 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The N-terminal (NT) domain of the connexins forms an essential transjunctional voltage (Vj) sensor and pore-forming domain that when truncated, tagged, or mutated often leads to formation of a nonfunctional channel. The NT domain is relatively conserved among the connexins though the α- and δ-group connexins possess a G2 residue not found in the β- and γ-group connexins. Deletion of the connexin40 G2 residue (Cx40G2Δ) affected the Vj gating, increased the single channel conductance (γj), and decreased the relative K+/Cl− permeability (PK/PCl) ratio of the Cx40 gap junction channel. The conserved α/β-group connexin D2/3 and W3/4 loci are postulated to anchor the NT domain within the pore via hydrophilic and hydrophobic interactions with adjacent connexin T5 and M34 residues. Cx40D3N and D3R mutations produced limited function with progressive reductions in Vj gating and noisy low γj gap junction channels that reduced the γj of wild-type Cx40 channels from 150 pS to < 50 pS when coexpressed. Surprisingly, hydrophobic Cx40 W4F and W4Y substitution mutations were not compatible with function despite their ability to form gap junction plaques. These data are consistent with minor and major contributions of the G2 and D3 residues to the Cx40 channel pore structure, but not with the postulated hydrophobic W4 intermolecular interactions. Our results indicate an absolute requirement for an amphipathic W3/4 residue that is conserved among all α/β/δ/γ-group connexins. We alternatively hypothesize that the connexin D2/3-W3/4 locus interacts with the highly conserved FIFR M1 motif to stabilize the NT domain within the pore.
Introduction
The cytoplasmic N-terminus (NT) of the connexins is proposed to fold back toward the inner membrane and line the gap junction pore via inclusion of a “glycine hinge” near the midpoint of this NT domain.Citation1,2 Functional evidence in favor of this NT domain pore-lining configuration includes identification of NT amino acid residues that regulate the transjunctional voltage (Vj) gating polarity and sensitivity of connexin-specific gap junctions, modulation of gap junction channel conductance (γj) and rectification, and spermine block of Cx40 gap junctions.Citation3-6 This NT pore structure is proposed to be stabilized by amino acid side chain interactions with the first transmembrane (M1) and NT domains of an adjacent connexin protomer.Citation2 The common connexin membrane topology consists of cytoplasmic amino- and carboxyl- (NT and CT) termini, 2 extracellular loops (E1 and E2), 4 transmembrane domains (M1-M4), and one cytoplasmic loop (CL) linking the M2 and M3 domains. A hexamer of homomeric or heteromeric connexins forms a hemichannel within each cell membrane and the extracellular domains irreversibly dock to form an intercellular gap junction channel that permits the flow of intracellular ions, metabolites, and signaling molecules between adjacent cells. The human connexin (Cx) family of gap junction proteins is subdivided into 3 groups (a.k.a. subfamilies, groups, or classes) based on their primary sequence homologies: the α-group consisting of Cx43, Cx37, Cx40, Cx46, Cx50, Cx59, and Cx62 (GJA-1,4,5,3,8,9,10 respectively); the β-group consisting of Cx32, Cx26, Cx31, Cx30.3, Cx31.1, Cx30, and Cx25 (GJB1-7 respectively); and the less homologous δ/γ/ε-group connexins Cx36, Cx31.9, Cx40.1 (GJD-2,3,4), Cx45, Cx47, Cx30.2 (GJC1-3), and Cx23 (GJE1).Citation7
The NT domain is relatively conserved among these connexins, though there are discrete sequence differences that impart different molecular structures and functions to their respective connexins. Characteristic of the α-group connexins is the insertion of a G2 residue, while the β-group connexins uniquely possess the position 11–12 glycine hinge (). The D2/N2 locus of Cx26 and Cx32 reverses the gating polarity of these 2 connexins, but the corresponding D3 locus is strictly conserved among the α-group connexins despite differences in gating polarities.Citation8,9 This polar D/N locus and the adjacent conserved amphipathic W residue are postulated to be integral to the NT pore structure via hydrophilic and hydrophobic interactions with M1 domain residues.Citation2 The NT domains of all 3 α/β/γ-group connexins finish with a K/R-I/V-W motif thought to delineate the beginning of the M1 domain. Substitution of the β-group connexin S/G-G hinge into the midpoint of the α-group connexin NT domain at positions 12 and 13 nullified the Vj gated inactivation of Cx43 and Cx40 gap junctions without loss of electrical coupling or dye transfer.Citation10 Insertion of the β-group connexin glycine hinge did not, however, enable Cx26 to heteromerize with Cx43 or Cx40.
Figure 1. Frequency histogram sequence logos, generated using weblogo v2.8.2, for the amino-terminal (NT) domains of 7 α-group, 6 β-group, 2 delta-group, and 2 gamma-group connexins based on the current connexin classification nomenclature.Citation7,41 Connexin sequences not included in the analyses are indicated in parentheses. Disparate (e.g., G2) and conserved α/β-group (e.g D2/3 and W3/4) connexin NT sequences were subjected to site-directed mutagenesis and analyzed for function.
