?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Undenatured (native) type II collagen is a dietary supplement ingredient reported to support joint health in healthy individuals by providing relief from symptoms of stiffness and discomfort and improving mobility. This benefit is thought to occur through oral tolerance, a mechanism whereby the immune system distinguishes between innocuous material in the gut and potentially harmful foreign invaders. The presence of antigenic epitopes in undenatured type II collagen, but not in denatured (hydrolyzed) collagen, is thought to be the basis for the therapeutic benefits. The purpose of this study was to investigate the physicochemical and analytical characteristics of type II collagen supplements currently available on the market and to explore whether they might be sufficiently similar in their physical properties to yield similar benefits in promoting joint health. Collagen type II supplement powders (raw material) and capsules (products in the market) were examined for color, particle size, quality profiles, fatty acid profiles, electron microscopy, and were analyzed for amino acid content as well as antigenic potential via an ELISA assay. Powders labeled as undenatured type II collagen were found to have markedly different properties, including the size of collagen fibers as per electron microscopy and antigenic configuration as per the ELISA assay. As significant differences were found between products, it allows consumers and practitioners to not assume that products labeled as undenatured (native) type II collagen are interchangeable.
Introduction
Undenatured (native) type II collagen has become popular as a dietary supplement ingredient consumed by those who wish to improve their joint health by providing relief from symptoms of stiffness and discomfort while concomitantly and/or subsequently improving mobility. According to Innova Market Insights, 35% of dietary supplement products launched globally in 2019 featured collagen in multiple categories, including joint health, bone health, and nutritional cosmetics (Innova-Market-Insights Citation2020). According to United States marketing data for the natural products industry, cross-channel sales growth of collagen in 2019 was 82.7%, reaching $147 million for the year (Krawiec Citation2020).
A recent meta-analysis of human studies conducted on the effect of orally administered collagen supplements on symptoms of osteoarthritis concluded that there was a significant reduction in symptom scores, as measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Visual Analog Scale (VAS) (García-Coronado Citation2019). The meta-analysis included five randomized controlled studies; four of which were conducted using hydrolyzed collagen in doses of 2 g/day (one study) and 10 g/day (three studies). One study was conducted with undenatured collagen type II taken in a dose of 40 mg per day (Lugo et al. Citation2015). An obvious quantitative difference between the different collagen supplements is the size of the dosage, which is in grams for hydrolyzed collagen compared to milligrams for undenatured collagen.
Hydrolyzed collagen or denatured collagen is collagen that has been broken into its peptide components by means of enzymes, heat, or pH (Woo et al. Citation2017). In contrast, undenatured collagen type II retains its native triple helix conformation. The molecular weight for undenatured collagen is approximately 300 kDa whereas that for collagen hydrolysates ranges from 2 to 9 kDa (Prabhoo and Billa Citation2018). Not only are the structures different, the two forms are thought to have different modes of action. Hydrolyzed collagen, which is more easily absorbed due to its lower molecular weight, is thought to aid in cartilage regeneration by providing material for the synthesis of collagen in situ (García-Coronado et al. Citation2019). In contrast, preclinical studies suggest that undenatured collagen influences the humoral and cellular immune response via interactions with gut-associated lymphoid tissues (GALT) in a process known as oral tolerance (Bagchi et al. Citation2002). Given the differences in physical characteristics, dosage, and mode of action, it seems inappropriate that studies on hydrolyzed collagen and undenatured collagen would be combined in the same meta-analysis (García-Coronado et al. Citation2019).
Type II collagen is the principal constituent of articular cartilage, a connective tissue that covers the ends of long bones and protects against the friction and wear associated with the motion of the joint (Gottardi et al. Citation2016). The collagen fiber network is infused with a hydrated proteoglycan matrix and together they provide protection and cushioning in the joint. Cartilage degradation is considered to be the central feature in osteoarthritis; with a loss of cartilage homeostasis resulting in degradation of the collagen- and proteoglycan-rich extracellular matrices, and erosion of the articular surface (He et al. Citation2020). Studies in mice indicated that undenatured type II collagen protects against experimentally induced arthritis in animal model through a mechanism of oral tolerance (Nagler-Anderson et al. Citation1986). The studies showed that intragastric administration of soluble type II collagen suppressed the development of arthritis induced by administration of type II collagen plus adjuvant. The experiment used collagen prepared from bovine articular cartilage using “limited proteolysis with pepsin.” The above experiment was repeated using denatured type II collagen, prepared by incubation at 56 °C for 45 min. In contrast to the previous results, administration of the denatured collagen had no observable effect on the incidence or severity of the arthritis. The authors explained that, although the same amino acids were present in both forms, the tertiary and quaternary structures present in native collagen were likely destroyed in the denatured form (Nagler-Anderson et al. Citation1986). Thus, the structural configurations of the undenatured form of collagen appeared to be required for benefit via oral tolerance.
