Abstract
Omega-3 polyunsaturated fatty acids (PUFAs) and vitamins exert multiple beneficial effects on host health, some of which may be mediated through the gut microbiome. We investigated the prebiotic potential of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and lipid-soluble phylloquinone (vitamin K1), each at 0.2x, 1x and 5x using the simulator of the human intestinal microbial ecosystem (SHIME®) to exclude in vivo systemic effects and host-microbe interactions.Microbial community composition and, diversity [shotgun metagenomic sequencing] and microbial activity [pH, gas pressure, and production of short-chain fatty acids (SCFAs)] were measured over a period of 48 h. Fermentations supernatants were used to investigate the effect on gut barrier integrity using a Caco-2/goblet cell co-culture model.We found that EPA, DHA and vitamin K1 increased alpha-diversity at 24 h when compared with control. Moreover, there was an effect on beta-diversity with changes in gut microbial composition, such as an increase in the Firmicutes/Bacteroidetes (F/B) ratio and a consistent increase in Veillonella and Dialister abundances with all treatments. DHA, EPA, and vitamin K1 also modulated metabolic activity of the gut microbiome by increasing total SCFAs which was related mainly to an increase in propionate (highest with EPA and vitamin K1 at 0.2x). Finally, we found that EPA and DHA increased gut barrier integrity with DHA at 1x and EPA at 5x (p < 0.05, respectively). In conclusion, our in vitro data further establish a role of PUFAs and vitamin K to modulate the gut microbiome with effects on the production of SCFAs and barrier integrity.
Introduction
The composition and function of the gut microbiota are affected by host genotypes and several exogenous environmental factors such as diet, antibiotics, and xenobiotics (Bajinka et al. Citation2020). Undoubtedly, diet is the most influencing factor driving the composition and function of the gut microbiota by providing nutrients to both the host and microbes. Numerous studies have shown that supplementation of pre and probiotics as well as dietary fibers can modulate the gut microbiome and produce short-chain fatty acids (SCFAs), particularly butyrate and propionate, which are known to positively correlate with various health benefits such as digestive and metabolic health or increased protection against pathogens (McNabney and Henagan Citation2017; Baxter et al. Citation2019; Kolodziejczyk et al. Citation2019; Kocsis et al. Citation2020). In addition, lipids and micronutrients are emerging factors able to influence the gut microbiota. Recent studies suggest that both lipids (Menni et al. Citation2017; Vijay et al. Citation2021) and vitamins (Fangmann et al. Citation2018; Steinert et al. Citation2020; von Martels et al. Citation2020) can modulate the gut microbiota either directly when reaching the colon (due to overdosing or targeted colon delivery) or indirectly via systemic effects (Pham et al. Citation2021).
In the case of omega three polyunsaturated fatty acids (PUFAs) previous in vivo studies in humans report that they can modulate the gut microbiota by shifting the Firmicutes/Bacteroidetes (F/B) ratio and enhancing the abundance of butyrate-promoting/producing bacterial groups such as Bifidobacterium, Lachnospira, Roseburia, Lactobacillus, and Lachnospiraceae (Balfegó et al. Citation2016; Costantini et al. Citation2017; Watson et al. Citation2018). In addition, in a recent randomized 6 weeks dietary intervention in healthy humans, omega-3 PUFA supplementation (165 mg EPA and 110 mg DHA) led to significant changes in Coprococcus spp., Bacteroides spp, and Collinsella spp., furthermore, the increase in Coprococcus was positively associated with isobutyric acid and negatively with triglyceride-rich lipoproteins (Vijay et al. Citation2021). We are not aware of any in vitro fermentation experiments that allow a more detailed evaluation of the isolated effects of PUFA on gut microbiota and metabolic activity excluding systemic mechanisms and host-microbe interactions such as in vivo absorption of SCFA. Of note only a minor proportion of SCFA (<10%) is excreted in feces in vivo which usually limits interpretation of fecal SCFA concentrations as a marker of prebiotic activity.
Vitamins are co-factors for multiple enzymes, including those facilitating fat and carbohydrate metabolism, and have direct and indirect antioxidant properties. Unlike lipids, water-soluble B-vitamin and vitamin K2 (menaquinones) are produced by members of the gut microbiota and act as a source to the host (Magnúsdóttir et al. Citation2015) upon colonic absorption (Ichihashi et al. Citation1992). Although the role of vitamin K in blood coagulation has been thoroughly explored (Halder et al. Citation2019), there is growing interest in its role in brain function, especially in cognition (Alisi et al. Citation2019). However, whether this is related to effects on the gut microbiota has not yet been explored. Our group has recently shown that colon delivered vitamins, especially vitamin C, B2 and D can modulate the gut microbiome in terms of phylogeny, diversity and microbial metabolites (Pham et al. Citation2021). and Fangmann et al. previously found that vitamin B3, when delivered directly to the colon, increased the abundance of Bacteroidetes, which was associated with improvements in insulin sensitivity in prediabetic subjects (Fangmann et al. Citation2018). However, no such data are available yet for vitamin K.
Given that the role of PUFAs and vitamins as non-carbohydrate based nutrients to modulate the gut microbiota has recently been recognized also by the International Scientific Association for Probiotic and Prebiotics (ISAPP) (Gibson et al. Citation2017), we sought to further investigate the effect of both PUFAs and the lipid-soluble vitamin K1 (phylloquinone- present in green leafy vegetables) on the composition and metabolic activity of the gut microbiota. We used an established in vitro batch fermentation model, the Human Intestinal Microbial Ecosystem (SHIME®)to exclude systemic effects of lipids and vitamins on gut microbial communities and systemic absorption of SCFA and subsequently investigated the impact of fermentation metabolites on intestinal barrier function in a Caco-2/goblet cell co-culture model.
