2 along with the intervening introns were replaced with cDNAs encoding various robo variants, including full-length robo2 coding sequences [Citation5,Citation26]. The robo2myc-robo2 allele reported here also deletes these intronic sequences. We have shown here that GMR27F07, GMR28D05 and GMR27D07 can promote expression in the embryonic VNC (). However, in both our and Spitzweck et al.’s robo2robo2 alleles, introns 2–13 were deleted without any detectable effect on the midline repulsion or lateral positioning activities of robo2. Santiago and colleagues also detected no defects in robo2-dependent motor neuron innervation of ventral muscles 6/7 in animals homozygous for the robo2robo2 knock-in allele [Citation10]. These results suggest that any expression conferred by these fragments is dispensable for those axon guidance activities, at least to the extent that can be measured by examining FasII-positive axons in the embryonic VNC. It may be the case that the expression patterns conferred by enhancers within introns 2–13 are redundant with patterns conferred by other enhancers that were not deleted in the modified alleles. Alternatively, the ventral nerve cord expression patterns conferred by these enhancers may be necessary for axon guidance or other developmental outcomes that were not examined in the studies cited above. Importantly, we have not examined all robo2-dependent developmental contexts in these gene replacement backgrounds, and thus cannot rule out the possibility that introns 2–13 and any associated enhancer elements may be required for some of robo2’s developmental roles.
Developmental timing of Robo2-positive vs FasII-positive longitudinal axon pathway formation
We have shown here that enhancers within the GMR28F02 and GMR28G05 fragments drive expression in distinct subsets of lateral longitudinal neurons. We note that GFP-positive longitudinally projecting axons are present in the lateral region of the neuropile in GMR28F02/UAS-TMG embryos by stage 13, and form a continuous lateral pathway well before the lateral FasII-positive pathways begin forming (). Myc-tagged Robo2 protein is also detectable within the lateral neuropile in robo2myc-robo2 embryos by stage 13 (), consistent with the idea that Robo2-positive lateral axon tracts form before FasII-positive lateral pathways. The x,z cross sections presented in reveal that at least some of these Robo2-positive tracts remain distinct from FasII-positive pathways through stage 17. GFP-positive lateral axons in GMR28G05/UAS-TMG embryos are also distinct from FasII lateral pathways in cross-sections, but GMR28G05 axons reach lateral positions later than GMR28F02 and are not detectable in the lateral zone until FasII-positive lateral pathways begin forming, around stage 15 ().
We and others have previously shown that the lateral positions of Robo2-expressing axons are not affected in robo2robo1 or robo2robo2∆Ig1 embryos, while lateral FasII pathways fail to form correctly in these embryos [Citation3,Citation26]. Robo2 protein expression appears to overlap with lateral FasII pathways in robo2myc-robo2 embryos, and the generally accepted model of Robo2’s lateral positioning activity is that it guides FasII-positive axons to the lateral region by acting cell-autonomously as a Slit receptor. However, it remains unclear which, if any, FasII-positive lateral axons also express Robo2. Our results suggest that at least some subsets of Robo2-positive lateral axons are distinct from FasII-positive lateral pathways. The GMR28F02 and GMR28G05 enhancer fragments described here provide a method for labelling Robo2-expressing axons independently of their Robo2 expression. This should allow us to directly examine which (if any) of these subsets depend on Robo2 expression for their guidance to the lateral zone, which may help clarify whether Robo2’s function in promoting lateral pathway formation is a cell-autonomous or non-autonomous function.
Locations of specific enhancer element(s) within each identified enhancer region
Most of the fragments tested here are quite large (3 kb) relative to the average size of a single enhancer element or transcription factor binding site. Importantly, our analysis here does not identify specific transcription factor binding sites within any of the identified enhancer regions, or reveal how many sites are present in each fragment, or their specific location(s). In some cases, the overlapping nature of the tested fragments may help narrow down the likely location of specific enhancer regions. For example, fragments GMR28A12 and GMR28F02 overlap with each other and both exhbit similar strong expression in the visceral mesoderm, suggesting that the relevant enhancer sequence(s) for this expression may be located within the 1,020 bp overlap region shared by these two fragments. In contrast, the neuronal expression of GMR28F02 is not shared by GMR28A12, suggesting that any enhancer(s) active in lateral longitudinal neurons are likely to be located within the 2,715 bp region of GMR28F02 that does not overlap with GMR28A12. It is more difficult to determine whether GMR28F02’s lateral axon expression is shared by GMR28E10, which also overlaps with GMR28F02 but has much broader neuronal expression. Similarly, it is difficult to determine if GMR28G05’s lateral neuron expression is shared by its overlapping fragments GMR28D10 or GMR28D12, because the expression patterns of these two fragments are also very broad. Further studies will be necessary to sub-divide each of these regions to determine the number, identity and location of specific enhancer elements within each.
Acknowledgments
Stocks obtained from the Bloomington Drosophila Stock Center (National Institutes of Health [NIH] grant P40 OD- 018537) were used in this study. Monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the Eunice Kennedy Shriver National Institute of 940 Child Health and Human Development of the NIH and maintained at The Department of Biology, University of Iowa, Iowa City, IA 52242. This work was supported by NIH grant R15 NS-098406 (T.A.E.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Additional information
Funding
References
- Rajagopalan S, Nicolas E, Vivancos V, et al. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000a;28(3):767–777.
- Rajagopalan S, Vivancos V, Nicolas E, et al. 2000b Selecting a longitudinal pathway: robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000b;103(7):1033–1045.