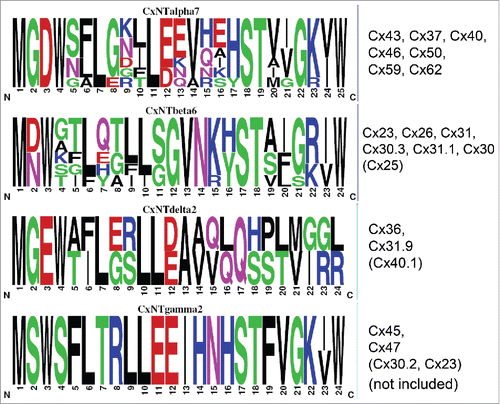
Given the G2 insertion, non-Vj-gating polarity reversing D3, and the strict conservation of W3/4 locus of the α-group connexins relative to the β-group connexins on which 3-dimensional structure of the connexin gap junction channel is based, we chose to examine the functional consequences of these 3 inter-group connexin NT domain substitutions/deletions. We hypothesized that the G2 insertion is effectively neutral other than increasing the distance between the adjacent β-group connexin M1 and D/N loci; that D3 charge neutralizing or reversing mutations, if functional, would alter the gap junction channel conductance (γj) and Vj gating of the α-group connexins; and test the ability of other aromatic amino acids to substitute for the conserved connexin W3/4 locus in the putative hydrophobic NT-M1 domain interactions that anchor the NT domain within the pore. Cx43 G2V and D3N oculodentodigital dysplasia (ODDD) mutations and a W4A substitution reportedly result in loss-of-function due to changes in NT terminal structure.Citation11-13 Hydrophobic amino acids phenylalanine (F) or tyrosine (Y) participate in cation-π, carboxylate salt bridge, and membrane-spanning hydrophobic “girdle” interactions in a manner analogous to tryptophan (W), whereas substitution with a hydrophobic but non-aromatic alanine (A) residue does not support these secondary structural interactions.Citation14-17
Not surprisingly, deletion of the G2 residue resulted in a functional Cx40 gap junction with modestly increased γj, permeability, and Vj gating properties. Surprizingly, we found that substitution of the highly conserved W3/4 residue of the α/β/δ/γ-group connexins with F or Y residues was not compatible with function despite a limited ability to form gap junction plaques. When Cx40 or Cx43 W4Y mutant proteins were homologously or heterologously expressed with their wild-type counterparts, they functioned in a dominant-negative manner to reduce macroscopic gap junction conductance (gj) by 50–100%. We also report the functional consequences of charge neutralization (N) or reversal (R) of the Cx40 D3 locus common to the α/β/δ-group connexins. Charge neutralization or reversal of the D3 residue progressively decreased the Vj gating sensitivity of Cx40 gap junctions. Together, these α-group connexin NT position 2–4 amino acids seem to be located inside the gap junction channel pore within the ion conduction pathway and likely interact with transmembrane amino acids via a combination of hydrophobic and hydrophilic side chain interactions near a polar-nonpolar interface to position the connexin NT domain within the cytoplasmic pore in a Vj-dependent manner. These conformational interactions exhibit an absolute requirement for a tryptophan residue since other hydrophobic ring amino acid substitutions were nonfunctional and suppressed normal gap junction function in a dominant-negative manner.
Results
Deletion of the α-connexin G2 locus
The functional relevance of the α/δ-group connexin-specific G2 site was tested by deletion of this locus from the Cx40 sequence. The Cx40 G2 deletion mutant protein (Cx40G2Δ) was capable of forming gap junction plaques when transfected into HeLa cells () and, when transfected into N2a cells, resulted in functional gap junction coupling. The Vj-dependent gating of the G2Δ gap junctions was determined by stepping the Vj gradient to ±120 mV in 1 mV, 200 msec increments. The normalized junctional conductance – voltage (Gj-Vj) curve exhibited minor decreases in the half-inactivation voltage (V½) and gating charge valence (z) of 9 mV and 0.6 q respectively (p > 0.05; , ). Single channel currents, recorded from 7 G2Δ cell pairs, yielded an average gap junction channel current (ij) –Vj relationship slope conductance (γj) of 174 ±1 pS (), which was not significantly different from the wild-type Cx40 γj of 150 ± 2 pS.Citation6 Given the modest 16% increase in γj with the G2 deletion, we assessed the change in relative ionic conductance and permeabilities using the Kgluconate/KCl γj ratio and asymmetric KCl gradient K+/Cl− permeability (PK/PCl) ratio methods as previously described.Citation6 The γj of the G2Δ gap junction channel was 152 pS in the 140 mM Kgluconate pipette solution (data not shown). This corresponds to a cation/anion conductance ratio of 4.0, slightly lower than the ratio of 4.5 for the wild-type (wt) Cx40 gap junction channel and indicative of a modest reduction in the cation selectively of the channel. Thus we performed 70:140 mM asymmetric KCl pipette solution experiments which produced a reversal potential (Erev) of −13.5 mV (n = 6), again slightly less than the published value of −14.4 mV for this wt Cx40 gap junction channel (). This ≈1 mV lower Erev corresponds to a PK/PCl value of 5.3, 15% lower than the wt Cx40 gap junction channel PK/PCl ratio of 6.2.Citation6 Although the slopes of the 50% asymmetric KCl ij - Vj relationships from the wt and G2Δ Cx40 gap junction channels were not statistically different, the intercepts were significantly different (p < 0.05), thus indicating that the cation selectivity of the G2D channel was significantly reduced.