Materials and methods
Donor prescreening experiments
In a first step, three fecal donors (male, 26 years; female, 35 years; female, 29 years) were prescreened in short-term colonic batch fermentation experiments to select one donor with a balanced SCFA production profile to be included in the final fermentation experiments. Such an approach has provided valuable pilot information on microbial fermentation dynamics previously (Van den Abbeele et al. Citation2013). Of note, we chose not to pool all three donors for the final experiments because it has been argued frequently that by pooling fecal microbiota, interindividual differences are completely removed, and an ´artificial´ community with unpredictable competition and balance among taxa is created (Isenring et al. Citation2023). All donors were healthy, of normal weight, free from any known gastrointestinal disease, on a normal diet (i.e. no major dietary changes in the past 3 months; no eating disorders; no vegetarians or vegans; no additional vitamin supplements), and without any history of antibiotic use during the previous 6 months. Fecal material was collected according to the ethical approval of the University Hospital Ghent (reference number B670201836585) from each donor to prepare slurries in anaerobic phosphate buffer. These fecal slurries were added in a 10% (v/v) concentration to sugar-depleted nutritional colon medium mimicking colon basal nutrients and supplemented with NaCl, MgSO4.7H2O, CaCl2.2H2O, hemin, and bile salts. Glucose, starch, and cellobiose were added as carbon sources. Each incubation was performed in a single repetition. Incubation was performed for 48 h at 37 °C, under shaking (90 rpm) and anaerobic conditions. Samples were collected after 48 h.
Main fermentation experiments
The final short-term batch experiment was performed in a single reactor representing a modified simulation of the continuous Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). It has been used extensively for more than 20 years for both scientific and industrial projects to evaluate the health-promoting effects of numerous pre- and probiotic formulations and has been validated with in vivo parameters (Van den Abbeele et al. Citation2018; Salgaço et al. Citation2021). At the start of the intestinal batch fermentation incubation, all test ingredients were added from stock solutions to the modified nutritional medium, containing the following (g/l): 2.5 K2HPO4, 10.9 KH2PO4, 2 NaHCO3, two yeast extract, two peptone, 1 mucin, 0.5 cystein, 2 Tween-80, two glucose, two starch, 2 cellobiose, 0.1 NaCl, 0.01 MgSO4.7H2O, 0.01 CaCl2.6H2O, 0.05 hemin, and 0.5 bile salts as described previously (Pham et al. Citation2021). The test ingredients, vitamin K1, DHA, and EPA (DSM Nutritional Products, Ltd., Kaiseraugst, Switzerland) were dissolved in ethanol to prepare a stock solution. Each ingredient was tested at three concentrations (). 1x concentration was calculated based on the previous human studies using vitamin K1 10 mg/day (Craciun et al. Citation1998), DHA 300 mg/day (Harris et al. Citation2015) and EPA 1800 mg/day (Yokoyama et al. Citation2007), respectively. One vessel was treated with solvent ethanol only to serve as a control. A freshly prepared fecal suspension from the selected donor was added to the reactors as a source of the microbial community and each vessel had a volume of 70 ml. All tests including the control were performed in single repetition, resulting in 10 independent incubations. Incubation conditions were 48 h at 37 °C, under shaking (90 rpm) in anaerobic conditions. Effluent samples were collected from each fermentation vessel at baseline (0 h fermentation), at 24 h, and at the termination of the experiment (48 h). The collected effluent samples were filtered through 0.22 µm filters for SCFA analysis and in vitro cell culture experiments. Microbiome analysis was performed on effluent samples (24 h) without filtration steps.
Table 1. Doses used in fermentation experiments.
Microbial metabolic activity
pH (Senseline F410; ProSense, Oosterhout, The Netherlands), gas pressure (Hand-held pressure indicator CPH6200; Wika, Echt, The Netherlands), and SCFAs (acetate, propionate, and butyrate) were measured at the start of incubation, after 24 and after 48 h. SCFAs were extracted from the samples using diethyl ether, after the addition of 2-methyl hexanoic acid as an internal standard. Extracts were analyzed using a GC-2014 gas-chromatograph (Shimadzu, ‘s-Hertogenbosch, the Netherlands) equipped with a capillary fatty acid-free EC-1000 Econo-Cap column (dimensions: 25 mm × 0.53 mm, film thickness 1.2 µm; Alltech, Laarne, Belgium), a flame ionization detector, and a split injector. The injection volume was 1 µL, and the temperature profile was set from 110 to 160 °C, with a temperature increase of 6 °C min−1. The carrier gas was nitrogen at a flow rate 95.6 ml/min and the temperatures of the injector and detector were 100 and 220 °C, respectively. The production of unbranched and branched SCFAs was calculated by summing the molar concentrations of acetate, propionate, butyrate, valerate, and caproate and summing the molar concentrations of isobutyrate, isovalerate, and isocaproate, respectively. The total SCFA production was defined as the sum of unbranched and branched SCFAs (De Weirdt et al. Citation2010).
Microbiome composition analysis by shotgun sequencing
Total DNA was extracted from all samples collected throughout the study using the QIAamp DNA stool minikit (Qiagen, Crawley, United Kingdom) according to the manufacturer’s instructions, apart from addition of a bead-beating step and increasing the lysis temperature to 95 °C as described previously by McCormack et al., (McCormack et al. Citation2018). After DNA isolation, DNA was quantified using the Qubit High Sensitivity DNA assay (Thermo Fisher). Whole metagenome libraries were then prepared using the Illumina Nextera XT kit (Illumina) in accordance with the manufacturer’s instructions, but with the following modifications: first, the tagmentation time was increased to 7 min, and second, following incorporation of indices and Ampure purification of the products, the samples were each individually sized by running them on an Agilent High Sensitivity Chip (Agilent) and quantified using the Qubit High Sensitivity DNA assay (Thermo Fisher) in accordance with Teagasc sequencing platform standard. The samples were then pooled in equimolar concentrations and sequenced on the Illumina NextSeq 500 with a NextSeq 500/550 v2 high-output reagent kit (300 cycles). All sequencing was performed at the Teagasc (Oak Park, Carlow, R93 XE12, Ireland) sequencing facility based on the standards of Illumina sequencing protocols. Delivered raw FASTQ sequence files were quality checked based on poor quality and duplicate read removal, and trimming was implemented using a combination of SAM and Picard tools. Taxonomy was assigned to the reads using the METAgenomic PHyLogenetic ANalysis (metaphlan2) tool. Alpha and beta diversities were computed with PAST software version 2.17 (https://folk.uio.no/ohammer/past/). Metagenome sequence data is deposited under NCBI BioProject accession number PRJNA743357 (http://www.ncbi.nlm.nih.gov/bioproject/743357)
Cell culture and transepithelial electrical resistance assay
Caco-2 (ECACC 86010202) and the mucus secreting HT29-MTX-E12 cells (ECACC 12040401) were both purchased from the European Collection of Cell Cultures (Salisbury, UK). The two cell lines were cultured separately in Falcon tissue culture flasks (Corning Life Sciences B.V., Amsterdam, Netherlands) in an atmosphere of 5% CO2 at 37 °C in a complete growth medium consisting of high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4 mM sodium pyruvate, 1% minimum essential medium non-essential amino acids, 50 µg/mL gentamicin (all from Life Technologies Europe B.V., Zug, Switzerland), and 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, Buchs, Switzerland). Sub-confluent cells were detached using 0.25% Trypsin/EDTA (Life Technologies Europe B.V., Zug, Switzerland) and passaged twice a week. Cells between passages 57 and 68 were used for this study.