Oral tolerance is an immune process the body uses to distinguish between innocuous material in the gut (i.e. commensal organisms that make up the microbiome and food proteins) from potentially harmful foreign invaders. The screening of bacteria and food in the gut lumen is conducted by gut-associated lymphatic tissue (GALT), which is composed of mesenteric lymph nodes and patches of lymphoid tissue along the small intestine called Peyer’s patches. Peyer’s patches are composed of aggregated lymphoid follicles surrounded by an epithelium layer containing specialized cells named M (for microfold). M-cells transport substances to underlying immune cells (T-cells), which activate or inhibit the immune response leading to either tolerance or attack (Weiner et al. Citation2011).
Studies indicate that undenatured type II collagen is taken up by the Peyer’s patches, where naive T-cells are transformed into T-regulatory (Treg) cells that are specifically activated by type II collagen (Park et al. Citation2009). Treg cells circulate through the body via the blood. Upon recognition of their target (collagen type II) in joint tissue, the Treg cells secrete mediators that act to ameliorate the immune response in the joint. This response stimulates the release of anti-inflammatory cytokines, namely transforming growth factor-beta (TGF-beta), interleukin 4 (IL-4), and interleukin 10 (IL-10) (Tong et al. Citation2010). The release of these mediators results in a reduction in joint inflammation and associated discomfort.
Antigenic epitopes in undenatured glycosylated type II collagen, which are recognized by the T-cells, have been quantified using an enzyme-linked immunosorbent assay (ELISA) (Bagchi et al. Citation2002). A proprietary undenatured glycosylated type II collagen product (UC-II) was developed that uses an ELISA assay as a means to assure product integrity. The benefits of UC-II undenatured type II collagen for the support of joint health have been established in human clinical studies, that have demonstrated improvements in symptoms of stiffness and discomfort in the knee caused by strenuous exercise or osteoarthritis (Bagchi et al. Citation2002; Lugo et al. Citation2013, Citation2015). A randomized, double-blind, placebo-controlled study was conducted with 55 healthy subjects who had no prior history of arthritic disease or joint discomfort at rest, but experienced joint discomfort with physical activity. The study demonstrated that administration of UC-II in a dose of 40 mg daily for 120 days resulted in improvements in knee extension and the ability to exercise longer before experiencing any initial joint discomfort (Lugo et al. Citation2013). Another randomized, double-blind, placebo-controlled study evaluated the potential benefits of administration of UC-II compared to placebo and glucosamine hydrochloride plus chondroitin sulfate (GC) (Lugo et al. Citation2015). 191 volunteers with osteoarthritis of the knee were randomized into three groups receiving a daily dose of UC-II (40 mg), GC (1500 mg G & 1200 mg C), or placebo for 180 days. At the end of the study, the UC-II group demonstrated a significant reduction in overall WOMAC score compared to placebo and GC. Supplementation with UC-II resulted in significant changes for the WOMAC subscales of discomfort, stiffness, and physical function compared to placebo.
On the basis of positive clinical trials, health practitioners recommending nutraceutical products, and consumers who purchase them, have expectations of a beneficial effect. In order to achieve a similar beneficial effect to that reported in clinical studies, products need to be sufficiently similar. The evaluation of similarity begins with the physical and chemical profile of the ingredient, along with its dosage, means of administration, and product formulation. Comparison of similarity also involves review of the product’s bioavailability. Differences in the excipients and/or final formulation of the capsules or tablets may lead to faster or slower dissolution (release of chemical constituents) and/or absorption into the body.
It is natural for health practitioners and consumers to assume that ingredients with the same name are, in fact, the same. However, this is not necessarily the case. Type II collagen supplements for joint health are produced using different manufacturing processes and vary in containing hydrolyzed or non-hydrolyzed collagen. Product differences can be detected through particle size, microscopic examination of the collagen fibrils, quantities of total collagen, and ultimately the presence or absence of denatured or undenatured type II collagen as measured via an ELISA assay.
The purpose of this study was to investigate the physicochemical and analytical characteristics of undenatured (native) type II collagen supplements in the market and to explore whether they might be sufficiently similar in their physical properties to yield similar benefits to the UC-II brand in promoting joint health.