Figure 2. Functional expression of the Cx40 G2 deletion (G2Δ) mutation. (A and B) Immunocytochemical analysis of the wt Cx40 (A) and Cx40G2Δ (B) protein expressed in HeLa cells revealed that the mutant protein trafficked to the membrane and formed gap junction plaques (arrow). (C) The expression of the Cx40G2Δ protein in N2a cells revealed the ability to form functional gap junctions with shifted Vj gating properties (see ). (D) One example of a multi-channel Cx40G2Δ gap junction channel recording from an N2a cell pair. (E) Single gap junction channel current-voltage (ij-Vj) relationship from 7 Cx40G2Δ cell pairs with an average slope conductance (γj) of 174 ± 1 pS, a 16% increase from the published wtCx40 γj of 150 pS.Citation6 (F) The reversal potential (Erev) was measured in response to a 50% transjunctional [KCl] gradient across the Cx40G2Δ gap junction channel. The calculated relative K+/Cl− permeability ratio (PK/PCl) was 5.3, 15% lower than the wtCx40 PK/PCl ratio obtained using previously published procedures.Citation6
![Figure 2. Functional expression of the Cx40 G2 deletion (G2Δ) mutation. (A and B) Immunocytochemical analysis of the wt Cx40 (A) and Cx40G2Δ (B) protein expressed in HeLa cells revealed that the mutant protein trafficked to the membrane and formed gap junction plaques (arrow). (C) The expression of the Cx40G2Δ protein in N2a cells revealed the ability to form functional gap junctions with shifted Vj gating properties (see Table 1). (D) One example of a multi-channel Cx40G2Δ gap junction channel recording from an N2a cell pair. (E) Single gap junction channel current-voltage (ij-Vj) relationship from 7 Cx40G2Δ cell pairs with an average slope conductance (γj) of 174 ± 1 pS, a 16% increase from the published wtCx40 γj of 150 pS.Citation6 (F) The reversal potential (Erev) was measured in response to a 50% transjunctional [KCl] gradient across the Cx40G2Δ gap junction channel. The calculated relative K+/Cl− permeability ratio (PK/PCl) was 5.3, 15% lower than the wtCx40 PK/PCl ratio obtained using previously published procedures.Citation6](/cms/asset/bfd110fa-7cd8-448c-ab56-fadf81e9117f/kchl_a_1200775_f0002_c.gif)
Table 1. Vj-dependent Gating Parameters of NT mutant Cx40 Gap Junctions.
Charge substitution of the conserved α-connexin D3 locus
The position 2 aspartic acid (D) or asparagine residues (N) of Cx26 and Cx32 are known to switch the polarity of Vj gating for the β-connexin gap junction channels and modulate the rectification properties of their respective hemichannel currents.Citation3,4,Citation8 Although Cx40 and Cx43 gap junctions close with opposite Vj polarities, the D3 locus is conserved among all of the α-connexins and, therefore, cannot account for the Vj gating polarity and cation selectivity differences between Cx40 and Cx43 gap junction channels.Citation5,6,Citation18-20 Recently, a Cx43 D3N mutation identified in ODDD patients was found to form nonfunctional gap junction plaques and dominant-negatively inhibit Cx43 gj.Citation11,13,Citation21 We prepared Cx40 D3N and D3R mutations and transfected them into HeLa and N2a cells for immunohistochemical and electrophysiological analyses. The Cx40 D3N mutant protein readily formed gap junction plaques () and both D3 mutants formed functional gap junctions with dramatically reduced Vj gating properties (p < 0.0005, and ). Unitary gap junction channel recordings were difficult to obtain or resolve owing to increased noise and decreased amplitude of the Cx40 D3N ij (). Co-transfection of the D3N mutant into WT Cx40 N2a cells reduced gj to near zero and γj to < 50 pS (≈1/3rd of the normal Cx40 γj) in a dominant-negative manner (). Relative ionic conductance and permeability measurements were not attempted because of the poor signal-to-noise ratio.
Figure 3. Functional expression of the Cx40 D3N/R mutations. (A) The Cx40D3N mutant protein readily formed gap junction plaques when express in HeLa cells. (B) Gj-Vj curves for the Cx40 D3N and D3R reveal progressively shifted Vj gating properties including decreased gating charge valence (z), increased Gmin, and increased half-inactivation voltage (V½; see ). (C) Example of a homotypic Cx40D3N gap junction channel recording from an N2a cell pair. The slope γj from 6 cell pairs averaged 49 ± 1 pS (data not shown). (D) A heteromeric gap junction channel recording from a wtCx40 N2a cell pair co-expressing the mutant D3N protein. (E) The ij-Vj relationship from 6 wtCx40 + Cx40D3N cell pairs had an average γj of 45 ± 1 pS, indicative of a dominant-negative effect of the D3N mutation on Cx40 γj. The PK/PCl ratio of the homomeric or heteromeric Cx40D3N gap junction channel could not be measured because of the noisy channel phenotype. (F) The unilateral inhibition of Cx40 gj by 2 mM spermine was examined in 7 wtCx40, 5 Cx40D3N, and 7 Cx40D3R cell pairs using previously published procedures.Citation6 Both mutations reduced the maximum inhibition by 31%, from 80 to 55% of Cx40 gj.
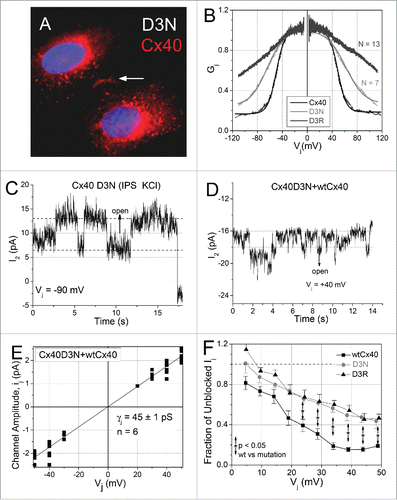
Cx40 gap junctions are blocked by intracellular spermine and 2 NT domain glutamate residues are essential for this Vj-dependent ionic blockade.Citation5,6 To assess the contribution of the D3 residue to the spermine block of Cx40 gap junctions, 2 mM spermine was added to the cell 1 patch pipette solution and the cationic Vj block protocol was applied as previously described. Mutation of the Cx40 D3 locus to N or R reduced the maximum spermine inhibition from 80% to 55% (p < 0.05 for Vj ≥ 25 mV; n = 5, 7 respectively), a loss of approximately 1/3rd of the spermine inhibition (). The 2 mM spermine inhibition curves are nearly identical despite the electrostatic charge differences of the D3N and D3R substitution mutations, suggesting that fixed anionic charges other than the D3 locus are directly involved in the binding of spermine within the Cx40 gap junction channel pore.