To assess the effect of batch fermentation supernatants on the intestinal barrier integrity, co-cultures of Caco-2 and HT29-MTX-E12 cells, closely mimicking the intestinal epithelium, were seeded at a density of 20,000 cells/well in a 7:3 ratio (Caco-2:HT29-MTX-E12) on the apical surface of Corning HTS Transwell-24 system PET membranes, with a 0.4 µm pore size and a cell growth area of 0.33 cm2/well (Corning Life Sciences B.V., Amsterdam, Netherlands), and cultured in the complete growth medium (Pham et al. Citation2021). The medium was renewed every second to third day. The integrity of the monolayers was determined by placing the plates containing the inserts on a 37 °C warming plate and measuring the transepithelial electrical resistance (TEER) using an EVOM2 Voltohmmeter (EVOM, World Precision Instruments, Berlin) equipped with STX100 Electrodes, as described (Pham et al. Citation2018). The cells were allowed to differentiate to confluent monolayers for 13 days post seeding. Initial TEER readings were done before the apical treatment with sterile filtered fermentation supernatants diluted 1:10 in a complete growth medium. Experiments were performed in duplicate on two separate transwell plates, each with three replicates per treatment and time point. Fermentation medium (Prodigest) diluted 1:10 in the complete growth medium was tested six-fold and served as an internal control. After 24 h incubation at 37 °C in an atmosphere of 5% CO2, the resistance across each cell monolayer was measured again, and the percentage change in TEER compared to initial TEER for each insert was calculated after subtracting the resistance value of the filters alone.
Statistical analysis
For effects on gut barrier integrity, statistical analysis was performed using SPSS Statistics 22.0 (IBM SPSS Inc., Chicago, IL, USA). TEER values were determined as the percentage change after treatments (tested supernatants) and were expressed as mean ± SEM. TEER values after the treatment with fermentation supernatants without any stressor were tested for normal distribution using the Shapiro–Wilk test, and were compared pairwise using a non-parametric Mann–Whitney test and the p-value equal to less or less than 0.05 are considered statistically significant. No formal statistics were performed for the effects on the gut microbiome and microbial metabolic activity given the lack of biological replicates and the use of only one donor fecal inoculum.
Results
Omega-3 fatty acids (EPA and DHA) and vitamin K1 alter microbial community
The observed number of bacterial genera (Sobs) and the Shannon index were quantified as a measure of alpha diversity. In the control vessel, the fermentation process led to a decrease in the number of observed taxa (from 32 to 25 over 24 h) as well as Shannon diversity (from 1.81 to 0.89 over 24 h) over 24 h. Interestingly, EPA, DHA, and vitamin K1 seem to compensate for this effect resulting in almost full recovery of the observed number of genera with vitamin K1 at 0.2x (Sobs;32) and EPA at 5x (Sobs;31) at 24 h in comparison with control (Sobs; 24) (). Likewise, partial improvement in Shannon diversity was observed in all treatment vessels (). Shannon diversity with DHA at 0.2x (1.29), EPA at 1x (1.32), and vitamin K1 at 0.2x (1.28) increased when compared to control (0.89) ().
Figure 1. Microbial alpha and beta diversity. (A) Observed number of bacterial taxa, (B) Shannon diversity and (C) Nonmetric dimensional scaling (nMDS) using Bray-Curtis distances in control vessels and vessel treated with EPA, DHA or vitamin K1 at baseline and after 24 h of fermentation.
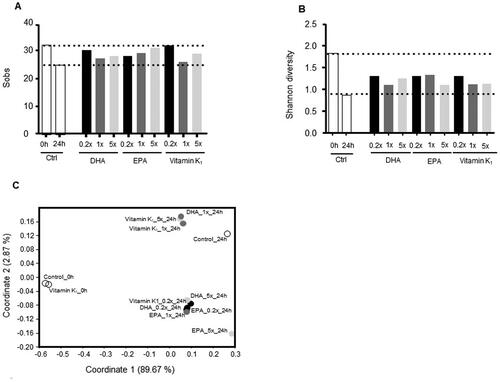
Rank-based nonmetric multidimensional scaling (nMDS) analysis based on Bray-Curtis distances was employed to assess the overall change in microbial communities in different samples. A sample from the untreated vessel (Control_0h) and one intervention vessel before treatment (vitamin K1_0h) clustered tightly reflecting a stable control at baseline. At 24 h, the untreated control vessel clustered clearly distant from baseline, indicating a marked impact of the fermentation process alone on microbial communities (). For EPA, DHA and vitamin K1, two different clusters were observed at 24 h. The first cluster consisted of EPA at 0.2x, 1x, 5x, DHA at 0.2x and 5x, and vitamin K1 at 0.2x with EPA at 5x being most distant from control. In contrast, the second cluster included vitamin K1 at 1x and 5x and DHA at 1x and was more similar to control ().
To further evaluate the effect of DHA, EPA, and vitamin K1 on microbial taxa, we calculated the top three enriched and depleted bacterial genera that were altered at all concentrations when compared to the control vessels (). Interestingly, two bacterial generaVeillonella and Dialister were enriched, while Bilophila was depleted in all three treatment groups when compared with control vessels. Furthermore, Eubacterium and Odoribacter were depleted in both DHA- and EPA-treated vessels, whereas Blautia was enriched with DHA and Eggerthella with EPA when compared to the control. In addition, in vitamin K1 vessels, Collinsella was enriched, while Dorea and Oscillibacter were depleted when compared with control.
Figure 2. Microbiome composition. Changes in abundances of the top three increased and decreased bacterial taxa as well as changes in akkermansia, bifidobacterium, faecalibacterium and lactobacillus abundances consistently observed at all three doses of (A) DHA, (B) EPA, and (C) vitamin K1 at 24 h. (D) F/B ratio in all treatment groups at 24 h.