Materials and methods
Type II collagen samples were obtained commercially in powdered form and in finished dosage (viz. capsule, tablet). The raw materials for b-2Cool (Bioberica, S.A.U.; Barcelona, Spain), were Lots no. 19-0010, no. 18/0008, and no. 20/0005, designated CII-X, X1, and X2, respectively. The raw materials for UC-II (Lonza Consumer Health Inc, USA) were Lots no. 19FS211801, no. 19FS093321, and no. 19FS122324, designated CII-Y, Y1, and Y2, respectively. These products are reported to be derived from chicken sternum.
The specifications and measured values for a typical lot of CII-X as shown on the Certificate of Analysis (CoA) provided by the manufacturer are: Total collagen ≥25% and native collagen type II content ≥4% . The specifications and measurements for a typical lot of CII-Y collagen as shown on its CoA are: Total collagen ≥25% and native collagen type II content ≥3% ().
Table 1. Type II Collagen assay methods and product specifications.
The collagen type II capsules are designated CII A–F, as follows:
Physical characteristics
Visual assessment of color and clumping were observed after spreading a small aliquot of product on a dark background. The odor of the products was assessed immediately after opening the sealed storage bags.
Hunter color
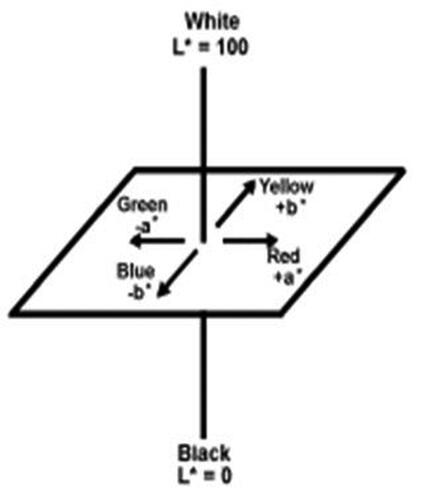
The color of raw materials CII-X and CII-Y were determined using CIE L*a*b* color scales measured in a Hunter ColorQuest XE instrument. The CIELAB color space was defined by the International Commission on Illumination (CIE) in 1976 as an expression of three values: L* for the lightness from black (0) to white (100), a* from green (−) to red (+), and b* from blue (−) to yellow (+) (). A numerical change in these values corresponds to roughly the same amount of visually perceived change. Measurements were taken from glass cuvettes filled with product. Two duplicate measurements were taken for each sample.
Density
The bulk volume of the product was determined by adding 50 ± 0.5 g of product to a funnel (free flow, without vibration or tapping) into a 100-mL volumetric cylinder. The tapped volume of the product was determined by placing a cylinder on the plate of a Stav 2003 Stamp volumeter, J Engelsmann AG which was actuated for 100 taps. The bulk and tapped densities were determined by dividing the volumes by sample weights.
Particle size distribution
The particle size distribution of the products was measured at the Food Science Department of Cornell University (Ithaca, NY), using a Brookhaven 90Plus Nanoparticle Size Analyzer, Brookhaven Instruments, NY. Samples, 60 ± 0.5 mg, were suspended in 25 mL of deionized water. Thoroughly mixed suspensions were transferred in 4 mL polystyrene cuvettes and immediately measured (particle sizing from <1 nm to 6 μm).
Quality analysis
The moisture content of the raw materials was measured by the National Food Lab, Ithaca, NY, using a Computrac Max 100 moisture analyzer (loss on drying); the results reported are averages of two duplicate measurements. Select chemical testing of raw materials was conducted by Eurofins Food Integrity & Innovation (Madison, WI, USA); the percentage nitrogen, and ash content were determined according to the AOAC Official Methods of Analysis 968.06-992.15 and 923.03, respectively (AOAC, Citation2002; Thiex et al. Citation2002, Citation2012). Heavy metals were analyzed by Eurofins Food Integrity & Innovation (Madison, WI, USA) using inductively coupled plasma–mass spectrometry (ICP-MS), as per AOAC Official Methods of Analysis 2011.19 and 993.14 (Pacquette et al. Citation2011). Microbiological testing of products was conducted by Eurofins Food Integrity & Innovation (Madison, WI, USA) according to the USP guidelines: Aerobic Plate count (Eurofins USPC 2021 Citation2020), E. coli (Eurofins USPE 2022 Citation2020), Enterobacteriaceae (Bile-Tolerant Gram-Negative Bacteria) (Eurofins USPN 2021 Citation2020), Salmonella (Eurofins USPS 2022 Citation2020), Staphylococcus aureus (Eurofins USPA 2022 Citation2020), and Yeast and Mold Count (Eurofins USPM 2021 Citation2020). The pH of a 5% dispersion of raw materials was determined by dispersing 1 g product in 20 mL water purified using reverse osmosis (RO). The pH was measured using an Accumet Research Ar25 pH meter (Fisher Scientific, NY, USA). Reported results were an average of two duplicate readings.