- Simpson JH, Kidd T, Bland KS, et al. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000a;28(3):753–766.
- Simpson JH, Bland KS, Fetter RD, et al. Short-range and long-range guidance by slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000b;103(7):1019–1032.
- Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for drosophila robo receptors. Cell. 2010;140(3):409–420.
- Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol. 2010;20(6):567–572.
- Evans TA, Santiago C, Arbeille E, et al. Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. Elife. 2015;4:e08407.
- Nüsslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster I. Zygotic loci on the second chromosome. Roux Arch Dev Biol. 1984;193(5):267–282.
- Schimmelpfeng K, Gögel S, Klämbt C. The function of leak and kuzbanian during growth cone and cell migration. Mech Dev. 2001;106(1-2):25–36.
- Santiago C, Labrador J-P, Bashaw GJ. The homeodomain transcription factor Hb9 controls axon guidance in Drosophila through the regulation of Robo receptors. Cell Rep. 2014;7(1):153–165.
- Kraut R, Zinn K. Roundabout 2 regulates migration of sensory neurons by signaling in trans. Curr Biol. 2004;14(15):1319–1329.
- Ordan E, Volk T. A non-signaling role of Robo2 in tendons is essential for Slit processing and muscle patterning. Development. 2015;142(20): 3512–3518. DOI:10.1242/dev.128157.
- Santiago-Martínez E, Soplop NH, Kramer SG. Lateral positioning at the dorsal midline: slit and Roundabout receptors guide Drosophila heart cell migration. Proc Natl Acad Sci USA. 2006;103(33):12441–12446.
- Englund C, Steneberg P, Falileeva L, et al. Attractive and repulsive functions of Slit are mediated by different receptors in the Drosophila trachea. Development. 2002;129(21):4941–4951.
- Parsons L, Harris K-L, Turner K, et al. Roundabout gene family functions during sensory axon guidance in the drosophila embryo are mediated by both Slit-dependent and Slit-independent mechanisms. Dev Biol. 2003;264(2):363–375.
- Kramer SG, Kidd T, Simpson JH, et al. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292(5517):737–740.
- Weyers JJ, Milutinovich AB, Takeda Y, et al. A genetic screen for mutations affecting gonad formation in Drosophila reveals a role for the slit/robo pathway. Dev Biol. 2011;353(2):217–228.
- Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7(6):1867–1875.
- Sasse S, Klämbt C. Repulsive epithelial cues direct glial migration along the nerve. Dev Cell. 2016;39(6):696–707.
- Stine RR, Greenspan LJ, Ramachandran KV, et al. Coordinate regulation of stem cell competition by slitrobo and JAK-STAT signaling in the Drosophila testis. In: Buszczak M, editor. PLoS Genet. 2014;10(11):e1004713.
- Brierley DJ, Blanc E, Reddy OV, et al. Dendritic targeting in the leg neuropil of Drosophila: the role of midline signalling molecules in generating a myotopic map. PLoS Biol. 2009;7(9):e1000199.
- Mellert DJ, Knapp J-M, Manoli DS, et al. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesexand the Roundabout receptors. Development. 2010;137(2):323–332.
- Jenett A, Rubin GM, Ngo T-TB, et al. A GAL4-driver line resource for Drosophila Neurobiology. Cell Rep. 2012;2(4):991–1001.
- Pfeiffer B D, Jenett A, Hammonds AS, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA. 2008;105(28):9715–9720.
- Port F, Chen H-M, Lee T, et al. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci USA. 2014;111(29):E2967–76.
- Howard LJ, Reichert MC, Evans TA. The Slit-binding Ig1 domain is required for multiple axon guidance activities of Drosophila Robo2. Genesis. 2021;59(9):e23443.
- Hayashi S, Ito K, Sado Y, et al. GETDB, a database compiling expression patterns and molecular locations of a collection of gal4 enhancer traps. Genesis. 2002;34(1-2):58–61.
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 1994;44:445–487.
- Haase A, Stern M, Wächtler K, et al. A tissue-specific marker of Ecdysozoa. Dev Genes Evol. 2001;211(8):428–433.
- Jan LY, Jan Y-N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci USA. 1982;79(8):2700–2704.
- Grenningloh G, Rehm EJ, Goodman CS. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991;67(1):45–57.
- Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682.
- Ito K, Urban J, Technau GM. Distribution, classification, and development ofDrosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux Arch Dev Biol. Rouxs Arch. Dev. Biol. 1995;204(5):284–307.
- Gorczyca MG, Phillis RW, Budnik V. The role of tinman, a mesodermal cell fate gene, in axon pathfinding during the development of the transverse nerve in Drosophila.10. Development. 1994;120(8):2143–2152.
- Noordermeer J N, Kopczynski CC, Fetter RD, et al. Wrapper, a novel member of the Ig superfamily, is expressed by midline glia and is required for them to ensheath commissural axons in Drosophila. Neuron. 1998;21(5):991–1001.
- Brown HE, Reichert MC, Evans TA. Slit binding via the Ig1 domain is essential for midline repulsion by drosophila robo1 but dispensable for receptor expression. Localization, and Regulation in Vivo. G3: Genes|Genomes|Genetics. 2015;5(11):2429–2439.
- Kidd T, Brose K, Mitchell KJ, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998b;92(2):205–215.
- Keleman K, Rajagopalan S, Cleppien D, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110(4):415–427.
- Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8(2):156–163.
- Kidd T, Russell C, Goodman CS, et al. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998a;20(1):25–33.