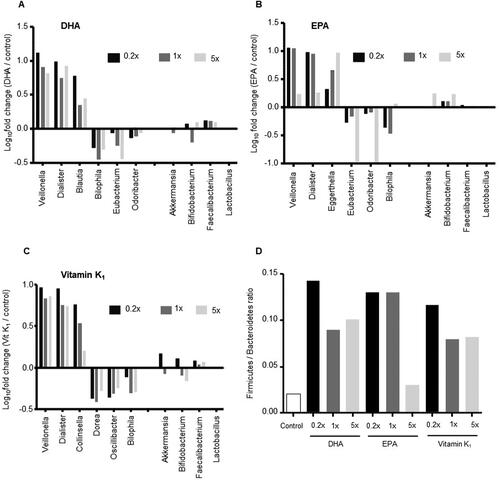
We also investigated the effects of EPA, DHA and vitamin K1 on the relative abundances of four important gut bacterial taxa—Akkermansia, Bifidobacterium, Faecalibacterium and Lactobacillus. We observed an increase in Faecalibacterium abundance (log10 changes treatment/control) upon DHA (0.2x;0.12, 1x;0.11, 5x;0.10) and vitamin K1 (0.2x;0.08, 1x;0.02, 5x;0.06) treatment at all tested doses, but not for EPA (0.2x;0.04, 1x;0.01, 5x;0.02). Moreover, there was an increase in Bifidobacterium abundance with DHA at 0.2x and 5x when compared to the control (). Finally, we compared changes in the overall F/B ratio in treatment groups vs. control vessels and found that all doses of DHA and vitamin K1 as well as EPA at 0.2x and 1x led to an increase. Of note, the donor microbiota was heavily dominated by the denominator taxa resulting in a low F/B ratio. ).
Omega-3 fatty acids (EPA and DHA) and vitamin K1 alter microbial activity
pH and gas production
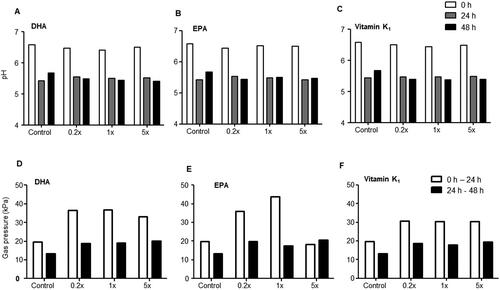
In the control vessel, a strong decrease in pH was observed at 24 h (5.4) when compared to 0 h (6.5) while pH increased slightly again at 48 h (5.6) (). A similar decrease in pH at 24 h was observed in the DHA (0.2x;5.54, 1x;5.50, 5x;5.51), EPA (0.2x;5.54, 1x;5.49, 5x;5.43), and vitamin K1-treated vessels at all tested concentrations. However, there was a slight but further decrease in pH in DHA (0.2x;5.48, 1x;5.44, 5x;5.41), EPA (0.2x;5.44, 1x;5.50, 5x;5.47), and vitamin K1 (0.2x;5.39, 1x;5.37, 5x;5.38) at 48 h (). Gas production was another measure of microbial activity. A distinct increase in gas pressure was observed at all three tested concentrations in vessels treated with DHA (0.2x;36.50 kPa, 1x;36.90 kPa, 5x;33.20 kPa), EPA (0.2x;36.10 kPa, 1x;43.90 kPa, 5x;18.40 kPa), and vitamin K1 (0.2x;30.70 kPa, 1x;30.40 kPa, 5x;30.30 kPa) at 0 to 24 h except with EPA at 5x when compared with control (19.75 kPa). This effect was less pronounced at 24 to 48 h in DHA (0.2x;18.80 kPa, 1x;19.20 kPa, 5x;20.30 kPa), EPA (0.2x;19.80 kPa, 1x;17.40 kPa, 5x;20.50 kPa) and vitamin K1 (0.2x;18.80 kPa, 1x;17.80 kPa, 5x;19.40 kPa) treated vessels with only a slight increase in gas pressure when compared with control (19.40 kPa)
Short-chain fatty acids
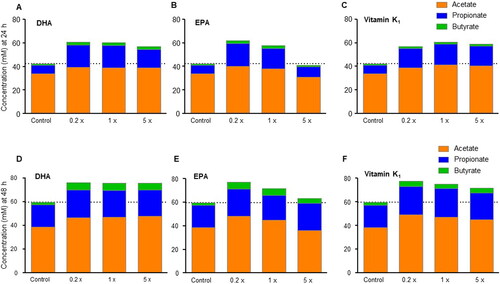
At 24 h, total SCFA concentrations were higher after treatments with DHA (0.2x;60.6 mM; 1x;60.4 mM, 5x; 57 mM), EPA (0.2x; 61.7 mM, 1x;57.6 mM) and vitamin K1 (0.2x;56.8 mM; 1x; 60.8 mM, 5x; 58.8 mM) at all tested concentrations except for EPA at 5x (41 mM) when compared with control (42.3 mM) (). Similarly, at 48 h, higher total SCFA concentration was observed with DHA (0.2x;76.0 mM; 1x; 75.7 mM, 5x; 75.8 mM), EPA (0.2x; 77 mM; 1x; 71.8 mM, 5x; 63.2 mM) and vitamin K1 (0.2x;77.5 mM; 1x; 75.2 mM, 5x; 72 mM)at all tested concentrations when compared with control (). Overall, the increase in SCFA concentrations was highest with the 0.2x doses and least with the 5x doses, particularly with EPA and vitamin K1; this result was largely attributed to a proportional increase in propionate, followed by butyrate and acetate concentrations.
Omega-3 fatty acids (EPA and DHA) and vitamin K1 fermentation supernatants enhance gut barrier integrity
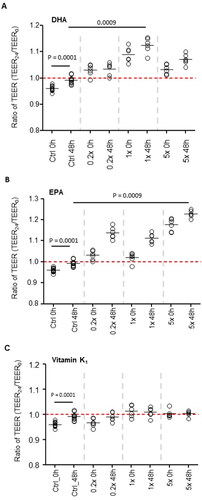
To measure effects on barrier integrity, TEER ratios (TEER24/TEER0) were compared from cells exposed to supernatants collected at baseline and 48 h. In the control vessels, TEER values were significantly higher (p = 0.0001) in cells treated with the 48-h fermentation supernatant when compared to baseline (), suggesting that metabolites produced during the fermentation process independent of DHA, EPA, and vitamin K1 had a beneficial effect on barrier integrity. When we compared the effect of DHA, EPA, and vitamin K1 fermentation supernatant against the effect seen in the control vessels at 48 h, we found an additional significant increase in TEER values in cells treated with DHA at 1x (, p < 0.001) and in cells treated with EPA at 5x (, p < 0.001). However, we failed to observe any such differences with supernatant from vitamin K1treated vessels ().