Conservation of the W3/4 locus
The W3/4 locus is conserved across all 4 α/β/δ/γ-connexin groups and is believed to stabilize the NT domain within the connexin channel pore via hydrophobic interactions with M1 residues M34 or L35.Citation2 Other than the report that the Cx43 W4A ODDD mutation formed nonfunctional gap junction plaques,Citation13 there is no information on the functional compatibility of hydrophobic amino acid substitutions at this conserved position. Since F and Y residues possess an aromatic ring side chain, these mutations should be functionally compatible based on protein chemical cation-π, carboxylate salt bridge, transmembrane hydrophobic girdle, or hydrogen bonding predictions.Citation2,14-17 The Cx40 W4F and W4Y mutations were prepared and transfected into HeLa cells for immunocytochemical localization and found that both mutant Cx40 proteins readily assembled into gap junctions (). However, dual whole cell patch clamp experiments failed to detect any electrical coupling, thus demonstrating that the resultant gap junctions were non-functional. Co-expression of the Cx40 W4Y protein with wtCx40 reduced gj by 99% (p < 0.00001). Cx40 channel currents were difficult to resolve from these poorly coupled wtCx40 + W4Y cell pairs and the ij- Vj relationship from 6 cell pairs yielded a slope γj of 34 pS (). These data suggest that the heteromeric Cx40 W4Y subunits exert a dominant-negative effect on the wtCx40 gap junction channel.
Figure 4. Expression of the Cx40 W4F/Y mutations (A-B) The Cx40W4F (A) and Cx40W4Y (B) mutant proteins formed gap junction plaques (arrows) between HeLa cells. (C-D) Homotypic mutant Cx40W4F and W4Y gap junction were, however, nonfunctional and reduced the wtCx40 channel currents (C) and γj (D) in a dominant-negative manner to minimal detectable levels. (E) Coexpression of the Cx40W4Y mutation with wtCx40 or wtCx43 in N2a cells reduced the average gj by 99% and 64% respectively, suggestive of a slight preference for Cx40W4Y subunits to heteromerize with homologous wtCx40 over heterologous Cx43 subunits. (F) The Cx43W4Y mutation was created and reciprocal wtCx40 and wtCx43 coexpression experiments were performed. The mutant Cx43W4Y subunit decreased wtCx43 gj by 90% despite the higher overall gj of wtCx43 cell pairs when these experiments were performed, suggesting an increased efficiency of functional knockdown of wtCx43 gj by the homologous mutant Cx43W4Y subunit.
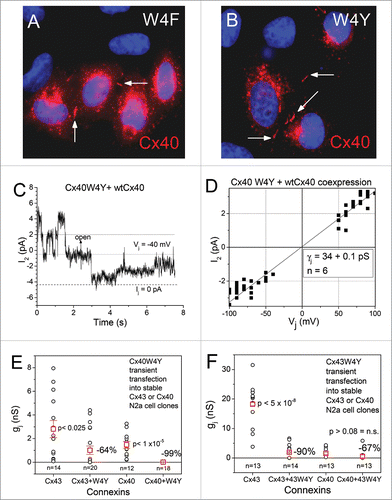
Since there is controversy about the ability of Cx40 and Cx43 subunits to functionally heteromerize,Citation22-25 we co-expressed the Cx40 W4Y mutant protein in our stable Cx43 N2a cells and measured the resultant gj. Cx43 gj was reduced by 64% from control wtCx43 measurements, about 2/3rd of the inhibitory effect achieved with homologous wtCx40 + mutant Cx40 W4Y co-expression (p < 0.025; ). Since our Cx43 N2a cells possessed approximately twice the gj of the stable Cx40 N2a cells on average (2.8 ± 0.7 and 1.5 ± 0.3 nS, mean ± SEM, n = 14, 12 respectively), the Cx43 W4Y mutation was prepared to reciprocally test the homologous and heterologous effects of this mutation on the wtCx40 and wtCx43 gap junctions. The Cx43 W4Y mutant protein reduced wtCx43 gj by 90% (p < 5x10−8) compared to 64% (p > 0.05) for the Cx40 W4Y mutation (Fig. F), thus suggesting that homologous Cx40 and Cx43 W4Y mutant interactions are favored (≥90% gj inhibition) over wt/mutant heterologous Cx40/Cx43 interactions (≈67% gj inhibition), i.e. the heteromerization of co-expressed Cx40 and Cx43 subunits is not entirely random.
Discussion
The first half of the NT domain of Cx26 is believed to fold toward the membrane and form a Vj-sensing pore domain that transduces the fast Vj-dependent gating of the gap junction channel from the fully open to a residual subconductance state.Citation1,8,Citation26 The connexin NT domain assumes this structure by virtue of the β-group connexin glycine hinge and intermolecular W3-M34 and D2-T5 hydrophobic and hydrophilic amino acid side chain interactions that positions and anchors the NT domain within the connexin channel pore.Citation2 The N-terminal M1 residue possesses a positive charge that contributes to the net NT pore domain gating charge and polarity of the fast Vj gating mechanism.Citation8,27 Charge modifying mutations to the NT domains of α-group connexins are consistent with the internal pore location and function of the connexin NT domain,Citation5,28,Citation29 although insertion of the glycine hinge motif into the NT domains of Cx40 and Cx43 nullified all Vj gating.Citation10 Mutations beyond the midpoint of the NT domain also influence the Vj-gating and spermine block without significantly altering the γj and PK/PCl ratio of the Cx40 gap junction channels.Citation6 Some hypothesize that the α-group connexin NT domain retains a kink or hinge near the position 12/13 midpoint despite the lack of a proline kink or glycine hinge motif and the negative functional consequences of substituting these α-helical disruptive motifs into the Cx40 or Cx43 NT domain.Citation10 Bioinformatic secondary structure predictions and NMR studies suggest that the α-group connexin NT domain possesses a predominantly α-helical pore domain structure between positions W4 and S18.Citation30,31 How the NT domain folds into the connexin channel pore in the absence of the β-group connexin glycine hinge may be fundamentally different for the α-group connexins.