The fatty acid profiles were determined by Eurofins Food Integrity & Innovation (Madison, WI, USA) according to the AOAC Official Methods of Analysis 996.06 (Folch et al. Citation1957; Geldenhuys et al. Citation2013).
Transmission electron microscopy of type II collagen
Transmission electron microscopy (TEM) was used to compare collagen type II raw materials, as described by Dahl et al. (Citation2007). Collagen powders were analyzed by thin-sectioning following fixation. Briefly, fixation began with 2.5% vol/vol fresh glutaraldehyde in 0.2 M sodium cacodylate, pH 7.4, followed by 0.2 M sodium cacodylate and 1% (wt/vol) osmium tetraoxide, the solution was replaced sequentially by ethanol, acetone, and then resin. Images were obtained at various magnifications for multiscale imaging of the material. Lower-magnification images are useful for assessing relative abundance of fibrils while higher-magnification was necessary for visualizing the morphology of individual filaments.
Total collagen assays and undenatured type II collagen
Collagen in raw materials and capsules were individually analyzed for total collagen content (amino acid analysis, AAA) and undenatured type II collagen content by methods established and validated at NEXT Bio Research Services, LLC (Chester, VA, USA).
Extraction of collagen from the test samples
Raw materials (CII-X, CII-X1, CII-X2 and CII-Y, CII-Y1 and CII-Y3) were extracted in duplicate samples of 3–5 g as follows. For capsules, the contents of five of each type (CII A–F) were pooled together (total nominal collagen content 200 mg) for each extraction and each capsule type was extracted in duplicate.
Collagen materials were washed with 3 M guanidine-HCl (to remove glycosaminoglycans) and then suspended in 50 mM acetic acid and the pH was adjusted to 2.8 with 70%(vol/vol) formic acid. Solid pepsin (Sigma Aldrich, catalog P7012) was added to allow for digestion of the sample. The pepsin digestion reactions were stopped by the addition of 10× Tris-buffered saline containing CaCl2, and then the pH of was adjusted to 8 with 5 N NaOH. A portion of each pepsin digest was added to 1× Tris-buffered saline containing CaCl2. Elastase (Chondrex Inc., Catalog 30047) was added to allow for another digestion. The samples were then centrifuged, and supernatants removed to a clean tube ELISA assay (for quantitative determination of the undenatured collagen content).
ELISA for undenatured type II collagen
ELISA assays were completed using a Type II Collagen Detection Kit (Chondrex Inc., Catalog 6018) as modified by NEXT Bio Research Services, LLC (Chester, VA, USA) (Lugo, Citation2019). Purified Type II avian collagen (Chondrex Inc., Catalog 9034) served as the reference standard. The percent (weight) undenatured type-II collagen content in the samples was calculated on the basis of mg undenatured collagen per g solid.
Amino acid analysis for total collagen
Total protein in each sample was determined by AAA. Briefly, a weighed aliquot of each test sample was hydrolyzed in 6 N HCl (20 h, 110 °C) in sealed evacuated glass tubes and then taken to dryness. The dried samples were dissolved in loading buffer, appropriately diluted, and a 1 μL aliquot was subjected to analysis using a Waters AccQ•TagTM amino acid analysis system (reverse-phase chromatography with fluorescence detection). Prior to chromatography, pre-column derivatization of the amino groups is performed using AccQTag (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate) chemistry under aqueous conditions. Following the pre-column derivatization of the analytes, separation and detection is achieved using a reversed-phase column and a multi wavelength fluorescence detector. Samples are automatically analyzed with assured performance methods and reports are generated using predefined software templates. Total collagen was determined based on the hydroxyproline (Hyp) content in the test samples, based on the published percent of mass for Hyp in Type II collagen (12.6%).
All experiments and analysis were conducted in duplicate, and data are expressed as the mean values in tables. Data was entered into Excel for a four-parameter fit equation.
Results
Physical characteristics
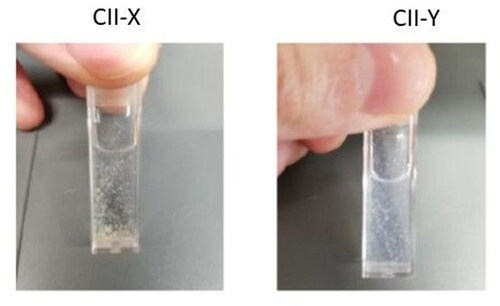
Powders for CII-Y and CII-X were examined for their physical characteristics. Visual inspection determined that powder CII-Y is off-white and contains some coarse particles, whereas powder CII-X is light brown and displays more coarse particles (; ). The Hunter color measurements determined that CII-Y has a high “L” reading compared to CII-X indicating it is slightly lighter, while CII-X is higher in “a” and “b” measurements indicating that it is more brownish.