Discussion
Here we investigated the lone effect of EPA, DHA, and lipid-soluble vitamin K1 on the gut microbiota using the SHIME in vitro batch fermentation model. We found that EPA, DHA, and vitamin K1 all can modulate microbial communities and metabolic activity and that DHA and EPA fermentation supernatants support intestinal barrier function.
There is growing evidence supporting the notion that PUFAs (Menni et al. Citation2017; Vijay et al. Citation2021) and vitamins (Fangmann et al. Citation2018; von Martels et al. Citation2020; Pham et al. Citation2021) can modulate the gut microbiome. In fact, the most recent definition of prebiotics by ISAPP, listed PUFAs as ‘candidate prebiotics’ (Rinninella and Costantini Citation2022) and vitamins as substances with the potential to affect the gut microbiome (Gibson et al. Citation2017; Cunningham et al. Citation2021). In both cases, however, little is known regarding the precise impact of these nutrients on gut microbial composition and metabolic activity, which warrants further investigation in in vitro fermentation models
Using the in vitro SHIME model, we observed a decrease in alpha diversity in control vessels over time, a phenomenon often seen with batch fermentation experiments (Fehlbaum et al. Citation2018; Parmanand et al. Citation2019). It likely reflects the lack of pH control as well as other controls for negative inhibitory feedback. Interestingly, we found that after 24 h of fermentation, alpha diversity increased in all EPA and DHA as well as vitamin K1-treated vessels suggesting that these nutrients may compensate or protect the decline in alpha diversity. This observation supports earlier findings in another in vitro fermentation model in response to human milk oligosaccharides (HMOs) showing a similar increase in microbial diversity [31]. An effect of lipid-rich almond snacking for eight weeks in a clinical study also led to an increase in alpha diversity (Dhillon et al. Citation2019), which further aligns with our findings and suggests that lipids may directly affect microbial diversity. However, the latter study should be interpreted with precaution because in addition to unsaturated fat, almonds are also a rich source of fiber and polyphenols, and all these nutrients are known to modulate the gut microbiota. In contrast, in a recent study in healthy subjects receiving omega-3 fatty acids (Vijay et al. Citation2021) there was no effect on microbial diversity. Given that reduced microbial diversity is a feature of many diseases associated with a westernized lifestyle such as obesity, type 2 diabetes, and inflammatory bowel diseases (IBD) [33] colon delivery of PUFAs and/or vitamin K1 may represent a promising measure to maintain or restore microbial diversity in diseased and at risk human subjects.
The effects observed on alpha diversity were further supported by our findings on beta-diversity. We found that EPA and DHA as well as and vitamin K1 at various concentrations clustered distinct from control samples, indicating a clear effect on microbial composition and structure. Prebiotics such as xylooligosaccharides (XOS) or non-digestible components from kiwifruits have similar effects in terms of altering the beta-diversity of microbial communities in animals and in vitro gut models (Blatchford et al. Citation2015; Pan et al. Citation2019).
We also observed several microbial changes at the genus level that require consideration. For instance, there was a characteristic enrichment in Veillonella levels after treatment with all doses of EPA, DHA and vitamin K1. An increase in Veillonella sp. has been associated with improved athlete performance by converting lactate into propionate (Scheiman et al. Citation2019). Moreover, Veillonella sp. are known to convert vitamin K1 to vitamin K2 (menaquinone) (Biesalski Citation2016); hence, increased abundance of Veillonella in vitamin K1 vessels may be a result of increased substrate availability. Given the many beneficial effects of microbial produced vitamin K2 such as the protective role for the vascular system, reduction in the risk of CVD, mitigation of cognitive diseases, and the suppression of inflammation (Alisi et al. Citation2019; Simes et al. Citation2020), it would be interesting to further study this effect, e.g. by measuring vitamin K2 concentrations in fermentation samples.
Notably, there was also a characteristic enrichment of Dialister after treatment with all doses of EPA, DHA, and vitamin K1, which is interesting because depletion of Dialister abundance was recently found to be associated with brain function, especially depression (Valles-Colomer et al. Citation2019). Taken together, these results suggest that administration of vitamin K1, DHA, and EPA could be a potential approach to enrich not only key microbial species such as Veillonella and Dialister but also luminal vitamin K2 levels.
We found a slight but consistent increase in Faecalibacterium abundance upon vitamin K1 and DHA treatments, while this was not observed after EPA treatment. An increase in Faecalibacterium was recently noted also by Holscher et al. in 18 healthy male and female subjects consuming walnuts for 3 weeks (Holscher et al. Citation2018), which is one of the prime sources of dietary PUFA. In contrast, a case study by Noriega et al. reported a decrease in Faecalibacterium after administration of a PUFA enriched diet for 14 days (Noriega et al. Citation2016) and there was no changes in Faecalibacterium in a study by Vijay et al. (Vijay et al. Citation2021) with supplementation of 165 mg EPA and 110 mg DHA. Although in none of these studies (Noriega et al. Citation2016; Holscher et al. Citation2018), the individual effects of DHA and EPA on gut microbiota was not investigated. Our study is in agreement with the Hoschner et al. observation. The current pilot study investigated effect of DHA and EPA independently but has its limitations, thus clinical studies are warranted to investigate better the individual effects of DHA and EPA on gut microbiota.
PUFAs have been shown to modulate the gut microbiota also by shifting the Firmicutes/Bacteroidetes (F/B) ratio and enhancing the abundance of butyrate-promoting/producing bacterial groups such as Bifidobacterium, Lachnospira, Roseburia, and Lactobacillus (Andersen et al. Citation2011; Watson et al. Citation2018). For example, Watson et al. reported an increase in Bifidobacterium, Roseburia, and Lactobacillus abundances in response to EPA and DHA supplementation for 8 weeks in 22 middle aged healthy volunteers (Watson et al. Citation2018). While an increase in these species is considered a beneficial effect in conditions such as obesity, aging and IBD (Kabeerdoss et al. Citation2015), changes in F/B ratio in health and disease is a matter of ongoing debate. In fact, there is presently no consensus to link the F/B ratio with a health status and more specifically to consider it as a marker of obesity (Magne et al. Citation2020). In line with Watson et al.(Watson et al. Citation2018), we also observed a consistent increase in Bifidobacterium abundance upon EPA treatments in all tested concentrations; moreover, we found a substantial rise in the F/B ratio upon DHA- and EPA- as well as vitamin K1 treatments. Interpretation of the F/B ratio, however, should be taken with caution because the donor microbiota was heavily dominated by the denominator taxa resulting in a low F/B ratio.