Molecular dynamic simulations of the Cx26 crystal structure have not supported the W3-M34 and D2-T5 amino acid side chain interactions proposed to anchor the NT domain within the pore.Citation32,33 Both MD simulation studies also placed the major pore restriction site and cation/anion energy barriers near the M1-E1 interface, away from the connexin N-terminal M1 residue. Thus, MD simulations of the Cx26 channel structure did not agree with some of the salient features of the pore structure advanced by the Maeda et al.Citation2 model. Deletion of the G2 residue should diminish the magnitude of the NT domain energy barrier by increasing the pore diameter and charge neutralization of the positive/negative M1/D3 interactions. Our experimental results indicate a ≈15% increase in γj and reduced cation selectivity associated with the Cx40 G2Δ mutation (), consistent with the interpretation of increased pore volume and less electrostatic charge in the vicinity of the N-terminus. Neutralization or charge reversal of the Cx40 D3 locus progressively decreased the magnitude and sensitivity of Vj-gating, lessened the inhibition by spermine, and produced a noisy (flickering) gap junction channel with dramatically reduced γj (). These results are consistent with destabilization of the open channel structure, reduced electrostatic (Vj-gating) charge and ionic conductance, but only partial loss of the inhibitory spermine binding site. Together, these results suggest that the α-group connexin G2 locus makes a minor contribution to the pore structure, primarily by increasing the flexibility and length of the N-terminus and increasing the M1 and D3 charge separation, while the D3 locus is essential to the Vj sensor, ionic conductance, and open configuration of the resultant connexin channel pore.
Cx43 G2V and D3N mutations were identified in ODDD patients and subsequent functional analysis revealed that both of these mutant Cx43 proteins form nonfunctional gap junction plaques, presumably due to disruption of the analogous Cx43 D3-S5 and W4-L35 interactions.Citation11-13 Their results suggest that substitution of the G2 residue with a hydrophobic V residue introduces localize hydrophobic bonds that disrupt the NT pore structure while our results show that deletion of the G2 locus does not. In contrast to the results with the Cx43 D3N ODDD mutation, we found that the Cx40 D3N and D3R mutations could support function, although the mutant Cx40 D3N protein dramatically reduced γj and gj when co-expressed with wtCx40. A congenital cataract D3Y mutation in Cx46 was also recently demonstrated to be nonfunctional, presumably owing to localized narrowing of the pore and reduced electrostatic negative charge.Citation34 D3N and D3E mutations incorporated into Cx46 or Cx50 form functional gap junctions with dramatically reduced γj values.Citation35,36
Shao et al.Citation13 also prepared a Cx32 W4A mutation that also formed nonfunctional gap junction plaques, this time by increasing the α-helicity of the α-group connexin NT pore domain and disrupting the essential W4 hydrophobic interaction. Substitution of F or Y residues at the W4 locus should still support this vital W4 hydrophobic interaction, as well as be permissive for cation-π, carboxylate salt bridge, and membrane bordering hydrophobic girdle interactions.Citation14-17 Surprisingly, none of the W4 hydrophobic amino acid substitutions resulted in functional Cx40 or Cx43 gap junction plaques, which leads to question the validity of the hydrophobic W3/4 connexin NT domain interaction advanced by Maeda et al.Citation2 Tryptophan is distinct among the natural amino acids in that its side chain indole ring is amphipathic, containing a hydrophobic benzene ring and a hydrophilic nitrogen-containing pyrrole ring. Our results suggest that the amphipathic nature of the W4 residue must be conserved to preserve connexin channel function. Recently, a conserved transmembrane W residue was proven to be essential to the gating, kinetics, and selectivity of a voltage-gated proton channel, presumably by stabilizing (i + 4) cation-π interactions with a conserved R residue.Citation15,37
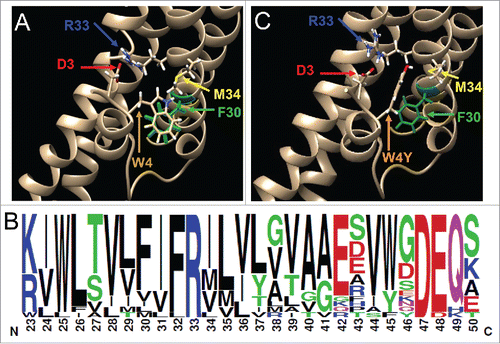
If W3/4 doesn't interact with M34/L35 as hypothesized by Maeda et al.,Citation2 what NT intra- and intermolecular domain interactions possibly exist to stabilize the NT structure within the pore? We homology mapped the Cx40 sequence onto the Cx26 crystal structure (Protein Data Bank accession code 2ZW3)Citation2 and then modeled the effect of the W4Y mutation on possible D3 and W4 interactions. The wild-type structure suggests possible ionic bond interactions between D3 and R33, alignment of the hydrophobic benzene rings of W4 and F30, and possibly a polar interaction of the pyrrole ring of W4 with M34, all within the same connexin subunit (). This mid-M1 domain FIFR motif is highly conserved among all connexins (), suggesting possible conserved intramolecular NT-M1 D/N-R and W-F ionic and hydrophobic interactions that stabilize the connexin NT domain within the channel pore. The absence of a pyrrole ring with the W4F and W4Y mutations shifts the alignment of the benzene rings between position 4 and F30, weakening this hydrophobic interaction without dramatically affecting the D3-R33 interaction (). The presence of a hydroxyl group with the W4Y mutation further lessens the interaction with the M1 (FIF) hydrophobic sequence. In conclusion, we propose an alternative NT-M1 DW-FIFR hypothesis that involves intramolecular interdomain polar and nonpolar interactions to stabilize the connexin N-terminus within the pore. Further studies, e.g., molecular dynamic simulations, are required to test the feasibility of these proposed interactions and additional questions remain to be solved, for example, how do α-group connexin NT domains bend toward membrane since they lack a glycine-hinge in the middle of NT domain? Ultimately, a high resolution crystal structure of Cx43 or Cx40 is necessary to determine the actual amino acid side-chain alignments that anchor the α-group connexin NT domain within the connexin pore.