Figure 2. Visual Appearance of CII-X and CII-Y powders. CII-Y is off-white and contains some coarse particles, while powder CII-X is light brown and displays coarser particles.

Table 2. Physical Characteristics of Collagen Powders.
Odor intensity was characteristic of collagen: mild for CII-X and faint for CII-Y. Tapped density measurements found that CII-X scored slightly higher than CII-Y, at 0.74 g/cc compared to 0.70. This is consistent with the visual appearance assessment that sample CII-X was slightly denser than CII-Y. Neither CII-X nor CII-Y was soluble in water. When placed into solution, the particles dispersed and overtime fell to the bottom of the container (). The particles in sample CII-X were large and lumpy in appearance. Those in CII-Y were finer, smaller, and homogeneous.
Chemical properties
The amount of moisture in CII-Y was measured at 6.55% and was higher in CII-X at 7.97% (). The pH results revealed differences between the two samples, CII-Y at 6.1 (slightly acidic) and CII-X at 7.5 (slightly alkaline). Results for measurements of heavy metal and microbial content for CII-X and CII-Y are listed in . The CII-X sample was reported to be higher in levels of chromium, copper, lead, and zinc compared to CII-Y. The total fatty acid content for sample CII-Y was 1.8 g/100 g whereas that for CII-X was 0.073/100 g (). Thus, CII-Y powder contained 25 times the quantity of fatty acids compared to CII-X powder.
Table 3. Fatty acid profile.
Microbial assays
Microbial assays indicate that CII-X has a higher content of aerobic organisms compared to CII-Y, however this level is still acceptable according to guidance in the European pharmacopeia ().
Table 4. Quality profiles for collagen powders.
Transmission electron microscopy
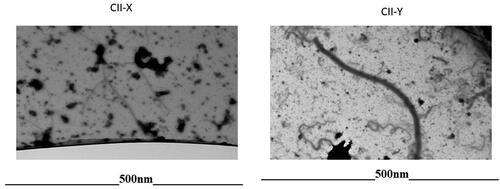
Transmission electron microscopy was used to detect the fibrils typical of type II collagen fiber in raw materials, CII-X and -Y. As shown in , CII-Y samples shows fibrils typical of type II collagen. In contrast, raw materials for CII-X failed to present with the typical fibril structures characteristic of intact type II collagen fibers. Instead, those samples displayed partially hydrolyzed collagen fibrils fibers suggesting that the collagen protein in CII-X is hydrolyzed.
Total collagen and undenatured type II collagen
Total collagen was determined based upon the hydroxyproline content in the test samples and the data are reported in . Overall, CII-X lots contain type II collagen protein at levels comparable to CII-Y lots. In contrast, the ELISA assay which measured the presence of undenatured type II collagen showed a sizable difference between the CII-Y and CII-X samples. As shown in , lots of CII-Y collagen contained an average of 9.33% undenatured collagen by weight (∼93 mg undenatured type II collagen per g solid), whereas lots of CII-X contained an average of 0.13% undenatured collagen. The amount of undenatured collagen in CII-Y is 71 times greater than that for CII-X.
Table 5. Type II collagen raw materials: total collagen and undenatured type II collagen.
Further experiments were conducted to see if there were any contents in the CII-X material that might interfere with the ELISA assay. A 1:1 mixture of samples CII-X and Y was analyzed using the same procedure. The idea being that, if there were no interference in the assay itself, the measured binding capacity would be approximately half that observed with the undiluted CII-Y material. This was, in effect, what was observed (). Thus, it was concluded that this lot of CII-X contained negligible amounts of undenatured collagen.
Table 6. ELISA analysis of a mixture of raw materials.
In similar fashion to the analysis of the raw materials, weighed aliquots of each of the collagen capsules (CII-A to F) were subjected to undenatured collagen type II. The results obtained are summarized in . More importantly, the UC-II capsules (CII-F) contain the highest level of undenatured collagen (on a per capsule basis).
Table 7. Capsules: total collagen and undenatured type II collagen.
Discussion
This study investigated the physicochemical and analytical characteristics of undenatured (native) type II collagen supplements in the market. The purpose of this inquiry was to determine whether or not the samples would contain similar amounts of undenatured type II collagen and whether or not any differences might correlate with differences in the beneficial properties of promoting joint health. Initial visual observations noted differences in the color of sample CII-X and CII-Y. The two samples were slightly different in coarse fractions, but the medium particles and fines were very close. Neither was homogeneous, and aqueous suspensions were similar as well.