The fermentation process also reduced the pH, which was consistent in all fermentation vessels. Part of this effect is likely the result of an increased production of SCFA, however, other factors may also account for this phenomenon such as the lack of pH control and absorption processes (Pham and Mohajeri Citation2018). In humans, colonic luminal pH is tightly controlled and buffered in a physiological range of 5.5 to 7.5 by several factors such as mucosal bicarbonate, microbial lactic acid production, absorption of SCFAs, and continuous intestinal transit of digesta (Nugent et al. Citation2001; Younes et al. Citation2001). Therefore, significant changes in colonic pH are often associated with gastrointestinal diseases (Perez et al. Citation1987).
With regard to gas production, which also corresponds to microbial activity, we observed an increased gas production particularly in the first 24 h in all treated vessels except in one vessel treated with a high dose of EPA. Increased gas production has been correlated with increased SCFA production during the in vitro fermentation of fibers (Hernot et al. Citation2009). However, as a side effect, consumption of dietary fiber causes uncomfortable gaseous symptoms such as bloating and abdominal distension and decreases gaseous flow (Gonlachanvit et al. Citation2004). Therefore, further investigation is warranted to directly compare gas production in response to EPA, DHA and vitamin K1, to that of dietary fiber to evaluate whether these ingredients could be a strategy to enhance colonic SCFA without the common side effects as seen with dietary fibers.
We also observed an increase in SCFAs which was related predominantly to an increased production of propionate, followed by butyrate. Previous studies in humans have not yet reported effects of PUFAs on SCFA production which is not surprising given that only a minor proportion of SCFA (<10%) is excreted in feces due to intestinal absorption. Thus, our data provide valuable additions to the existing in vivo literature of the effects of PUFA on gut microbiota and metabolic activity. Propionate has been linked to lower lipogenesis, serum cholesterol levels and carcinogenesis in extraintestinal tissue (Hosseini et al. Citation2011). Moreover, propionate has been shown to stimulate the production of gastrointestinal peptides such as peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) which are linked to appetite and blood glucose control (Chambers et al. Citation2015; Psichas et al. Citation2015). Butyrate is known for its anti-inflammatory properties and is known to increase the intestinal barrier integrity (Vinolo et al. Citation2011). In line with the latter effect, we found that DHA and EPA fermentation supernatants strengthened the intestinal integrity in our in vitro cell culture model and these effects were comparable to our earlier observations with fermentation supernatants of prebiotic fibers, including oat b-glucan, dried chicory root containing inulin, xylo-oligosaccharide, and inulin (Pham et al. Citation2018). However, it requires consideration that the observed TEER increase with EPA and DHA treated supernatants may include also a direct effect of PUFAs on barrier function which we did not control for (Xiao et al. Citation2020).
There are several limitations that require consideration when interpreting our experiments. 1) The experiments were performed in only one donor; thus, we lack biological replication which can limit the interpretation of our results in the light of interindividual variability of fecal inoculums. However, other studies have performed fermentation experiments using fresh inoculum from only one donor that has provided valuable pilot information on microbial fermentation dynamics (Van den Abbeele et al. Citation2013). Moreover, it is also important to consider the report in which the effect of arabinoxylan and inulin was tested using an inoculum prepared from a pooled donation using the validated TIM-2 model and from a single volunteer where a similar metabolic activity of the microbiota in both setups was observed (Van den Abbeele et al. Citation2013). Finally, we used three different doses for each tested ingredient which were performed as (in total 10) independent incubations, thus, these repetitions provide additional information of the consistency of the effects observed. 2) Whether the effects seen in our in vitro experiments translate into the same effects in humans is unknown. We used dosages of 10 mg, 300 mg and 1800 mg of vitamin K1, DHA and EPA, respectively when translated to the human colon. However, it requires consideration that this assumes full delivery of these nutrients to the gut microbiome which may require targeted colon delivery. We recently found that in both humans (using a colon targeting technology) and in vitro (using the same experimental approach with one donor as in the current study), vitamins C, B2, and D modulated microbial diversity and metabolic activity when compared to placebo in a similar pattern (Pham et al. Citation2021). 3) The extent to which the effects seen on the gut microbiome translate into host health benefits has been hardly investigated. We found that there was a beneficial effect on gut barrier function; however, additional studies on gut immune responses and gut brain axis signaling are warranted as well as studies looking into the combination of both EPA and DHA.
In conclusion, the present in vitro pilot study suggests that EPA, DHA and vitamins K1 may modulate the gut microbiome and metabolic activity. Most distinct were the increases in SCFAs, an effect on microbial diversity, and characteristic changes in gut microbial composition such as an increase in the F/B ratio and increases in the abundance of Dialister and Veillonella. Given the recent link between Dialister and depression, these data warrant further in vivo animal and human intervention studies to understand the physiological role of the observed changes on host health.
Author contributions
WS, VTP and RES conceptualized and designed the study. NS and NR performed the in vitro cellular experiment, performed analysis, data interpretation and visualization. AR performed the microbiome analysis. VTP, RES, and AR contributed to the data analysis and interpretation. AR and RES wrote the manuscript. All authors have read and agreed to the published version of the manuscript
Acknowledgements
We thank ProDigest (Gent, Belgium) for performing the in vitro experiment, collecting data and samples for analysis.
Conflicts of interest
All authors are employees of DSM Nutritional Products Ltd, Kaiseraugst, Switzerland.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
Notes on contributors
Ateequr Rehman
Ateequr Rehman has a PhD in biology (microbiology) and works as EMEA principal scientist in DSM Nutritional Products at Kaiseraugst, Switzerland. Rehman’s research investigates the interaction of diet and microbiota and their impact on host health in inflammatory diseases.