Figure 5. Structural model of the Cx40 NT-M1 domain interactions. (A) A molecular model of the wild-type Cx40 NT domain D3 and W4 interactions with conserved M1 domain R33 and F30 residues. The structure was derived by homology mapping the Cx40 sequence onto the Cx26 crystal structure from Maeda et al.Citation2 (B) A frequency histogram sequence logo of the M1 domain of 19 connexin proteins illustrating the highly conserved mid-M1 FIFR motif. (C) A molecular model of the Cx40 NT and M1 domain interactions after incorporation of the W4Y mutation. The hydrophobic ring interaction between W4Y and F30 is disrupted by the absence of the W4 pyrrole ring and insertion of a hydroxyl group. Residue D3 is pictured in red, R33 in blue, F30 in green, and W4 or Y4 in light brown or orange, and M34 in yellow.
Materials and methods
Site-directed mutagenesis
PCR-based site-directed mutagenesis was performed on rat Cx40 and Cx43 cDNA sequences as previously described, the defined Cx40 or Cx43 mutations were confirmed by the SUNY Upstate Medical University DNA Sequencing Core Facility, and the mutant Cx40 or Cx43 cDNAs were directionally cloned into the pTracer-CMV2 vector (ThermoFisherScientific, https://www.thermofisher.com/order/catalog/product/V88520) using 2 restriction endonucleases for transient expression in N2a cells.Citation6 Plasmids were prepared using the EndoFree plasmid maxi kit (Qiagen, https://www.qiagen.com/us/shop/sample-technologies/dna/dna-preparation/endofree-plasmid-kits#orderinginformation/12362) according to manufacturer's directions and stored at −20°C. Transient transfections were performed according to manufacturer's directions using 1 μg of plasmid DNA with Lipofectamine2000 and OptiMEM (ThermoFisher, https://www.thermofisher.com/order/catalog/product/11668030 and https://www.thermofisher.com/order/catalog/product/31985062) on 70–90% confluent 12-well parental N2a or HeLa cell cultures for homomeric expression studies and stable Cx40-N2a or Cx43-N2a clones for functional co-expression studies.
Immunocytochemistry
HeLa cells were grown in 10% fetal bovine serum (FBS) minimum essential media (MEM) to 80% confluency on 18 mm diameter poly-L-lysine coated glass coverslips and transiently transfected with 2 μg of the mutated Cx40-pTracer-CMV2 DNA as described above and incubated overnight in a humidified 37°C, 5% CO2 incubator. The next day the coverslips were rinsed in phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde in PBS, rinsed, permeabilized with 1% Triton X100 in PBS, and blocked with 2% goat serum in 1% Triton X100 PBS, all for 15 min at room temperature.Citation38 A 1 μl aliquot of rabbit polyclonal anti-Cx40 antibody (ThermoFisher, https://www.thermofisher.com/order/genome-database/antibody/Connexin-40-Antibody-Polyclonal/36-4900) was diluted in 200 μl of 2% goat serum, 1% Triton X100 PBS and added to the 12-well coverslip containing the transfected Cx40-HeLa cells and stored overnight in the refrigerator at 4°C. The next day the coverslips were thoroughly rinsed with PBS, incubated in the dark for 3 hours at room temperature with a 1:1500 dilution of goat anti-mouse Alexa Fluor555 secondary antibody in 10% goat serum PBS, rinsed with PBS, stained with DAPI in PBS for 10 min, rinsed, and mounted on a clean glass slide using ProLong Gold anti-fade reagent (ThermoFisher, https://www.thermofisher.com/order/genome-database/antibody/Goat-anti-Mouse-IgG-H-L-Secondary-Antibody-Polyclonal/A-21422, https://www.thermofisher.com/order/catalog/product/D1306, and https://www.thermofisher.com/order/catalog/product/P10144). After drying, the coverslips were sealed with nail polish and viewed on the stage of an Olympus IX-70 microscope equipped with a Sutter Lambda 10–2 filter wheel controller and LS 175 W Xenon arc lamp epifluorescence illumination system using a 555/25 nm band pass excitation filter and FITC/Cy3/Cy5 dichroic mirror/emitter filter set (Chroma Technology Corp, cat. #62005). Fluorescent micrographs were acquired with an Andor iXon 885 ECCD camera using Imaging Workbench 6.0 software (INDEC Systems, Santa Clara, CA). DNA staining was observed with a 365/10 nm excitation filter and DAPI 460/50 nm dichroic mirror/emitter filter set (Chroma, https://www.chroma.com/products/sets/31000v2-dapi-hoechst-amca). Exported TIF files were background subtracted (≈10%) and red/blue color processed using ImageJ software. Magnification was 600X using an Olympus PlanApo 1.40/0.17 aperture 60X oil immersion and 10X C-mount objectives.