More importantly, there were differences in the size of molecules in the different product observed via electron micrographs. As observed in , clear differences were observed in the electron micrographs of samples CII-X and CII-Y. The micrograph of sample CII-X displays mostly fragments, whereas that for sample CII-Y indicates the presence of the triple helix of native collagen. Undenatured type II collagen is a triple helix structure whereas the hydrolyzed collagen is composed of peptide components. This difference has been defined in measurements of molecular weights; the molecular weight for native/undenatured collagen is approximately 300 kDa whereas that for collagen hydrolysates ranges from 2 to 9 kDa (Prabhoo and Billa Citation2018).
The presence of undenatured collagen in the samples, measured using an ELISA assay, revealed a large difference between the two samples. CII-X lots (Lot no. 18/0008; no. 19/0010, and no. 20/0005) contained very little antigenic material (0.12 wt% or 1.2 mg/g solid), whereas CII-Y lots (no. 19FS211801 and no. 19FS122324) contained nearly at 9.9 wt.%. The ELISA measurement of undenatured type II collagen in capsules produced similar results. Sample CII-F was found to contain 2.35 mg of type II collagen whereas the other finished products contain less than 1 mg.
The possibility that there might be substances in the other products that interfered with the ELISA assay was considered. To explore this possibility, further experiments were conducted to see if there were any contents in the CII-X material that might interfere with the ELISA assay. An equal parts mixture of samples CII-X and Y was analyzed using the same procedure as before. If there was no interference in the assay itself, the measured undenatured type II collagen would be approximately half that observed with the undiluted CII-Y material. This was, in effect, what was observed (). Thus, it was concluded that the lot of CII-X contained very little antigenic material.
The effect of dosage on the effectiveness of the collagen type II in joint health has been explored in animal studies. A study in dogs found that 10 mg/day was more effective than 1 mg/day undenatured collagen type II at mitigating pain during limb manipulation (Peal et al. Citation2007). Likewise, a study with horses reported that doses of 80 to 160 mg/day were therapeutic whereas doses of 20 and 40 mg/day displayed little benefit in regard to reducing pain at rest and during limb manipulation (Bagchi et al. Citation2007). The lowest possible dosage in humans has yet to be determined, but 10 to a 100-fold decrease in the amount of undenatured type II collagen may be expected to have different pharmacological effects.
Assuming that the ELISA assay represents active antigenic site that leads to the mechanism of oral tolerance, then the clinical activities of lots CII-X and Y would not be expected to have similar activity. CII-X has been evaluated in a randomized, controlled study as a conjunctive therapy for acetaminophen in patients with osteoarthritis of the knee (Bakilan et al. Citation2016). Thirty-nine patients diagnosed with knee osteoarthritis were randomly distributed into two groups: one treated with 1500 mg/day of acetaminophen and the other treated with 1500 mg/day of acetaminophen plus 10 mg/day of CII-X for 3 months. Comparisons between the groups revealed a significant difference in joint discomfort (VAS) walking score in favor of the combination therapy as compared to acetaminophen only. There were no differences in biological markers of cartilage degradation. A limitation of this study is that there was no comparison of CII-X to placebo, which would have evaluated the effects of taking CII-X on its own.
As summarized in the Introduction section , CII-Y has been evaluated in several clinical trials demonstrating improvements in symptoms of stiffness and discomfort in the knee caused by strenuous exercise and osteoarthritis (Bagchi et al. Citation2002; Lugo et al. Citation2013, Citation2015) Two of those studies, which were randomized and double-blinded, demonstrated benefits compared to placebo for knee discomfort following strenuous exercise or osteoarthritis, with a dose of 40 mg taken for 120 or 180 days (Lugo et al. Citation2013, Citation2015). CII-Y has also demonstrated benefits to the joint health of companion animals, namely cats, dogs, and horses (Gencoglu et al. Citation2020). Furthermore, a US patent (No. 9,066,926) establishes the discovery of the benefit of CII-Y in treating exercise-induced joint discomfort in arthritis-free mammals.
Without a direct comparison of pharmacological properties of CII-X and CII-Y, it is unknown how these differences would affect the clinical benefit from these two powders. CII-Y was reported to be clinically beneficial with a dose of 40 mg per day delivering 1.2 mg of undenatured collagen (Lugo et al. Citation2015). CII-X was reported to be tested clinically in a dose of 10 mg per day (Bakilan et al. Citation2016). The ELISA measurements of undenatured collagen in this study suggest that the amount of undenatured collagen in CII-Y is more than 70 times that in CII-X.
