Abstract
Keywords:
Introduction
The Transient Receptor Potential Melastatin 3 (TRPM3) ion channel was cloned based on homology with other known TRP channels, and was considered an orphan channel for a while.Citation1 Various activators of TRPM3 emerged over the years, including the steroid pregenenolone sulfate (PregS), nifedipine and noxious heat.Citation1 Recently it was shown that clotrimazol when co-applied with PregS activates a second permeation pathway with characteristics different from that opened by heat or PregS alone.Citation2 What is the physiological chemical activator of TRPM3 is not really clear, but noxious heat is likely to be a biologically important stimulus. Little is known about regulation of TRPM3 by 2nd messenger pathways.
Most TRP channels require the membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] for activity, but regulation of many members of the TRPC and TRPV families is controversial; negative effects of phosphoinositides have been described for many of them.Citation3 Six out of 8 members of the TRPM family were shown to be positively regulated by PI(4,5)P2, with no negative effects reported for any of them.Citation3 The two missing members, where functional effects of PI(4,5)P2 had not been tested were TRPM1, a channel that is very difficult to study in expression systems, and TRPM3. Two recent articles concluded that TRPM3 is a PI(4,5)P2 sensitive ion channel,Citation4,5 making it very likely that mammalian TRPMs, similarly to inwardly rectifying K+ channels and voltage gated KCNQ K+ channels (Kv7), are a bona fide PI(4,5)P2 dependent ion channel family.
Assessing PI(4,5)P2 regulation of ion channels is not trivial, relying exclusively on one experimental technique, such as application of exogenous phosphoinositides in excised patches had resulted in controversies before.Citation6 Both Badheka et al.Citation4 and Tóth et al.Citation5 used an array of approaches in the excised inside out and in the whole-cell configuration, and drew very similar conclusions. These two papers together with an earlier publication showing binding of PI(4,5)P2 to the TRPM3 proteinCitation7 make a convincing case that this channel is a PI(4,5)P2 dependent ion channel. Below is a brief discussion of the results of the 2 papers; shows a schematic of the approaches used.
Figure 1. Cartoon illustrating the approaches used by the 2 discussed papers, see text for more details.
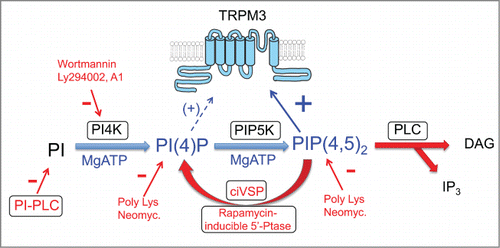
Excised inside out patch clamp experiments
TRPM3 channel activity in excised patches showed a marked rundown, and was reactivated by MgATP in both papers. This is a characteristic of PI(4,5)P2 dependent ion channels; the general view is that rundown is caused by unopposed activity of lipid phosphatases present in the membrane patch in an ATP free environment. When MgATP is applied to the patch, the balance shifts to the dominance of kinase enzymes, phosphatidylinositol 4-kinases (PI4K) and phosphatidylinositol 4-phosphate-5-kinases (PIP5K) that generate PI(4)P and PI(4,5)P2, respectively (). To demonstrate that MgATP indeed acted via the generation of PI(4,5)P2, both groups used various pharmacological agents to prevent formation these lipids.Citation4,5 This included inhibitors of PI4K, such as high concentrations of wortmannin, or LY294002, and the recently described specific PI4K inhibitor compound A1.Citation8 A phosphatidylinositol specific bacterial phospholipase C (PI-PLC) enzymeCitation4 and 2 different polycations poly-Lysine,Citation4,5 and neomycinCitation5 that chelate endogenous negatively charged lipids also inhibited the effect of MgATP. Overall, both papers concluded that MgATP stimulates TRPM3 activity in excised patches by inducing the formation of PI(4,5)P2.
To have a more direct evidence for the positive effect of phosphoinositides, both groups applied exogenous phosphoinositides to excised patches.Citation4,5 In both papers, the effect of the short acyl chain diC8 PI(4,5)P2 was smaller, compared to long acyl chain variants, such as the natural AASt PI(4,5)P2 or synthetic dipalmitoyl PI(4,5)P2. The two papers also found a very similar specificity profile, with PI(3,4,5)P3 being the most active, followed by PI(3,5)P2, PI(4,5)P2, PI(3,4)P2 and PI(4)P in order of decreasing effectiveness. As PI(4,5)P2 and PI(4)P are found in the plasma membrane in much higher concentrations than any of the other phosphoinositides, PI(4,5)P2 is likely to be the most important phosphoinositide regulating TRPM3 in a cellular context, with some potential contribution of PI(4)P, see discussion later. Note that for the excised patch measurements Badheka et al.Citation4 used a human TRPM3 isoform expressed in Xenopus laevis oocytes, whereas Tóth et al.Citation5 used a different mouse splice variant expressed in HEK cells, yet, the results and conclusions showed remarkable similarity between the 2 papers.