Van Pham
Van Pham is a Ph.D. holder in Biotechnology from ETH Zürich. Over the past decade, she has been actively working and publishing in the field of gut microbiome and gut health. Her expertise lies in in vitro intestinal fermentation models that provide proof of concept and elaborate on modes of action on microbiome modulators.
Nicole Seifert
Nicole Seifert holds a Bachelor of Science in biology. She has much experience in performing cell-based assays and developing new methodologies to explore the effect of the microbiome on gut barrier function.
Nathalie Richard
Nathalie Richard holds a Master’s degree in Plant Cell and Molecular Biology from Université Louis Pasteur, Strasbourg. She has more than 20 years of experience in developing in vitro models.
Wilbert Sybesma
Wilbert Sybesma has a PhD in metabolic engineering and (microbiology). Wilbert has been active for over 20 years in Nutrition, Health and Innovation. Wilbert’s main expertise relates to the development and commercialization of probiotics, prebiotics, fermented foods and other microbiome-modulating ingredients. At present, he works as an independent advisor for Microbiome Solutions GmbH.
Robert E. Steinert
Robert Steinert holds PhD in gastrointestinal physiology and Nutrition. He is also a part-time lecturer and research fellow at the University Hospital Zurich. His research interest is to study the effect of dietary ingredients, especially prebiotics and vitamins, on microbiota and their impact on intestinal health.
References
- Alisi L, Cao R, De Angelis C, Cafolla A, Caramia F, Cartocci G, Librando A, Fiorelli M. 2019. The relationships between vitamin K and cognition: a review of current evidence. Front Neurol. 10:239. doi:10.3389/fneur.2019.00239.
- Andersen AD, Mølbak L, Michaelsen KF, Lauritzen L. 2011. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J Pediatr Gastroenterol Nutr. 53(3):303–309. doi:10.1097/MPG.0b013e31821d298f.
- Bajinka O, Tan Y, Abdelhalim KA, Özdemir G, Qiu X. 2020. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express. 10(1):130. doi:10.1186/s13568-020-01066-8.
- Balfegó M, Canivell S, Hanzu FA, Sala-Vila A, Martínez-Medina M, Murillo S, Mur T, Ruano EG, Linares F, Porras N, et al. 2016. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. 15:78. doi:10.1186/s12944-016-0245-0.
- Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. 2019. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio. 10(1):e02566-18. doi:10.1128/mBio.02566-18.
- Biesalski HK. 2016. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 1372(1):53–64. doi:10.1111/nyas.13145.
- Blatchford P, Stoklosinski H, Walton G, Swann J, Gibson G, Gearry R, Ansell J. 2015. Kiwifruit fermentation drives positive gut microbial and metabolic changes irrespective of initial microbiota composition. Bioact Carbohydr Dietary Fibre. 6(1):37–45. doi:10.1016/j.bcdf.2015.07.001.
- Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. 2015. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 64(11):1744–1754. doi:10.1136/gutjnl-2014-307913.
- Costantini L, Molinari R, Farinon B, Merendino N. 2017. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 18(12):2645. doi:10.3390/ijms18122645.
- Craciun AM, Wolf J, Knapen MH, Brouns F, Vermeer C. 1998. Improved bone metabolism in female elite athletes after vitamin K supplementation. Int J Sports Med. 19(7):479–484. doi:10.1055/s-2007-971948.
- Cunningham M, Azcarate-Peril MA, Barnard A, Benoit V, Grimaldi R, Guyonnet D, Holscher HD, Hunter K, Manurung S, Obis D, et al. 2021. Shaping the future of probiotics and prebiotics. Trends Microbiol. 29(8):667–685. doi:10.1016/j.tim.2021.01.003.
- De Weirdt R, Possemiers S, Vermeulen G, Moerdijk-Poortvliet TCW, Boschker HTS, Verstraete W, Van de Wiele T. 2010. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol Ecol. 74(3):601–611. doi:10.1111/j.1574-6941.2010.00974.x.
- Dhillon J, Li Z, Ortiz RM. 2019. Almond snacking for 8 wk increases alpha-diversity of the gastrointestinal microbiome and decreases Bacteroides fragilis abundance compared with an isocaloric snack in college freshmen. Curr Dev Nutr. 3(8):nzz079. doi:10.1093/cdn/nzz079.
- Fangmann D, Theismann E-M, Türk K, Schulte DM, Relling I, Hartmann K, Keppler JK, Knipp J-R, Rehman A, Heinsen F-A, et al. 2018. Targeted microbiome intervention by microencapsulated delayed-release niacin beneficially affects insulin sensitivity in humans. Diabetes Care. 41(3):398–405. doi:10.2337/dc17-1967.
- Fehlbaum S, Prudence K, Kieboom J, Heerikhuisen M, van den Broek T, Schuren FHJ, Steinert RE, Raederstorff D. 2018. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int J Mol Sci. 19(10):3097. doi:10.3390/ijms19103097.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. 2017. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 14(8):491–502. doi:10.1038/nrgastro.2017.75.
- Gonlachanvit S, Coleski R, Owyang C, Hasler W. 2004. Inhibitory actions of a high fibre diet on intestinal gas transit in healthy volunteers. Gut. 53(11):1577–1582. doi:10.1136/gut.2004.041632.
- Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. 2019. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. 20(4):896. doi:10.3390/ijms20040896.
- Harris MA, Reece MS, McGregor JA, Wilson JW, Burke SM, Wheeler M, Anderson JE, Auld GW, French JI, Allen KGD. 2015. The effect of omega-3 docosahexaenoic acid supplementation on gestational length: randomized trial of supplementation compared to nutrition education for increasing n-3 intake from foods. Biomed Res Int. 2015:e123078. doi:10.1155/2015/123078.
- Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS, Fahey GC. 2009. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J Agric Food Chem. 57(4):1354–1361. doi:10.1021/jf802484j.
- Holscher HD, Guetterman HM, Swanson KS, An R, Matthan NR, Lichtenstein AH, Novotny JA, Baer DJ. 2018. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J Nutr. 148(6):861–867. doi:10.1093/jn/nxy004.
- Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. 2011. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev. 69(5):245–258. doi:10.1111/j.1753-4887.2011.00388.x.
- Ichihashi T, Takagishi Y, Uchida K, Yamada H. 1992. Colonic absorption of menaquinone-4 and menaquinone-9 in rats. J Nutr. 122(3):506–512. doi:10.1093/jn/122.3.506.