In addition to the distinctions in their collagen profiles, the CII-Y powder had 25 times the quantity of fatty acids compared to CII-X. This difference suggests differing manufacturing procedures, with CII-X being exposed to a step that removed the lipid contents.
Our primary finding is that the physical characteristics of the supplements are different enough that it allows consumers and practitioners not to assume that products labeled as undenatured (native) type II collagen are interchangeable.
The physicochemical and analytical characteristics of undenatured (native) type II collagen supplements in the market were examined to determine whether they might be expected to yield similar benefits to UC-II brand in promoting joint health. The difference in undenatured content in the material influences efficacy and health benefits of the product. It is observed that CII-X reported values in certification of analysis are less than the analyzed values for undenatured collagen. Significant differences were found in the presence of antigenic epitopes which are thought to be the active moiety for type II collagen supplements in establishing oral tolerance; the mechanism whereby undenatured type II collagen is thought to reduce joint inflammation and associated pain.
Author contributions
RH conducted the study and interpreted the results. MS, JW, CO were involved in writing the manuscript, interpreted the results, and FF interpreted and reviewed the manuscript.
Acknowledgement
We would like to acknowledge NEXT Bio-Research Services, LLC and Eurofin for doing the analysis.
Disclosure statement
All authors declare no conflict of interest in this article.
Additional information
Notes on contributors
Robert B. Harris
Robert B. Harris, PhD is the founder of NEXT Bio-Research Services LLC in Richmond, VA.
Fernando L. A Fonseca
Fernando L. A. Fonseca, PhD is a research department member at Faculdade de Medicina do ABC in Santo Andre, Brazil.
Matthew H. Sharp
Matthew H. Sharp, MS, is the Vice President of Research at the Applied Science and Performance Institute in Tampa, FL.
Charlie R. Ottinger
Charlie R. Ottinger, MS, is the Director of Human Performance at the Applied Science and Performance Institute in Tampa, FL.
References
- AOAC. 2002. Official methods of analysis (OMA). 17th ed. Arlington, VA: AOAC Inc.
- Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, Fafard RD, Preuss HG. 2002. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 22(3–4):101–110.
- Bagchi M, Stocker A, Burke R, Wedgeford K, Gupta RC, Canerdy TD, Goad JT, Barnett D, Bagchi D. 2007. Efficacy and safety of undenatured type II collagen (UC-II) in arthritic horses. Toxicol Lett. 172:S223. doi:10.1016/j.toxlet.2007.05.563.
- Bakilan F, Armagan O, Ozgen M, Tascioglu F, Bolluk O, Alatas O. 2016. Effects of native type II collagen treatment on knee osteoarthritis: a randomized controlled trial. Eurasian J Med. 48(2):95–101. doi:10.5152/eurasianjmed.2015.15030.
- Dahl SLM, Vaughn ME, Niklason LE. 2007. An ultrastructural analysis of collagen in tissue engineered arteries. Ann Biomed Eng. 35(10):1749–1755. doi:10.1007/s10439-007-9340-8. Epub 2007 Jun 14
- Eurofins. 2020. Test Method USPN 2021 for Enterobacteriaceae (Bile-Tolerant Gram-Negative Bacteria). Based on United States Pharmacopeia current revision, Chapter 2021. Eurofins.com.
- Eurofins. 2020. Test Method USPC 2021 for Aerobic Plate Count. Based on United States Pharmacopeia current revision, Chapter 2021. Eurofins.com.
- Eurofins. 2020. Test Method USPM 2021 for Yeast and Mold Count. Based on United States Pharmacopeia current revision, Chapter 2021. Eurofins.com.
- Eurofins. 2020. Test Method USPA 2022 for Staphylococcus. Based on United States Pharmacopeia current revision, Chapter 2022. Eurofins.com.
- Eurofins. 2020. Test Method USPS 2022 for Salmonella. Based on United States Pharmacopeia current revision, Chapter 2022. Eurofins.com.
- Eurofins. 2020. Test Method USPE 2022 for E. coli. Based on United States Pharmacopeia current revision, Chapter 2022. Eurofins.com.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226(1):497–509.
- García-Coronado JM, Martínez-Olvera L, Elizondo-Omaña RE, Acosta-Olivo CA, Vilchez-Cavazos F, Simental-Mendía LE, Simental-Mendía M. 2019. Effect of collagen supplementation on osteoarthritis symptoms: a meta-analysis of randomized placebo-controlled trials. Int Orthop (Sicot). 43(3):531–538. Epub 2018 Oct 27. doi:10.1007/s00264-018-4211-5.
- Geldenhuys G, Hoffman LC, Muller N. 2013. Aspects of the nutritional value of cooked Egyptian goose (Alopochen aegyptiacus) meat compared with other well-known fowl species. Poult Sci. 92(11):3050–3059. doi:10.3382/ps.2013-03342.