Gap junction electrophysiology
One well of a 12-well plate of transiently transfected N2a cells were lightly trypsinized after 4 hours of transfection and transferred to 2 35 mm culture dishes for overnight 5% CO2, 37°C incubation in 10% FBS/MEM culture media. The next day each dish was rinsed and bathed in serum-free saline (in mM: NaCl 142, KCl 1.3, CsCl 4, TEACl 2, MgSO4 0.8, NaH2PO4 0.9, CaCl2 1.8, dextrose 5.5, Hepes 10, pH 7.4) for whole cell patch clamp procedures.Citation6 4–6 MΩ patch pipettes were filled with normal KCl internal patch pipette solution (in mM: KCl 140, CsCl 4, TEACl 2, MgCl2 1, CaCl2 3, BAPTA 5, Hepes 25, pH 7.4) and gap junction conductance (gj) measurements were performed using series-resistance corrected dual whole cell patch clamp procedures as previously described.Citation39 A 50% [KCl] internal pipette solution was prepared using raffinose (Sigma Chemical) to maintain osmotic balance (310 mosm) for transjunctional reversal potential (Erev) measurements.Citation6 Spermine inhibition was examined by adding 2 mM spermine to the cell 1 patch pipette solution and applying -/+/- Vj steps of 30 sec duration each in 5 mV increments to ±50 mV as previously described.Citation5,40 Whole cell currents were low pass filtered at 500 Hz with a Warner Instruments (Hamden, CT) LPF-2 4 pole Bessel filter and digitally sampled at 4 kHz using a Digidata 1320A A/D converter and pClamp8.2 software (Molecular Devices, Sunnyvale, CA). Normalized gj (Gj = gj/gj,max) – transjunctional voltage (Vj) curves were fitted with the Boltzmann z-delta function: where z is negative for negative Vj and positive for positive Vj values using the Levenberg-Marquart search and sum of squared errors minimization methods in Clampfit8.2. Gap junction channel currents were recorded with a gain of 50 mV/pA, low pass filtered at 100 Hz, and digitized at 1 kHz. Current amplitude histograms were generated using Origin7.5 or 8.6 and fitted with multiple Gaussian peaks to determine the mean current amplitudes of all channels that could be resolved from each recording.
Molecular modeling
The atomic coordinates for the Cx26 crystal structure was downloaded from the Protein Data Bank using the accession code 2ZW3 from Maeda et al.Citation2 The wild type and W4Y mutant rat Cx40 sequences were homology mapped onto the Cx26 crystal structure using the Molecular Operation Environment (MOE, Chemical Computing Group, version 2014.09). The wild type and W4Y mutant rat Cx40 sequences were homology mapped onto the Cx26 protomer crystal structure separately.
Statistics
Gap junction channel conductance (γj) values are presented as the average linear slope ± SE of the fitted channel I-V relationship. Statistical comparisons of the fitted datasets (linear I-V relationships, Bolzmann Gj-Vj curves) was performed using the F-test comparison of Two Data sets function in Origin7.5. Average values (gj, fraction of unblocked Ij) represent the mean ± SEM and statistical analyses were performed by one-way ANOVA using the Bonferroni method in Origin 8.6.
Abbreviations
| CL | = | cytoplasmic loop |
| CT | = | carboxyl terminus |
| Cx# | = | connexin# (e.g.Cx40 = connexin40) |
| Erev | = | reversal potential |
| E1 and E2 | = | first and second extracellular loops |
| FBS | = | fetal bovine serum |
| Gj | = | normalized gj (Gj = gj/gj,max) |
| gj | = | macroscopic gap junction conductance |
| γj | = | single gap junction channel conductance |
| ij | = | single gap junctional channel current |
| MEM | = | minimum essential media |
| M1 | = | first transmembrane domain |
| M1-M4 | = | transmembrane domains 1–4 |
| NT | = | N-terminus |
| ODDD | = | oculodentodigital dysplasia |
| PBS | = | phosphate-buffered saline |
| PK/PCl | = | relative K+/Cl− permeability ratio |
| Vj | = | transjunctional voltage |
| V½ | = | half-inactivation voltage |
| wt | = | wild-type |
| z, q, | = | valence, elementary charge |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Li Gao for her technical assistance with N2a cell cultures and Edward (Tedd) Fenn for preparing the Cx40 D3N and D3R mutations. We wish to thank Dr. Debashis Ghosh and Chinaza Egbuta for access to the Molecular Operation Environment (MOE) software and their help performing the molecular modeling of the wt and mutant Cx40 structures.
Funding
This work was supported by NIH grant HL-042220 to RDV.
References
- Purnick PEM, Benjamin DC, Verselis VK, Bargiello TA. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys 2000; 381:181-90; PMID:11032405; http://dx.doi.org/10.1006/abbi.2000.1989
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 2009; 458:597-602; PMID:19340074; http://dx.doi.org/10.1038/nature07869
- Oh S, Rubin JB, Bennett MVL, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol 1999; 114:339-64; PMID:10469726; http://dx.doi.org/10.1085/jgp.114.3.339
- Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol 2000; 116:13-31; PMID:10871637; http://dx.doi.org/10.1085/jgp.116.1.13
- Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol 2004; 557:863-78; PMID:15107469; http://dx.doi.org/10.1113/jphysiol.2003.059386
- Lin X, Fenn E, Veenstra RD. An amino terminal lysine residue of connexin40 that is required for spermine block. J Physiol 2006; 570:251-69; PMID: 16284078; http://dx.doi.org/10.1113/jphysiol.2005.097188
- Beyer EC, Berthoud VM. The family of connexin genes. In: Harris AL, Locke D, editors. Connexins: A Guide. Totowa, NJ: Humana Press; 2009. pp.3-26.
- Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature 1994; 368:348-51; PMID:8127371; http://dx.doi.org/10.1038/368348a0
- Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res 2000; 86(2):E42-9; PMID:10666425; http://dx.doi.org/10.1161/01.RES.86.2.e42
- Gemel J, Lin X, Veenstra RD, Beyer EC. N-terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J Cell Sci 2006; 119:2258-68; PMID:16723732; http://dx.doi.org/10.1242/jcs.02953
- Paznekas WA, Karczaski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Maldergem LV, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Human Mutation 2009; 30:724-33; PMID:19338053; http://dx.doi.org/10.1002/humu.20958
- de la Parra DR, Zenteno JC. A new GJA1 (connexin 43) mutation causing oculodentodigital dysplasia associated to uncommon features. Ophthalmic Genet 2007; 28:198-202; PMID:18161618; http://dx.doi.org/10.1080/13816810701538620
- Shao Q, Liu Q, Lorentz R, Gong X-Q, Bai D, Gary S. Shaw GS, Laird DW. Structure and functional studies of N-terminal Cx43 mutants linked to oculodentodigital dysplasia. Mol Biol Cell 2012; 23:3312-21; PMID:22809623; http://dx.doi.org/10.1091/mbc.E12-02-0128
- Landolt-Marticorena C, Williams KA, Dber CM, Reithmeier RAF. Non-random distribution of amino acids in the transmembrane segments of human type 1 single span membrane proteins. J Mol Biol 1993; 229:602-8; PMID:8433362; http://dx.doi.org/10.1006/jmbi.1993.1066
- Gallivan JP, Dougherty DA. Cation-p interactions in structural biology. Proc Natl Acad Sci USA 1999; 96:9459-64; PMID:10449714; http://dx.doi.org/10.1073/pnas.96.17.9459
- Mall S, Broadbridge R, Sharma RP, Lee AG, East JM. Effects of aromatic residues at the ends of transmembrane a-helicies on helix interactions with lipid bilayers. Biochemistry 2000; 39:2071-8; PMID:10684657; http://dx.doi.org/10.1021/bi992205u
- Thompson SE, Smithrud DB. Carboxylates stacked over aromatic rings promote salt bridge formation in water. J Am Chem Soc 2001; 124:442-9; PMID:11792215; http://dx.doi.org/10.1021/ja011973h
- Harris AL. Voltage-sensing and substate rectification: moving parts of connexin channels. J Gen Physiol 2002; 119:165-9; PMID:11815666; http://dx.doi.org/10.1085/jgp.119.2.165
- Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J 2004; 87:912-28; PMID:15298899; http://dx.doi.org/10.1529/biophysj.103.038448
- Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomembr 1996; 28:327-37; PMID:8844330; http://dx.doi.org/10.1007/BF02110109
- Churko JM, Shao Q, Gong XQ, Swoboda KJ, Bai D, Sampson J, Laird DW. Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects. Hum Mut 2011; 32:456-66; PMID:21305658; http://dx.doi.org/10.1002/humu.21472
- Beyer EC, Lin X, Veenstra RD. Interfering amino-terminal peptides and functional implications for heteromeric gap junction formation. Front Pharmacol 2013; 4:67; PMID:23734129; http://dx.doi.org/10.3389/fphar.2013.00067
- Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol. 2001; 281:C1559-67; PMID:11600419
- Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol Cell Physiol. 2002; 282:C1469-82, 2002; PMID:11997262; http://dx.doi.org/10.1152/ajpcell.00484.2001
- Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 2001; 281:H1675-89; PMID:11557558
- Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin32. Biophys J 2000; 79:2403-15; PMID:11053119; http://dx.doi.org/10.1016/S0006-3495(00)76485-X
- Bargiello T, Brink P. Voltage-gating mechanisms of connexin channels. In: Harris AL, Locke D, editors. Connexins: A Guide. Totowa, NJ: Humana Press; 2009. pp.103-28.
- Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol 2006; 576:787-99; PMID:16931554; http://dx.doi.org/10.1113/jphysiol.2006.113837
- Tong JJ, Liu X, Dong L, Ebihara L. Exchange of gating properties between rat Cx46 and chicken Cx45.6. Biophys J 2004; 87:2397-406; PMID:15454438; http://dx.doi.org/10.1529/biophysj.104.039594
- Kyle JW, Berthoud VM, Kurutz J, Minogue PJ, Greenspan M, Hanck DA, Beyer EC. The N terminus of connexin37 contains an alpha-helix that is required for channel function. J Biol Chem 2009; 284:20418-27; PMID:19478091; http://dx.doi.org/10.1074/jbc.M109.016907
- Veenstra RD, Lin X. More on the pore of gap junction channels. Recent Res Devel Biophys 2006; 5:187-221.
- Kwon T, Harris AL, Rossi A, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: Evaluation of structural models with Brownian dynamics. J Gen Physiol 2011; 138:475-93; PMID:22006989; http://dx.doi.org/10.1085/jgp.201110679
- Zonta F, Polles G, Zanotti G, Mammano F. Permation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J Biomolec Struct & Dynamics 2012; 29:985-98; PMID:22292956; http://dx.doi.org/10.1080/073911012010525027
- Tong JJ, Sohn BCH, Lam A, Walters DE, Vertel BM, Ebihara L. Properties of two cataract-associated mutations located in the NH2 terminus of Cx46. Am J Physiol 2013; 304:C823-32; PMI:23302783; http://dx.doi.org/10.1152/ajpcell.00344.2012
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verseslis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 2005; 88:1725-39, 2005; PMID:15596513; http://dx.doi.org/10.1529/biophysj.104.054023
- Xin L, Nakagawa S, Tsukihara T, Bai D. Aspartic acid residue D3 critically determines Cx50 gap junction channel transjunctional voltage-dependent gating and unitary conductance. Biophys J 2012; 102:1022-31; PMID:22404924; http://dx.doi.org/10.1016/j.bpj.2012.02.008
- Cherny VV, Morgan D, Musset B, Chaves G, Smith SME, DeCoursey TE. Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J Gen Physiol 2015; 146:343-56; PMID:26458876; http://dx.doi.org/10.1085/jgp.201511456
- Lin X, Gemel J, Glass A, Zemlin CW, Beyer EC, Veenstra RD. Connexin40 and connexin43 determine gating properties of atrial gap junction channels. J Mol Cell Cardiol 2010; 48:238-45; PMID:19486903; http://dx.doi.org/10.1016/j.yjmcc.2009.05.014
- Veenstra RD. Voltage clamp limitations of dual whole-cell gap junction current and voltage recordings. I. Conductance measurements. Biophys J 2001; 80:2231-47; PMID:11325726; http://dx.doi.org/10.1016/S0006-3495(01)76196-6
- Musa H, Veenstra RD. Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys J 2003; 84:205-19; PMID:12524276; http://dx.doi.org/10.1016/S0006-3495(03)74843-7
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Research 2004; 14:1188-90; PMID:15173120; http://dx.doi.org/10.1101/gr.849004