Whole-cell patch clamp experiments
Inducible phosphatases became the gold standard to demonstrate phosphoinositide dependence of ion channels in whole-cell patch clamp experiments.Citation6 Both papers used 2 different variants of these tools: voltage sensitive and chemically inducible phosphatases to demonstrate that the mouse TRPM3 expressed in HEK cells requires PI(4,5)P2 for activity. The agreement again was quite remarkable. Both papers found that voltage sensitive phosphatases ciVSP and drVSP inhibited the channel, but the inhibition was incomplete.Citation4,5 Similarly, when using a rapamycin-inducible 5′-phosphatase, both papers found a significant, but partial inhibition.Citation4,5 While these data qualitatively confirm the excised patch experiments, there is a quantitative discrepancy from the conclusion of excised patch experiments. The essentially full rundown and relatively moderate affinity for PI(4,5)P2 found in both papers in excised patches predicted a stronger inhibition by the 5′-phosphatases. The complex environment of a cell differs in many aspects from the reductionist system of the excised patch, thus a quantitative difference between the 2 settings is not surprising, and it could be due to many reasons. Badheka et al.Citation4 offered a simple potential explanation, which we briefly discuss here. Even though diC8 PI(4)P essentially did not activate the channel,Citation4,5 the long acyl chain AASt PI(4)P was partially active.Citation4 Both inducible phosphatases described before remove the 5′-phosphate from PI(4,5)P2, generating PI(4)P (); if PI(4)P activates the channel, we expect partial or no inhibition. This hypothesis was tested by Badheka et al.Citation4 with the combined 4′ and 5′ phosphatase pseudojanin, which depletes both PI(4,5)P2 and PI(4)P. They showed that the fully active pseudojanin inhibited TRPM3 more than pseudojanin with only the 5′-phosphatase activity, concluding that despite its quite low efficiency in excised patches, endogenous PI(4)P may contribute to channel activity in intact cells.
Both papers found that activation of muscarinic M1 receptors coupled to Gq and phospholipase Cβ (PLCβ) inhibited TRPM3 activity in HEK cells expressing M1 receptors. Tóth et al. also showed inhibition of endogenous PregS-induced currents by a muscarinic agonist in insulinoma cells.Citation5 These results are consistent with the PI(4,5)P2 dependence of TRPM3, but they do not exclude possible contribution of other signaling pathways downstream of PLC activation.
Conclusions
In conclusion, both papers convincingly demonstrated that TRPM3 requires PI(4,5)P2 for activity, and that depletion of this lipid upon PLC activation may regulate its activity. As with any seemingly complete studies, outstanding questions remain, for example, it remains to be seen if other activation modes of this channel, such as heat or the second permeation pathway also require PI(4,5)P2. The role of downstream effectors other than PI(4,5)P2 depletion upon PLC activation has also not been thoroughly addressed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Oberwinkler J, Philipp SE. Trpm3. Handb Exp Pharmacol 2014; 222:427-59; PMID:24756716; http://dx.doi.org/10.1007/978-3-642-54215-2_17
- Vriens J, et al. Nat Chem Biol 2014; 10:188-95; PMID:24390427; http://dx.doi.org/10.1038/nchembio.1428
- Rohacs T. Handb Exp Pharmacol 2014; 233:1143-76; PMID:24961984; http://dx.doi.org/10.1007/978-3-319-05161-1_18
- Badheka D, et al. Journal of General Physiology 2015; 146:65-77; PMID:26123195; http://dx.doi.org/10.1085/jgp.201411336
- Tóth BI, et al. J Gen Physiol 2015; 146:51-63; PMID:26123194; http://dx.doi.org/10.1085/jgp.201411339
- Hilgemann DW. J Gen Physiol 2012; 140:245-8; PMID:22930801; http://dx.doi.org/10.1085/jgp.201210874
- Holendova B, et al. Channels 2012; 6:479-82; PMID:22989896; http://dx.doi.org/10.4161/chan.22177
- Bojjireddy N, et al. J Biol Chem 2014; 289:6120-32; PMID:24415756; http://dx.doi.org/10.1074/jbc.M113.531426