- Isenring J, Bircher L, Geirnaert A, Lacroix C. 2023. In vitro human gut microbiota fermentation models: opportunities, challenges, and pitfalls. Microbiome Res Rep. 2(1):2. doi:10.20517/mrr.2022.15.
- Kabeerdoss J, Jayakanthan P, Pugazhendhi S, Ramakrishna BS. 2015. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res. 142(1):23–32. doi:10.4103/0971-5916.162091.
- Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, Garami A, Soós A, Márta K, Solymár M. 2020. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep. 10(1):11787. doi:10.1038/s41598-020-68440-1.
- Kolodziejczyk AA, Zheng D, Elinav E. 2019. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 17(12):742–753. doi:10.1038/s41579-019-0256-8.
- Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. 2020. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 12(5):1474. doi:10.3390/nu12051474.
- Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. 2015. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 6:148. doi:10.3389/fgene.2015.00148.
- McCormack UM, Curião T, Wilkinson T, Metzler-Zebeli BU, Reyer H, Ryan T, Calderon-Diaz JA, Crispie F, Cotter PD, Creevey CJ, et al. 2018. Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring. mSystems. 3(3):e00134-17–e00117. doi:10.1128/mSystems.00134-17.
- McNabney SM, Henagan TM. 2017. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 9(12):1348. doi:10.3390/nu9121348.
- Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM. 2017. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 7(1):11079. doi:10.1038/s41598-017-10382-2.
- Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. 2016. Understanding the impact of omega-3 rich diet on the gut microbiota. Case Rep Med. 2016:3089303. doi:10.1155/2016/3089303.
- Nugent SG, Kumar D, Rampton DS, Evans DF. 2001. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 48(4):571–577. doi:10.1136/gut.48.4.571.
- Pan J, Yin J, Zhang K, Xie P, Ding H, Huang X, Blachier F, Kong X. 2019. Dietary xylo-oligosaccharide supplementation alters gut microbial composition and activity in pigs according to age and dose. AMB Express. 9(1):134. doi:10.1186/s13568-019-0858-6.
- Parmanand BA, Kellingray L, Le Gall G, Basit AW, Fairweather-Tait S, Narbad A. 2019. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J Nutr Biochem. 67:20–27. doi:10.1016/j.jnutbio.2019.01.010.
- Perez GO, Oster JR, Rogers A. 1987. Acid-base disturbances in gastrointestinal disease. Dig Dis Sci. 32(9):1033–1043. doi:10.1007/BF01297195.
- Pham VT, Dold S, Rehman A, Bird JK, Steinert RE. 2021. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr Res. 95:35–53. doi:10.1016/j.nutres.2021.09.001.
- Pham VT, Fehlbaum S, Seifert N, Richard N, Bruins MJ, Sybesma W, Rehman A, Steinert RE. 2021. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome – a pilot study. Gut Microbes. 13(1):1–20. doi:10.1080/19490976.2021.1875774.
- Pham VT, Mohajeri MH. 2018. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Benef Microbes. 9(5):725–742. doi:10.3920/BM2017.0164.
- Pham VT, Seifert N, Richard N, Raederstorff D, Steinert RE, Prudence K, Mohajeri MH. 2018. The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ. 6:e5288. doi:10.7717/peerj.5288.
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. 2015. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 39(3):424–429. doi:10.1038/ijo.2014.153.
- Rinninella E, Costantini L. 2022. Polyunsaturated fatty acids as prebiotics: innovation or confirmation? Foods. 11(2):146. doi:10.3390/foods11020146.
- Salgaço MK, Perina NP, Tomé TM, Mosquera EMB, Lazarini T, Sartoratto A, Sivieri K. 2021. Probiotic infant cereal improves children’s gut microbiota: insights using the Simulator of Human Intestinal Microbial Ecosystem (SHIME®). Food Res Int. 143:110292. doi:10.1016/j.foodres.2021.110292.
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham L-D, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, et al. 2019. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 25(7):1104–1109. doi:10.1038/s41591-019-0485-4.
- Simes DC, Viegas CSB, Araújo N, Marreiros C. 2020. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients. 12(1):138. doi:10.3390/nu12010138.
- Steinert RE, Lee Y-K, Sybesma W. 2020. Vitamins for the gut microbiome. Trends Mol Med. 26(2):137–140. doi:10.1016/j.molmed.2019.11.005.
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, et al. 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 4(4):623–632. doi:10.1038/s41564-018-0337-x.
- Van den Abbeele P, Taminiau B, Pinheiro I, Duysburgh C, Jacobs H, Pijls L, Marzorati M. 2018. Arabinoxylo-oligosaccharides and inulin impact inter-individual variation on microbial metabolism and composition, which immunomodulates human cells. J Agric Food Chem. 66(5):1121–1130. doi:10.1021/acs.jafc.7b04611.
- Van den Abbeele P, Venema K, Van de Wiele T, Verstraete W, Possemiers S. 2013. Different human gut models reveal the distinct fermentation patterns of Arabinoxylan versus inulin. J Agric Food Chem. 61(41):9819–9827. doi:10.1021/jf4021784.
- Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. 2021. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes. 13(1):1–11. doi:10.1080/19490976.2020.1863133.
- Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. 2011. Regulation of inflammation by short chain fatty acids. Nutrients. 3(10):858–876. doi:10.3390/nu3100858.
- von Martels JZH, Bourgonje AR, Klaassen MAY, Alkhalifah HAA, Sadaghian Sadabad M, Vich Vila A, Gacesa R, Gabriëls RY, Steinert RE, Jansen BH, et al. 2020. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP study]. J Crohns Colitis. 14(5):595–607. doi:10.1093/ecco-jcc/jjz208.
- Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL, et al. 2018. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 67(11):1974–1983. doi:10.1136/gutjnl-2017-314968.
- Xiao K, Liu C, Qin Q, Zhang Y, Wang X, Zhang J, Odle J, Lin X, Hu C-AA, Liu Y. 2020. EPA and DHA attenuate deoxynivalenol-induced intestinal porcine epithelial cell injury and protect barrier function integrity by inhibiting necroptosis signaling pathway. FASEB J. 34(2):2483–2496. doi:10.1096/fj.201902298R.
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369(9567):1090–1098. doi:10.1016/S0140-6736(07)60527-3.
- Younes H, Coudray C, Bellanger J, Demigné C, Rayssiguier Y, Rémésy C. 2001. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 86(4):479–485. doi:10.1079/bjn2001430.