- Gencoglu H, Orhan C, Sahin E, Sahin K. 2020. Undenatured type II collagen (UC-II) in joint health and disease: a review on the current knowledge of companion animals. Animals (Basel). 10(4):697. doi:10.3390/ani10040697.
- Gottardi R, Hansen U, Raiteri R, Hansen U, Raiteri R, Loparic M, Düggelin M, Mathys D, Friederich NF, Bruckner P, Stolz M. 2016. Supramolecular organization of collagen fibrils in healthy and osteoarthritic human knee and hip joint cartilage. PLoS One. 11(10):e0163552. doi:10.1371/journal.pone.0163552. eCollection 2016
- He Y, Li Z, Alexander PG, Ocasio-Nieves BD, Yocum L, Lin H, Tuan RS. 2020. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology (Basel). 9(8):194. doi:10.3390/biology9080194.
- Innova-Market-Insights. 2020. Collagen moves into the mainstream. Nutrition Insight [accessed 2020 Oct]. https://www.nutritioninsight.com/nutrition-focus/Collagen-moves-into-the-mainstream.html.
- Krawiec S. 2020. Joint Health: collagen & Turmeric are two of the forward drivers. Nutr Outlook. 23(2):1.
- Lugo JP. 2019. Letter to the editor UC-II® undenatured type II collagen: update to analytical methods. J Int Soc Sports Nutr. 16(1):29. doi:10.1186/s12970-019-0298-3.
- Lugo JP, Saiyed ZM, Lane NE. 2015. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr J. 15(1):14. doi:10.1186/s12937-016-0130-8.
- Lugo JP, Saiyed ZM, Lau FC, Molina JP, Pakdaman MN, Shamie AN, Udani JK. 2013. Undenatured type II collagen (UC-II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J Int Soc Sports Nutr. 10(1):48. doi:10.1186/1550-2783-10-48.
- Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. 1986. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci USA. 83(19):7443–7446. doi:10.1073/pnas.83.19.7443.
- Pacquette LH, Szabo A, Thompson JJ. 2011. Simultaneous determination of chromium, selenium, and molybdenum in nutritional products by inductively coupled plasma/mass spectrometry: single-laboratory validation. J AOAC Int. 94(4):1240–1252. doi:10.1093/jaoac/94.4.1240.
- Park KS, Park MJ, Cho ML, Kwok SK, Ju JH, Ko HJ, Park SH, Kim HY. 2009. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol. 19(6):581–589. doi:10.1007/s10165-009-0210-0.
- Peal A, D'Altilio M, Simms C, Alvey M, Gupta RC, Goad JT, Canerdy TD, Bagchi M, Bagchi D. 2007. Therapeutic efficacy and safety of undenatured type-II collagen (UC-II) alone or in combination with (–)-hydroxycitric acid and chromemate in arthritic dogs. J Vet Pharmacol Ther. 30(3):275–278. doi:10.1111/j.1365-2885.2007.00844.x.
- Prabhoo R, Billa G. 2018. Undenatured collagen type II for the treatment of osteoarthritis: a review. Int J Res Orthop. 4(5):684–689. DOI:http://dx.doi.org/10.18203/issn. doi:10.18203/issn.2455-4510.IntJResOrthop20183386.
- Thiex N, Novotny L, Crawford A. 2012. Determination of ash in animal feed: AOAC official method 942.05 revisited. J AOAC Int. 95(5):1392–1397. doi:10.5740/jaoacint.12-129.
- Thiex NJ, Erem Tv, Amundson D, Boucher J, Clark A, Clark D, Coco P, Dow D, Fahey P, Hoffmann H, et al. 2002. Determination of water (moisture) and dry matter in animal feed, grain, and forage (plant tissue) by Karl Fischer titration: collaborative study. J AOAC Int. 85(2):318–327. doi:10.1093/jaoac/85.2.318.
- Tong T, Zhao W, Wu YQ, Chang Y, Wang QT, Zhang LL, Wei W. 2010. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res. 59(5):369–377. doi:10.1007/s00011-009-0109-4. Epub 2009 Oct 28
- Weiner HL, da Cunha AP, Quintana F, Wu H. 2011. Oral tolerance. Immunol Rev. 241(1):241–259. doi:10.1111/j.1600-065X.2011.01017.x.
- Woo T, Lau L, Cheung N, Chan P, Tan K, Gardner A. 2017. Efficacy of oral collagen in joint pain – osteoarthritis and rheumatoid arthritis. J Arthritis. 6(02):1–4. doi:10.4172/2167-7921.1000233.